Preparation and Evaluation of Inhalable Microparticles with Improved Aerodynamic Performance and Dispersibility Using L-Leucine and Hot-Melt Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ITZ Microparticles
2.2.1. Preparation of ITZ Microparticles Using Co-Jet Milling
2.2.2. Preparation of ITZ Microparticles Using HME and Jet Milling
2.3. Physicochemical Characterization
2.3.1. Particle Size Distribution (PSD) by Laser Diffraction
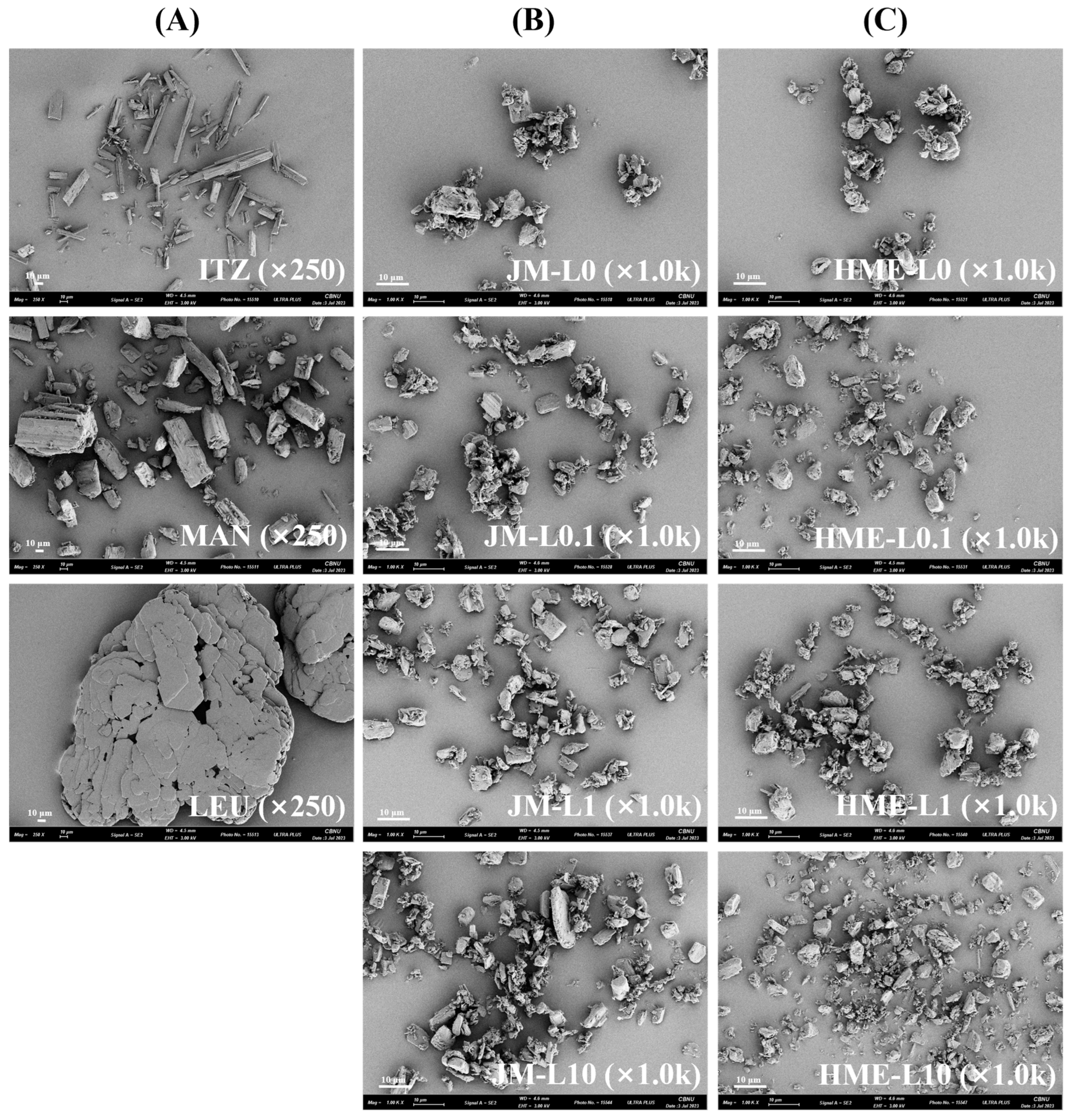
2.3.2. Scanning Electron Microscopy (SEM)
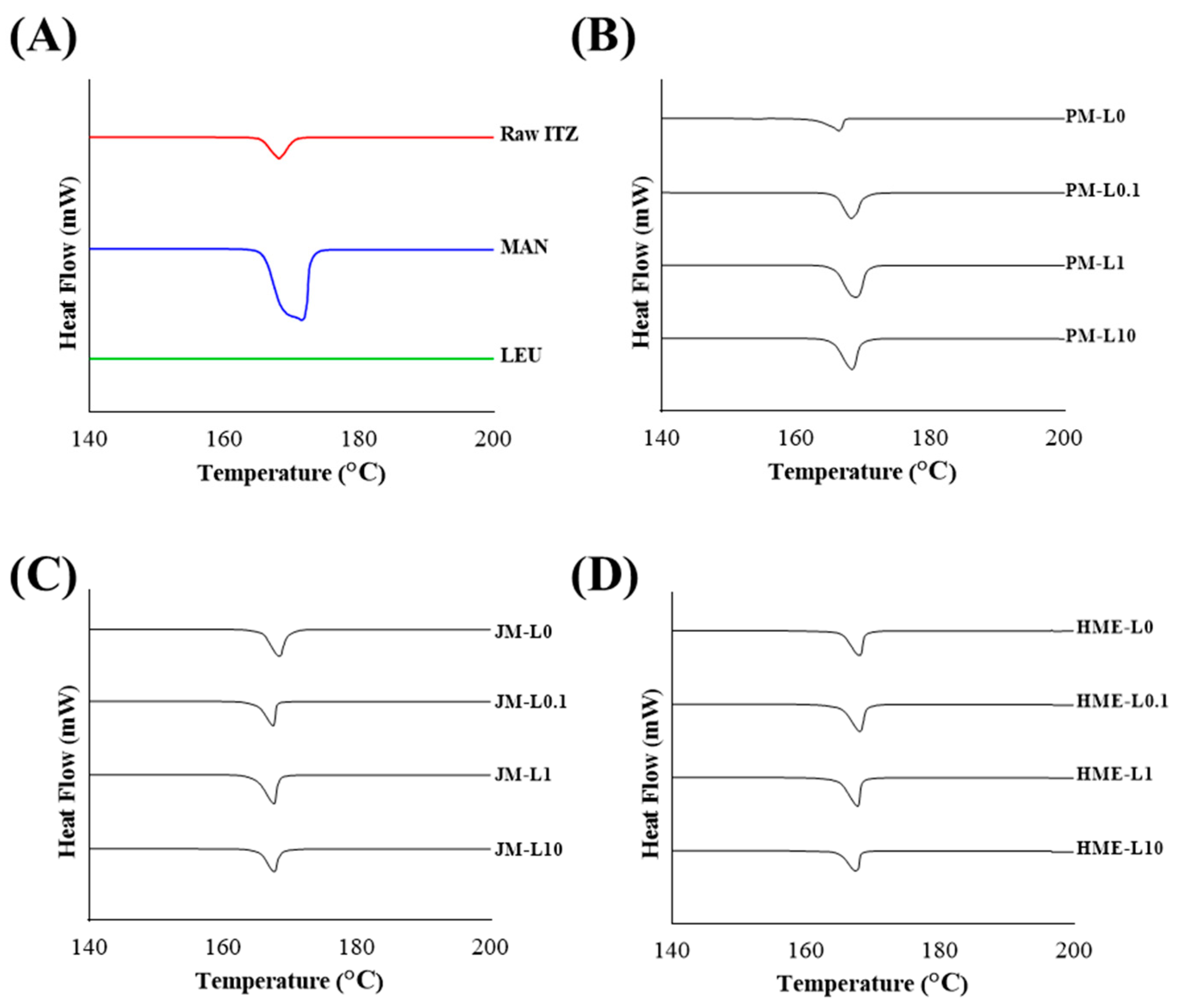
2.3.3. Thermal Analysis Differential Scanning Calorimeter (DSC)
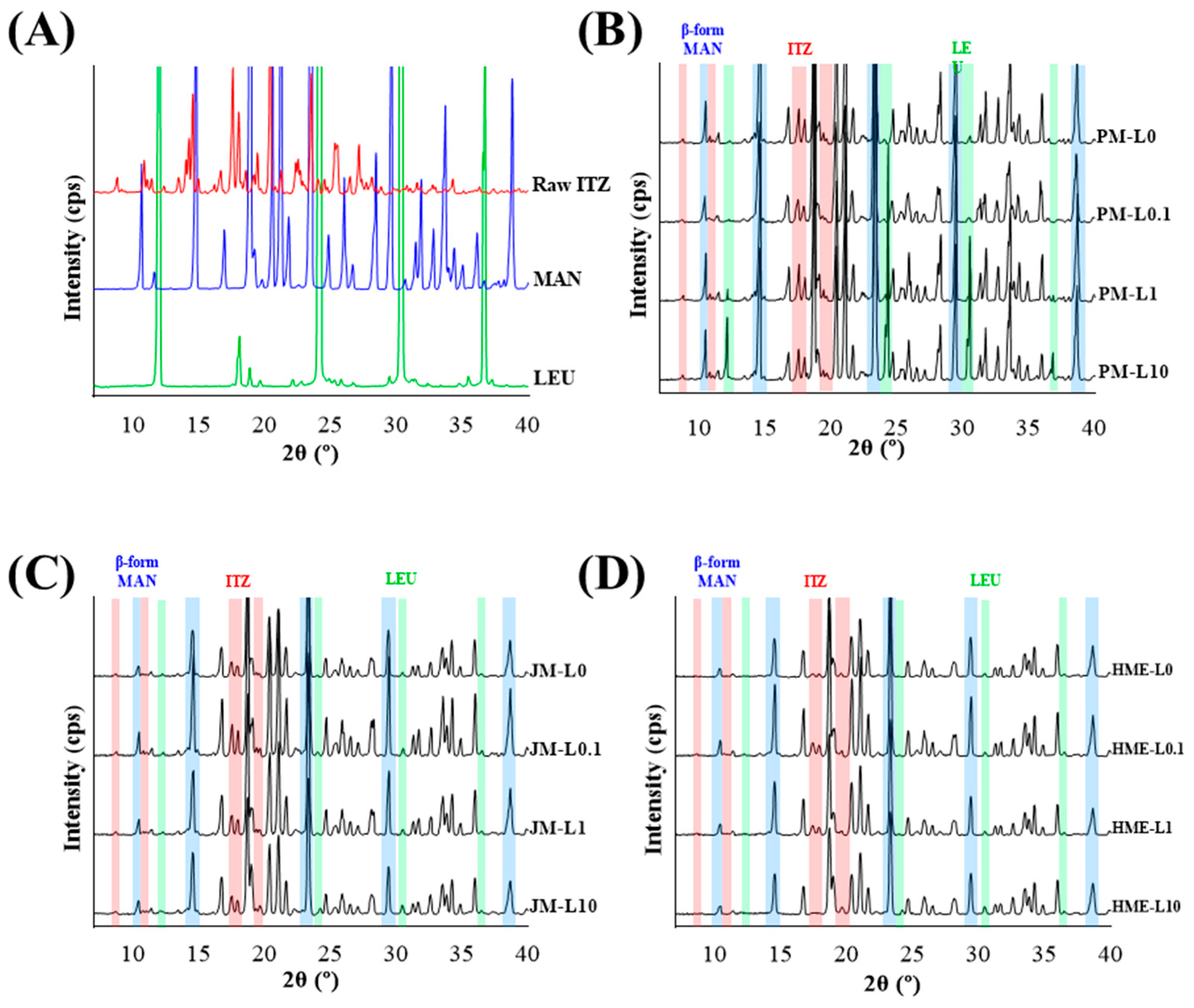
2.3.4. Powder X-ray Diffraction Analysis (PXRD)
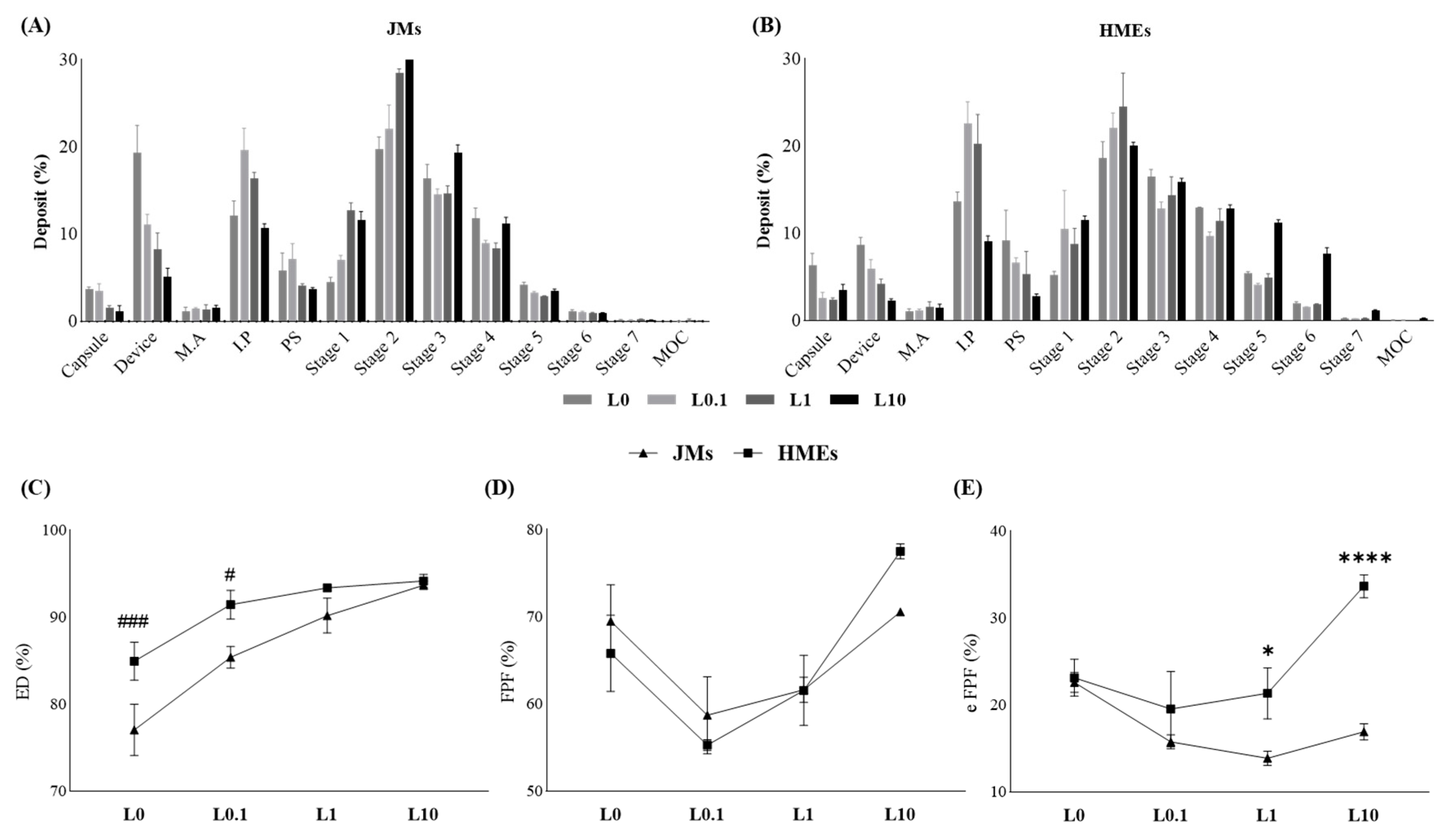
2.4. In Vitro Aerodynamic Performance Study
2.5. In Vitro Dissolution Behavior and Solubility
2.5.1. Solubility
2.5.2. In Vitro Release Study
2.5.3. Dynamic Vapor Sorption (DVS)
2.5.4. Contact Angle
2.6. Raman Microscopy
2.7. HPLC Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. Particle Size Distribution (PSD) Using Laser Diffraction
3.1.2. Morphology
3.1.3. Crystallinity
3.2. In Vitro Aerodynamic Performance Study
3.3. In Vitro Dissolution Behavior and Solubility
3.3.1. Solubility
3.3.2. In Vitro Release Study
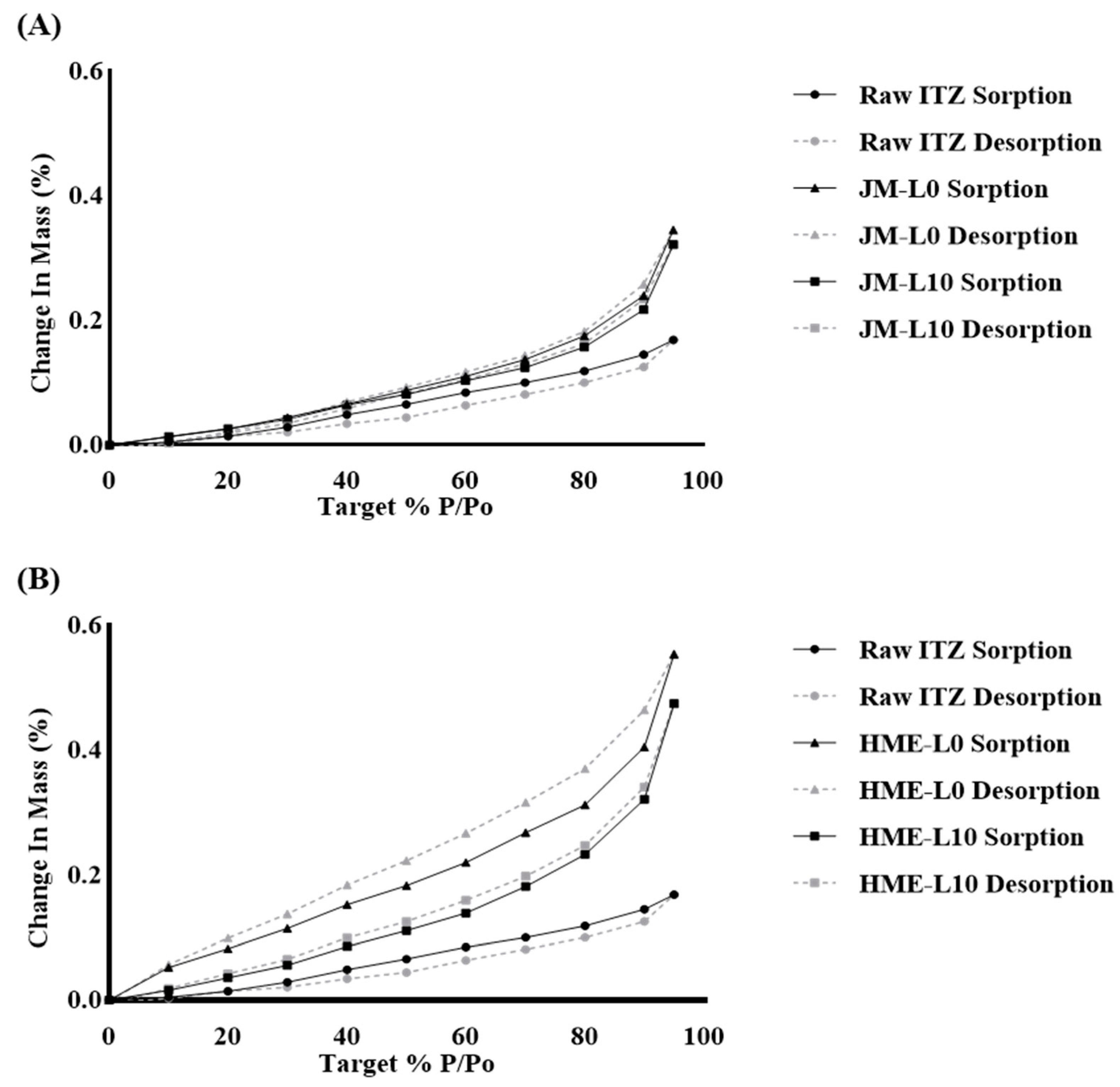
3.3.3. Dynamic Vapor Sorption (DVS)
3.3.4. Contact Angle
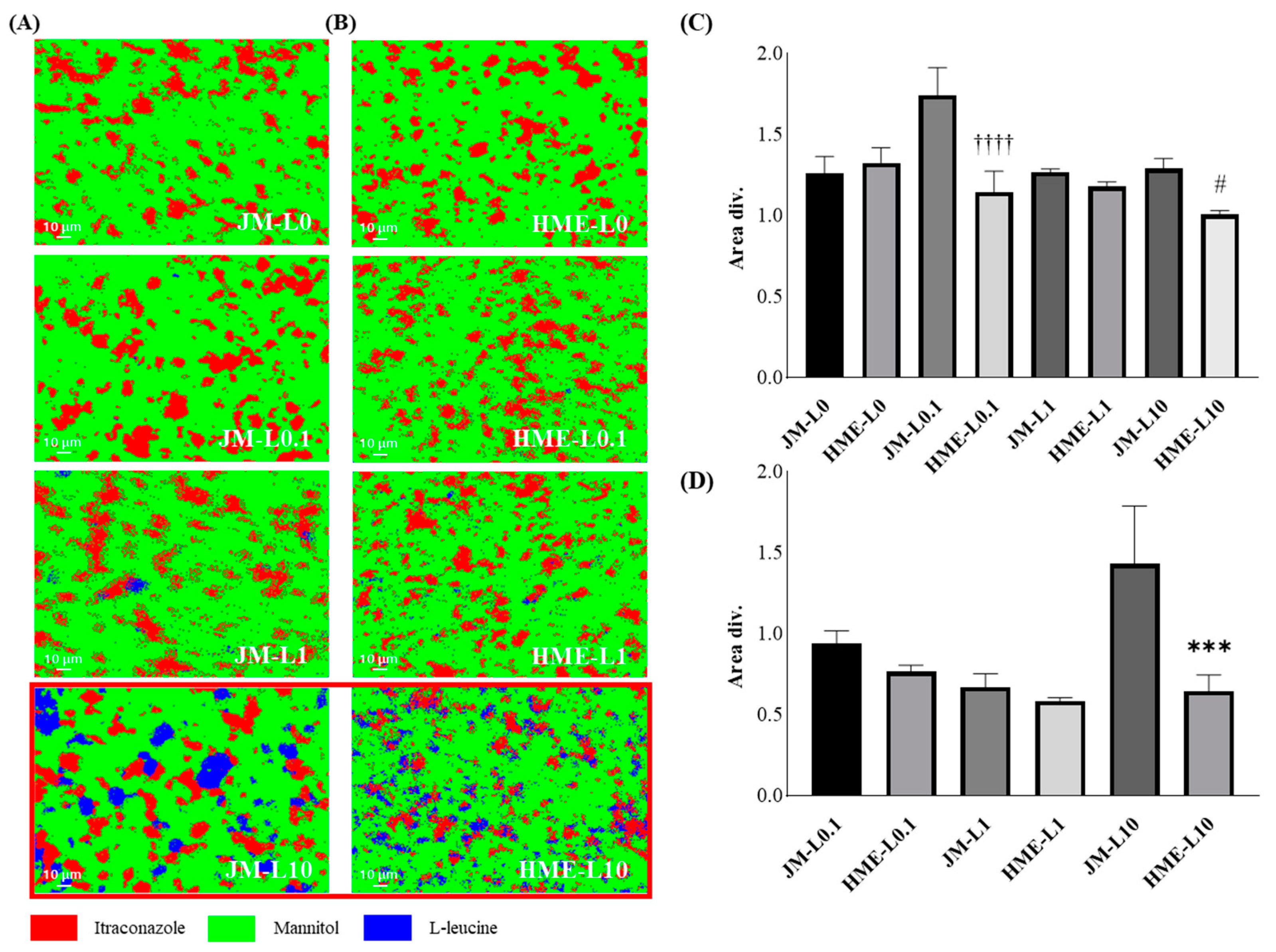
3.4. Raman Imaging and Dispersibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patton, J.S.; Fishburn, C.S.; Weers, J.G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 338–344. [Google Scholar] [CrossRef]
- Sou, T.; Bergström, C.A. Contemporary formulation development for inhaled pharmaceuticals. J. Pharm. Sci. 2021, 110, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Slavin, R.G. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. J. Immunol. Res. 2011, 2011, 843763. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.; Neeskens, P.; Tollenaere, J.P.; Van Remoortere, P.; Brewster, M.E. Characterization of the interaction of 2-hydroxypropyl-β-cyclodextrin with itraconazole at pH 2, 4, and 7. J. Pharm. Sci. 2002, 91, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Billaud, E.M.; Lestner, J.; Denning, D.W. Therapeutic drug monitoring for triazoles. Curr. Opin. Infect. Dis. 2008, 21, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Maghrabi, F.; Denning, D.W. The management of chronic pulmonary aspergillosis: The UK National aspergillosis centre approach. Curr. Fungal Infect. Rep. 2017, 11, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Saez-Lacy, D.; Wynkoop, W.; Walsh, T.J. Successful treatment of allergic bronchopulmonary aspergillosis with isavuconazole: Case report and review of the literature. Open Forum Infect. Dis. 2017, 4, ofx040. