Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. For the IL Preparation
2.1.2. Materials for Cell Culturing
2.2. Methods
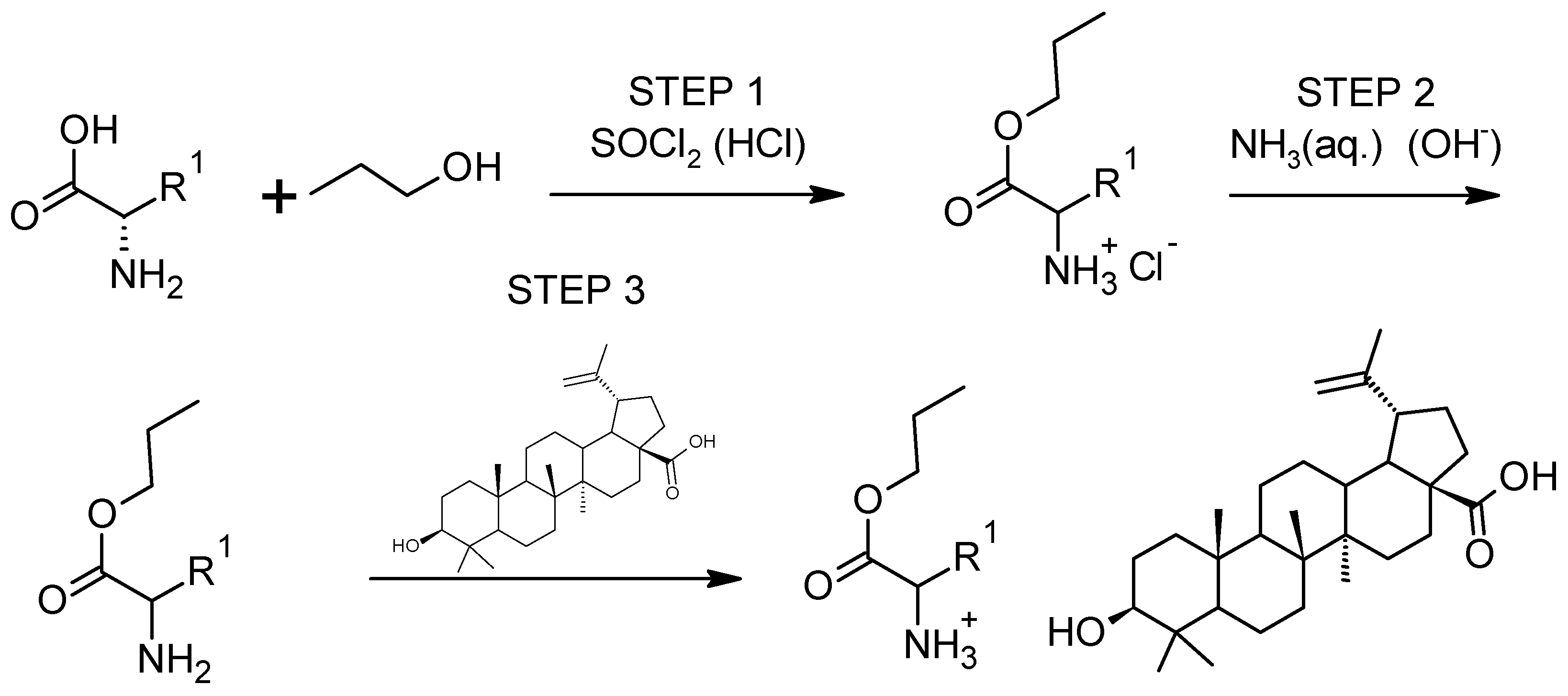
