Enrofloxacin Pharmaceutical Formulations through the Polymer-Free Electrospinning of β-Cyclodextrin–oligolactide Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
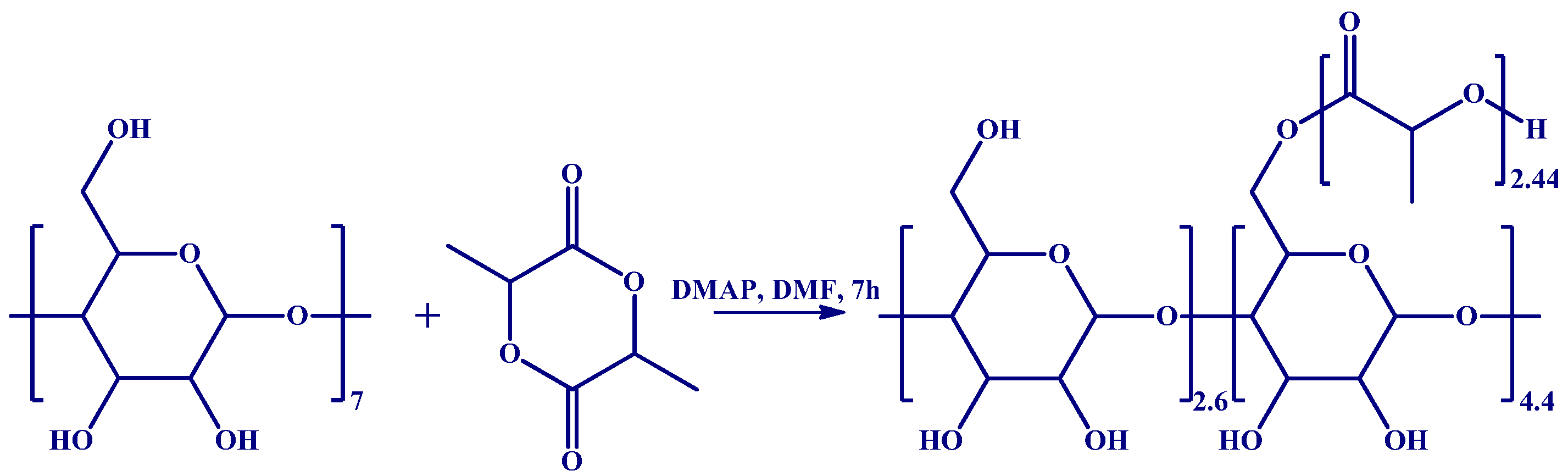
2.2. Methods
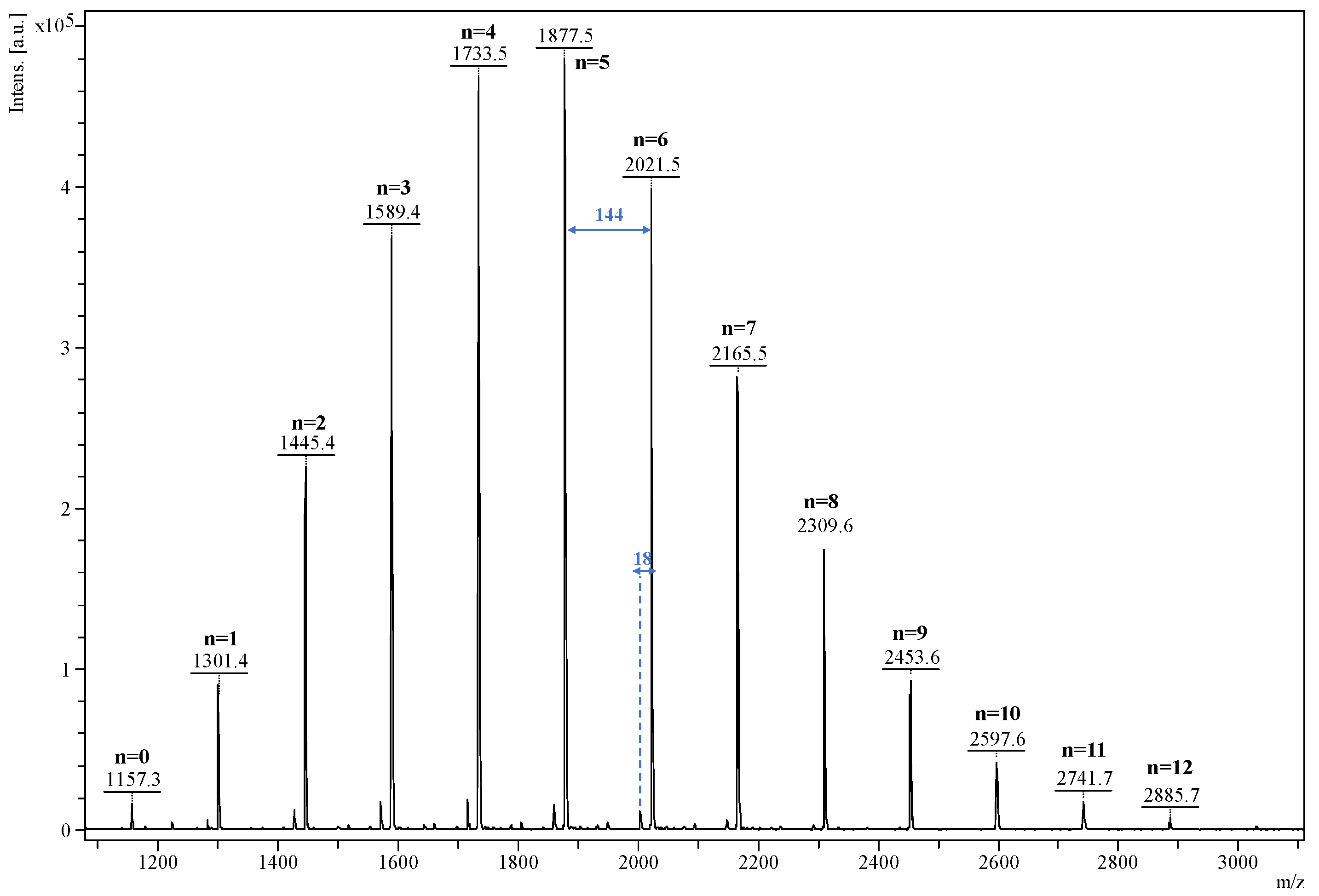
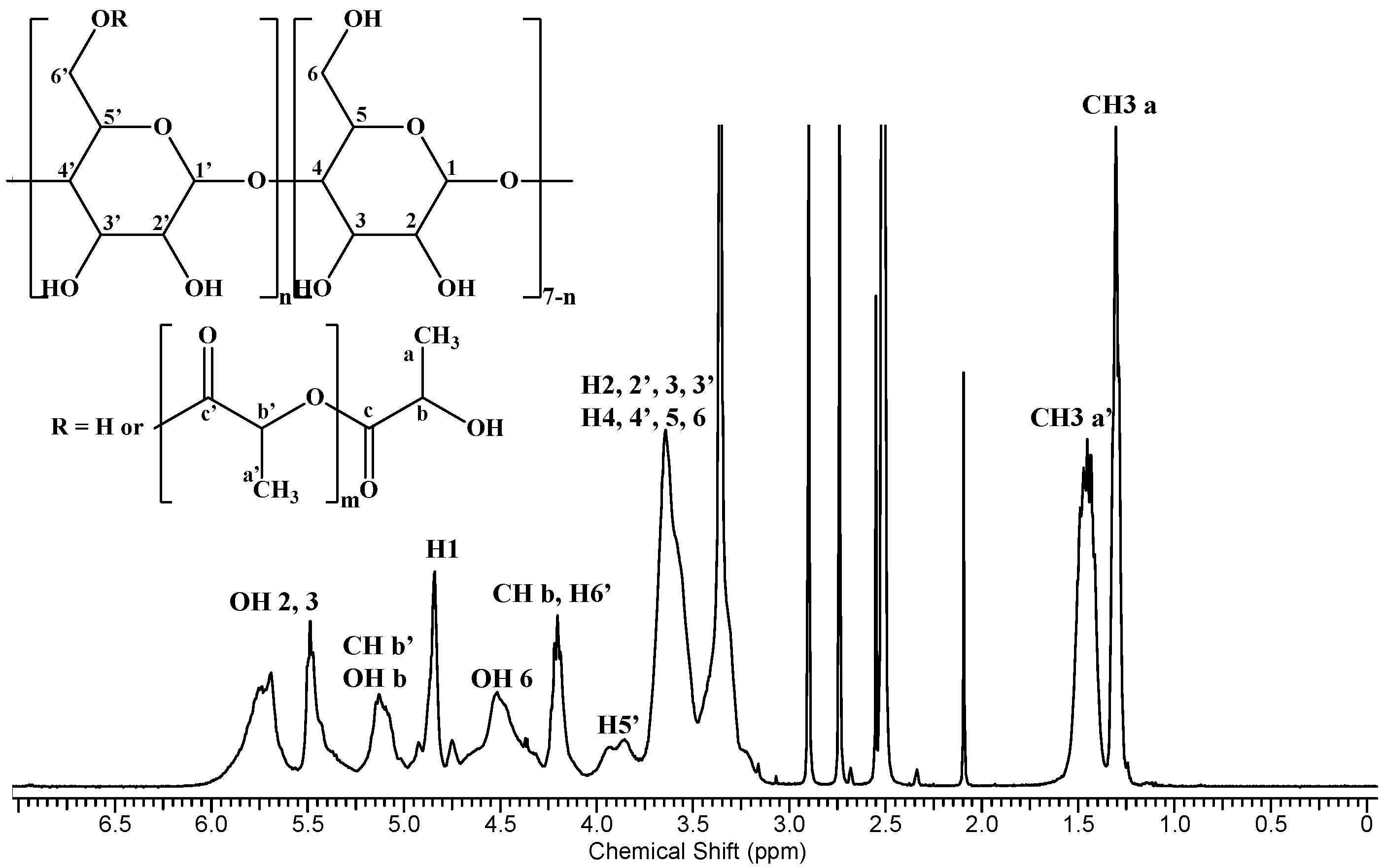
2.3. Characterization
- Ii—monoisotopic peak intensity corresponding to the m/z ratio;
- mi—m/z value of the corresponding i peak, with z = 1.
- ICH3-a′ and ICH3-a—integral values for chain (1.49–1.44 ppm) and chain-end (1.31–1.29 ppm) methyl groups of attached oligolactide.
3. Results and Discussion
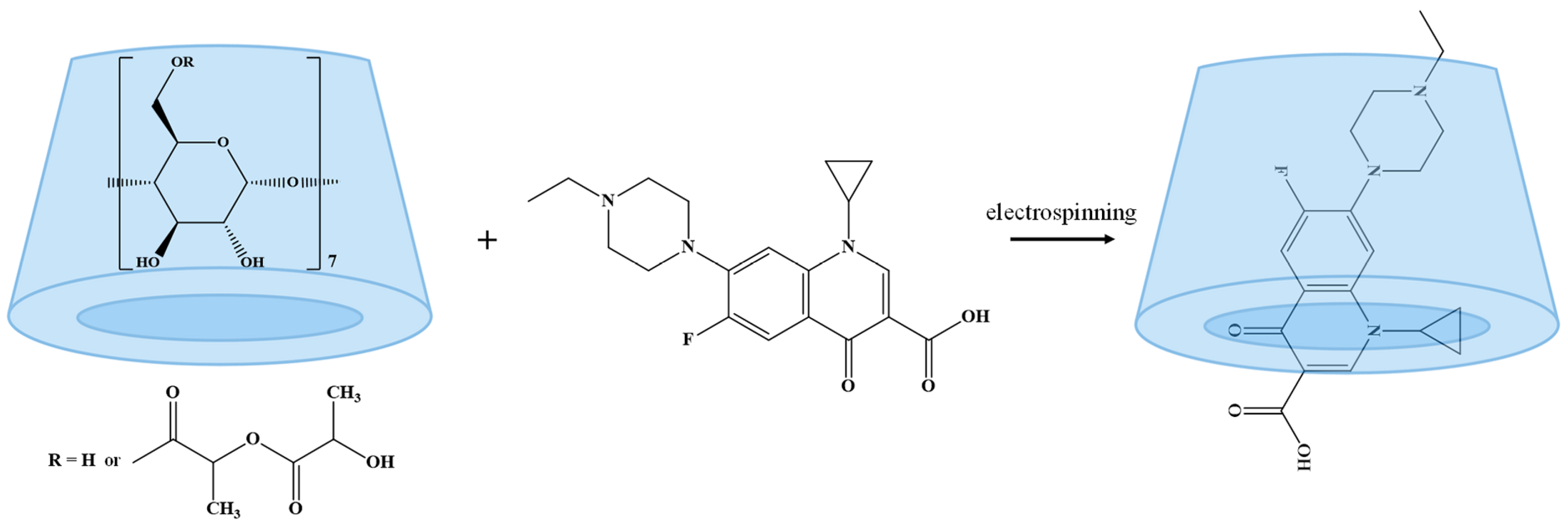
3.1. Electrospinning
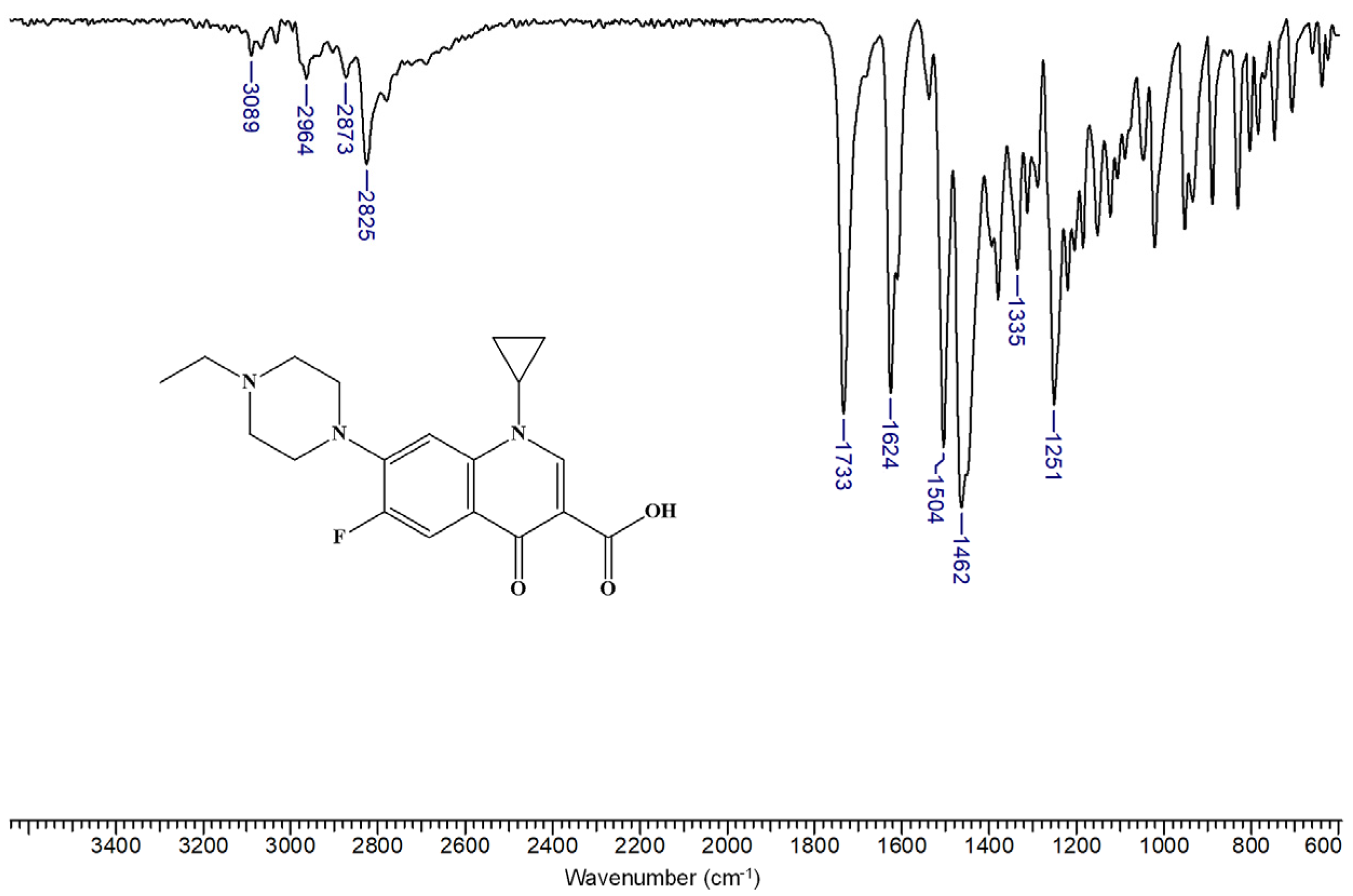
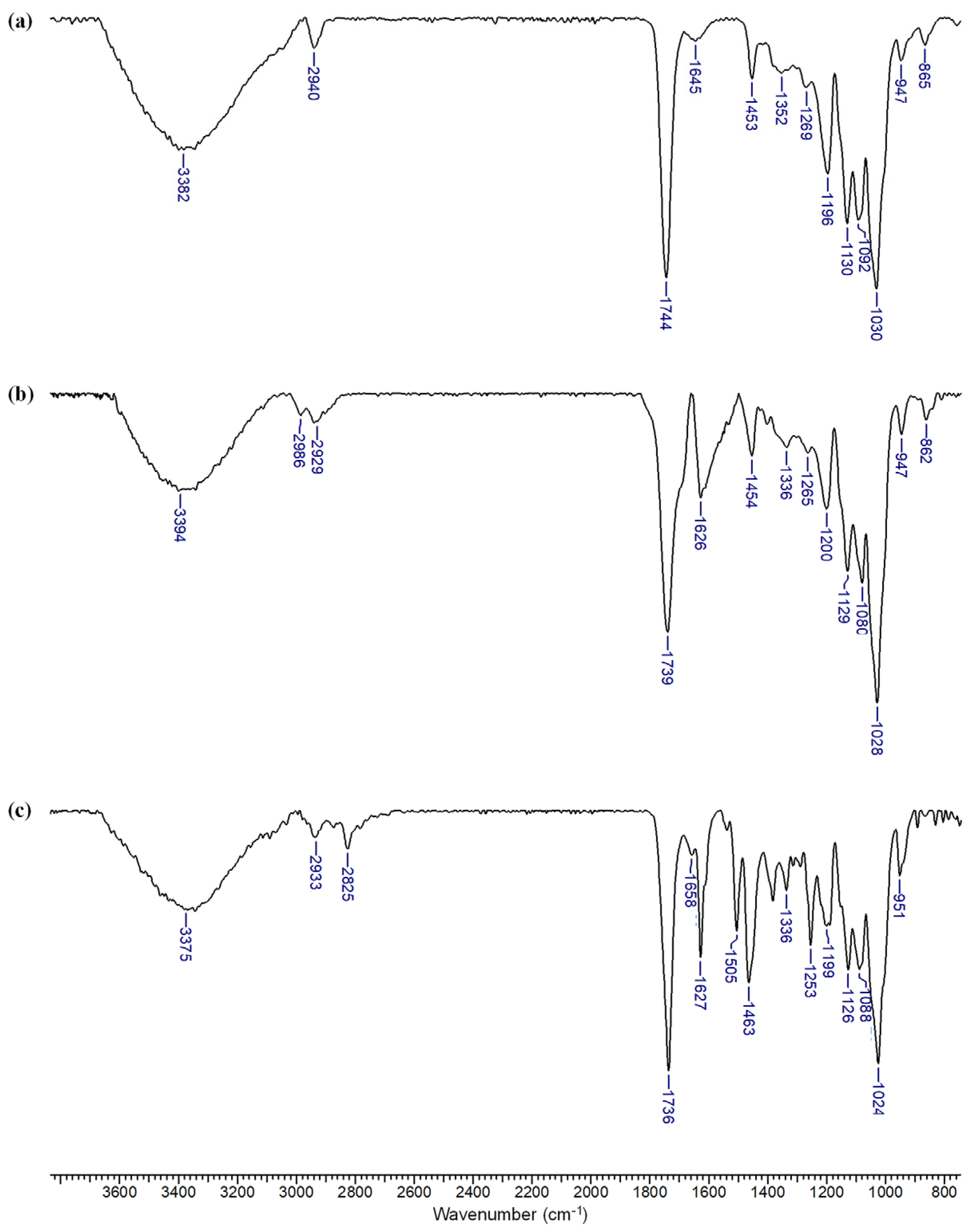
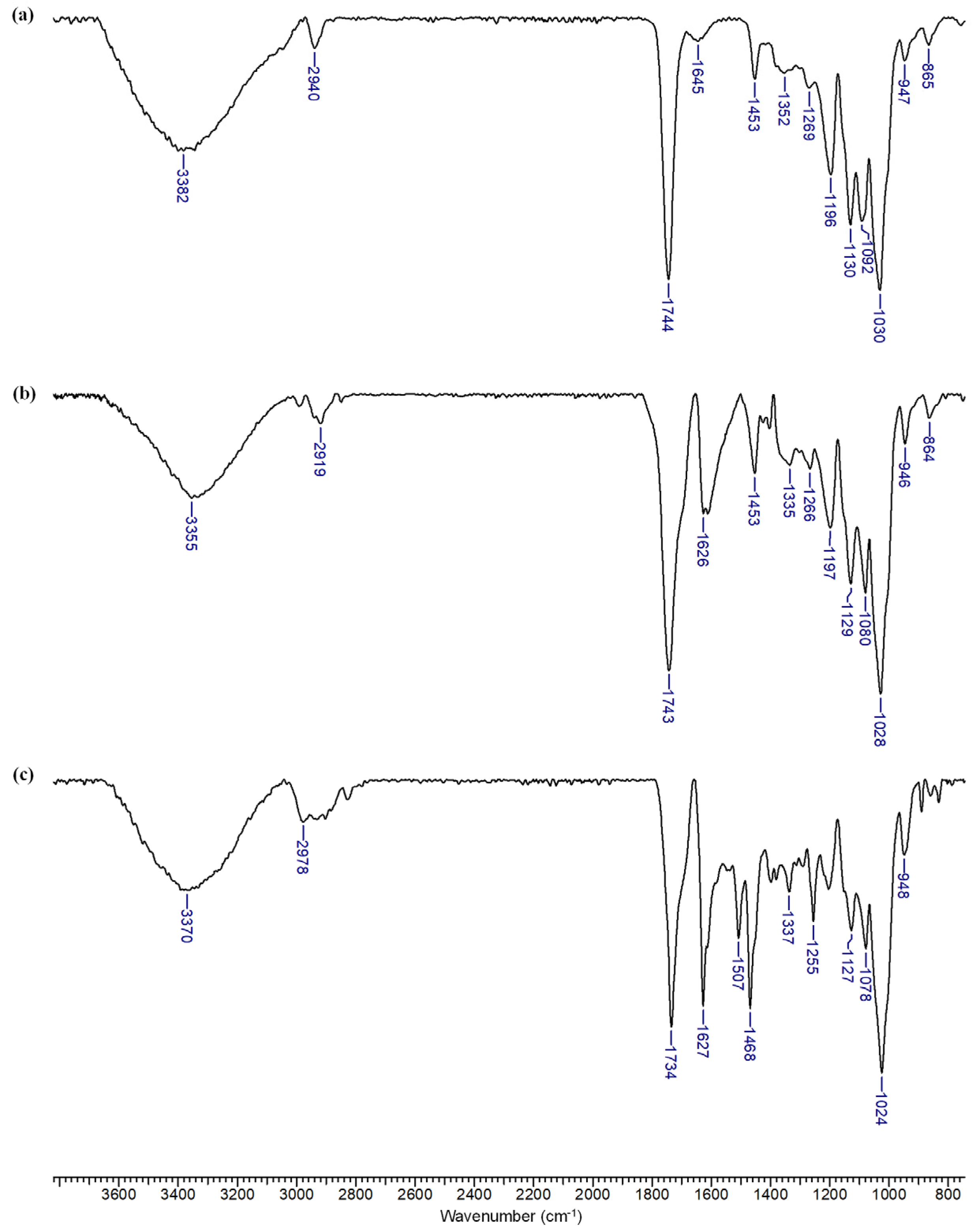
3.2. Characterization
3.3. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishna, S.; Fujihara, K.; Teo, W.; Lim, T.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Company: Singapore, 2005. [Google Scholar]
- Greiner, A.; Wendorff, J. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-Free Cyclodextrin and Natural Polymer-Cyclodextrin Electrospun Nanofibers: A Comprehensive Review on Current Applications and Future Perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef]
- Costoya, A.; Concheiro, A.; Alvarez-Lorenzo, C. Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins). Molecules 2017, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Shen, J.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Aliphatic Polyester Nanofibers Functionalized with Cyclodextrins and Cyclodextrin-Guest Inclusion Complexes. Polymers 2018, 10, 428. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant Activity and Photostability of α-Tocopherol/β-Cyclodextrin Inclusion Complex Encapsulated Electrospun Polycaprolactone Nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug Delivery System Based on Cyclodextrin-Naproxen Inclusion Complex Incorporated in Electrospun Polycaprolactone Nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Masoumi, S.; Amiri, S.; Bahrami, S.H. PCL-Based Nanofibers Loaded with Ciprofloxacin/Cyclodextrin Containers. J. Text. Inst. 2018, 109, 1044–1053. [Google Scholar] [CrossRef]