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Barrons, R.; Pegram, A.; Borries, A. Inhaler device selection: Special considerations in elderly patients with chronic obstructive pulmonary disease. Am. J. Health Syst. Pharm. 2011, 68, 1221–1232. [Google Scholar] [CrossRef]
- Hickey, A.J. Dry Powder Inhalers: An Overview. J. Aerosol Med. Pulm. Drug Deliv. 2023, 36, 316–323. [Google Scholar] [CrossRef]
- Thakkar, V.; Pandey, E.; Pandya, T.; Shah, P.; Patel, A.; Trivedi, R.; Gohel, M.; Baldaniya, L.; Gandhi, T. Formulation of dry powder inhaler of anti-tuberculous drugs using spray drying technique and optimization using 23 level factorial design approach. Curr. Drug Ther. 2019, 14, 239–260. [Google Scholar] [CrossRef]
- Rattanupatam, T.; Srichana, T. Budesonide dry powder for inhalation: Effects of leucine and mannitol on the efficiency of delivery. Drug Deliv. 2014, 21, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Karner, S.; Maier, M.; Littringer, E.; Urbanetz, N.A. Surface roughness effects on the tribo-charging and mixing homogeneity of adhesive mixtures used in dry powder inhalers. Powder Technol. 2014, 264, 544–549. [Google Scholar] [CrossRef]
- Smyth, H.D.C.; Hickey, A.J. Carriers in Drug Powder Delivery: Implications for inhalation system design. Am. J. Drug Deliv. 2005, 3, 117–132. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Armstrong, B.; Larson, I.; Stewart, P.J.; Morton, D.A. Understanding the influence of powder flowability, fluidization and de-agglomeration characteristics on the aerosolization of pharmaceutical model powders. Eur. J. Pharm. Sci. 2010, 40, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Z.G.; Watson, K. Two-step model for contact charge accumulation. J. Electrost. 2001, 51, 313–318. [Google Scholar] [CrossRef]
- Karner, S.; Urbanetz, N.A. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J. Aerosol Sci. 2011, 42, 428–445. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Reitz, E.; Vervaet, C.; Neubert, R.H.; Thommes, M. Solid crystal suspensions containing griseofulvin—Preparation and bioavailability testing. Eur. J. Pharm. Biopharm. 2013, 83, 193–202. [Google Scholar] [CrossRef]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.; Zia, H. Hot-melt extrusion technique: A review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar] [CrossRef]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef]