2.2.1. Synthesis of L-Amino Acid Ethyl Ester Betulinates
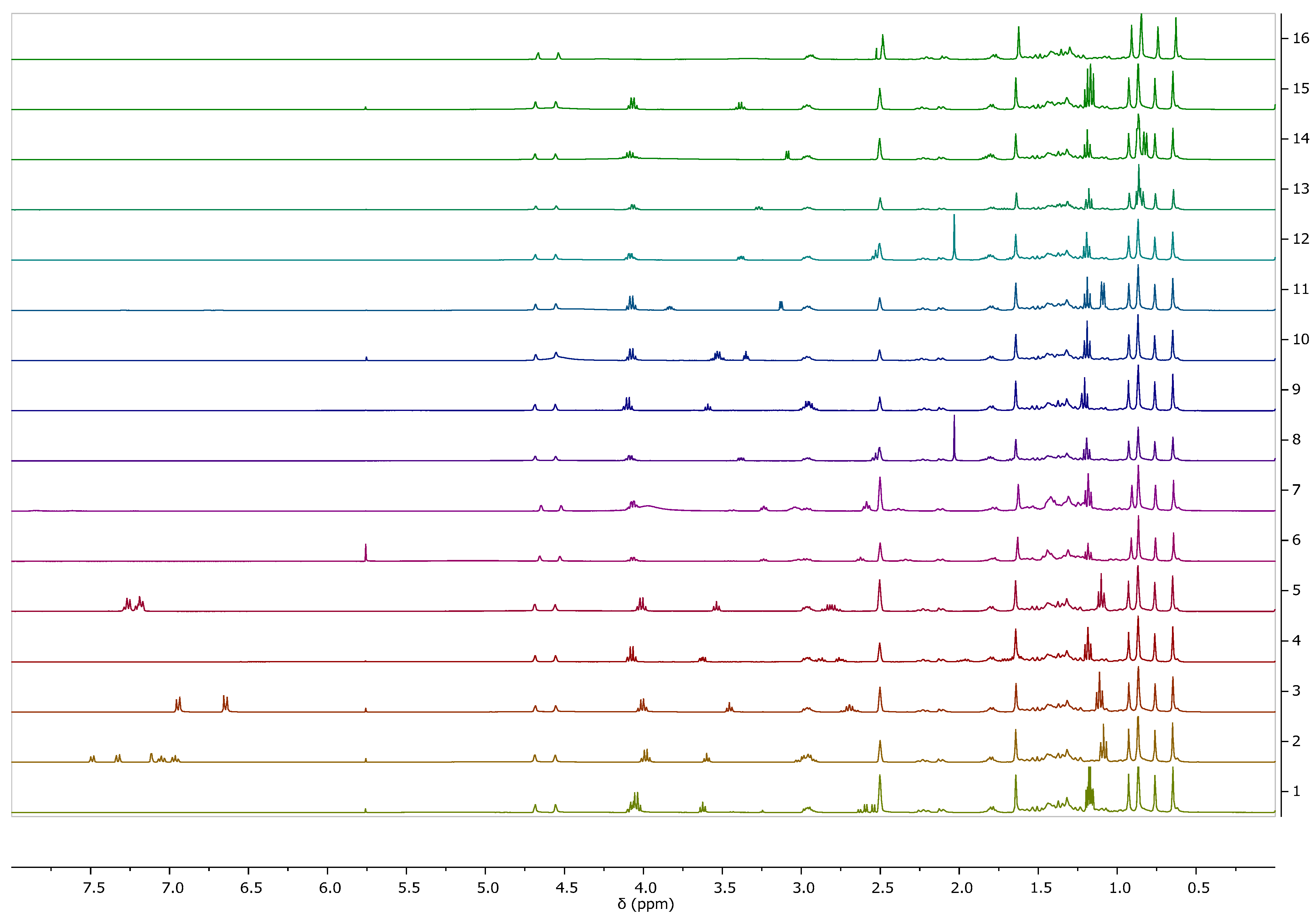
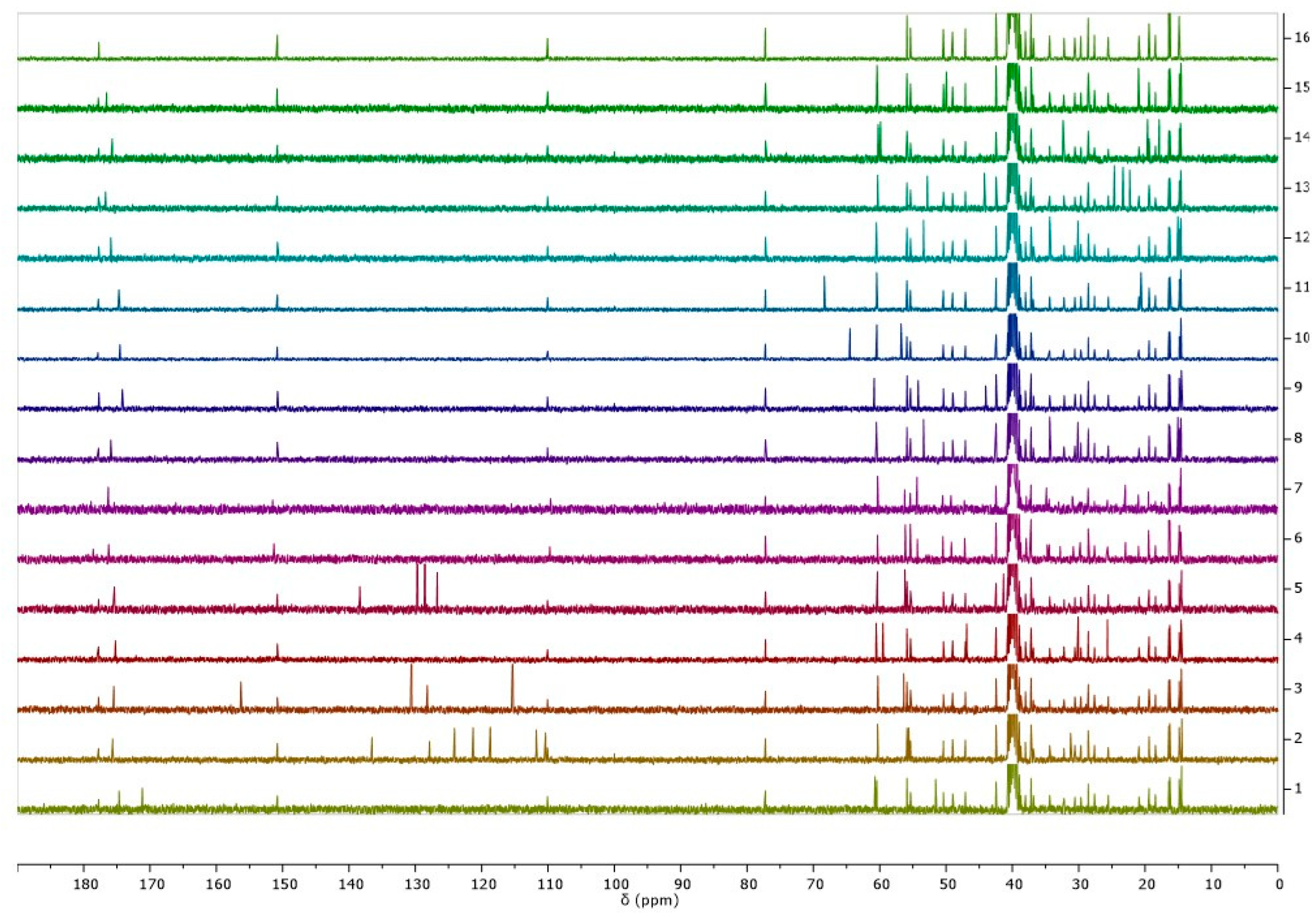
