Exploring Microemulsion Systems for the Incorporation of Glucocorticoids into Bacterial Cellulose: A Novel Approach for Anti-Inflammatory Wound Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Pseudo-Ternary Phase Diagram and ME Components
2.2. Preparation of ME Formulations and Glucocorticoid Loading
- ME-A; ME-B; ME-C; ME-D; ME-E (blank MEs without API).
- ME-A-HC; ME-B-HC; ME-C-HC; ME-D-HC; ME-E-HC (HC-loaded MEs).
- ME-A-DEX; ME-B-DEX; ME-C-DEX; ME-D-DEX; ME-E-DEX (DEX-loaded MEs).
2.3. Microemulsion Characterization
2.3.1. Rheology
2.3.2. Dynamic Light Scattering and Zeta Potential
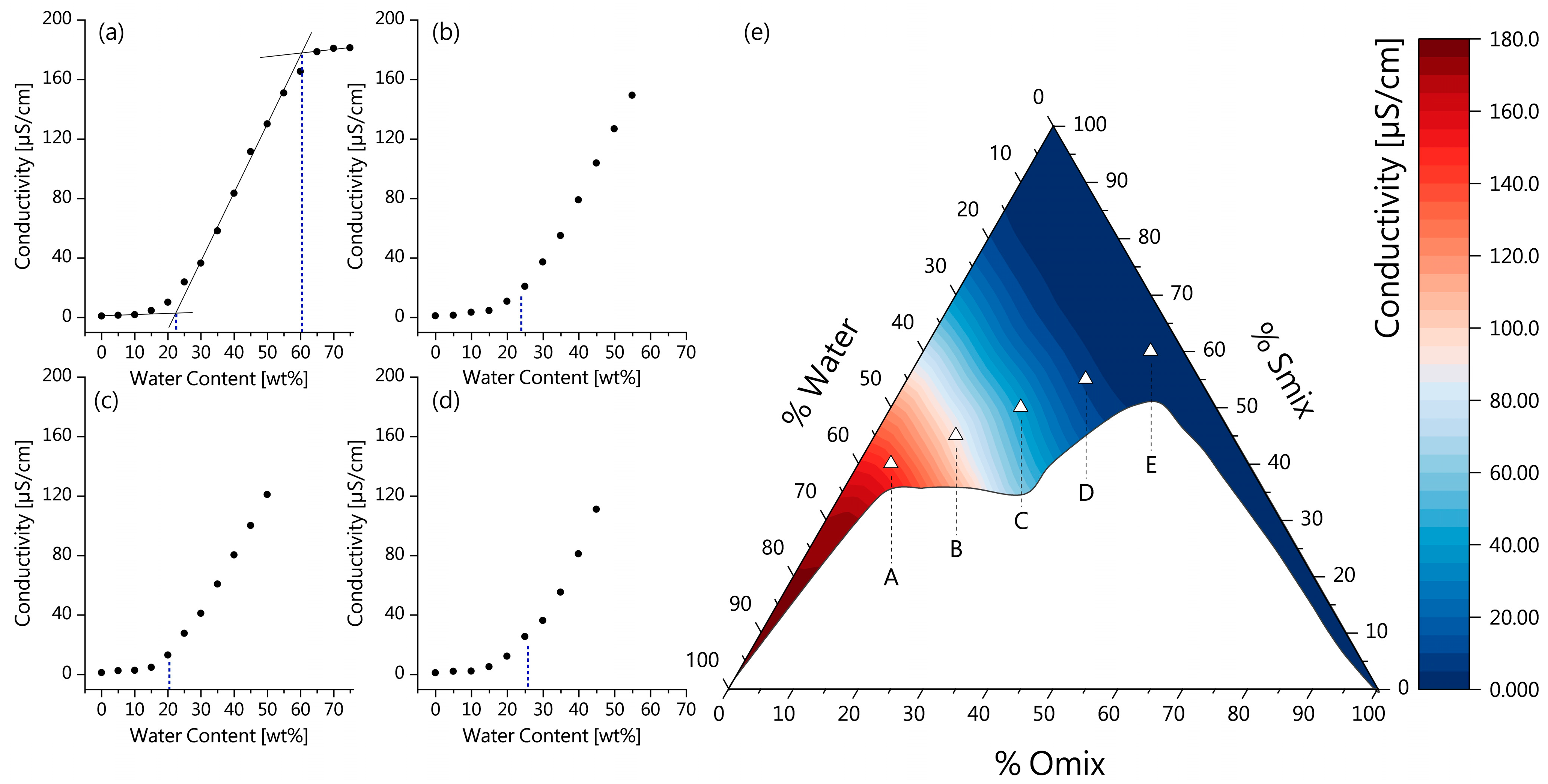
2.3.3. Electrical Conductivity
2.3.4. pH, Refractive Index and Isotropy
2.3.5. Thermodynamic Stability
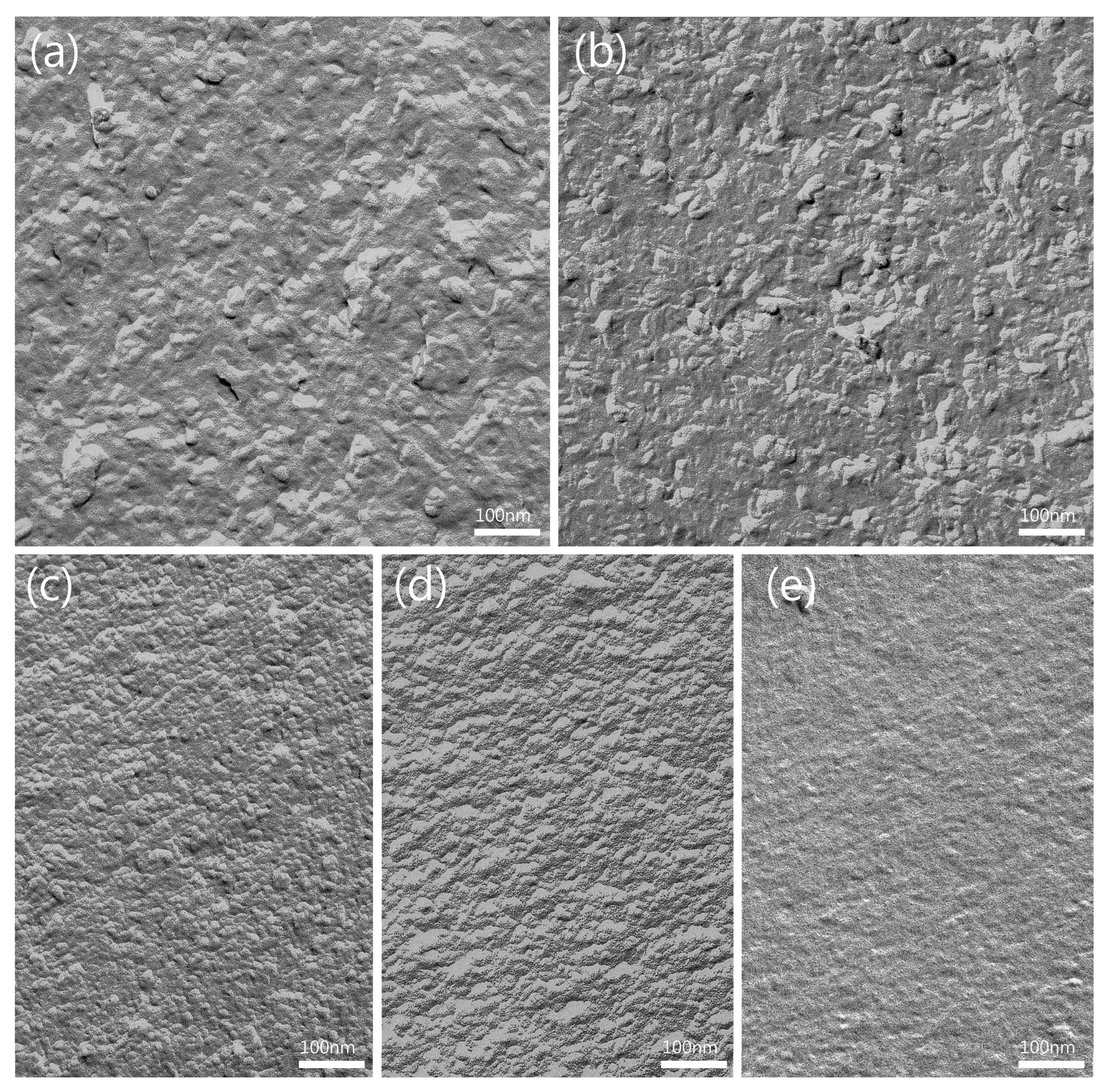
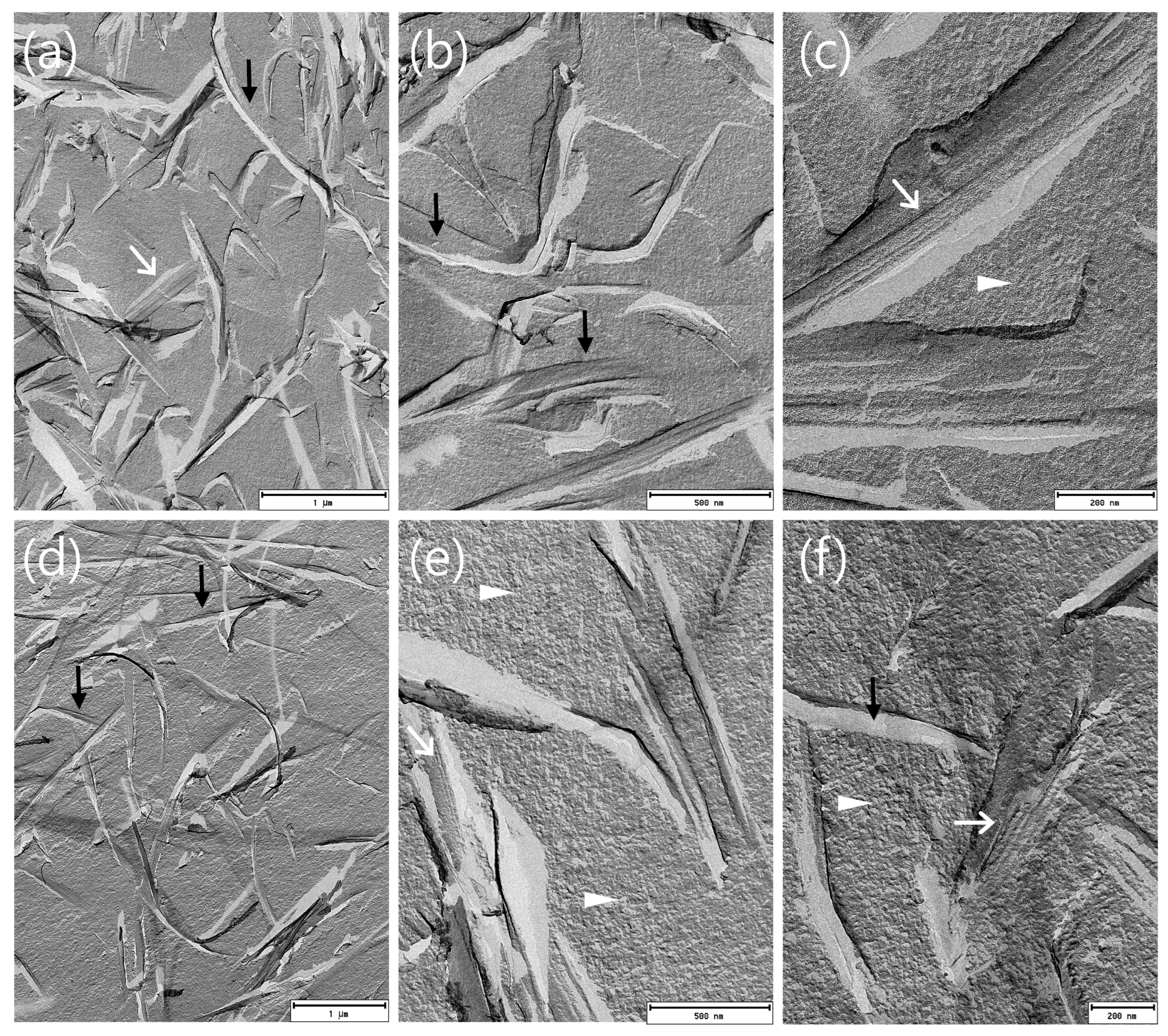
2.3.6. Freeze–Fracture Transmission Electron Microscopy (FF-TEM)
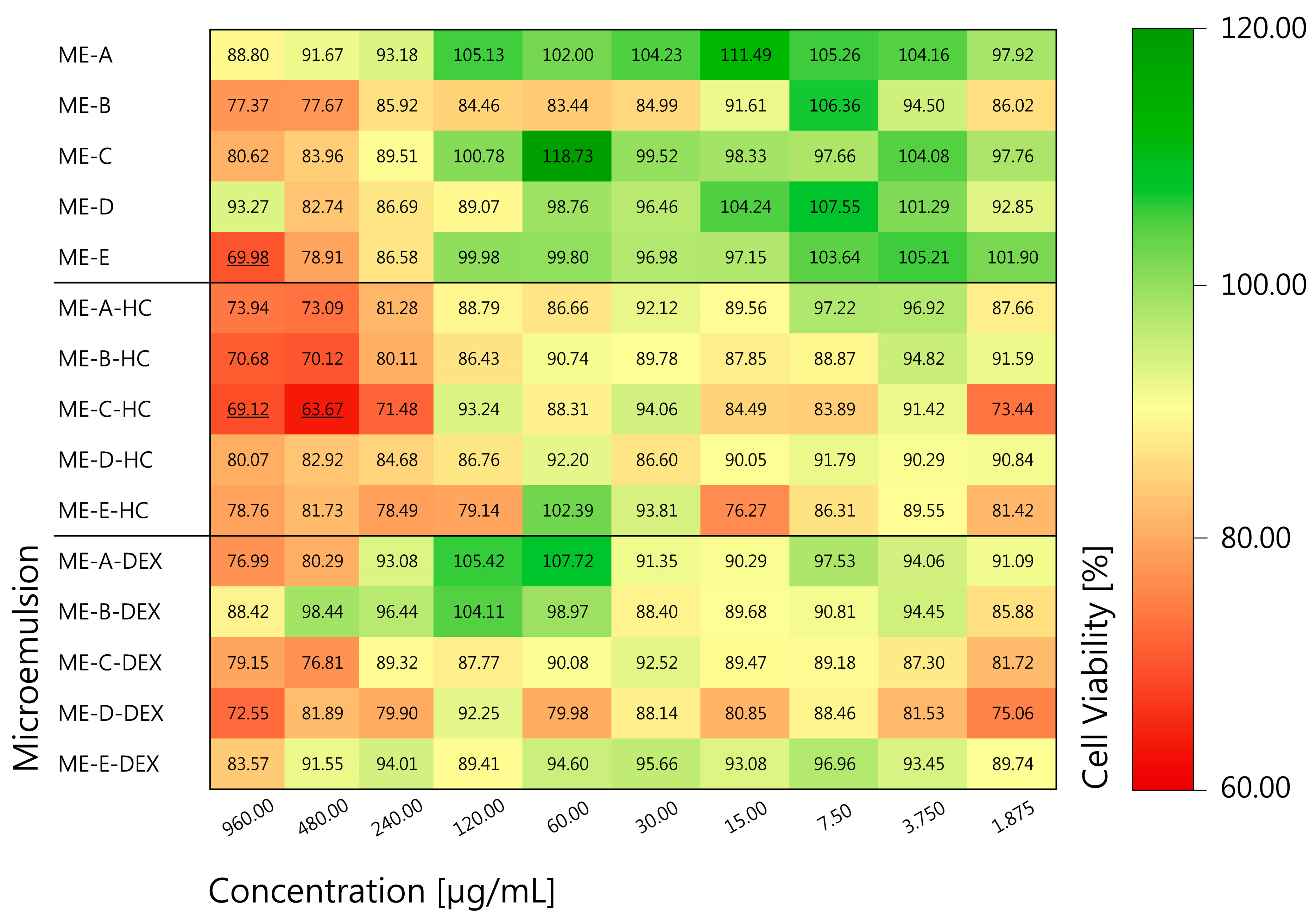
2.3.7. In Vitro Cytotoxicity
2.4. Stability Testing
2.4.1. Storage Stability
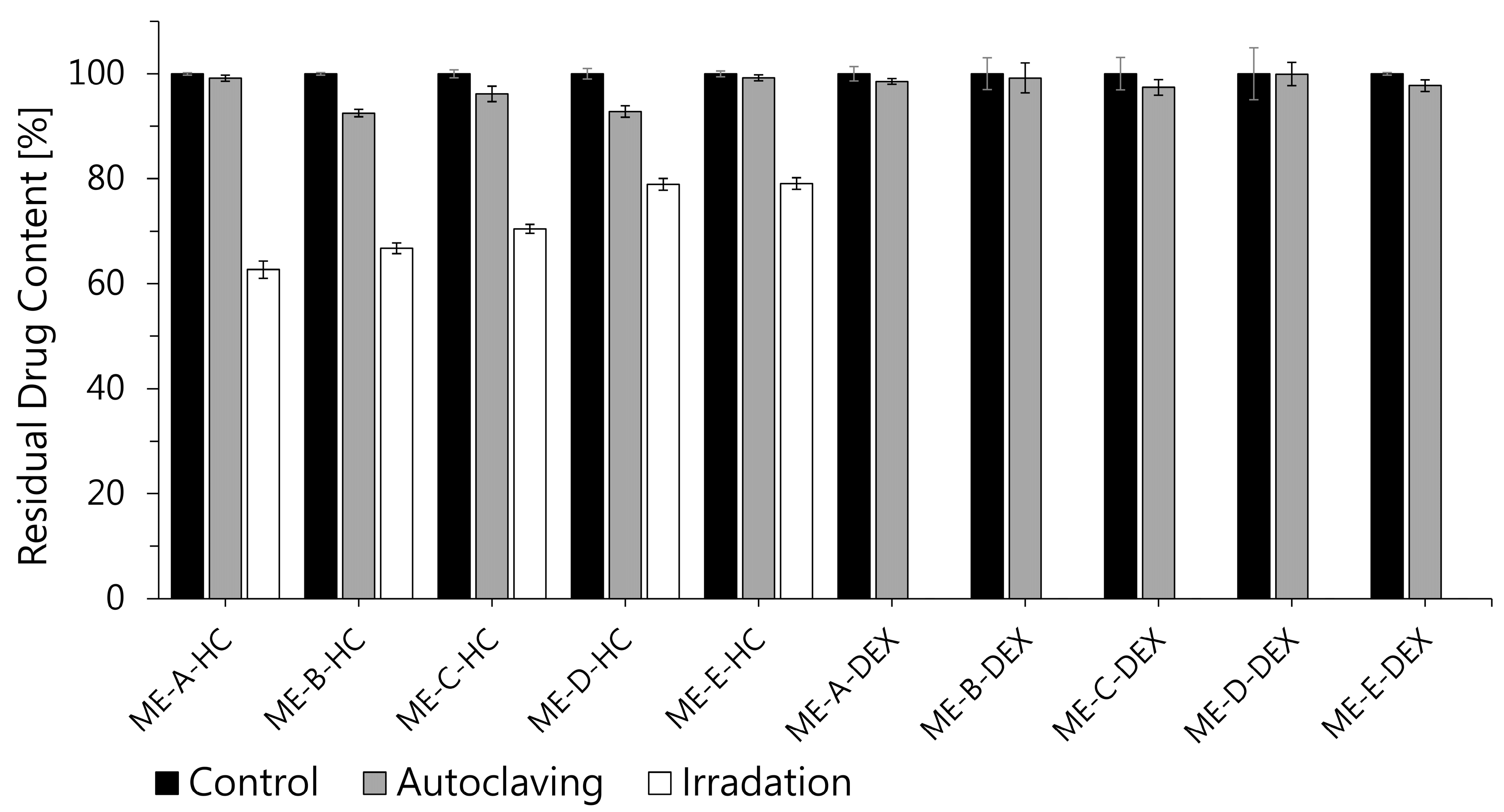
2.4.2. Sterilization Stability
2.5. Preparation and Microemulsion Loading of BC
- BC-ME-A; BC-ME-B; BC-ME-C; BC-ME-D; BC-ME-E (ME-loaded BC without API).
- BC-ME-A-HC; BC-ME-B-HC; BC-ME-C-HC; BC-ME-D-HC; BC-ME-E-HC (ME-loaded BC containing HC).
- BC-ME-A-DEX; BC-ME-B-DEX; BC-ME-C-DEX; BC-ME-D-DEX; BC-ME-E-DEX (ME-loaded BC containing DEX).
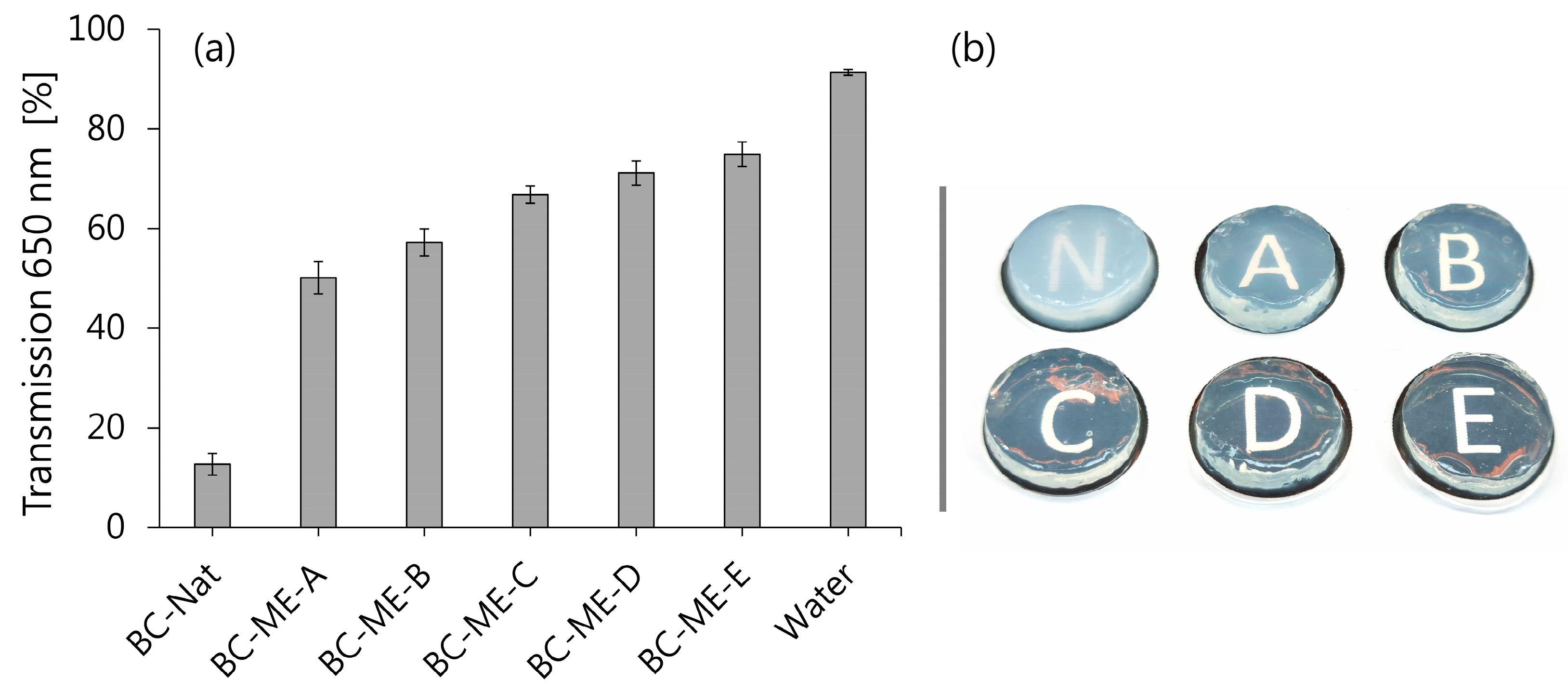
2.5.1. Transparency
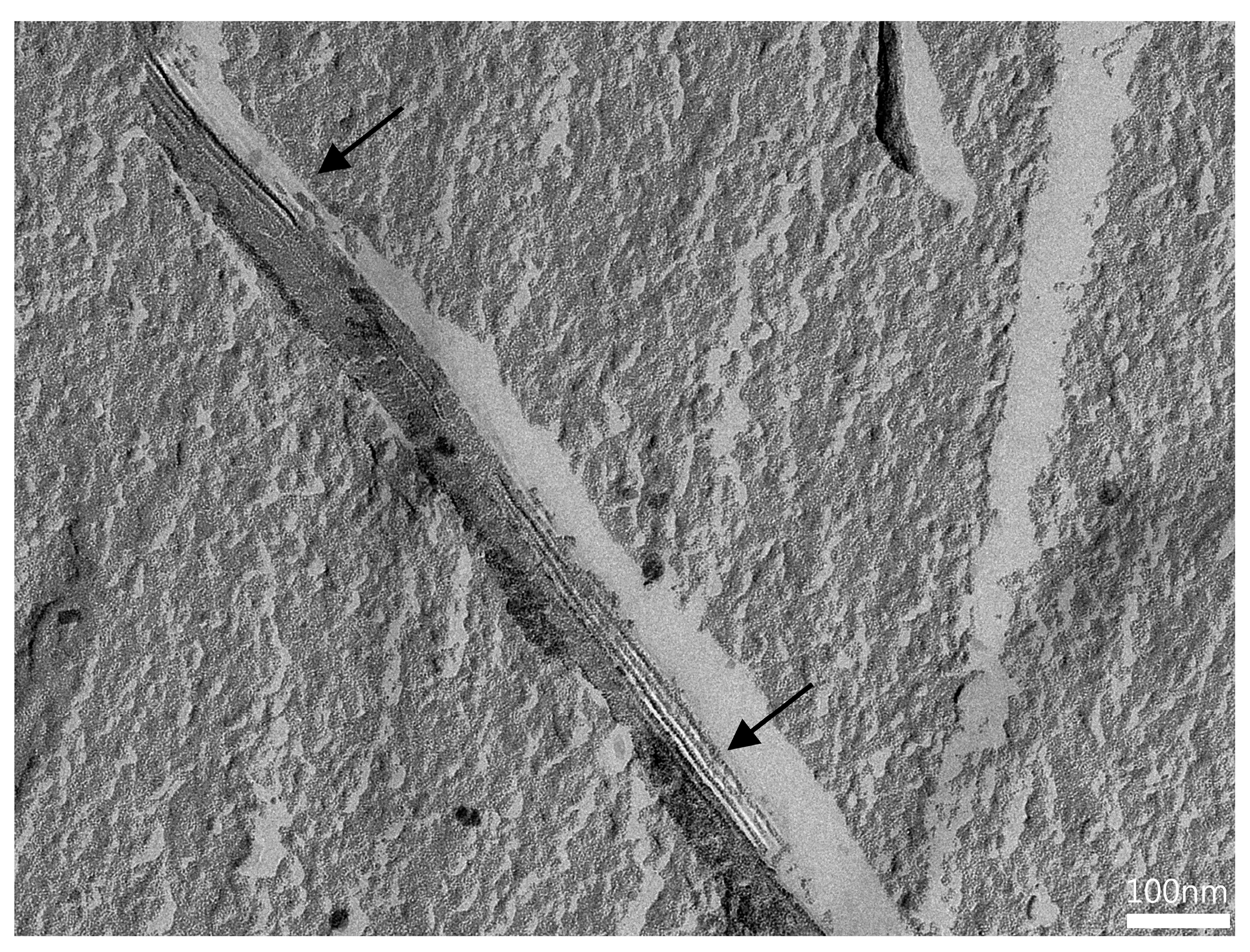
2.5.2. FF-TEM
2.6. In Vitro Strat-M® Permeation and Anti-Inflammatory Activity
2.6.1. Strat-M® Membrane Permeation Testing
2.6.2. API Release and Permeation from BC-ME
2.6.3. Monocyte Incubations and Determination of TNFα Release
2.7. Quantification of Glucocorticoids DEX and HC
3. Results and Discussion
3.1. Microemulsion Characterization
3.1.1. Pseudo-Ternary Phase Diagram
3.1.2. Electrical Conductivity and ME Microstructure
3.1.3. Physicochemical Characterization
3.1.4. In Vitro Cytotoxicity
3.1.5. FF-TEM of ME Formulations
3.2. Stability Testing
3.2.1. Storage Stability