- Park, J.-B.; Kang, C.-Y.; Kang, W.-S.; Choi, H.-G.; Han, H.-K.; Lee, B.-J. New investigation of distribution imaging and content uniformity of very low dose drugs using hot-melt extrusion method. Int. J. Pharm. 2013, 458, 245–253. [Google Scholar] [CrossRef]
- Edwards, D.A.; Dunbar, C. Bioengineering of Therapeutic Aerosols. Annu. Rev. Biomed. Eng. 2002, 4, 93–107. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2014, 66, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-S.; Kang, J.-H.; Kim, D.-W.; Park, C.-W. Recent developments in dry powder inhalation (DPI) formulations for lung-targeted drug delivery. J. Pharm. Investig. 2023, 54, 113–130. [Google Scholar] [CrossRef]
- Lu, W.; Rades, T.; Rantanen, J.; Chan, H.-K.; Yang, M. Amino acids as stabilizers for spray-dried simvastatin powder for inhalation. Int. J. Pharm. 2019, 572, 118724. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.-K. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Gregson, F.K.; Wang, H.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. On the particle formation of leucine in spray drying of inhalable microparticles. Int. J. Pharm. 2021, 592, 120102. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an excipient in spray dried powder for inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef] [PubMed]
- Ferdynand, M.S.; Nokhodchi, A. Co-spraying of carriers (mannitol-lactose) as a method to improve aerosolization performance of salbutamol sulfate dry powder inhaler. Drug Deliv. Transl. Res. 2020, 10, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Haser, A.; Huang, S.; Listro, T.; White, D.; Zhang, F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int. J. Pharm. 2017, 524, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Repka, M.A. Melt extrusion in drug delivery: Three decades of progress. In Melt Extrusion: Materials, Technology and Drug Product Design; Springer: New York, NY, USA, 2013; pp. 3–46. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.T.; Sun, S.-P.; Denman, J.A.; Gengenbach, T.R.; Barraud, N.; Rice, S.A.; Li, J.; Yang, M.; Chan, H.-K. Effects of surface composition on the aerosolisation and dissolution of inhaled antibiotic combination powders consisting of colistin and rifampicin. AAPS J. 2016, 18, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Wauthoz, N.; Sebti, T.; Vanderbist, F.; Amighi, K. Solid dispersions of itraconazole for inhalation with enhanced dissolution, solubility and dispersion properties. Int. J. Pharm. 2012, 428, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Neves, F.; Costa, E. Impact of jet-milling and wet-polishing size reduction technologies on inhalation API particle properties. Powder Technol. 2016, 298, 90–98. [Google Scholar] [CrossRef]
