Comparative Study of the Potential Cell-Penetrating Peptide ∆M4 on Apoptosis Cell Signaling in A375 and A431 Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Determination of Apoptosis Protein Expression
2.3. ROS Production Assay
2.4. Determination of Antioxidant Activity
2.5. Western Blot Analysis
2.6. Caspase 3/7 Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Expression of Apoptotic Proteins by ∆M4 Treatment
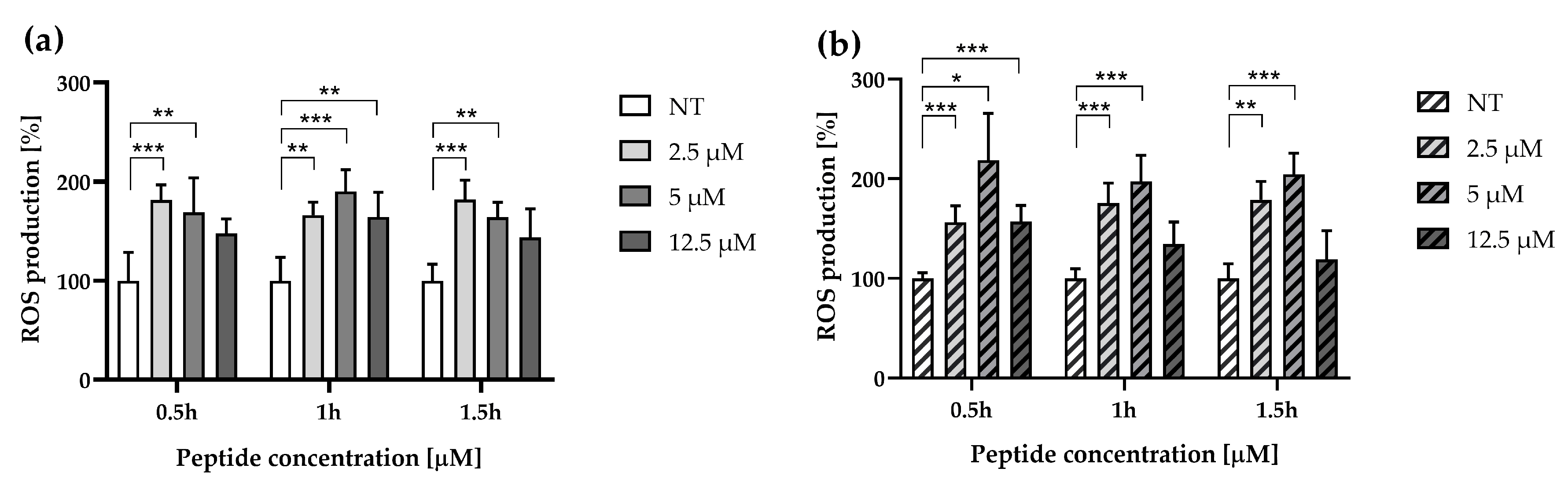
3.2. ∆M4 Increases Intracellular ROS Production
3.3. ∆M4 Lowers the Cu/ZnSOD Activity in Melanoma and Epidermoid Carcinoma Cells
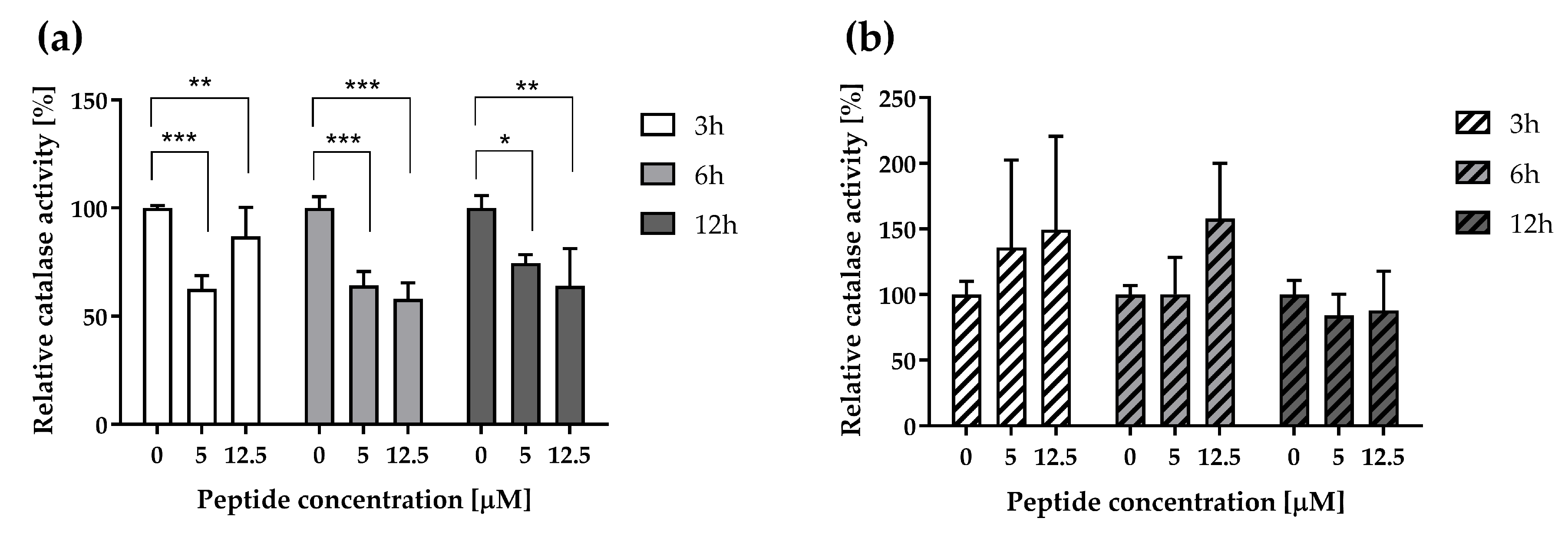
3.4. ∆M4 Decreases Catalase Activity in A375 Whereas It Has No Significant Effect in A431
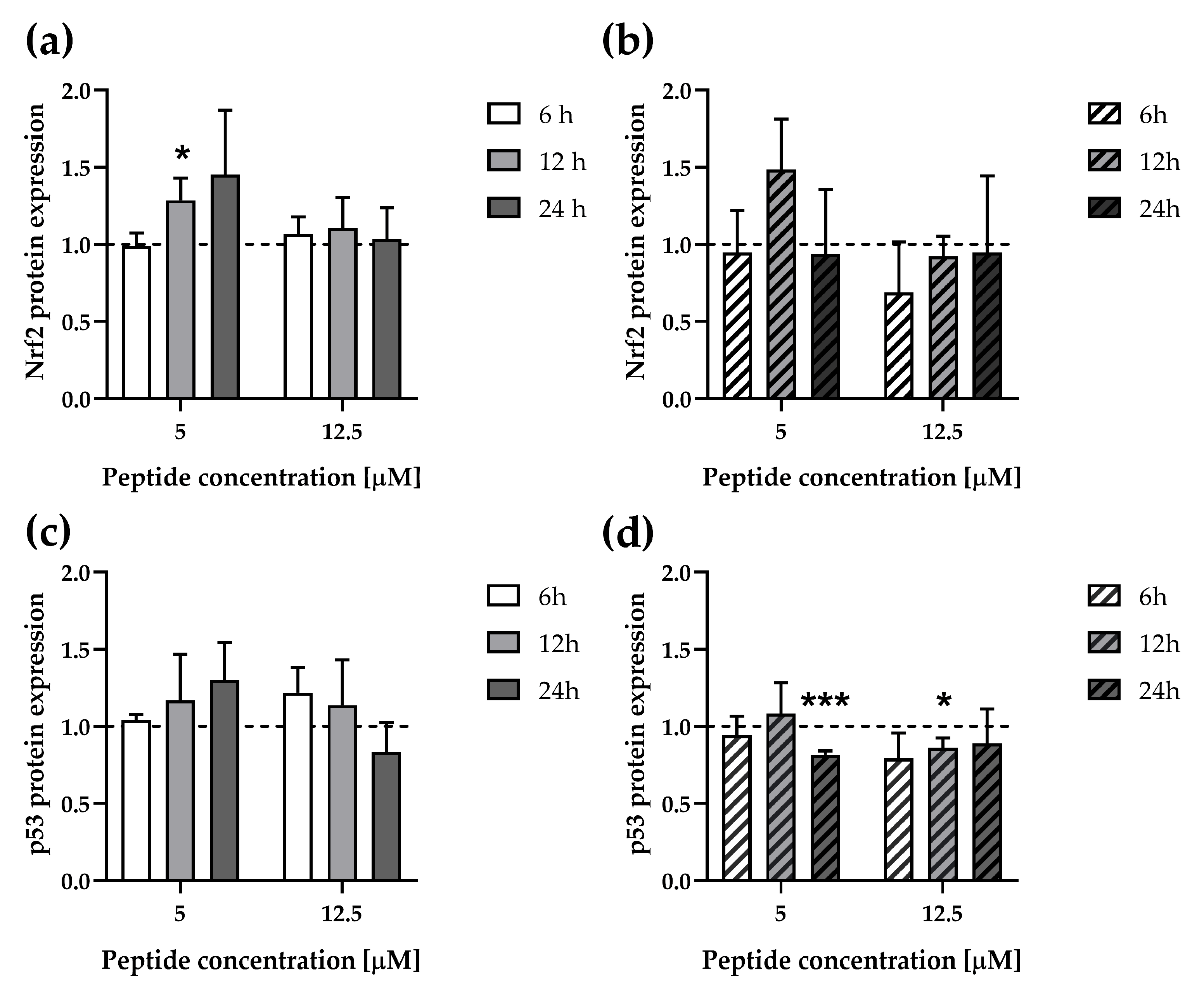
3.5. ∆M4 Change Expression of the Stress–Response Transcription Factors in A375 and A431 Cell Lines
3.6. ∆M4 Induces Activation of Effector Caspases 3/7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deber, C.M. Interaction of designed cationic antimicrobial peptides with the outer membrane of gram-negative bacteria. Sci. Rep. 2024, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Di Pisa, M.; Chassaing, G.; Swiecicki, J.-M. Translocation Mechanism(s) of Cell-Penetrating Peptides: Biophysical Studies Using Artificial Membrane Bilayers. Biochemistry 2015, 54, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Langel, Ü. Cell-Penetrating Peptides, 2nd ed.; Springer Nature: Cham, Switzerland, 2023; Chapter 14; pp. 359–372. ISBN 978-3-031-38731-9. [Google Scholar]