3.2.2. Sterilization Stability
3.3. Loading, Permeation and Anti-Inflammatory Activity
3.3.1. Loading Behavior
3.3.2. Transparency
3.3.3. FF-TEM of BC-ME
3.3.4. In Vitro Permeation
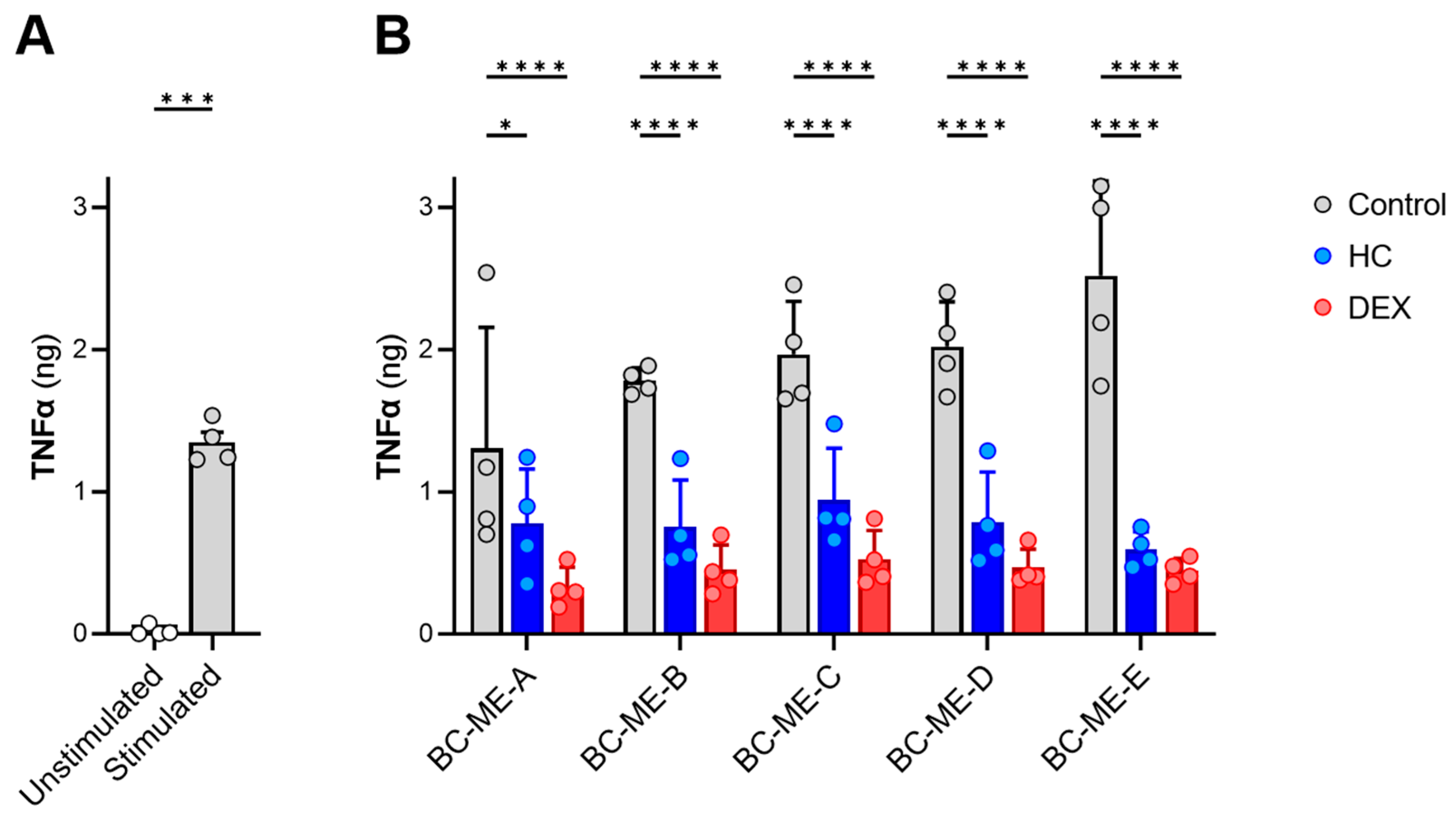
3.3.5. Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Terms
| AFM | atomic force microscopy |
| API(s) | active pharmaceutical ingredient(s) |
| BC | bacterial cellulose |
| DEX | dexamethasone |
| DEXA-LAW | “DEXAMETHASON Creme LAW, 0.05%”, commercial dexamethasone cream |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DSMZ | Deutsche Sammlung von Mikroorganismen und Zellkulturen (German Collection of Microorganisms and Cell Cultures) |
| EBENOL® | “EBENOL® 0.5% Creme”, commercial hydrocortisone cream |
| EDTA | ethylene diamine tetraacetic acid (sodium salt dihydrate) |
| ELISA | enzyme-linked immunosorbent assay |
| FCS | fetal calf serum |
| FFT | fast Fourier transform |
| FF-TEM | freeze–fracture transmission electron microscopy |
| GR | glucocorticoid receptor |
| GRC | glucocorticoid receptor complex |
| GRE | glucocorticoid response element |
| HaCaT | human epidermal keratinocyte cell line |
| HC | hydrocortisone |
| HSM | Hestrin–Schramm culture medium |
| IL | interleukin |
| K. xylinus | Komagataeibacter xylinus |
| LPS | lipopolysaccharide |
| ME(s) | microemulsion(s) |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NFκB | nuclear factor κB |
| Omix | oil phase mixture |
| PBMC | peripheral blood mononuclear cells |
| RH | relative humidity |
| rpm | revolutions per minute |
| RPMI | RPMI 1640 cell culture medium |
| Smix | surfactant/cosurfactant mixture |
| TNFα | tumor necrosis factor alpha |
References
- Alavi, A.; French, L.E.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and Bioequivalence of Topical Glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Buttgereit, F. Genomic and Nongenomic Effects of Glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, A.; Feliciani, C.; Tulli, A.; Amerio, P. Pharmacotherapy of Inflammatory and Pruritic Manifestations of Corticosteroid-Responsive Dermatoses Focus on Clobetasol Propionate. Clin. Med. Insights Ther. 2010, 2, CMT.S1993. [Google Scholar] [CrossRef]