- Begat, P.; Morton, D.A.; Shur, J.; Kippax, P.; Staniforth, J.N.; Price, R. The role of force control agents in high-dose dry powder inhaler formulations. J. Pharm. Sci. 2009, 98, 2770–2783. [Google Scholar] [CrossRef] [PubMed]
- Raula, J.; Thielmann, F.; Naderi, M.; Lehto, V.-P.; Kauppinen, E.I. Investigations on particle surface characteristics vs. dispersion behaviour of l-leucine coated carrier-free inhalable powders. Int. J. Pharm. 2010, 385, 79–85. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q. Physico-chemical properties, aerosolization and dissolution of co-spray dried azithromycin particles with l-leucine for inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef]
- Borgers, M.; Van de Ven, M. Mode of action of itraconazole: Morphological aspects. Mycoses 1989, 32 (Suppl. 1), 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nykamp, G.; Carstensen, U.; Müller, B. Jet milling—A new technique for microparticle preparation. Int. J. Pharm. 2002, 242, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Robins, E.; Flament, M. Agglomerate behaviour of fluticasone propionate within dry powder inhaler formulations. Eur. J. Pharm. Biopharm. 2012, 80, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.A.; El-Nabarawi, M.A.; El-Setouhy, D.A.; Alsammit, S.A. Formulation and stability testing of itraconazole crystalline nanoparticles. AAPS Pharmscitech 2011, 12, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Chen, L.; Chen, D.; Chan, H.-K. Overcoming challenges for development of amorphous powders for inhalation. Expert Opin. Drug Deliv. 2020, 17, 1583–1595. [Google Scholar] [CrossRef]
- Adhikari, S.; Kar, T. Bulk single crystal growth and characterization of l-leucine—A nonlinear optical material. Mater. Chem. Phys. 2012, 133, 1055–1059. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Hu, A.; Nie, T.; Cheng, Z.; Liu, W. A critical review on engineering of d-mannitol crystals: Properties, applications, and polymorphic control. Crystals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Cares-Pacheco, M.; Vaca-Medina, G.; Calvet, R.; Espitalier, F.; Letourneau, J.; Rouilly, A.; Rodier, E. Physicochemical characterization of d-mannitol polymorphs: The challenging surface energy determination by inverse gas chromatography in the infinite dilution region. Int. J. Pharm. 2014, 475, 69–81. [Google Scholar] [CrossRef]