- Reissmann, S.; Filatova, M.P. New generation of cell-penetrating peptides: Functionality and potential clinical application. J. Pept. Sci. 2021, 27, e3300. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 5627. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Asoodeh, A.; Klonisch, T.; Halayko, A.J.; Kadkhoda, K.; Kroczak, T.J.; Gibson, S.B.; Booy, E.P.; Naderi-Manesh, H.; Los, M. Brevinin-2R1 semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J. Cell. Mol. Med. 2008, 12, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Krishnan, H.B.J. BG-4, a novel anticancer peptide from bitter gourd (Momordica charantia), promotes apoptosis in human colon cancer cells. Sci. Rep. 2016, 6, 33532. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.M.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 2013, 8, e63641, published correction appears in PLoS ONE 2015, 10, e0131750. [Google Scholar] [CrossRef]
- Tanner, J.D.; Deplazes, E.; Mancera, R.L.J. The biological and biophysical properties of the spider peptide gomesin. Molecules 2018, 23, 1733. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xi, X.; Ma, C.; Chen, X.; Zhou, M.; Burrows, J.F.; Chen, T.; Wang, L.J. A novel dermaseptin isolated from the skin secretion of phyllomedusa tarsius and its cationicity-enhanced analogue exhibiting effective antimicrobial and anti-proliferative activities. Biomolecules 2019, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Santa-González, G.A.; Patiño-González, E.; Manrique-Moreno, M. Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells. Molecules 2020, 25, 5684. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Jang, B.-Y.; Bu, K.-B.; Lee, S.-H.; Han, D.-H.; Oh, J.W.; Sung, J.