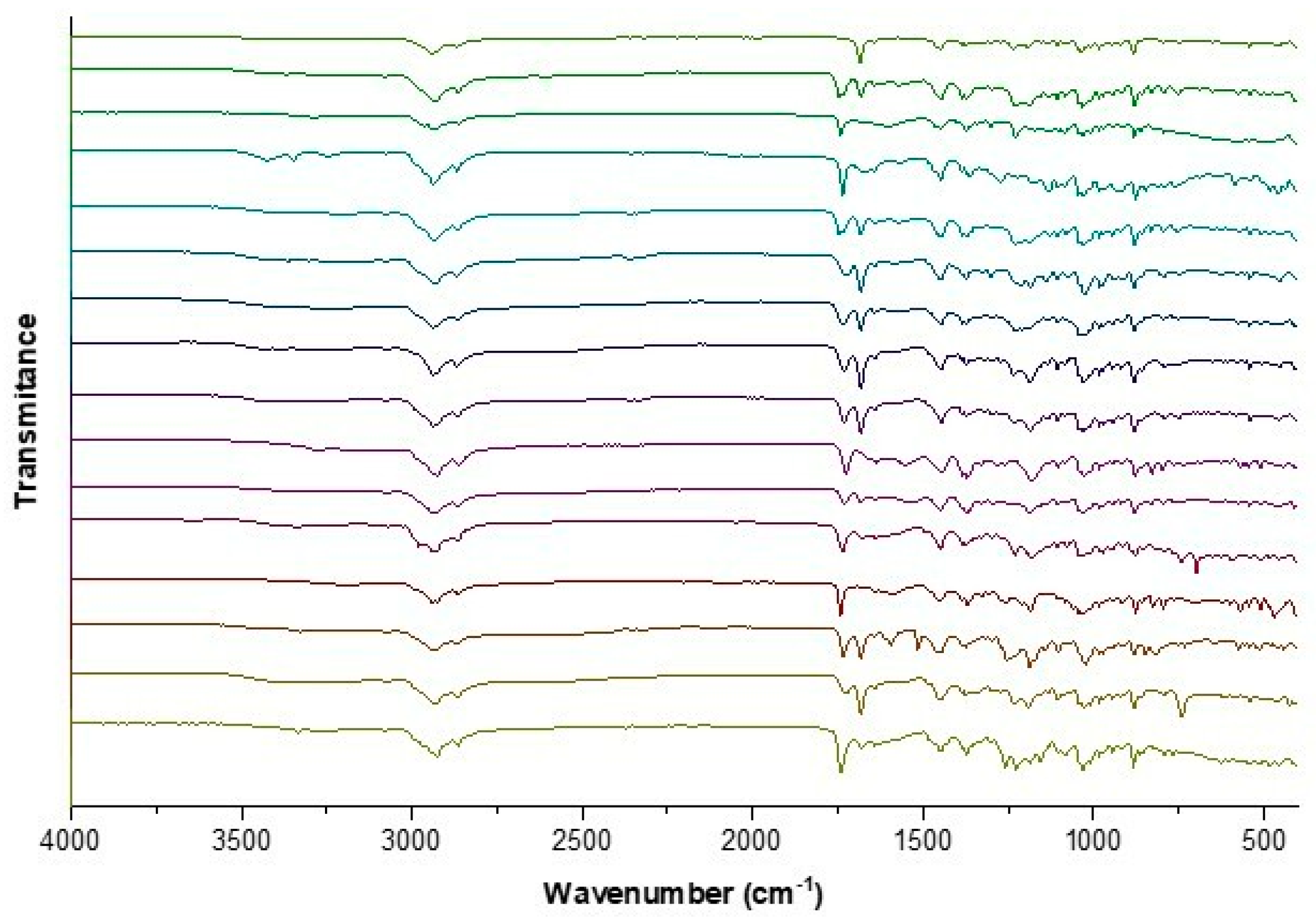
2.2.2. Identification and Characterization of [AAOEt][BA]
NMR
FTIR–ATR
Elemental Analysis
TG
DSC of BA-ILs
Specific Rotation
