Enhanced In Vitro Antiviral Activity of Ivermectin-Loaded Nanostructured Lipid Carriers against Porcine Epidemic Diarrhea Virus via Improved Intracellular Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical, Cells, and Viruses
2.2. Preparation of IVM-NLCs
2.3. Characterization of IVM-NLCs
2.3.1. Hydrodynamic Diameter (HD), Polydispersity Index (PDI), and Zeta Potential (ZP)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.4. Entrapment Efficiency (EE) and Drug Loading (DL)
2.4. Cell Culture
2.5. Cell Viability Evaluation
2.6. In Vitro Cellular Uptake
2.7. TCID50 Assay
2.8. Antiviral Assay
2.9. One-Step Growth Curve
2.10. RT-qPCR
2.11. Western Blot Analysis
2.12. IFA
2.13. Determination of Reactive Oxygen Species (ROS) Generation
2.14. Mitochondrial Membrane Potential (MMP) Analysis
2.15. Apoptosis Assay
2.16. Statistical Analysis
3. Results and Discussion
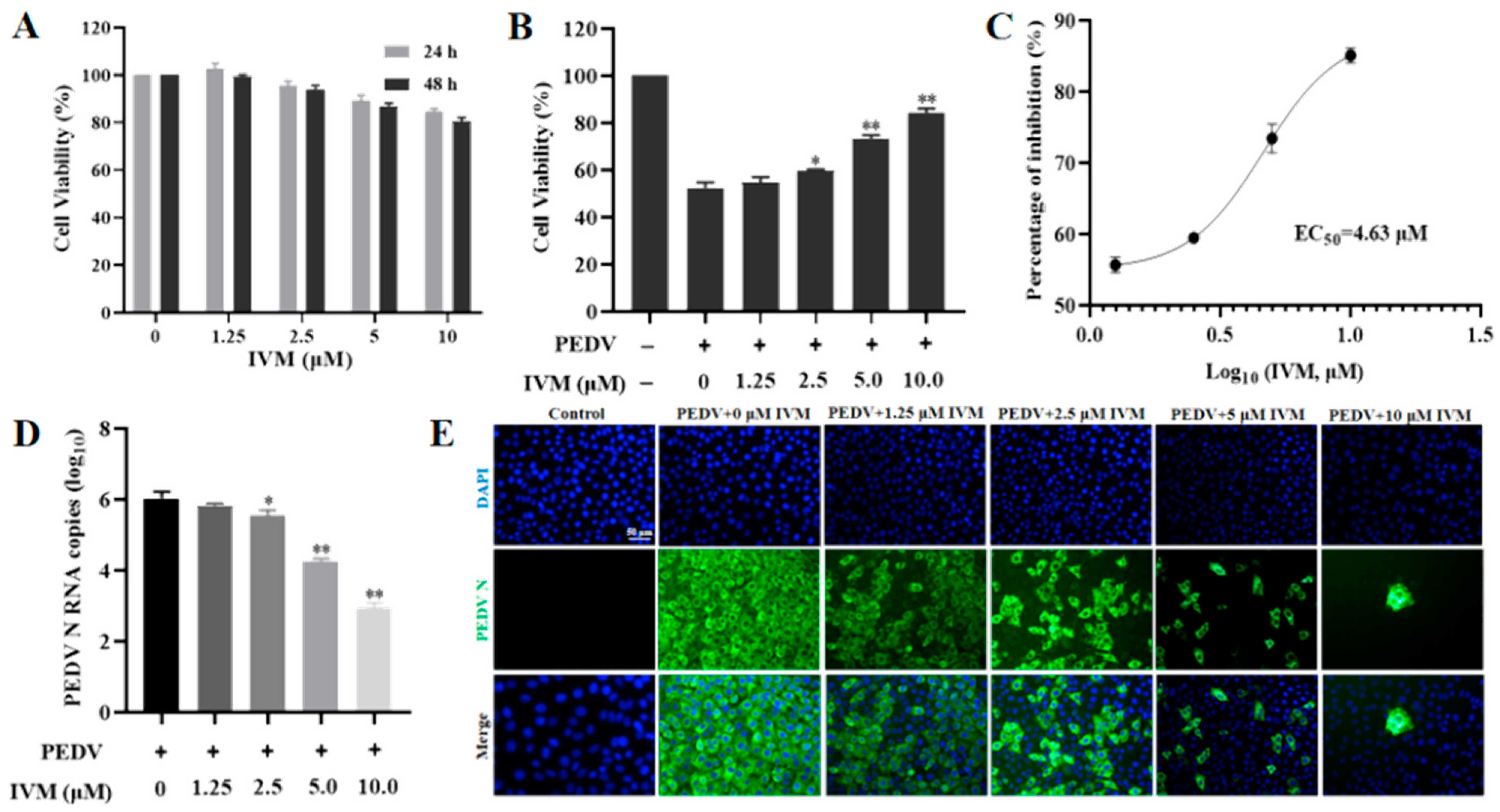
3.1. IVM Inhibited the Infectivity of PEDV
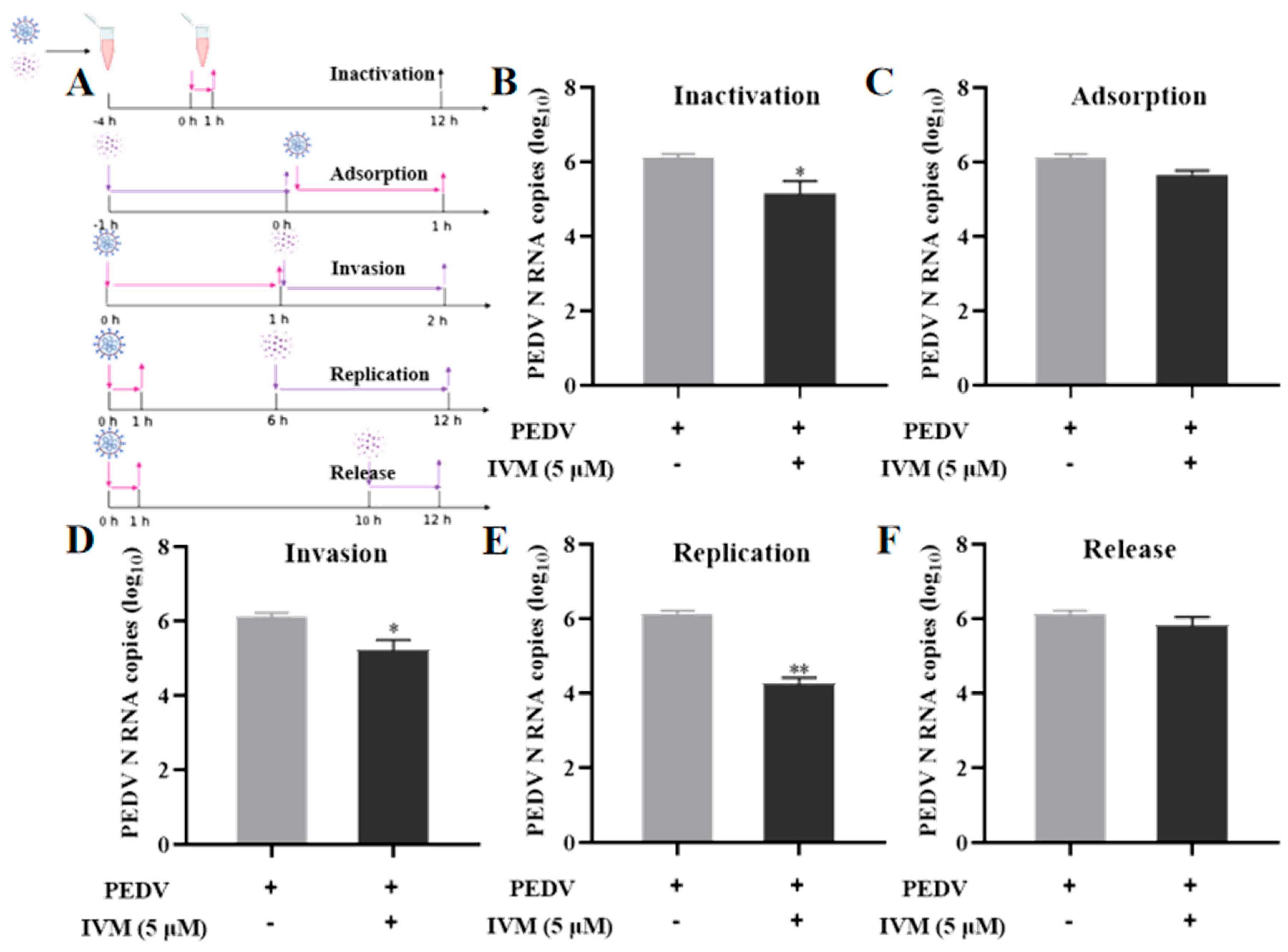
3.2. Effect of IVM in Diverse Stages of PEDV Life Cycle
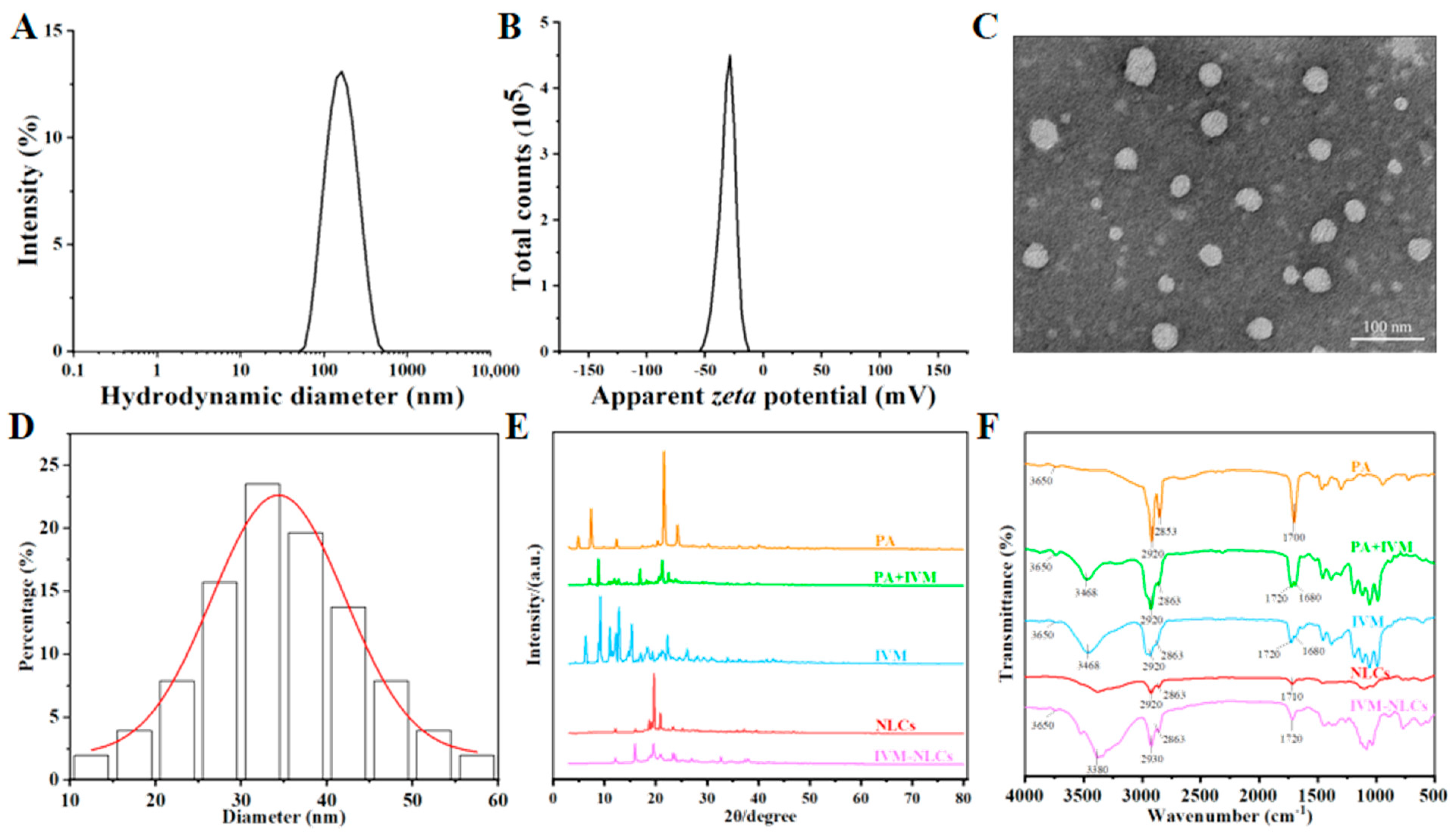
3.3. Characterization of IVM-Loaded Nanostructured Lipid Carriers
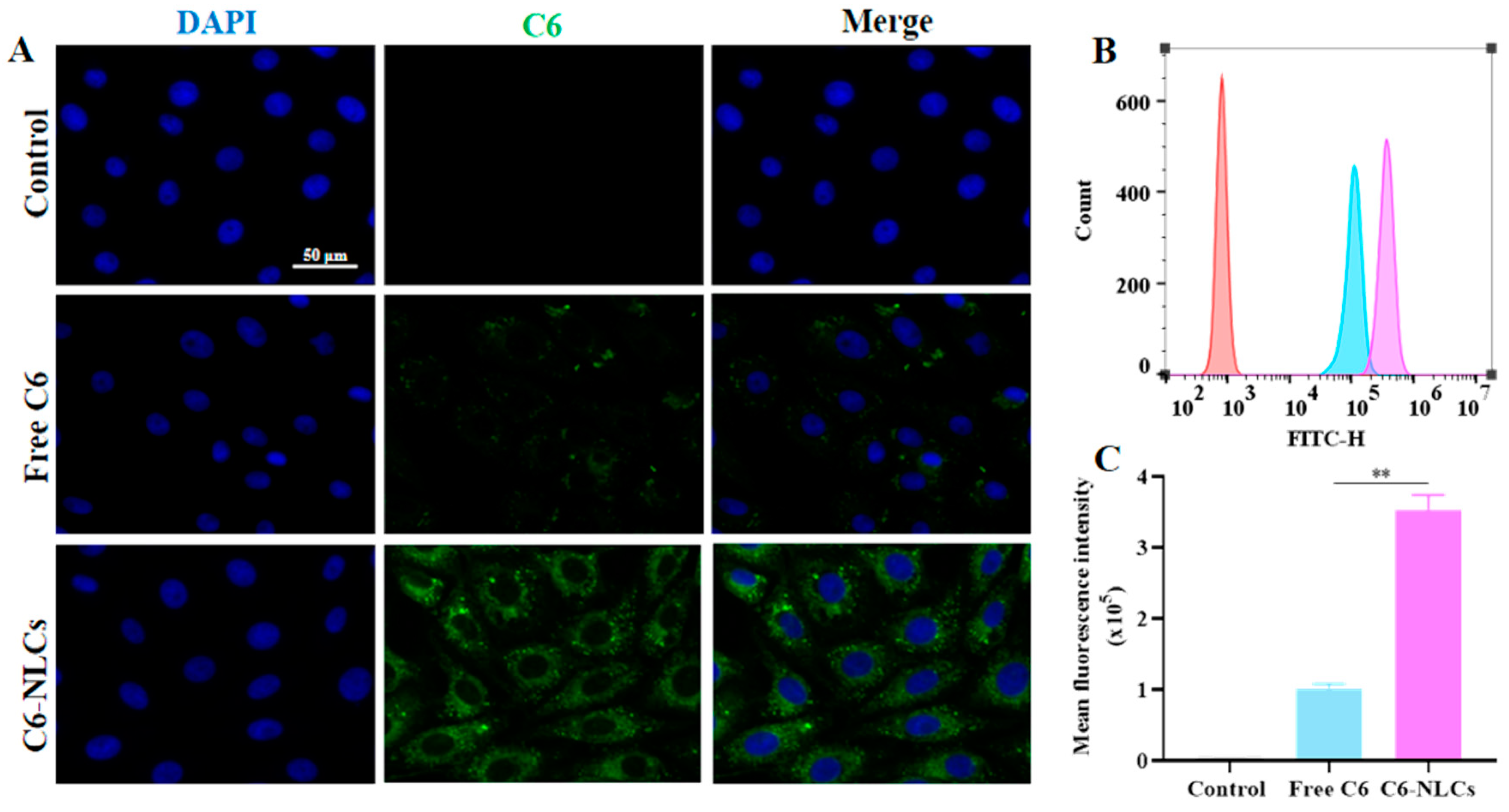