- Barnes, P.J. Anti-Inflammatory Actions of Glucocorticoids: Molecular Mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Drouin, J.; Sun, Y.L.; Chamberland, M.; Gauthier, Y.; De Léan, A.; Nemer, M.; Schmidt, T.J. Novel Glucocorticoid Receptor Complex with DNA Element of the Hormone-Repressed POMC Gene. EMBO J. 1993, 12, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Mikics, E.; Makara, G.B. The Effects of Non-Genomic Glucocorticoid Mechanisms on Bodily Functions and the Central Neural System. A Critical Evaluation of Findings. Front. Neuroendocrinol. 2008, 29, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Schäcke, H.; Rehwinkel, H.; Asadullah, K.; Cato, A.C.B. Insight into the Molecular Mechanisms of Glucocorticoid Receptor Action Promotes Identification of Novel Ligands with an Improved Therapeutic Index. Exp. Dermatol. 2006, 15, 565–573. [Google Scholar] [CrossRef]
- Buttgereit, F.; Scheffold, A. Rapid Glucocorticoid Effects on Immune Cells. Steroids 2002, 67, 529–534. [Google Scholar] [CrossRef]
- Croxtall, J.D.; Choudhury, Q.; Flower, R.J. Glucocorticoids Act within Minutes to Inhibit Recruitment of Signalling Factors to Activated EGF Receptors through a Receptor-dependent, Transcription-independent Mechanism. Br. J. Pharmacol. 2000, 130, 289–298. [Google Scholar] [CrossRef]
- Cato, A.C.B.; Nestl, A.; Mink, S. Rapid Actions of Steroid Receptors in Cellular Signaling Pathways. Sci. STKE 2002, 2002, re9. [Google Scholar] [CrossRef]
- Hafezi-Moghadam, A.; Simoncini, T.; Yang, Z.; Limbourg, F.P.; Plumier, J.-C.; Rebsamen, M.C.; Hsieh, C.-M.; Chui, D.-S.; Thomas, K.L.; Prorock, A.J.; et al. Acute Cardiovascular Protective Effects of Corticosteroids Are Mediated by Non-Transcriptional Activation of Endothelial Nitric Oxide Synthase. Nat. Med. 2002, 8, 473–479. [Google Scholar] [CrossRef]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and Wound Healing: Clinical