Estimation of the Water Solubility
2.2.3. Cell Culture
2.2.4. MTT Assay
2.2.5. Colony Formation Assay
2.2.6. Cell Morphology Analysis
2.2.7. DSC of MCF-7 Cells
3. Results and Discussion
3.1. Synthesis and Characterization of [AAOEt][BA]
3.2. Cytotoxic Effect of [AAOEt][BA] on MCF-7 Cells
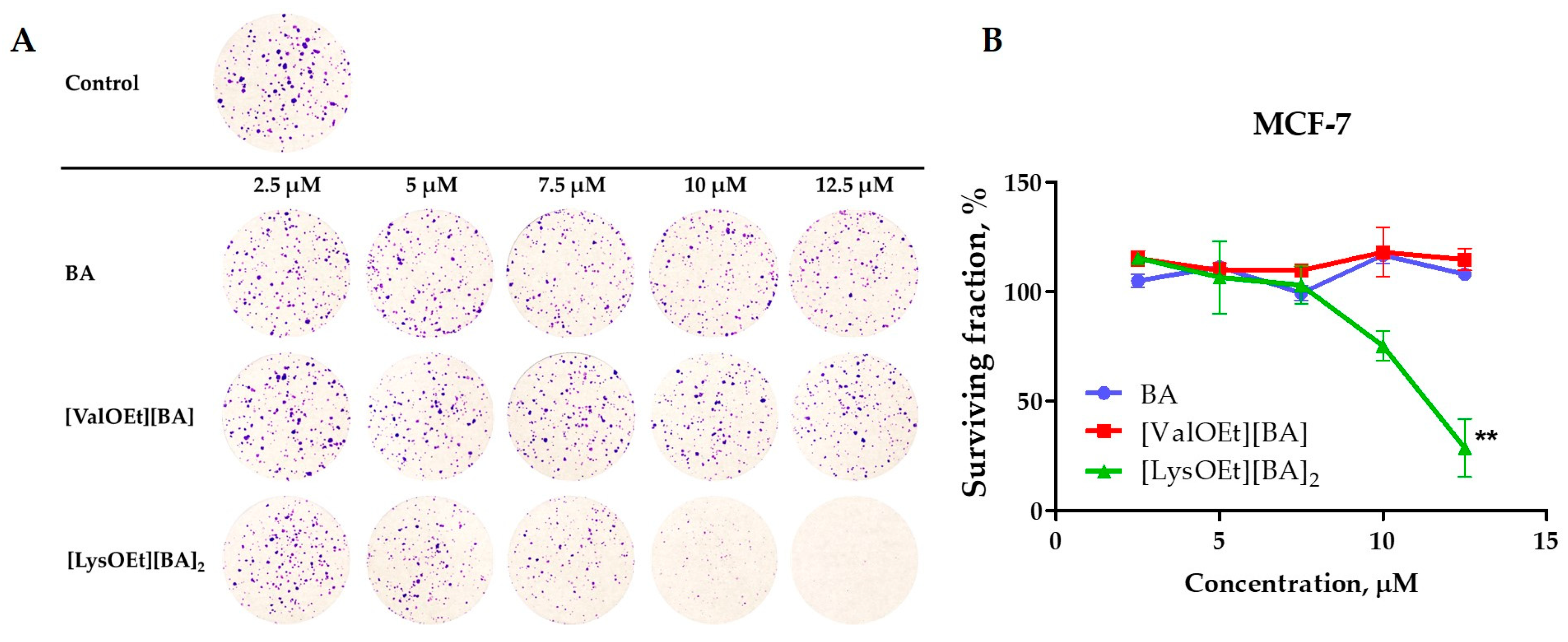