3.4. NLCs Improved Cellular Uptake of IVM
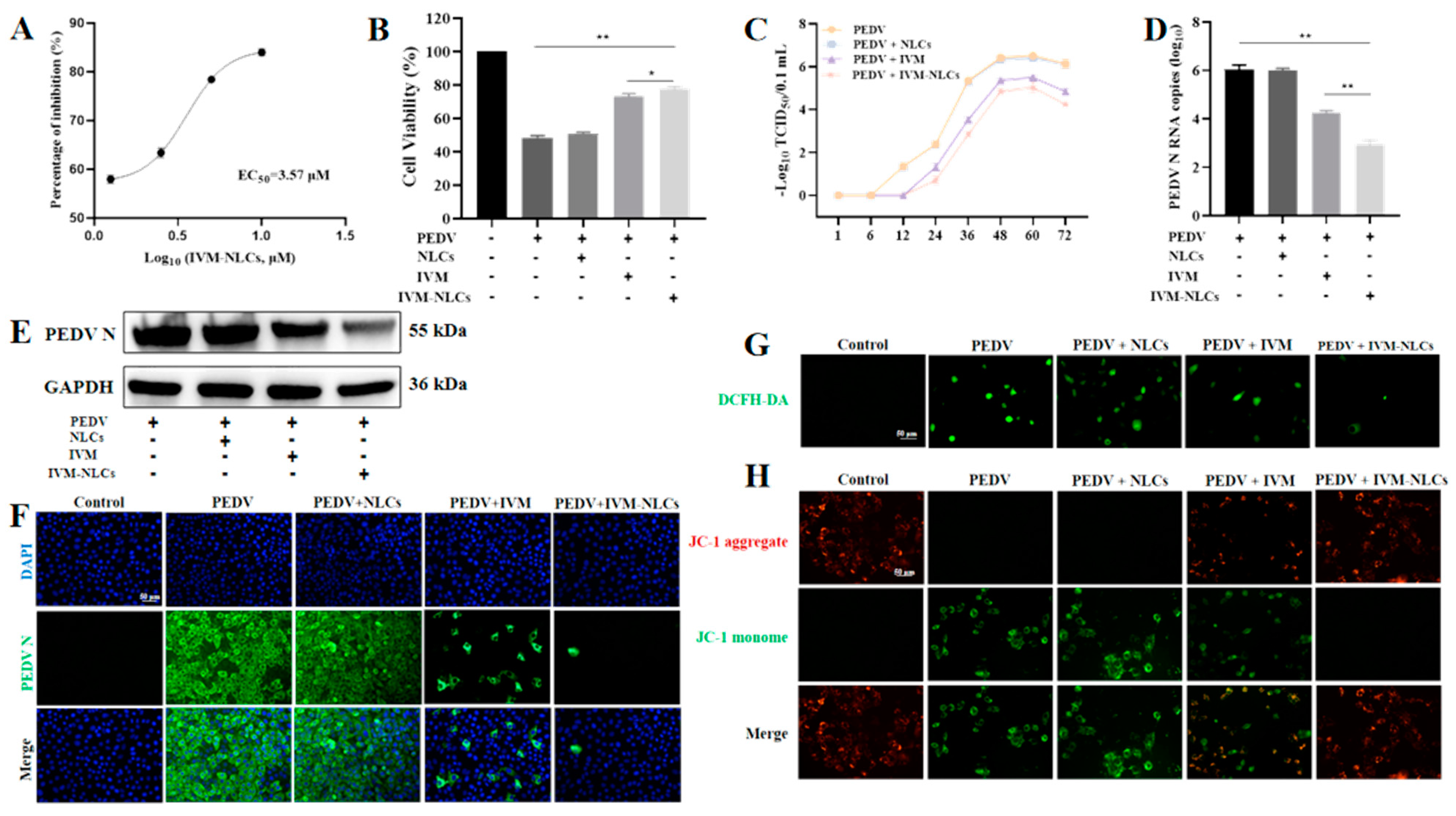
3.5. NLCs Enhanced the Antiviral Activity of IVM against PEDV
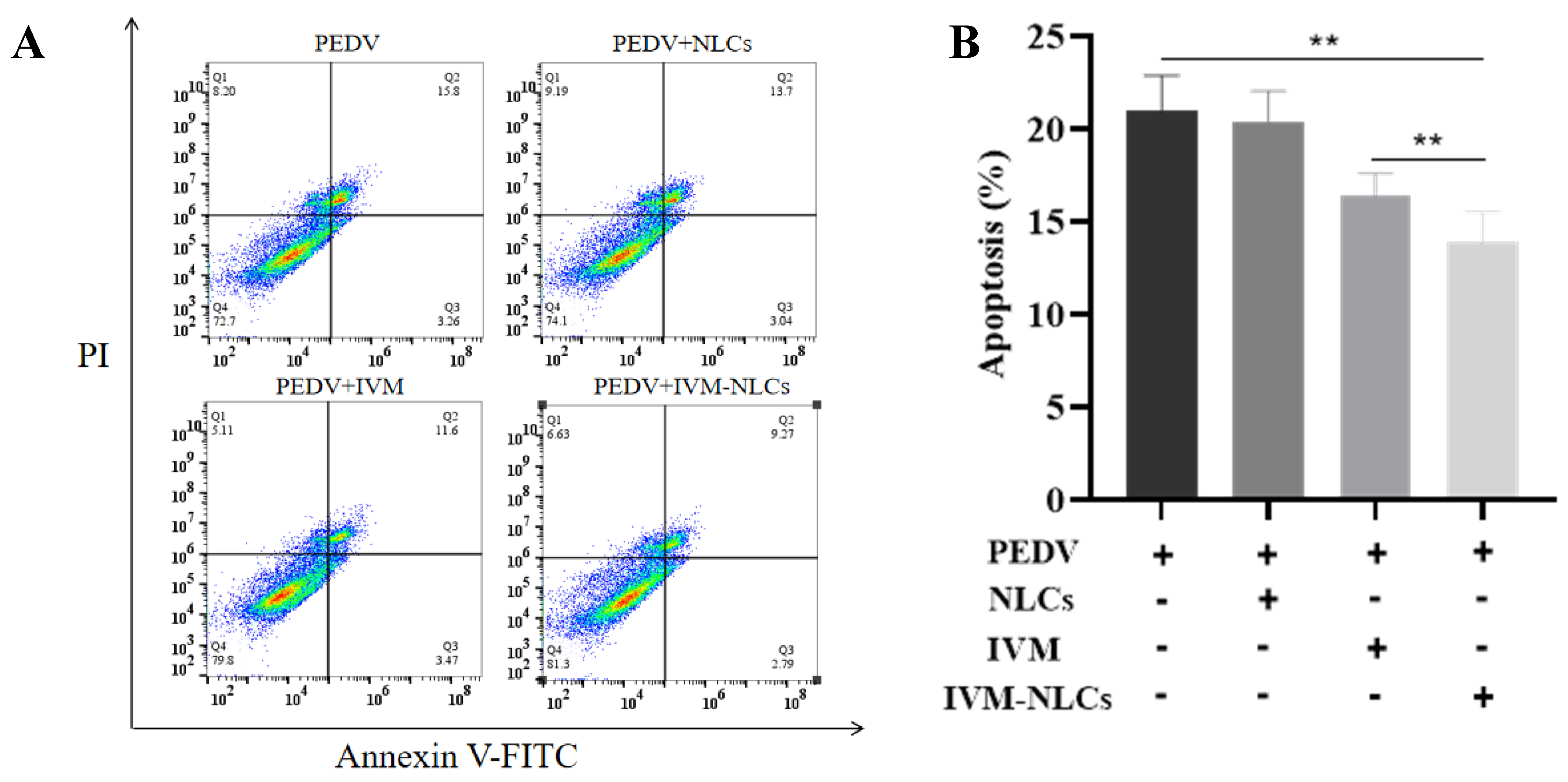
3.6. Effect of IVM-NLCs on the Apoptosis Rate in PEDV-Infected Vero Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.-J.; Jung, Y.-S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344-20. [Google Scholar] [CrossRef] [PubMed]
- Jung, K. Porcine epidemic diarrhea virus (PEDV)_An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y. In situ structure and dynamics of an alphacoronavirus spike protein by cryo-ET and cryo-EM. Nat. Commun. 2022, 13, 4877. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O.-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019, 93, e00406-19. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wang, N.; Jiao, H.; Zhang, J.; Li, C.; Ren, W.; Reiter, R.J.; Su, S. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 2021, 71, e12754. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, N.; Jiao, Y.; Dong, S.; Sun, D.; Chen, X.; Zheng, H.; Tong, W.; Yu, H.; Yu, L.; et al. EGR1 Suppresses Porcine Epidemic Diarrhea Virus Replication by Regulating IRAV to Degrade Viral Nucleocapsid Protein. J. Virol. 2021, 95, e00645-21. [Google Scholar] [CrossRef] [PubMed]