Considerations in the Perioperative Period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Werth, V.P. Prevention and Treatment of Systemic Glucocorticoid Side Effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AMWF) S1 Leitlinie Pyoderma Gangrenosum (German S1 Guideline on Pyoderma Gangraenosum). Available online: https://register.awmf.org/de/leitlinien/detail/013-091 (accessed on 5 July 2023).
- Reichrath, J.; Bens, G.; Bonowitz, A.; Tilgen, W. Treatment Recommendations for Pyoderma Gangrenosum: An Evidence-Based Review of the Literature Based on More than 350 Patients. J. Am. Acad. Dermatol. 2005, 53, 273–283. [Google Scholar] [CrossRef]
- Pachuau, L. Recent Developments in Novel Drug Delivery Systems for Wound Healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocellulosen: Eine neue Familie naturbasierter Materialien. Angew. Chem. 2011, 123, 5550–5580. [Google Scholar] [CrossRef]
- Carvalho, T.; Guedes, G.; Sousa, F.L.; Freire, C.S.R.; Santos, H.A. Latest Advances on Bacterial Cellulose-Based Materials for Wound Healing, Delivery Systems, and Tissue Engineering. Biotechnol. J. 2019, 14, 1900059. [Google Scholar] [CrossRef]
- Geyer, U.; Heinze, T.; Stein, A.; Klemm, D.; Marsch, S.; Schumann, D.; Schmauder, H.-P. Formation, Derivatization and Applications of Bacterial Cellulose. Int. J. Biol. Macromol. 1994, 16, 343–347. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial Cellulose as a Material for Wound Treatment: Properties and Modifications. A Review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brownjr, R. Microbial Cellulose—The Natural Power to Heal Wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Zahel, P.; Beekmann, U.; Eberlein, T.; Schmitz, M.; Werz, O.; Kralisch, D. Bacterial Cellulose—Adaptation of a Nature-Identical Material to the Needs of Advanced Chronic Wound Care. Pharmaceuticals 2022, 15, 683. [Google Scholar] [CrossRef]
- Colenci, R.; Miot, H.A.; Marques, M.E.A.; Schmitt, J.V.; Basmaji, P.; Jacinto, J.S.; Abbade, L.P.F. Cellulose Biomembrane versus Collagenase Dressing for the Treatment of Chronic Venous Ulcers: A Randomized, Controlled Clinical Trial. Eur. J. Dermatol. 2019, 29, 387–395. [Google Scholar] [CrossRef]