- Aytac, Z.; Sen, H.S.; Durgun, E.; Uyar, T. Sulfisoxazole/Cyclodextrin Inclusion Complex Incorporated in Electrospun Hydroxypropyl Cellulose Nanofibers as a Drug Delivery System. Colloids Surf. B Biointerfaces 2015, 128, 331–338. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Savas, H.B.; Gultekin, F. Improved Catalytic Activity by Catalase Immobilization Using γ-Cyclodextrin and Electrospun PCL Nanofibers. J. Appl. Polym. Sci. 2017, 134, 44404. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Savas, H.B.; Gultekin, F. Enzymatic Behavior of Laccase Following Interaction with γ-CD and Immobilization into PCL Nanofibers. Anal. Biochem. 2017, 528, 13–18. [Google Scholar] [CrossRef]
- Narayanan, G.; Aguda, R.; Hartman, M.; Chung, C.-C.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Fabrication and Characterization of Poly(ε-caprolactone)/α-Cyclodextrin Pseudorotaxane Nanofibers. Biomacromolecules 2015, 17, 271–279. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Cyclodextrin Nanofibers by Electrospinning. Chem. Commun. 2010, 46, 6903–6905. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Lo Nostro, P.; Baglioni, P. Self-Assembly of β-Cyclodextrin in Water. Part 1: Cryo-TEM and Dynamic and Static Light Scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Kurkov, S.; Jansook, P.; Loftsson, T. Self-Assembled Cyclodextrin Aggregates and Nanoparticles. Int. J. Pharm. 2010, 387, 199. [Google Scholar] [CrossRef]
- Dubes, A.; Degobert, G.; Fessi, H.; Parrot-Lopez, H. Synthesis and Characterisation of Sulfated Amphiphilic α-, β- and γ-Cyclodextrins: Application to the Complexation of Acyclovir. Carbohydr. Res. 2003, 338, 2185–2193. [Google Scholar] [CrossRef]
- Dubes, A.; Bouchu, D.; Lamartine, R.; Parrot-Lopez, H. An Efficient Regio-Specific Synthetic Route to Multiply Substituted Acyl-Sulphated β-Cyclodextrins. Tetrahedron Lett. 2001, 42, 9147–9151. [Google Scholar] [CrossRef]
- Silva, O.F.; Fernandez, M.A.; Pennie, S.L.; Gil, R.R.; de Rossi, R.H. Synthesis and Characterization of an Amphiphilic Cyclodextrin, a Micelle with Two Recognition Sites. Langmuir 2008, 24, 3718–3726. [Google Scholar] [CrossRef]
- Kieken, F.; West, C.; Keddadouche, K.; Elfakir, C.; Choisnard, L.; Geze, A.; Wouessidjewe, D. Characterization of Complex Amphiphilic Cyclodextrin Mixtures by High-Performance Liquid Chromatography and Mass Spectrometry. J. Chromatogr. A 2008, 1189, 385–391. [Google Scholar] [CrossRef]
- Stancanelli, R.; Løjkner, L.D.; Lambertsen Larsen, K.; Guardo, M.; Cannavà, C.; Tommasini, S.; Villari, V. Structural and Spectroscopic Features of Lutein/Butanoyl-β-Cyclodextrin Nanoassemblies. J. Pharm. Biomed. Anal. 2012, 71, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Yun, D.; Jeong, D.; Im, J.; Kim, H.; Dindulkar, S.D.; Jung, S. Regioselective Self-Acylating Cyclodextrins in Organic Solvent. Sci. Rep. 2016, 6, 23740. [Google Scholar] [CrossRef] [PubMed]