- Roessler, H.I.; Knoers, N.V.A.M.; van Haelst, M.M.; van Haaften, G. Drug Repurposing for Rare Diseases. Trends Pharmacol. Sci. 2021, 42, 255–267. [Google Scholar] [CrossRef]
- Ng, Y.L.; Salim, C.K.; Chu, J.J.H. Drug repurposing for COVID-19: Approaches, challenges and promising candidates. Pharmacol. Ther. 2021, 228, 107930. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Yzet, C.; Bossuyt, P.; Baert, F.J.; Vanasek, T.; D’Haens, G.R.; Joustra, V.W.; Panaccione, R.; Novacek, G.; Reinisch, W.; et al. Deep Remission at 1 Year Prevents Progression of Early Crohn’s Disease. Gastroenterology 2020, 159, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Aref, Z.F.; Bazeed, S.E.E.S.; Hassan, M.H.; Hassan, A.S.; Rashad, A.; Hassan, R.G.; Abdelmaksoud, A.A. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. IJN 2021, 16, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Li, M.; Wen, Y.; Song, Y.; Li, J. Construction of ivermectin producer by domain swaps of avermectin polyketide synthase in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 2006, 72, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, W.; Dou, Q.; Chen, H.; Li, Q.; Nice, E.C.; Huang, C. Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. Autophagy 2016, 12, 2498–2499. [Google Scholar] [CrossRef]