- Alvarez, O.M.; Phillips, T.J.; Menzoian, J.O.; Patel, M.; Andriessen, A. An RCT to Compare a Bio-Cellulose Wound Dressing with a Non-Adherent Dressing in VLUs. J. Wound Care 2012, 21, 448–453. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled Extended Octenidine Release from a Bacterial Nanocellulose/Poloxamer Hybrid System. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Blume, G.; Thamm, J.; Steiniger, F.; Kralisch, D.; Fischer, D. Overcoming the Hydrophilicity of Bacterial Nanocellulose: Incorporation of the Lipophilic Coenzyme Q10 Using Lipid Nanocarriers for Dermal Applications. Eur. J. Pharm. Biopharm. 2021, 158, 106–112. [Google Scholar] [CrossRef]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech Nanocellulose: A Review on Progress in Product Design and Today’s State of Technical and Medical Applications. Carbohydr. Polym. 2021, 254, 117313. [Google Scholar] [CrossRef]
- Karl, B.; Alkhatib, Y.; Beekmann, U.; Bellmann, T.; Blume, G.; Steiniger, F.; Thamm, J.; Werz, O.; Kralisch, D.; Fischer, D. Development and Characterization of Bacterial Nanocellulose Loaded with Boswellia Serrata Extract Containing Nanoemulsions as Natural Dressing for Skin Diseases. Int. J. Pharm. 2020, 587, 119635. [Google Scholar] [CrossRef]
- Bellmann, T.; Thamm, J.; Beekmann, U.; Kralisch, D.; Fischer, D. In Situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics 2023, 15, 559. [Google Scholar] [CrossRef]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1719. [Google Scholar] [CrossRef]
- Beekmann, U.; Zahel, P.; Karl, B.; Schmölz, L.; Börner, F.; Gerstmeier, J.; Werz, O.; Lorkowski, S.; Wiegand, C.; Fischer, D.; et al. Modified Bacterial Cellulose Dressings to Treat Inflammatory Wounds. Nanomaterials 2020, 10, 2508. [Google Scholar] [CrossRef]
- ICH Q3C Impurities—ICH Quality Guidelines—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118971147.ch7 (accessed on 24 August 2023).