-S. De novo design of AC-P19M, a novel anticancer peptide with apoptotic effects on lung cancer cells and anti-angiogenic activity. Int. J. Mol. Sci. 2022, 23, 15594. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Fandiño-Devia, E.; Santa-González, G.A.; Klaiss-Luna, M.C.; Guevara-Lora, I.; Tamayo, V.; Manrique-Moreno, M. ΔM4: Membrane-Active Peptide with Antitumoral Potential against Human Skin Cancer Cells. Membranes 2023, 13, 671. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar]
- Brankiewicz, A.; Trzos, S.; Mrożek, M.; Opydo, M.; Szostak, E.; Dziurka, M.; Tuleja, M.; Łoboda, A.; Pocheć, E. Cytotoxic and Antioxidant Activity of Hypericum perforatum L. Extracts against Human Melanoma Cells from Different Stages of Cancer Progression, Cultured under Normoxia and Hypoxia. Molecules 2023, 28, 1509. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska-Sendo, A.; Kozik, A.; Guevara-Lora, I.J.P.O. Influence of bradykinin B2 receptor and dopamine D2 receptor on the oxidative stress, inflammatory response, and apoptotic process in human endothelial cells. PLoS ONE 2018, 13, e0206443. [Google Scholar] [CrossRef] [PubMed]
- Hernández Borrero, L.J.H.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Reyna, D.E.; Gitego, N.; Kopp, F.; Zhou, H.; Miranda-Roman, M.A.; Nordstrøm, L.U.; Narayanagari, S.-R.; Chi, P.; Vilar, E.J.; et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat. Commun. 2022, 13, 1199. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-inducible factors and cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Iwakuma, T.J. Non-canonical cell death induced by p53. Int. J. Mol. Sci. 2016, 17, 2068. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Serritelli, E.N.; Salvolini, E.; Schiavoni, V.; Cecati, M.; Sartini, D.; Pozzi, V.; Emanuelli, M.J. Contribution of the Paraoxonase-2 Enzyme to Cancer Cell Metabolism and Phenotypes. Biomolecules 2024, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, L.; Matsumoto, M.; Iwata, K.; Umemura, A.; He, F.J. Reactive oxygen species and NRF2 signaling, friends or foes in cancer? Biomolecules 2023, 13, 353. [Google Scholar] [CrossRef]
- Seta, F.; Bellner, L.; Rezzani, R.; Regan, R.F.; Dunn, M.W.; Abraham, N.G.; Gronert, K.; Laniado-Schwartzman, M.J.T. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am. J. Pathol. 2006, 169, 1612–1623. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, H.; Cao, L.J. Recent advances in heat shock proteins in cancer diagnosis, prognosis, metabolism and treatment. Biomed. Pharmacother. 2021, 142, 112074. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D.J. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L.; Liu, Y.; Han, P.; Hong, D.; Li, S.; Ma, A.; Jia, Y.J. Brevilaterin B from Brevibacillus laterosporus has selective antitumor activity and induces apoptosis in epidermal cancer. World J. Microbiol. Biotechnol. 2022, 38, 201. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Fong, Y.; Tsai, E.-M.; Chang, Y.-G.; Chou, H.L.; Wu, C.-Y.; Teng, Y.-N.; Liu, T.-C.; Yuan, S.-S.; Chiu, C.-C. Exogenous C8-Ceramide induces apoptosis by overproduction of ROS and the switch of superoxide dismutases SOD1 to SOD2 in human lung cancer cells. Int. J. Mol. Sci. 2018, 19, 3010. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Zhao, J.; Shi, J.; Wang, M.; Qiu, S.; Hu, Y.; Xu, Y.; Cui, Y.; Liu, C.J. The specific inhibition of SOD1 selectively promotes apoptosis of cancer cells via regulation of the ROS signaling network. Oxid. Med. Cell Longev. 2019, 2019, 9706792. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X.J. SOD1 promotes cell proliferation and metastasis in non-small cell lung cancer via an miR-409-3p/SOD1/SETDB1 epigenetic regulatory feedforward loop. Front. Cell. Dev. Biol. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D.J. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, H.; Piao, C.; Lee, K.; Hyun, J.; Chang, I.; You, H.J. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death Differ. 2013, 20, 117–129. [Google Scholar] [CrossRef]
- Bauer, G.J. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012, 32, 2599–2624. [Google Scholar] [PubMed]
- Bechtel, W.; Bauer, G. Catalase protects tumor cells from apoptosis induction by intercellular ROS signaling. Anticancer Res. 2009, 29, 4541–4557. [Google Scholar] [PubMed]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Medicine, Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Menegon, S.; Columbano, A.; Giordano, S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Caamano, J.; Zhang, S.; Rosvold, E.; Bauer, B.; Klein-Szanto, A.J. p53 alterations in human squamous cell carcinomas and carcinoma cell lines. Am. J. Pathol. 1993, 142, 1131. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [PubMed]
- Marquette, A.; Bechinger, B. Biophysical investigations elucidating the mechanisms of action of antimicrobial peptides and their synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
 ) or decrease (
) or decrease ( ) in protein expression.