- Choisnard, L.; Geze, A.; Yameogo, B.G.J.; Putaux, J.-L.; Wouessidjewea, D. Miscellaneous Nanoaggregates Made of β-CD Esters Synthesized by an Enzymatic Pathway. Int. J. Pharm. 2007, 344, 26–32. [Google Scholar] [CrossRef]
- Opalkova Siskova, A.; Sacarescu, L.; Opalek, A.; Mosnacek, J.; Peptu, C. Electrospinning of Cyclodextrin–Oligolactide Derivatives. Biomolecules 2023, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Blaj, D.-A.; Balan-Porcarasu, M.; Peptu, C.A.; Harabagiu, V. Custom-Modified Oligolactide-Cyclodextrin Derivatives for Electrospun Drug Formulations. Eur. Polym. J. 2023, 196, 112234. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Diaconu, A.-D.; Danu, M.; Peptu, C.A.; Cristea, M.; Harabagiu, V. The Influence of the Hydroxyl Type on Crosslinking Process in Cyclodextrin-Based Polyurethane Networks. Gels 2022, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, A.-D.; Logigan, C.-L.; Peptu, C.A.; Ibanescu, C.; Harabagiu, V.; Peptu, C. Polyurethane Degradable Hydrogels Based on Cyclodextrin-Oligocaprolactone Derivatives. Gels 2023, 9, 755. [Google Scholar] [CrossRef]
- Choi, S.H.; Chung, J.W.; Priestley, R.D.; Kwak, S.-Y. Functionalization of Polysulfone Hollow Fiber Membranes with Amphiphilic β-Cyclodextrin and Their Applications for the Removal of Endocrine Disrupting Plasticizer. J. Membr. Sci. 2012, 409–410, 75–81. [Google Scholar] [CrossRef]
- Lumholdt, L.; Nielsen, T.T.; Lambertsen Larsen, K. Surface Modification Using Self-Assembled Layers of Amphiphilic Cyclodextrins. J. Appl. Polym. Sci. 2014, 131, 41047. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, H.; Liu, B.; Yuan, J.Y. Synthesis and MALDI-TOF Characterization of β-CD Core ATRP Initiators and RAFT Chain Transfers with Different Degrees of Substitution. Chin. J. Polym. Sci. 2015, 33, 36–48. [Google Scholar] [CrossRef]
- Cosola, A.; Conti, R.; Rana, V.K.; Sangermano, M.; Chiappone, A.; Levalois-Grutzmacher, J.; Grutzmacher, H. Synthesis of c-Cyclodextrin Substituted Bis(acyl)phosphane Oxide Derivative (BAPO-γ-CyD) Serving as Multiple Photoinitiator and Crosslinking Agent. Chem. Commun. 2020, 56, 4828. [Google Scholar] [CrossRef]
- Takashima, Y.; Osaki, M.; Harada, A. Cyclodextrin-Initiated Polymerization of Cyclic Esters in Bulk: Formation of Polyester-Tethered Cyclodextrins. J. Am. Chem. Soc. 2004, 126, 13588–13589. [Google Scholar] [CrossRef]
- Kost, B.; Brzezinski, M.; Socka, M.; Basko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef]