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for Drug Delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Gradzielski, M.; Duvail, M.; de Molina, P.M.; Simon, M.; Talmon, Y.; Zemb, T. Using Microemulsions: Formulation Based on Knowledge of Their Mesostructure. Chem. Rev. 2021, 121, 5671–5740. [Google Scholar] [CrossRef]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Zhang, J.; Froelich, A.; Michniak-Kohn, B. Topical Delivery of Meloxicam Using Liposome and Microemulsion Formulation Approaches. Pharmaceutics 2020, 12, 282. [Google Scholar] [CrossRef]
- Heuschkel, S.; Goebel, A.; Neubert, R.H.H. Microemulsions—Modern Colloidal Carrier for Dermal and Transdermal Drug Delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef]
- Schmalfuß, U.; Neubert, R.; Wohlrab, W. Modification of Drug Penetration into Human Skin Using Microemulsions. J. Control. Release 1997, 46, 279–285. [Google Scholar] [CrossRef]
- Teichmann, A.; Heuschkel, S.; Jacobi, U.; Presse, G.; Neubert, R.H.H.; Sterry, W.; Lademann, J. Comparison of Stratum Corneum Penetration and Localization of a Lipophilic Model Drug Applied in an o/w Microemulsion and an Amphiphilic Cream. Eur. J. Pharm. Biopharm. 2007, 67, 699–706. [Google Scholar] [CrossRef]
- Kreilgaard, M. Influence of Microemulsions on Cutaneous Drug Delivery. Adv. Drug Deliv. Rev. 2002, 54, S77–S98. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, Characterization and Pre-Clinical Trials of an Innovative Wound Healing Dressing Based on Propolis (EPP-AF®)-Containing Self-Microemulsifying Formulation Incorporated in Biocellulose Membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug Release from Nanoparticles Embedded in Four Different Nanofibrillar Cellulose Aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Rojewska, A.; Karewicz, A.; Baster, M.; Zając, M.; Wolski, K.; Kępczyński, M.; Zapotoczny, S.; Szczubiałka, K.; Nowakowska, M. Dexamethasone-Containing Bioactive Dressing for Possible Application in Post-Operative Keloid Therapy. Cellulose 2019, 26, 1895–1908. [Google Scholar] [CrossRef]
- Fini, A.; Bergamante, V.; Ceschel, G.C.; Ronchi, C.; De Moraes, C.A.F. Control of Transdermal Permeation of Hydrocortisone Acetate from Hydrophilic and Lipophilic Formulations. AAPS PharmSciTech 2008, 9, 762–768. [Google Scholar] [CrossRef]
- Sae Yoon, A.; Sakdiset, P. Development of Microemulsions Containing Glochidion Wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid. Sci. Pharm. 2020, 88, 53. [Google Scholar] [CrossRef]
- Chen, B.; Hou, M.; Zhang, B.; Liu, T.; Guo, Y.; Dang, L.; Wang, Z. Enhancement of the Solubility and Antioxidant Capacity of α-Linolenic Acid Using an Oil in Water Microemulsion. Food Funct. 2017, 8, 2792–2802. [Google Scholar] [CrossRef]
- Panoutsopoulou, E.; Zbytovská, J.; Vávrová, K.; Paraskevopoulos, G. Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery. Pharmaceuticals 2022, 15, 515. [Google Scholar] [CrossRef]
- Ryu, K.-A.; Park, P.J.; Kim, S.-B.; Bin, B.-H.; Jang, D.-J.; Kim, S.T. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics 2020, 12, 332. [Google Scholar] [CrossRef]
- Abruzzo, A.; Parolin, C.; Rossi, M.; Vitali, B.; Cappadone, C.; Bigucci, F. Development and Characterization of Azithromycin-Loaded Microemulsions: A Promising Tool for the Treatment of Bacterial Skin Infections. Antibiotics 2022, 11, 1040. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization/ANSI. ISO: Geneva, Switzerland, 2009.
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-Made Material Characteristics of Bacterial Cellulose for Drug Delivery Applications in Dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Beekmann, U.; Schmölz, L.; Lorkowski, S.; Werz, O.; Thamm, J.; Fischer, D.; Kralisch, D. Process Control and Scale-up of Modified Bacterial Cellulose Production for Tailor-Made Anti-Inflammatory Drug Delivery Systems. Carbohydr. Polym. 2020, 236, 116062. [Google Scholar] [CrossRef]
- Bellmann, T.; Luber, R.; Kischio, L.; Karl, B.; Pötzinger, Y.; Beekmann, U.; Kralisch, D.; Wiegand, C.; Fischer, D. Bacterial Nanocellulose Patches as a Carrier for Hydrating Formulations to Improve the Topical Treatment of Nail Diseases. Int. J. Pharm. 2022, 628, 122267. [Google Scholar] [CrossRef]
- Abruzzo, A.; Armenise, N.; Bigucci, F.; Cerchiara, T.; Gösser, M.B.; Samorì, C.; Galletti, P.; Tagliavini, E.; Brown, D.M.; Johnston, H.J.; et al. Surfactants from Itaconic Acid: Toxicity to HaCaT Keratinocytes In Vitro, Micellar Solubilization, and Skin Permeation Enhancement of Hydrocortisone. Int. J. Pharm. 2017, 524, 9–15. [Google Scholar] [CrossRef]
- Kozlina, F.; Meštrović, I.; Novak, V.; Marjanović, N.; Cetina-Cizmek, B. Development of Fiber Optic In Vitro Release Testing Method for Dexamethasone Release from the Oil Solutions. ADMET DMPK 2022, 10, 315–329. [Google Scholar] [CrossRef]
- Czapka, A.; König, S.; Pergola, C.; Grune, C.; Vougogiannopoulou, K.; Skaltsounis, A.-L.; Fischer, D.; Werz, O. The Indirubin Derivative 6-Bromoindirubin-3′-Glycerol-Oxime Ether (6BIGOE) Potently Modulates Inflammatory Cytokine and Prostaglandin Release from Human Monocytes through GSK-3 Interference. Biochem. Pharmacol. 2020, 180, 114170. [Google Scholar] [CrossRef]
- Zang, J.; Feng, M.; Zhao, J.; Wang, J. Micellar and Bicontinuous Microemulsion Structures Show Different Solute–Solvent Interactions: A Case Study Using Ultrafast Nonlinear Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 19938–19949. [Google Scholar] [CrossRef]