- Sharif, S.; Muneer, S.; Izake, E.L.; Islam, N. Impact of leucine and magnesium stearate on the physicochemical properties and aerosolization behavior of wet milled inhalable ibuprofen microparticles for developing dry powder inhaler formulation. Pharmaceutics 2023, 15, 674. [Google Scholar] [CrossRef]
- Buttini, F.; Brambilla, G.; Copelli, D.; Sisti, V.; Balducci, A.G.; Bettini, R.; Pasquali, I. Effect of flow rate on in vitro aerodynamic performance of NEXThaler® in comparison with Diskus® and Turbohaler® dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 167–178. [Google Scholar] [CrossRef]
- Adams, W.P.; Lee, S.L.; Plourde, R.; Lionberger, R.A.; Bertha, C.M.; Doub, W.H.; Bovet, J.-M.; Hickey, A.J. Effects of device and formulation on in vitro performance of dry powder inhalers. AAPS J. 2012, 14, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Marriott, C.; Zeng, X.-M.; Martin, G.P. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: A comparison of three impactors. Int. J. Pharm. 2010, 388, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Jetzer, M.; Morrical, B.; Schneider, M.; Edge, S.; Imanidis, G. Probing the particulate microstructure of the aerodynamic particle size distribution of dry powder inhaler combination products. Int. J. Pharm. 2018, 538, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Fine Particle Fraction: The Good and the Bad. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, H.; Liu, D.; Liu, Y.; Meng, X.; Chen, B.; Zou, Z. Cyclosporine A-loaded chitosan extra-fine particles for deep pulmonary drug delivery: In vitro and in vivo evaluation. J. Control. Release 2023, 362, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Sosnowski, T.R.; Tobita, S.; Tachibana, I.; Tse, J.Y.; Uchiyama, H.; Tozuka, Y. A particle technology approach toward designing dry-powder inhaler formulations for personalized medicine in respiratory diseases. Adv. Powder Technol. 2020, 31, 219–226. [Google Scholar] [CrossRef]
- Heyder, J. Alveolar deposition of inhaled particles in humans. Am. Ind. Hyg. Assoc. J. 1982, 43, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bariwal, J.; Narang, A.S.; Tso, J.; Cheong, J.; Mahato, R. Functional similarity of modified cascade impactor to deposit drug particles on cells. Int. J. Pharm. 2020, 583, 119404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Thakur, A.K.; Chaudhari, P.; Banerjee, N. Particle size reduction techniques of pharmaceutical compounds for the enhancement of their dissolution rate and bioavailability. J. Pharm. Innov. 2022, 17, 333–352. [Google Scholar] [CrossRef]
- Yun, F.; Kang, A.; Shan, J.; Zhao, X.; Bi, X.; Li, J.; Di, L. Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int. J. Pharm. 2014, 465, 436–443. [Google Scholar] [CrossRef]