- Garcia, A.; Leonardi, D.; Salazar, M.O.; Lamas, M.C. Modified β-Cyclodextrin Inclusion Complex to Improve the Physicochemical Properties of Albendazole. Complete In Vitro Evaluation and Characterization. PLoS ONE 2014, 9, e88234. [Google Scholar] [CrossRef]
- Kang, S.; Park, E.; Kim, Y.; Lee, S.; Kwon, J.; Cho, H.; Lee, Y. A Medusa-like β-Cyclodextrin with 1-Methyl-2-(2′-Carboxyethyl) Maleic Anhydrides, a Potential Carrier for pH-Sensitive Drug Delivery. J. Drug Target. 2014, 22, 658–668. [Google Scholar] [CrossRef]
- Lis-Cieplak, A.; Charuk, F.; Sobczak, M.; Zgadzaj, A.; Drobniewska, A.; Szeleszczuk, L.; Oledzka, E. Development and Evaluation of Matrices Composed of β-Cyclodextrin and Biodegradable Polyesters in the Controlled Delivery of Pindolol. Pharmaceutics 2020, 12, 500. [Google Scholar] [CrossRef]
- Shen, Z.; Hai, A.; Du, G.; Zhang, H.; Sun, H. A Convenient Preparation of 6-Oligo(Lactic Acid)Cyclomaltoheptaose as Kinetically Degradable Derivative for Controlled Release of Amoxicillin. Carbohydr. Res. 2008, 343, 2517–2522. [Google Scholar] [CrossRef]
- Tamba, B.I.; Ancuceanu, V.R.; Harabagiu, V.; Peptu, C.; Rotaru, R.; Peptu, C.A.; Stan, C.S.; Leon-Constantin, M.M.; Alexa-Stratulat, T. Procedeu de Obtinere a Unei Formulari Farmaceutice Care Cuprinde Sisteme Complexe pe baza de Lidocaina, Derivati de Ciclodextrina si Lipozomi. Romanian Patent RO132702B1. filed 07 September 2017, and issued 30 May 2024.
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the Design and Development of “Fast-Dissolving” Electrospun Nanofibers Based Drug Delivery Systems—A Systematic Review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Kost, B.; Svyntkivska, M.; Brzezinski, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczynska, A.; Biela, T. PLA/β-CD-Based Fibres Loaded with Quercetin as Potential Antibacterial Dressing Materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef]
- Kiss, K.; Vass, P.; Farkas, A.; Hirsch, E.; Szabó, E.; Mező, G.; Nagy, Z.K.; Marosi, G. A Solid Doxycycline HP-β-CD Formulation for Reconstitution (i.v. Bolus) Prepared by Scaled-Up Electrospinning. Int. J. Pharm. 2020, 586, 119539. [Google Scholar] [CrossRef]
- Chen, M.; Nielsen, S.R.; Uyar, T.; Zhang, S.; Zafar, A.; Dong, M.; Besenbacher, F. Electrospun UV-Responsive Supramolecular Nanofibers from a Cyclodextrin Azobenzene Inclusion Complex. J. Mater. Chem. C 2013, 1, 850–855. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of Polymer-Free Nanofibers from Cyclodextrin Inclusion Complexes. Langmuir 2011, 27, 6218–6226. [Google Scholar] [CrossRef]