- Fanun, M. Conductivity, Viscosity, NMR and Diclofenac Solubilization Capacity Studies of Mixed Nonionic Surfactants Microemulsions. J. Mol. Liq. 2007, 135, 5–13. [Google Scholar] [CrossRef]
- Zhang, J.; Michniak-Kohn, B. Investigation of Microemulsion Microstructures and Their Relationship to Transdermal Permeation of Model Drugs: Ketoprofen, Lidocaine, and Caffeine. Int. J. Pharm. 2011, 421, 34–44. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal Delivery Enhancement of Carvacrol from Origanum vulgare L. Essential Oil by Microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Lund, W. The Pharmaceutical Codex: Principles and Practice of Pharmaceutics (British Pharmaceutical Codex), 12th ed.; Pharmaceutical Press: London, UK, 1994. [Google Scholar]
- Trissel, L.A.; Ashworth, L.D.; Ashworth, J. Trissel’s Stability of Compounded Formulations, 6th ed.; APhA Publications; American Pharmacists Association: Washington, DC, USA, 2018. [Google Scholar]
- Aparicio, R.M.; José García-Celma, M.; Pilar Vinardell, M.; Mitjans, M. In Vitro Studies of the Hemolytic Activity of Microemulsions in Human Erythrocytes. J. Pharm. Biomed. Anal. 2005, 39, 1063–1067. [Google Scholar] [CrossRef]
- Shah, R.; Magdum, C.; Patil, S.; Niakwade, N. Preparation and Evaluation of Aceclofenac Topical Microemulsion. Iran. J. Pharm. Res. 2010, 9, 5–11. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.; da Silva, J.A.; Lira, A.A.M.; Conceição, T.M.F.; Nunes, R.S.; de Albuquerque, R.L.C., Jr.; Sarmento, V.H.V.; Leal, L.B.; de Santana, D.P. Evaluation of Microemulsion and Lamellar Liquid Crystalline Systems for Transdermal Zidovudine Delivery. J. Pharm. Sci. 2016, 105, 2188–2193. [Google Scholar] [CrossRef]
- Bubic Pajic, N.; Nikolic, I.; Mitsou, E.; Papadimitriou, V.; Xenakis, A.; Randjelovic, D.; Dobricic, V.; Smitran, A.; Cekic, N.; Calija, B.; et al. Biocompatible Microemulsions for Improved Dermal Delivery of Sertaconazole Nitrate: Phase Behavior Study and Microstructure Influence on Drug Biopharamaceutical Properties. J. Mol. Liq. 2018, 272, 746–758. [Google Scholar] [CrossRef]
- Alany, R.G.; Tucker, I.G.; Davies, N.M.; Rades, T. Characterizing Colloidal Structures of Pseudoternary Phase Diagrams Formed by Oil/Water/Amphiphile Systems. Drug Dev. Ind. Pharm. 2001, 27, 31–38. [Google Scholar] [CrossRef]
- Todosijević, M.N.; Cekić, N.D.; Savić, M.M.; Gašperlin, M.; Ranđelović, D.V.; Savić, S.D. Sucrose Ester-Based Biocompatible Microemulsions as Vehicles for Aceclofenac as a Model Drug: Formulation Approach Using D-Optimal Mixture Design. Colloid Polym. Sci. 2014, 292, 3061–3076. [Google Scholar] [CrossRef]
- Gattefossé SAS Plurol® Oleique CC 497 Product Website. Available online: https://www.gattefosse.com/pharmaceuticals-products/plurol-oleique-cc-497 (accessed on 3 July 2023).
- Lapasin, R.; Grassi, M.; Coceani, N. Effects of Polymer Addition on the Rheology of o/w Microemulsions. Rheol. Acta 2001, 40, 185–192. [Google Scholar] [CrossRef]
- Feng, J.-L.; Wang, Z.-W.; Zhang, J.; Wang, Z.-N.; Liu, F. Study on Food-Grade Vitamin E Microemulsions Based on Nonionic Emulsifiers. Colloids Surf. Physicochem. Eng. Asp. 2009, 339, 1–6. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial Functionalization of Bacterial Nanocellulose by Loading with Polihexanide and Povidone-Iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.Cfm (accessed on 3 July 2023).
- Wolf, L.; Hoffmann, H.; Richter, W.; Teshigawara, T.; Okamoto, T. Dynamic Properties of Microemulsions in the Single-Phase Channels. J. Phys. Chem. B 2011, 115, 11081–11091. [Google Scholar] [CrossRef]
- Gottwald-Hostalek, U.; Uhl, W.; Wolna, P.; Kahaly, G.J. New Levothyroxine Formulation Meeting 95–105% Specification over the Whole Shelf-Life: Results from Two Pharmacokinetic Trials. Curr. Med. Res. Opin. 2017, 33, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Bundgaard, H. Studies on the Stability of Corticosteroids V. The Degradation Pattern of Hydrocortisone in Aqueous Solution. Int. J. Pharm. 1980, 6, 307–319. [Google Scholar] [CrossRef]
- Matter, B.; Ghaffari, A.; Bourne, D.; Wang, Y.; Choi, S.; Kompella, U.B. Dexamethasone Degradation in Aqueous Medium and Implications for Correction of In Vitro Release from Sustained Release Delivery Systems. AAPS PharmSciTech 2019, 20, 320. [Google Scholar] [CrossRef]
- Van Heugten, A.J.P.; De Boer, W.; De Vries, W.S.; Pieters, R.J.; Vromans, H. Topically Used Corticosteroids: What Is the Big Picture of Drug Product Degradation? Eur. J. Pharm. Sci. 2018, 117, 1–7. [Google Scholar] [CrossRef]
- Yip, Y.W.; Po, A.L.W.; Irwin, W.J. Kinetics of Decomposition and Formulation of Hydrocortisone Butyrate in Semiaqueous and Gel Systems. J. Pharm. Sci. 1983, 72, 776–781. [Google Scholar] [CrossRef]
- Davies, N.M.; Wang, G.; Tucker, I.G. Evaluation of a Hydrocortisone/Hydroxypropyl-β-Cyclodextrin Solution for Ocular Drug Delivery. Int. J. Pharm. 1997, 156, 201–209. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Wilhelms, T.; Schulze, D.; Norgauer, J.; Hipler, U.-C. Effect of the Sterilization Method on the Performance of Collagen Type I on Chronic Wound Parameters In Vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 710–719. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Bosela, A.A. Investigation of Self-Microemulsifying and Microemulsion Systems for Protection of Prednisolone from Gamma Radiation. Pharm. Dev. Technol. 2011, 16, 237–242. [Google Scholar] [CrossRef]