- Colombo, M.; Orthmann, S.; Bellini, M.; Staufenbiel, S.; Bodmeier, R. Influence of drug brittleness, nanomilling time, and freeze-drying on the crystallinity of poorly water-soluble drugs and its implications for solubility enhancement. AAPS Pharmscitech 2017, 18, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Hiramatsu, T.; Suzuki, R.; Okamoto, R.; Shibagaki, K.; Fujita, K.; Takahashi, C.; Kawashima, Y.; Yamamoto, H. Improvement in the water solubility of drugs with a solid dispersion system by spray drying and hot-melt extrusion with using the amphiphilic polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer and d-mannitol. Eur. J. Pharm. Sci. 2018, 111, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Radivojev, S.; Zellnitz, S.; Paudel, A.; Fröhlich, E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int. J. Pharm. 2019, 556, 45–56. [Google Scholar] [CrossRef] [PubMed]
- May, S.; Jensen, B.; Wolkenhauer, M.; Schneider, M.; Lehr, C.M. Dissolution techniques for in vitro testing of dry powders for inhalation. Pharm. Res. 2012, 29, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Tan, R.B.; Ng, W.K.; Shen, S.; Zhou, Q.; Heng, P.W. Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler. J. Aerosol Sci. 2008, 39, 510–524. [Google Scholar] [CrossRef]
- Ng, W.K.; Kwek, J.W.; Tan, R.B.H. Anomalous particle size shift during post-milling storage. Pharm. Res. 2008, 25, 1175–1185. [Google Scholar] [CrossRef]
- Lau, M.; Young, P.M.; Traini, D. Co-milled API-lactose systems for inhalation therapy: Impact of magnesium stearate on physico-chemical stability and aerosolization performance. Drug Dev. Ind. Pharm. 2017, 43, 980–988. [Google Scholar] [CrossRef]
- Sheokand, S.; Modi, S.R.; Bansal, A.K. Dynamic vapor sorption as a tool for characterization and quantification of amorphous content in predominantly crystalline materials. J. Pharm. Sci. 2014, 103, 3364–3376. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Xie, X. Water vapor sorption properties of cellulose nanocrystals and nanofibers using dynamic vapor sorption apparatus. Sci. Rep. 2017, 7, 14207. [Google Scholar] [CrossRef] [PubMed]
- Bley, O.; Siepmann, J.; Bodmeier, R. Characterization of Moisture-protective polymer coatings using differential scanning calorimetry and dynamic vapor sorption. J. Pharm. Sci. 2009, 98, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Young, P.M.; Edge, S.; Staniforth, J.N.; Steele, D.F.; Price, R. Dynamic vapor sorption properties of sodium starch glycolate disintegrants. Pharm. Dev. Technol. 