) in protein expression.
 ) or decrease (
) or decrease ( ) in protein expression.
) in protein expression.
 ) or decrease (
) or decrease ( ) in protein expression.
) in protein expression.
 ) or decrease (
) or decrease ( ) in protein expression.
) in protein expression.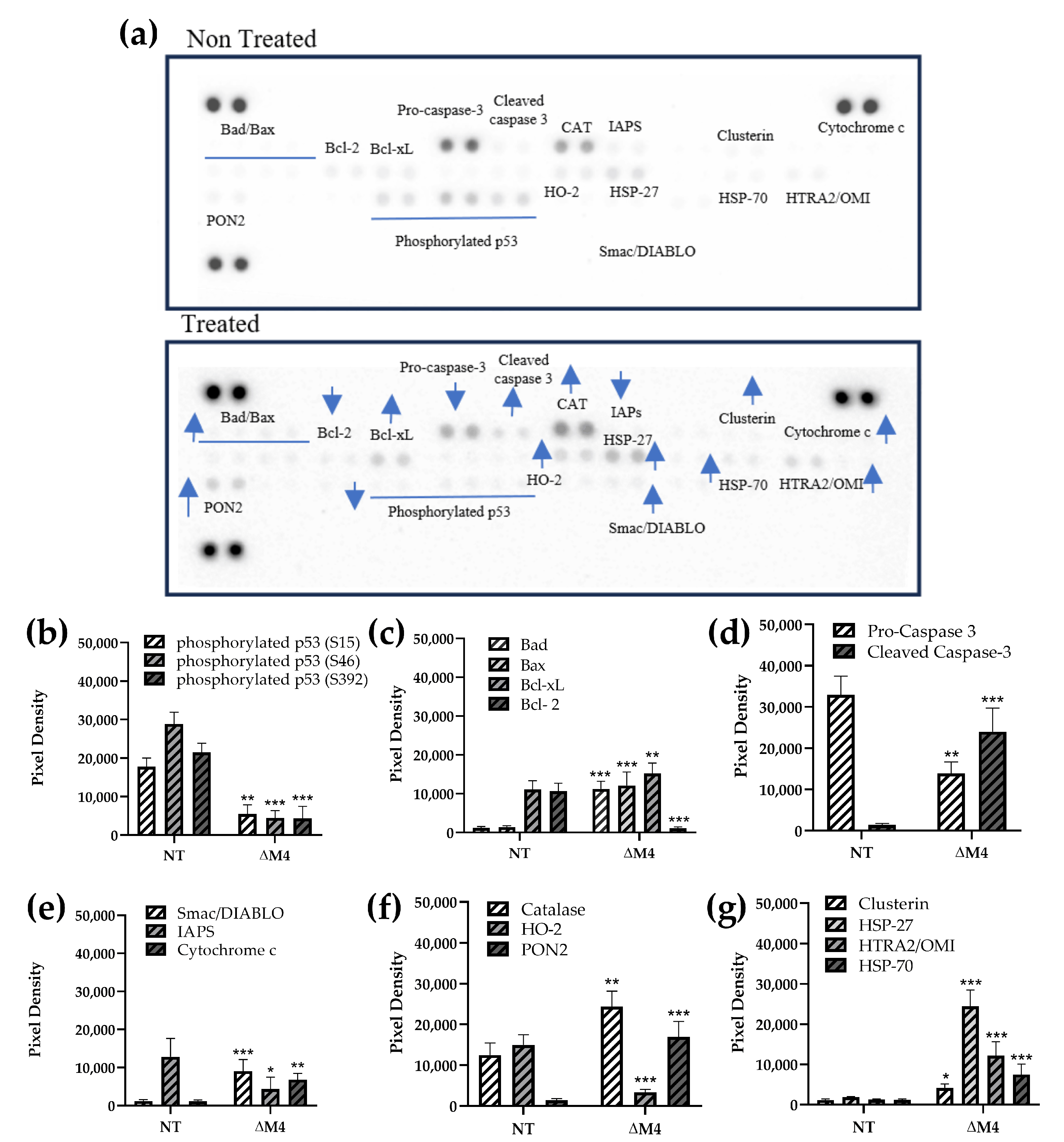


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fandiño-Devia, E.; Brankiewicz, A.; Santa-González, G.A.; Guevara-Lora, I.; Manrique-Moreno, M. Comparative Study of the Potential Cell-Penetrating Peptide ∆M4 on Apoptosis Cell Signaling in A375 and A431 Cancer Cell Lines. Pharmaceutics 2024, 16, 775. https://doi.org/10.3390/pharmaceutics16060775
Fandiño-Devia E, Brankiewicz A, Santa-González GA, Guevara-Lora I, Manrique-Moreno M. Comparative Study of the Potential Cell-Penetrating Peptide ∆M4 on Apoptosis Cell Signaling in A375 and A431 Cancer Cell Lines. Pharmaceutics. 2024; 16(6):775. https://doi.org/10.3390/pharmaceutics16060775
Chicago/Turabian StyleFandiño-Devia, Estefanía, Aleksandra Brankiewicz, Gloria A. Santa-González, Ibeth Guevara-Lora, and Marcela Manrique-Moreno. 2024. "Comparative Study of the Potential Cell-Penetrating Peptide ∆M4 on Apoptosis Cell Signaling in A375 and A431 Cancer Cell Lines" Pharmaceutics 16, no. 6: 775. https://doi.org/10.3390/pharmaceutics16060775
APA StyleFandiño-Devia, E., Brankiewicz, A., Santa-González, G. A., Guevara-Lora, I., & Manrique-Moreno, M. (2024). Comparative Study of the Potential Cell-Penetrating Peptide ∆M4 on Apoptosis Cell Signaling in A375 and A431 Cancer Cell Lines. Pharmaceutics, 16(6), 775. https://doi.org/10.3390/pharmaceutics16060775