- Martin, R.J.; Robertson, A.P.; Choudhary, S. Ivermectin: An Anthelmintic, an Insecticide, and Much More. Trends Parasitol. 2021, 37, 48–64. [Google Scholar] [CrossRef]
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.; Wang, X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018, 159, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.F.; Fraser, J.E.; Chan, W.K.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Götz, V.; Magar, L.; Dornfeld, D.; Giese, S.; Pohlmann, A.; Höper, D.; Kong, B.-W.; Jans, D.A.; Beer, M.; Haller, O.; et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 2016, 6, 23138. [Google Scholar] [CrossRef]
- Thomas, D.R.; Lundberg, L.; Pinkham, C.; Shechter, S.; DeBono, A.; Baell, J.; Wagstaff, K.M.; Hick, C.A.; Kehn-Hall, K.; Jans, D.A. Identification of novel antivirals inhibiting recognition of Venezuelan equine encephalitis virus capsid protein by the Importin α/β1 heterodimer through high-throughput screening. Antivir. Res. 2018, 151, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Polli, J.E. Prediction of positive food effect: Bioavailability enhancement of BCS class II drugs. Int. J. Pharm. 2016, 506, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Garciafuentes, M.; Torres, D.; Alonso, M. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int. J. Pharm. 2005, 296, 122–132. [Google Scholar] [CrossRef]
- Valencia-Lazcano, A.A.; Hassan, D.; Pourmadadi, M.; Shamsabadipour, A.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. 5-Fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur. J. Med. Chem. 2023, 246, 114995. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, H.; Sahito, B.; Li, X.; Peng, L.; Gao, X.; Ji, H.; Wang, L.; Jiang, S.; Guo, D. Nanostructured lipid carriers with exceptional gastrointestinal stability and inhibition of P-gp efflux for improved oral delivery of tilmicosin. Colloids Surf. B Biointerfaces 2020, 187, 110649. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-loaded solid lipid nanoparticles: Preparation, characterisation, stability and transdermal behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 Attachment, Entry, and Cell-to-Cell Spread by Functionalized Multivalent Gold Nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Jiang, Y.; Yang, W.; Huang, H.; Shi, C.; Yang, G.; Wang, C. Surface-Displayed