- Celebioglu, A.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial Electrospun Nanofibers from Triclosan/Cyclodextrin Inclusion Complexes. Colloids Surf. B Biointerfaces 2014, 116, 612–619. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; San Keskin, N.O.; Tekinay, T.; Uyar, T. Electrospinning of Polymer-Free Cyclodextrin/Geraniol-Inclusion Complex Nanofibers: Enhanced Shelf-Life of Geraniol with Antibacterial and Antioxidant Properties. RSC Adv. 2016, 6, 46089–46099. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of Camphor in Cyclodextrin Inclusion Complex Nanofibers via Polymer-Free Electrospinning: Enhanced Water Solubility, High Temperature Stability, and Slow Release of Camphor. J. Mater. Sci. 2018, 53, 5436–5449. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; San Keskin, N.O.; Kusku, S.I.; Durgun, E.; Tekinay, T.; Uyar, T. Fast-Dissolving, Prolonged Release, and Antibacterial Cyclodextrin/Limonene-Inclusion Complex Nanofibrous Webs via Polymer-Free Electrospinning. J. Agric. Food Chem. 2016, 64, 7325–7334. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of Cyclodextrin/Linalool-Inclusion Complex Nanofibers: Fast Dissolving Nanofibrous Web with Prolonged Release and Antibacterial Activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Fabrication of Electrospun Eugenol/Cyclodextrin Inclusion Complex Nanofibrous Webs for Enhanced Antioxidant Property, Water Solubility, and High Temperature Stability. J. Agric. Food Chem. 2018, 66, 457–466. [Google Scholar] [CrossRef]
- Aytac, Z.; Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Efficient Encapsulation of Citral in Fast-Dissolving Polymer-Free Electrospun Nanofibers of Cyclodextrin Inclusion Complexes: High Thermal Stability, Longer Shelf-Life, and Enhanced Water Solubility of Citral. Nanomaterials 2018, 8, 793. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/Cyclodextrin Inclusion Complex Nanofibrous Webs: Enhanced Water Solubility, High Thermal Stability and Antioxidant Property of Thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Menthol/Cyclodextrin Inclusion Complex Nanofibers: Enhanced Water-Solubility and High Temperature Stability of Menthol. J. Food Eng. 2018, 224, 27–36. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-Dissolving Antioxidant Curcumin/Cyclodextrin Inclusion Complex Electrospun Nanofibrous Webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Uyar, T. Fast-Dissolving Electrospun Nanofibrous Films of Paracetamol/Cyclodextrin Inclusion Complexes. Appl. Surf. Sci. 2019, 492, 626–633. [Google Scholar] [CrossRef]