- Bosela, A.A.; Salamah, K.K.; Alsarra, I.A.; El-Bagory, I.M. Reactivity of Prednisolone to Gamma Radiation in Aqueous and Organic Solutions. J. Drug Deliv. Sci. Technol. 2010, 20, 225–229. [Google Scholar] [CrossRef]
- Marciniec, B.; Ogrodowczyk, M.; Dettlaff, K. Search for the Effect of E-Beam Irradiation on Some Steroids. Radiat. Phys. Chem. 2005, 72, 517–524. [Google Scholar] [CrossRef]
- Crucq, A.-S.; Deridder, V.; Tilquin, B. Radiostability of Pharmaceuticals under Different Irradiation Conditions. Radiat. Phys. Chem. 2005, 72, 355–361. [Google Scholar] [CrossRef]
- Jay-Gerin, J.-P.; Ferradini, C. A New Estimate of the ⋅OH Radical Yield at Early Times in the Radiolysis of Liquid Water. Chem. Phys. Lett. 2000, 317, 388–391. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, A.; Guo, Q.; Rui, M.; Zhao, Y.; Zhang, H.; Zhu, S. Decomposition of Dexamethasone by Gamma Irradiation: Kinetics, Degradation Mechanisms and Impact on Algae Growth. Chem. Eng. J. 2017, 307, 722–728. [Google Scholar] [CrossRef]
- Kane, M.P.; Tsuji, K. Radiolytic Degradation Scheme for 60Co-Irradiated Corticosteroids. J. Pharm. Sci. 1983, 72, 30–35. [Google Scholar] [CrossRef]
- Wildner, W.; Drummer, D. Nanofiller Materials for Transparent Polymer Composites: Influences on the Properties and on the Transparency—A Review. J. Thermoplast. Compos. Mater. 2019, 32, 1547–1565. [Google Scholar] [CrossRef]
- Babi, M.; Williams, A.; Reid, M.; Grandfield, K.; Bassim, N.D.; Moran-Mirabal, J.M. Unraveling the Supramolecular Structure and Nanoscale Dislocations of Bacterial Cellulose Ribbons Using Correlative Super-Resolution Light and Electron Microscopy. Biomacromolecules 2023, 24, 258–268. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Suchenek, A.; Wilkowski, D. Optimization and Evaluation of the In Vitro Permeation Parameters of Topical Products with Non-Steroidal Anti-Inflammatory Drugs through Strat-M® Membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with In Vivo-In Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane Properties for Permeability Testing: Skin versus Synthetic Membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W. Diethylene Glycol Monoethyl Ether: An Emerging Solvent in Topical Dermatology Products. J. Cosmet. Dermatol. 2011, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Streit, M.; Beleznay, Z.; Braathen, L.R. Topical Application of the Tumour Necrosis Factor-α Antibody Infliximab Improves Healing of Chronic Wounds. Int. Wound J. 2006, 3, 171–179. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
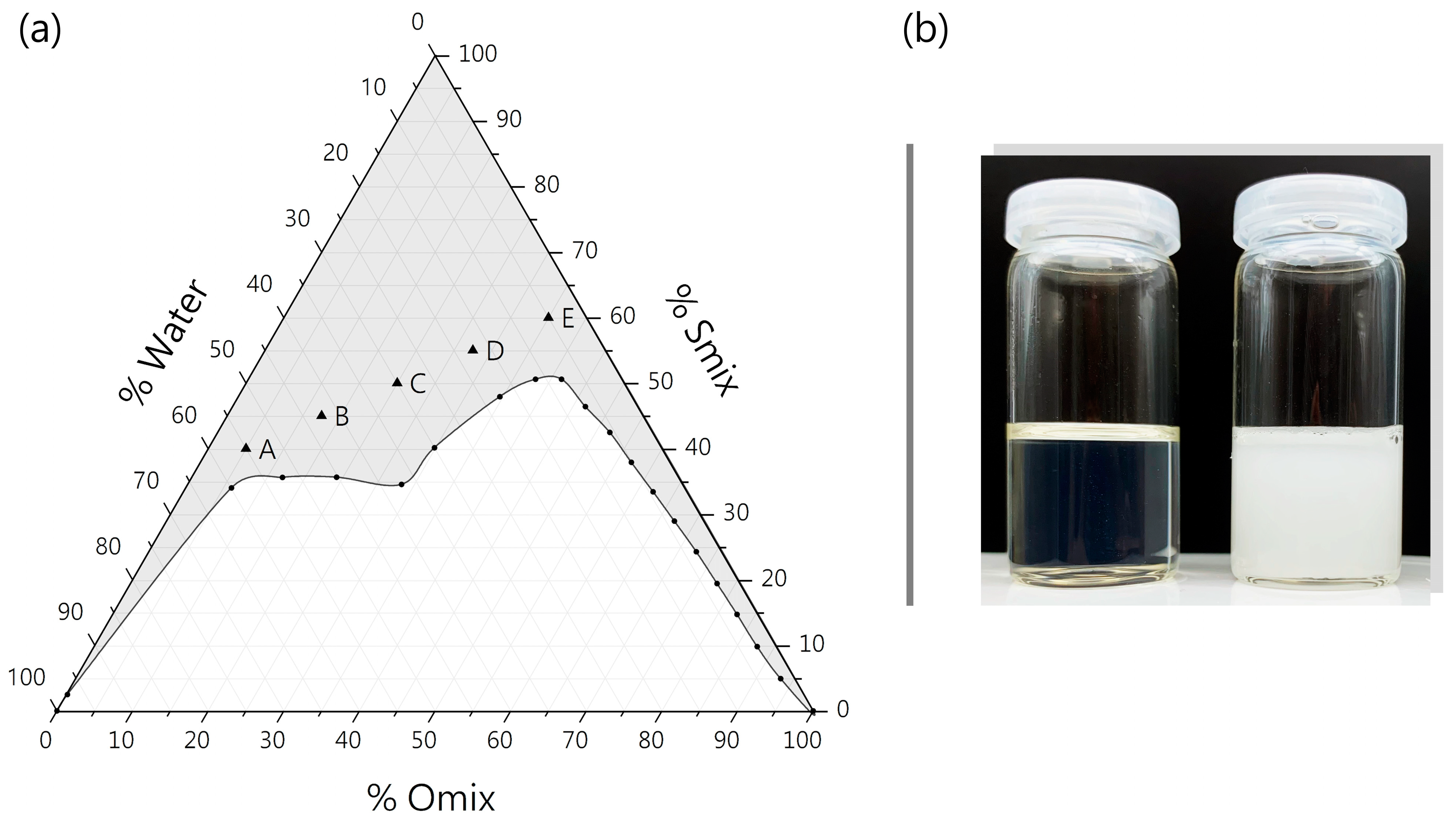


| Microemulsion | Smix 1 [wt%] | Water [wt%] | Omix 2 [wt%] |
|---|---|---|---|
| ME-A | 40.0 | 55.0 | 5.0 |
| ME-B | 45.0 | 42.5 | 12.5 |
| ME-C | 50.0 | 30.0 | 20.0 |
| ME-D | 55.0 | 17.5 | 27.5 |
| ME-E | 60.0 | 5.0 | 35.0 |
| Microemulsion | Smix [wt%] | Water [wt%] | Omix [wt%] | Conductivity [µS/cm] |
|---|---|---|---|---|
| ME-A | 40.0 | 55.0 | 5.0 | 142.07 ± 0.98 |
| ME-B | 45.0 | 42.5 | 12.5 | 89.86 ± 0.90 |
| ME-C | 50.0 | 30.0 | 20.0 | 38.79 ± 0.76 |
| ME-D | 55.0 | 17.5 | 27.5 | 7.04 ± 1.92 |
| ME-E | 60.0 | 5.0 | 35.0 | 3.65 ± 2.75 |
| Microemulsion | pH | Refractive Index | Z-Ave [nm] | Zeta Potential [mV] | Dynamic Viscosity [mPa·s] |
|---|---|---|---|---|---|
| ME-A | 3.16 ± 0.02 | 1.3938 | 13.22 ± 1.54 | 0.129 ± 0.470 | 20.90 ± 0.17 |
| ME-B | 3.18 ± 0.04 | 1.4115 | 6.98 ± 0.90 | −0.081 ± 0.223 | 44.70 ± 0.20 |
| ME-C | 3.20 ± 0.06 | 1.4275 | 3.72 ± 0.09 | 0.169 ± 0.533 | 69.05 ± 0.66 |
| ME-D | 3.30 ± 0.02 | 1.4432 | 7.19 ± 0.18 | 0.044 ± 0.525 | 77.13 ± 0.86 |
| ME-E | 3.25 ± 0.06 | 1.4560 | 11.24 ± 0.63 | 0.010 ± 0.454 | 78.08 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahel, P.; Bruggink, V.; Hülsmann, J.; Steiniger, F.; Hofstetter, R.K.; Heinzel, T.; Beekmann, U.; Werz, O.; Kralisch, D. Exploring Microemulsion Systems for the Incorporation of Glucocorticoids into Bacterial Cellulose: A Novel Approach for Anti-Inflammatory Wound Dressings. Pharmaceutics 2024, 16, 504. https://doi.org/10.3390/pharmaceutics16040504
Zahel P, Bruggink V, Hülsmann J, Steiniger F, Hofstetter RK, Heinzel T, Beekmann U, Werz O, Kralisch D. Exploring Microemulsion Systems for the Incorporation of Glucocorticoids into Bacterial Cellulose: A Novel Approach for Anti-Inflammatory Wound Dressings. Pharmaceutics. 2024; 16(4):504. https://doi.org/10.3390/pharmaceutics16040504
Chicago/Turabian StyleZahel, Paul, Vera Bruggink, Juliana Hülsmann, Frank Steiniger, Robert K. Hofstetter, Thorsten Heinzel, Uwe Beekmann, Oliver Werz, and Dana Kralisch. 2024. "Exploring Microemulsion Systems for the Incorporation of Glucocorticoids into Bacterial Cellulose: A Novel Approach for Anti-Inflammatory Wound Dressings" Pharmaceutics 16, no. 4: 504. https://doi.org/10.3390/pharmaceutics16040504
APA StyleZahel, P., Bruggink, V., Hülsmann, J., Steiniger, F., Hofstetter, R. K., Heinzel, T., Beekmann, U., Werz, O., & Kralisch, D. (2024). Exploring Microemulsion Systems for the Incorporation of Glucocorticoids into Bacterial Cellulose: A Novel Approach for Anti-Inflammatory Wound Dressings. Pharmaceutics, 16(4), 504. https://doi.org/10.3390/pharmaceutics16040504