Porcine IFN-λ3 in Lactobacillus plantarum Inhibits Porcine Enteric Coronavirus Infection of Porcine Intestinal Epithelial Cells. J. Microbiol. Biotechnol. 2020, 30, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Kong, N.; Zhang, Y.; Song, Y.; Qin, W.; Yang, X.; Ye, C.; Ye, M.; Tong, W.; Liu, C.; et al. N protein of PEDV plays chess game with host proteins by selective autophagy. Autophagy 2023, 19, 2338–2352. [Google Scholar] [CrossRef] [PubMed]
- Jaru-Ampornpan, P.; Jengarn, J.; Wanitchang, A.; Jongkaewwattana, A. Porcine Epidemic Diarrhea Virus 3C-Like Protease-Mediated Nucleocapsid Processing: Possible Link to Viral Cell Culture Adaptability. J. Virol. 2017, 91, e01660-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, J.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Colloid properties of hydrophobic modified alginate: Surface tension, ζ-potential, viscosity and emulsification. Carbohydr. Polym. 2018, 181, 56–62. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle size distribution quantification from transmission electron microscopy (TEM) of ruthenium tetroxide stained polymeric nanoparticles. J. Colloid. Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, L.; Peers, S.; Dalverny, C.; Ladavière, C. Tunable morphology of lipid/chitosan particle assemblies. J. Colloid. Interface Sci. 2019, 534, 105–109. [Google Scholar] [CrossRef]
- Pina, M.F.; Pinto, J.F.; Sousa, J.J.; Craig, D.Q.M.; Zhao, M. Generation of hydrate forms of paroxetine HCl from the amorphous state: An evaluation of thermodynamic and experimental predictive approaches. Int. J. Pharm. 2015, 481, 114–124. [Google Scholar] [CrossRef]
- Ud-Din, A.I.M.S.; Roujeinikova, A. Cloning, purification, crystallization and X-ray crystallographic analysis of the periplasmic sensing domain of Pseudomonas fluorescens chemotactic transducer of amino acids type A (CtaA). BST 2016, 10, 320–324. [Google Scholar] [CrossRef][Green Version]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Livney, Y.D.; Assaraf, Y.G. Targeted nanomedicine modalities for prostate cancer treatment. Drug Resist. Updates 2021, 56, 100762. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Gao, S.; Zuo, Q.; Gong, J.; Song, X.; Liu, Y.; Xiao, J.; Zhai, X.; Sun, H.; Zhang, M.; et al. Enhanced In Vitro Antiviral Activity of Ivermectin-Loaded Nanostructured Lipid Carriers against Porcine Epidemic Diarrhea Virus via Improved Intracellular Delivery. Pharmaceutics 2024, 16, 601. https://doi.org/10.3390/pharmaceutics16050601