- Celebioglu, A.; Kayaci-Senirmak, F.; Ipek, S.; Durgun, E.; Uyar, T. Polymer-Free Nanofibers from Vanillin/Cyclodextrin Inclusion Complexes: High Thermal Stability, Enhanced Solubility and Antioxidant Property. Food Funct. 2016, 7, 3141–3153. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Uyar, T. Polymer-Free Electrospun Nanofibers from Sulfobutyl Ether7-Beta-Cyclodextrin (SBE7-β-CD) Inclusion Complex with Sulfisoxazole: Fast-Dissolving and Enhanced Water-Solubility of Sulfisoxazole. Int. J. Pharm. 2017, 531, 550–558. [Google Scholar] [CrossRef]
- Vass, P.; Demuth, B.; Farkas, A.; Hirsch, E.; Szabo, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; et al. Continuous Alternative to Freeze Drying: Manufacturing of Cyclodextrin-Based Reconstitution Powder from Aqueous Solution Using Scaled-Up Electrospinning. J. Control Release 2019, 298, 120–127. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Fast-Dissolving Antibacterial Nanofibers of Cyclodextrin/Antibiotic Inclusion Complexes for Oral Drug Delivery. J. Colloid. Interface Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef]
- Baluja, S.; Bhalodia, R.; Bhatt, M.; Vekariya, N.; Gajera, R. Solubility of Enrofloxacin Sodium in Various Solvents at Various Temperatures. J. Chem. Eng. Data 2008, 53, 2897–2899. [Google Scholar] [CrossRef]
- Scheer, M. Studies on the Antibacterial Activity of Baytril. Vet. Med. Rev. 1987, 2, 90–98. [Google Scholar]
- Seedher, N.; Agarwal, P. Various Solvent Systems for Solubility Enhancement of Enrofloxacin. Indian. J. Pharm. Sci. 2009, 71, 82–87. [Google Scholar] [CrossRef]
- Calsavara, L.P.V.; Zanin, G.M.; de Moraes, F.F. Enrofloxacin Inclusion Complexes with Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 219–224. [Google Scholar] [CrossRef]
- Ding, Y.; Pang, Y.; Vara Prasad, C.V.N.S.; Wang, B. Formation of Inclusion Complex of Enrofloxacin with 2-Hydroxypropyl-β-Cyclodextrin. Drug Deliv. 2020, 27, 334–343. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Zhao, J.; Tan, M.; Zhai, S.; Wei, Y.; Wang, L.; Dai, T. Electrospun Fibrous Membrane Containing a Cyclodextrin Covalent Organic Framework with Antibacterial Properties for Accelerating Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 3898–3907. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 2nd ed.; Approved Guideline; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2009. [Google Scholar]
- Blaj, D.A.; Balan-Porcarasu, M.; Petre, B.A.; Harabagiu, V.; Peptu, C. MALDI Mass Spectrometry Monitoring of Cyclodextrin-Oligolactide Derivatives Synthesis. Polymer 2021, 233, 124186. [Google Scholar] [CrossRef]