2005, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; D’urso, B.; Liu, H.; Li, L. Study on the surface energy of graphene by contact angle measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.-M.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Goebel, M.-O.; Bachmann, J.; Woche, S.K.; Fischer, W.R.; Horton, R. Water potential and aggregate size effects on contact angle and surface energy. Soil Sci. Soc. Am. J. 2004, 68, 383–393. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Forny, L.; Saleh, K.; Denoyel, R.; Pezron, I. Contact angle assessment of hydrophobic silica nanoparticles related to the mechanisms of dry water formation. Langmuir 2010, 26, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.; De Palma, R.; Verlaak, S.; Heremans, P.; Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films 2006, 515, 1433–1438. [Google Scholar] [CrossRef]
- Wang, H.; Barona, D.; Oladepo, S.; Williams, L.; Hoe, S.; Lechuga-Ballesteros, D.; Vehring, R. Macro-Raman spectroscopy for bulk composition and homogeneity analysis of multi-component pharmaceutical powders. J. Pharm. Biomed. Anal. 2017, 141, 180–191. [Google Scholar] [CrossRef]
- Tian, F.; Zeitler, J.; Strachan, C.; Saville, D.; Gordon, K.; Rades, T. Characterizing the conversion kinetics of carbamazepine polymorphs to the dihydrate in aqueous suspension using Raman spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Spahn, J.E.; Hefnawy, A.; Smyth, H.D.; Zhang, F. Development of a novel method for the continuous blending of carrier-based dry powders for inhalation using a co-rotating twin-screw extruder. Int. J. Pharm. 2022, 623, 121914. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kang, C.-Y.; Park, J.-B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J. Pharm. Investig. 2017, 47, 123–132. [Google Scholar] [CrossRef]
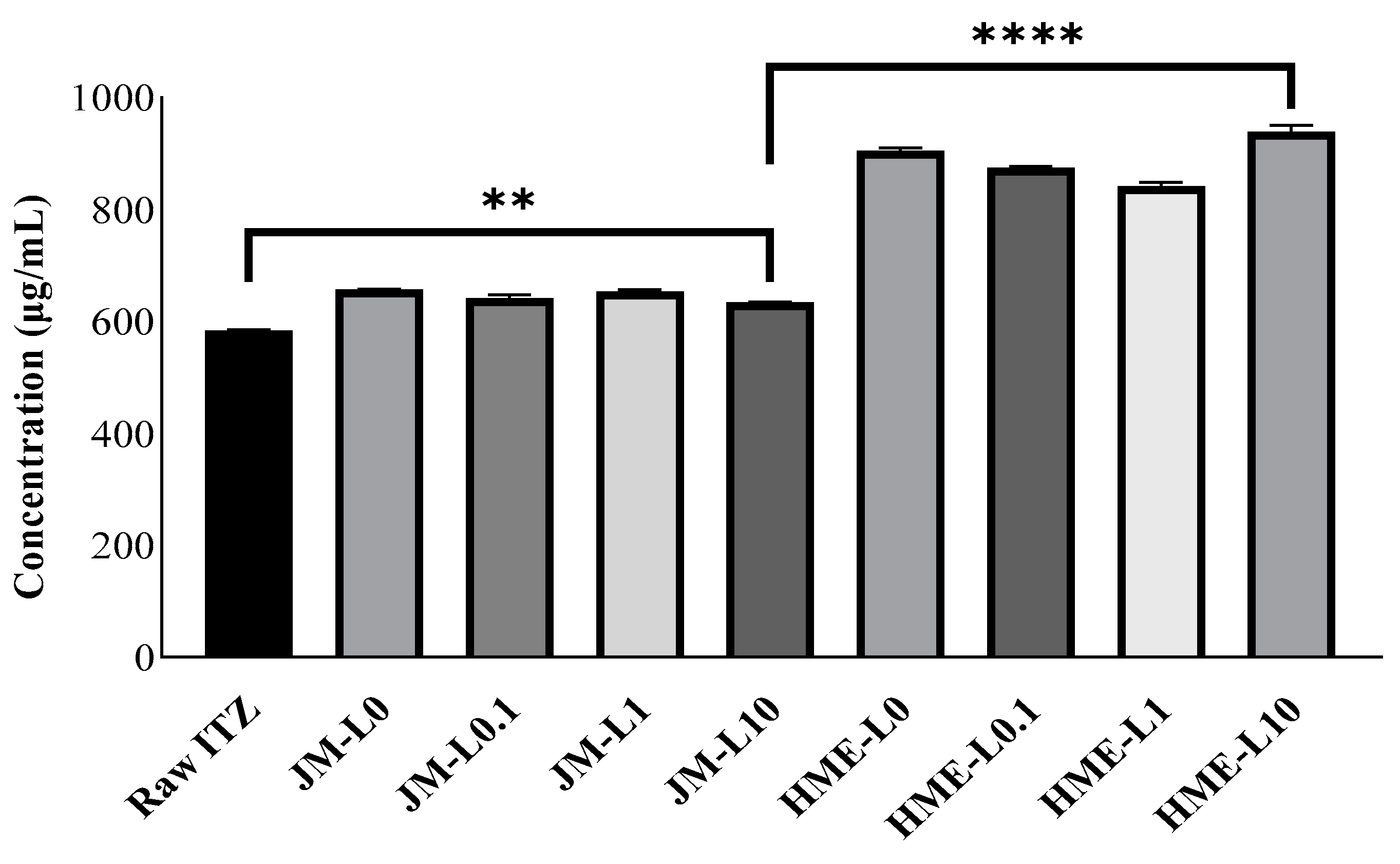


| Code | Process | Formulation Ratio (%) | ||
|---|---|---|---|---|
| Itraconazole (ITZ) | Mannitol (MAN) | L-Leucine (LEU) | ||
| JM-L0 | Co-jet mill | 20.00 | 80.00 | - |
| JM-L0.1 | 19.98 | 79.92 | 0.10 | |
| JM-L1 | 19.80 | 79.20 | 1.00 | |
| JM-L10 | 18.00 | 72.00 | 10.00 | |
| HME-L0 | Jet mill after HME | 20.00 | 80.00 | - |
| HME-L0.1 | 19.98 | 79.92 | 0.10 | |
| HME-L1 | 19.80 | 79.20 | 1.00 | |
| HME-L10 | 18.00 | 72.00 | 10.00 | |
| Formulation | Dv (10) (μm) | Dv (50) (μm) | Dv (90) (μm) | Span | |
|---|---|---|---|---|---|
| Co-jet mill | JM-L0 | 1.06 ± 0.01 | 3.41 ± 0.04 | 7.30 ± 0.04 | 1.83 ± 0.05 |
| JM-L0.1 | 1.14 ± 0.02 | 3.83 ± 0.06 | 8.02 ± 0.16 | 1.77 ± 0.05 | |
| JM-L1 | 1.15 ± 0.01 | 3.99 ± 0.02 | 8.45 ± 0.17 | 1.83 ± 0.03 | |