Xu X, Gao S, Zuo Q, Gong J, Song X, Liu Y, Xiao J, Zhai X, Sun H, Zhang M, et al. Enhanced In Vitro Antiviral Activity of Ivermectin-Loaded Nanostructured Lipid Carriers against Porcine Epidemic Diarrhea Virus via Improved Intracellular Delivery. Pharmaceutics. 2024; 16(5):601. https://doi.org/10.3390/pharmaceutics16050601
Chicago/Turabian StyleXu, Xiaolin, Shasha Gao, Qindan Zuo, Jiahao Gong, Xinhao Song, Yongshi Liu, Jing Xiao, Xiaofeng Zhai, Haifeng Sun, Mingzhi Zhang, and et al. 2024. "Enhanced In Vitro Antiviral Activity of Ivermectin-Loaded Nanostructured Lipid Carriers against Porcine Epidemic Diarrhea Virus via Improved Intracellular Delivery" Pharmaceutics 16, no. 5: 601. https://doi.org/10.3390/pharmaceutics16050601
APA StyleXu, X., Gao, S., Zuo, Q., Gong, J., Song, X., Liu, Y., Xiao, J., Zhai, X., Sun, H., Zhang, M., Gao, X., & Guo, D. (2024). Enhanced In Vitro Antiviral Activity of Ivermectin-Loaded Nanostructured Lipid Carriers against Porcine Epidemic Diarrhea Virus via Improved Intracellular Delivery. Pharmaceutics, 16(5), 601. https://doi.org/10.3390/pharmaceutics16050601