- Meimoun, J.; Phuphuak, Y.; Miyamachi, R.; Miao, Y.; Bria, M.; Rousseau, C.; Nogueira, G.; Valente, A.; Favrelle-Huret, A.; Zinck, P. Cyclodextrins Initiated Ring-Opening Polymerization of Lactide Using 4-Dimethylaminopyridine (DMAP) as Catalyst: Study of DMAP/β-CD Inclusion Complex and Access to New Structures. Molecules 2022, 27, 1083. [Google Scholar] [CrossRef]
- Blaj, D.-A.; Balan-Porcarasu, M.; Harabagiu, V.; Peptu, C. Synthesis of β-Cyclodextrin Derivatives Substituted at Larger or Smaller Rims via Amine-Catalyzed Ring-Opening Oligomerization of ε-Caprolactone. Carbohydr. Polym. 2024, 334, 122032. [Google Scholar] [CrossRef]
- Peptu, C.; Danchenko, M.; Skultety, L.; Mosnacek, J. Structural Architectural Features of Cyclodextrin Oligoesters Revealed by Fragmentation Mass Spectrometry Analysis. Molecules 2018, 23, 2259. [Google Scholar] [CrossRef]
- Peptu, C.; Balan-Porcarasu, M.; Siskova, A.; Skultety, L.; Mosnacek, J. Cyclodextrins Tethered with Oligolactides—Green Synthesis and Structural Assessment. Beilstein J. Org. Chem. 2017, 13, 779–792. [Google Scholar] [CrossRef]
- Liu, M.-J.; Fu, H.-L.; Yin, D.-P.; Zhang, Y.-L.; Lu, C.-C.; Cao, H.; Zhou, J.-Y. Measurement and Correlation of the Solubility of Enrofloxacin in Different Solvents from (303.15 to 321.05) K. J. Chem. Eng. Data 2014, 59, 2070–2074. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Manasco, J.L.; Saquing, C.D.; Tang, C.; Khan, S.A. Cyclodextrin Fibers via Polymer-Free Electrospinning. RSC Adv. 2012, 2, 3778–3784. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Zha, B.; Diao, G. Correlation of Polymer-Like Solution Behaviors with Electrospun Fiber Formation of Hydroxypropyl-β-Cyclodextrin and the Adsorption Study on the Fiber. Phys. Chem. Chem. Phys. 2012, 14, 9729–9737. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef]
- Bar, R.; Ulitzur, S. Bacterial Toxicity of Cyclodextrins: Luminous Escherichia coli as a Model. Appl. Microbiol. Biotechnol. 1994, 41, 574–577. [Google Scholar] [CrossRef]
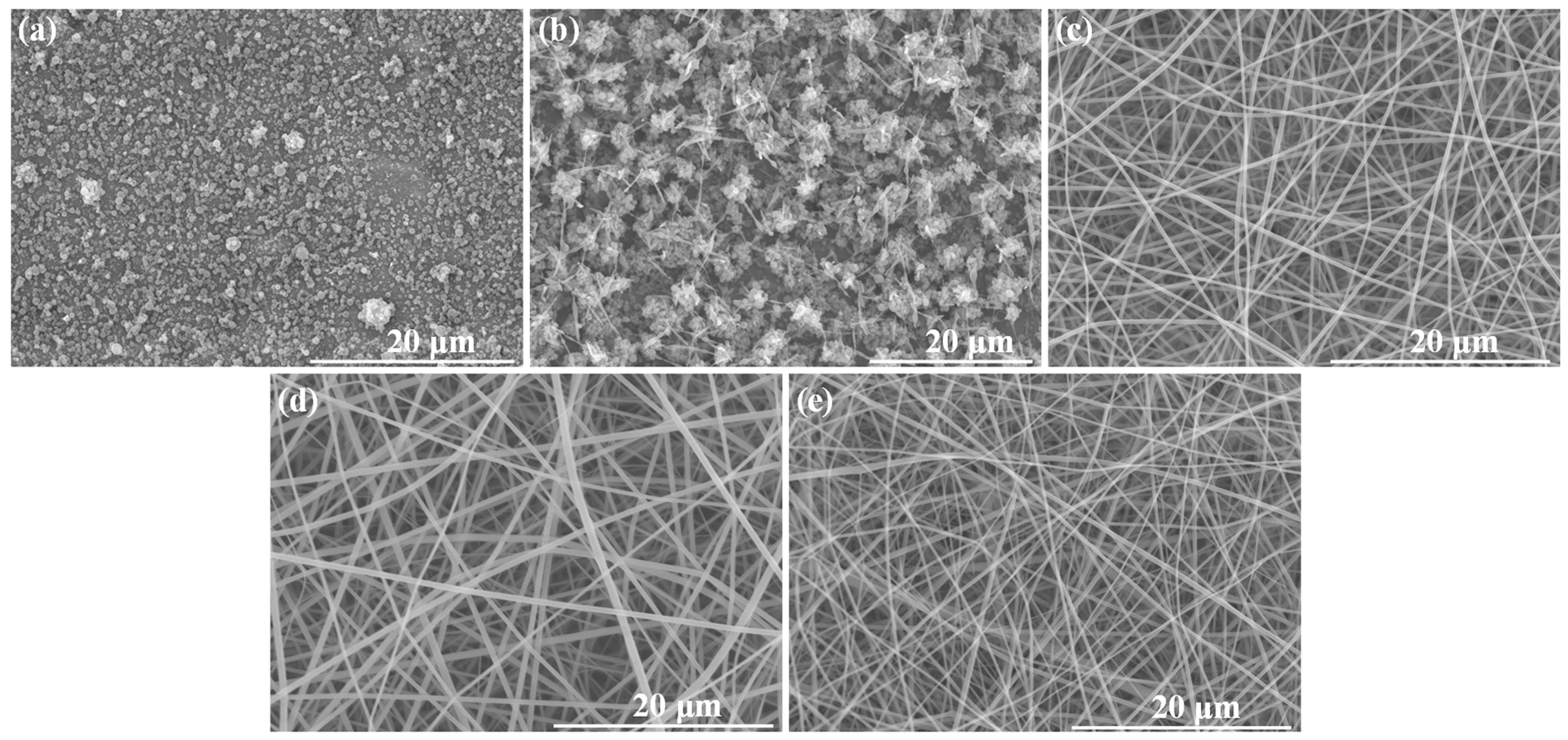
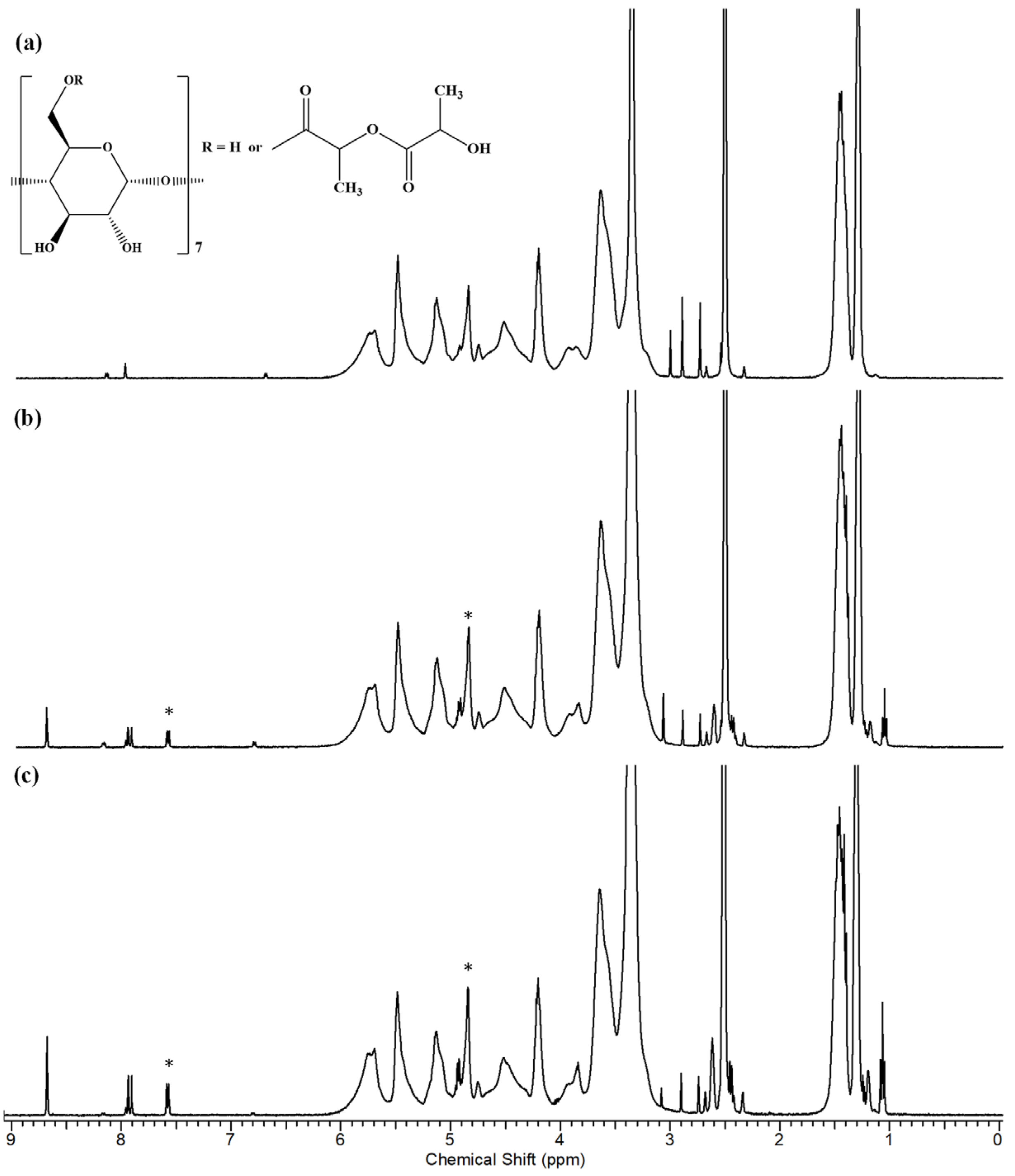
| Composition | β-CDLA/ENR Molar Ratio | CDLA Concentration (w/v%) | ENR Amount (% wt.) | Viscosity (±0.05 Pa·s) | Morphology | Diameter (nm) |
|---|---|---|---|---|---|---|
| β-CDLA/ENR | 1/1 | 180 | 15.6 | 1.85 | particles | - |
| β-CDLA/ENR | 1/1 | 200 | 15.6 | 2.34 | fibers, particles | - |
| β-CDLA/ENR | 1/1 | 220 | 15.6 | 3.04 | fibers | 342 ± 59 |