| JM-L10 | 1.08 ± 0.01 | 3.67 ± 0.03 | 7.25 ± 0.19 | 1.68 ± 0.04 | |
| Jet mill after HME | HME-L0 | 0.98 ± 0.01 | 3.23 ± 0.01 | 6.24 ± 0.04 | 1.63 ± 0.01 |
| HME-L0.1 | 1.07 ± 0.01 | 3.98 ± 0.11 | 8.09 ± 0.51 | 1.76 ± 0.08 | |
| HME-L1 | 1.05 ± 0.01 | 3.94 ± 0.02 | 8.34 ± 0.09 | 1.85 ± 0.03 | |
| HME-L10 | 0.97 ± 0.01 | 3.21 ± 0.03 | 7.44 ± 0.19 | 2.02 ± 0.06 | |
| Formulation | ED (%) | FPF (%) <4.46 μm | eFPF (%) <1.66 μm | MMAD (μm) | GSD | |
|---|---|---|---|---|---|---|
| Co-jet mill | JM-L0 | 77.06 ± 2.93 | 69.50 ± 4.17 | 22.61 ± 1.13 | 3.91 ± 0.06 | 1.50 ± 0.66 |
| JM-L0.1 | 85.40 ± 1.25 | 58.72 ± 4.42 | 15.77 ± 0.79 | 4.50 ± 0.12 | 1.40 ± 0.58 | |
| JM-L1 | 90.19 ± 2.00 | 61.64 ± 1.44 | 13.88 ± 0.82 | 5.10 ± 0.13 | 1.44 ± 0.59 | |
| JM-L10 | 93.68 ± 0.46 | 70.57 ± 0.35 | 16.92 ± 0.92 | 4.74 ± 0.14 | 1.42 ± 0.57 | |
| HME + jet mill | HME-L0 | 84.95 ± 2.19 ### | 65.83 ± 4.36 | 23.14 ± 2.13 | 3.71 ± 0.14 | 1.94 ± 0.01 |
| HME-L0.1 | 91.45 ± 1.66 # | 55.29 ± 0.62 | 19.54 ± 4.33 | 4.68 ± 0.43 | 1.34 ± 0.92 | |
| HME-L1 | 93.39 ± 0.39 | 61.57 ± 4.02 | 21.34 ± 2.94 * | 4.42 ± 0.31 | 2.08 ± 0.39 | |
| HME-L10 | 94.18 ± 0.74 | 77.53 ± 0.87 | 33.68 ± 1.31 **** | 3.46 ± 0.06 | 2.11 ± 1.31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-H.; Kim, J.-S.; Choi, Y.-R.; Shin, D.H.; Kang, J.-H.; Kim, D.-W.; Park, Y.-S.; Park, C.-W. Preparation and Evaluation of Inhalable Microparticles with Improved Aerodynamic Performance and Dispersibility Using L-Leucine and Hot-Melt Extrusion. Pharmaceutics 2024, 16, 784. https://doi.org/10.3390/pharmaceutics16060784
Jeong J-H, Kim J-S, Choi Y-R, Shin DH, Kang J-H, Kim D-W, Park Y-S, Park C-W. Preparation and Evaluation of Inhalable Microparticles with Improved Aerodynamic Performance and Dispersibility Using L-Leucine and Hot-Melt Extrusion. Pharmaceutics. 2024; 16(6):784. https://doi.org/10.3390/pharmaceutics16060784
Chicago/Turabian StyleJeong, Jin-Hyuk, Ji-Su Kim, Yu-Rim Choi, Dae Hwan Shin, Ji-Hyun Kang, Dong-Wook Kim, Yun-Sang Park, and Chun-Woong Park. 2024. "Preparation and Evaluation of Inhalable Microparticles with Improved Aerodynamic Performance and Dispersibility Using L-Leucine and Hot-Melt Extrusion" Pharmaceutics 16, no. 6: 784. https://doi.org/10.3390/pharmaceutics16060784
APA StyleJeong, J.-H., Kim, J.-S., Choi, Y.-R., Shin, D. H., Kang, J.-H., Kim, D.-W., Park, Y.-S., & Park, C.-W. (2024). Preparation and Evaluation of Inhalable Microparticles with Improved Aerodynamic Performance and Dispersibility Using L-Leucine and Hot-Melt Extrusion. Pharmaceutics, 16(6), 784. https://doi.org/10.3390/pharmaceutics16060784