| β-CDLA/ENR | 2/1 | 220 | 7.8 | 2.62 | fibers | 377 ± 92 |
| β-CDLA | - | 220 | - | 1.84 | fibers | 307 ± 77 |
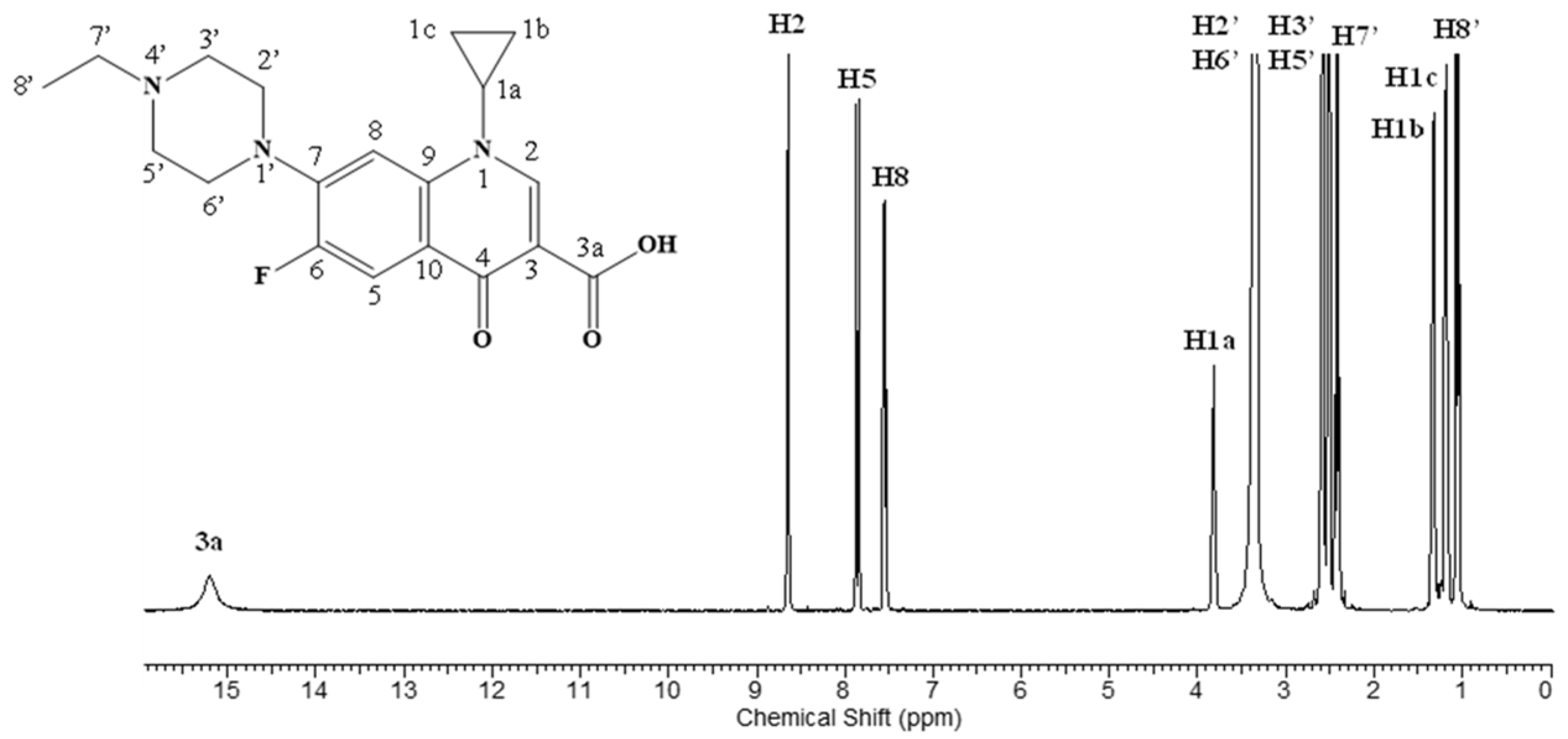
| 3a (1H, s) | H2 (1H, s) | H5 (1H, d) | H8 (1H, s) | H1a (1H, m) | H2′, H6′ (4H, m) | H3′, H5′ (4H, m) | H7′ (2H, s) | H1b (2H, d) | H1c (2H, d) | H8′ (3H, t) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ENR | 15.20 | 8.64 | 7.87–7.84 | 7.55–7.53 | 3.83–3.80 | 3.34–3.32 | 2.59–2.57 | 2.44–2.39 | 1.35–1.30 | 1.20–1.16 | 1.07–1.03 |
| 1/1 1 | 15.24 | 8.67 | 7.93–7.90 | 7.58–7.56 | 3.85–3.83 | 3.36 * | 2.62–2.60 | 2.47–2.43 | 1.30 * | 1.22–1.17 | 1.08–1.04 |
| 2/1 1 | 15.24 | 8.67 | 7.93–7.90 | 7.58–7.56 | 3.85–3.84 | 3.36 * | 2.62–2.61 | 2.45–2.41 | 1.30 * | 1.24–1.19 | 1.08–1.04 |
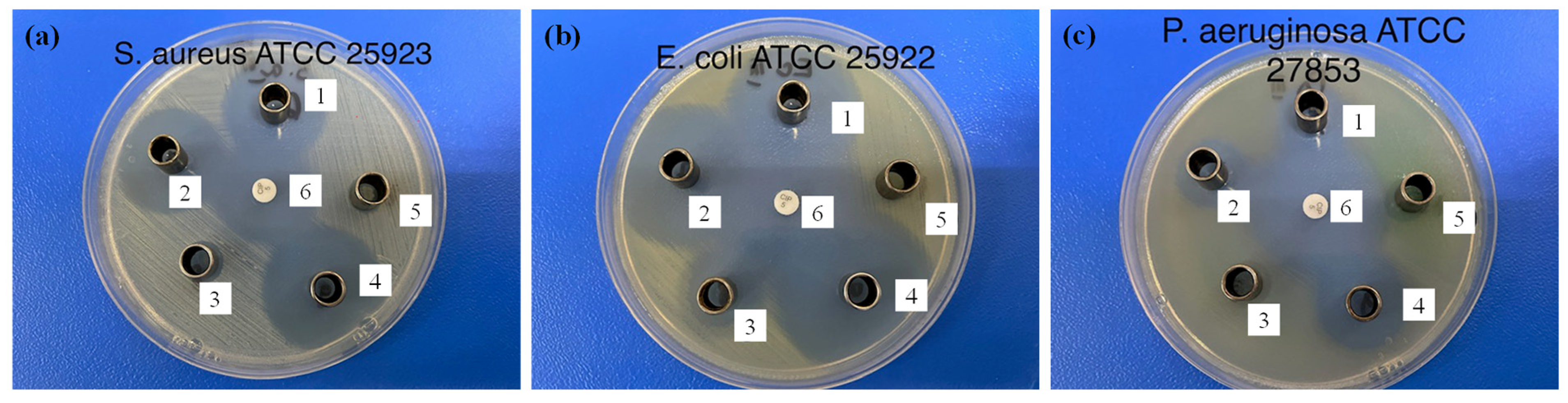
| Sample | Diameter of the Inhibition Zone (mm) | ||
|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | |
| β-CDLA/ENR 1/1 | 26.10 ± 0.05 | 31.70 ± 0.06 | 17.10 ± 0.05 |
| β-CDLA/ENR 2/1 | 27.00 ± 0.00 | 35.10 ± 0.05 | 18.00 ± 0.00 |
| β-CDLA | 0 | 15.00 ± 0.00 | 0 |
| ENR | 29.00 ± 0.00 | 34.10 ± 0.05 | 21.00 ± 0.00 |
| Water/DMSO (3/1 v/v) | 0 | 0 | 0 |
| Ciprofloxacin | 26.70 ± 0.06 | 31.00 ± 0.00 | 32.30 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaj, D.-A.; Peptu, C.A.; Danu, M.; Harabagiu, V.; Peptu, C.; Bujor, A.; Ochiuz, L.; Tuchiluș, C.G. Enrofloxacin Pharmaceutical Formulations through the Polymer-Free Electrospinning of β-Cyclodextrin–oligolactide Derivatives. Pharmaceutics 2024, 16, 903. https://doi.org/10.3390/pharmaceutics16070903
Blaj D-A, Peptu CA, Danu M, Harabagiu V, Peptu C, Bujor A, Ochiuz L, Tuchiluș CG. Enrofloxacin Pharmaceutical Formulations through the Polymer-Free Electrospinning of β-Cyclodextrin–oligolactide Derivatives. Pharmaceutics. 2024; 16(7):903. https://doi.org/10.3390/pharmaceutics16070903
Chicago/Turabian StyleBlaj, Diana-Andreea, Cătălina Anișoara Peptu, Maricel Danu, Valeria Harabagiu, Cristian Peptu, Alexandra Bujor, Lăcrămioara Ochiuz, and Cristina Gabriela Tuchiluș. 2024. "Enrofloxacin Pharmaceutical Formulations through the Polymer-Free Electrospinning of β-Cyclodextrin–oligolactide Derivatives" Pharmaceutics 16, no. 7: 903. https://doi.org/10.3390/pharmaceutics16070903
APA StyleBlaj, D.-A., Peptu, C. A., Danu, M., Harabagiu, V., Peptu, C., Bujor, A., Ochiuz, L., & Tuchiluș, C. G. (2024). Enrofloxacin Pharmaceutical Formulations through the Polymer-Free Electrospinning of β-Cyclodextrin–oligolactide Derivatives. Pharmaceutics, 16(7), 903. https://doi.org/10.3390/pharmaceutics16070903