3.3. Effect of BA and Two Selected [AAOEt][BA] on the Clonogenic Activity of MCF-7 Cells
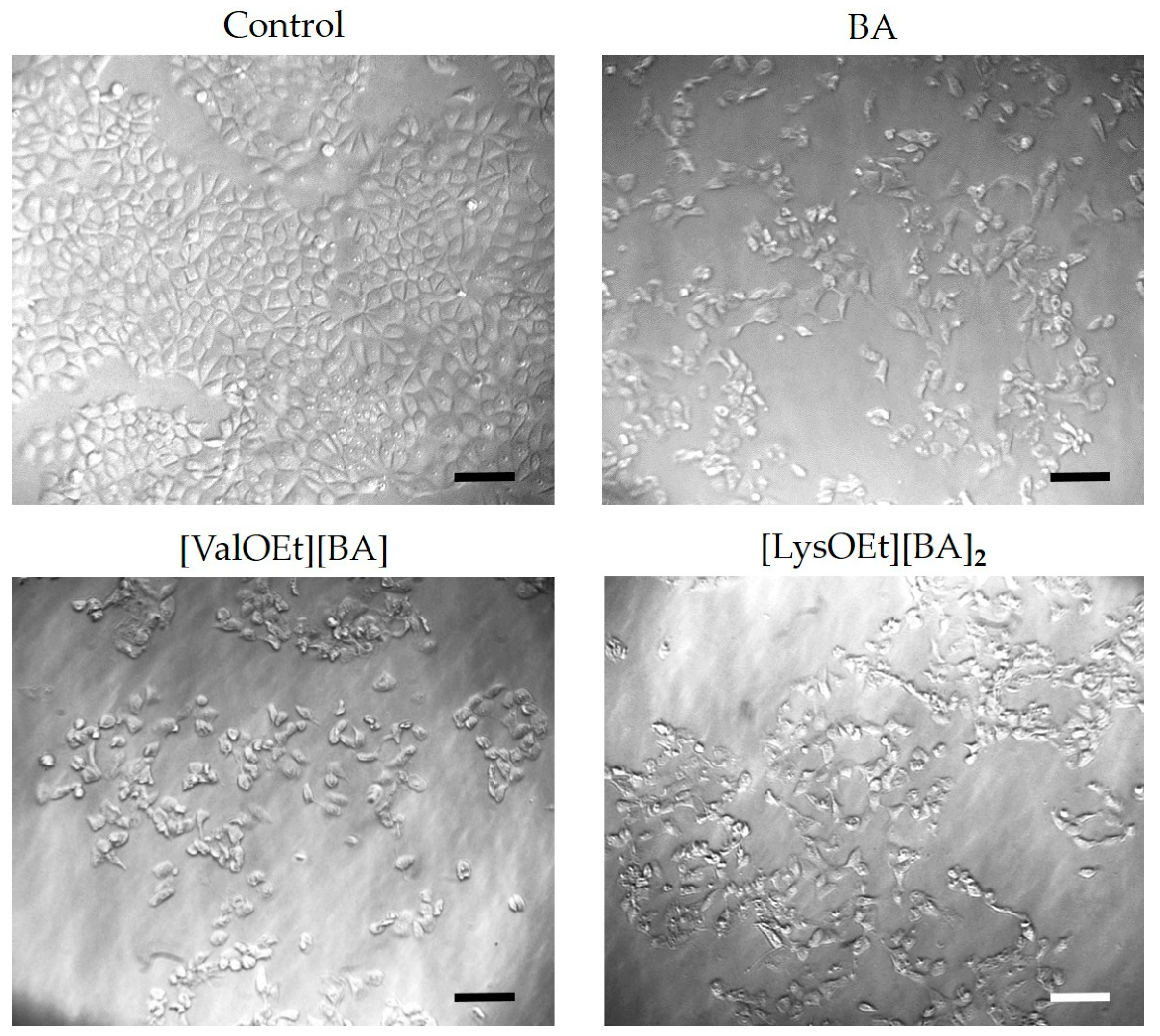
3.4. Cell Morphology Analysis
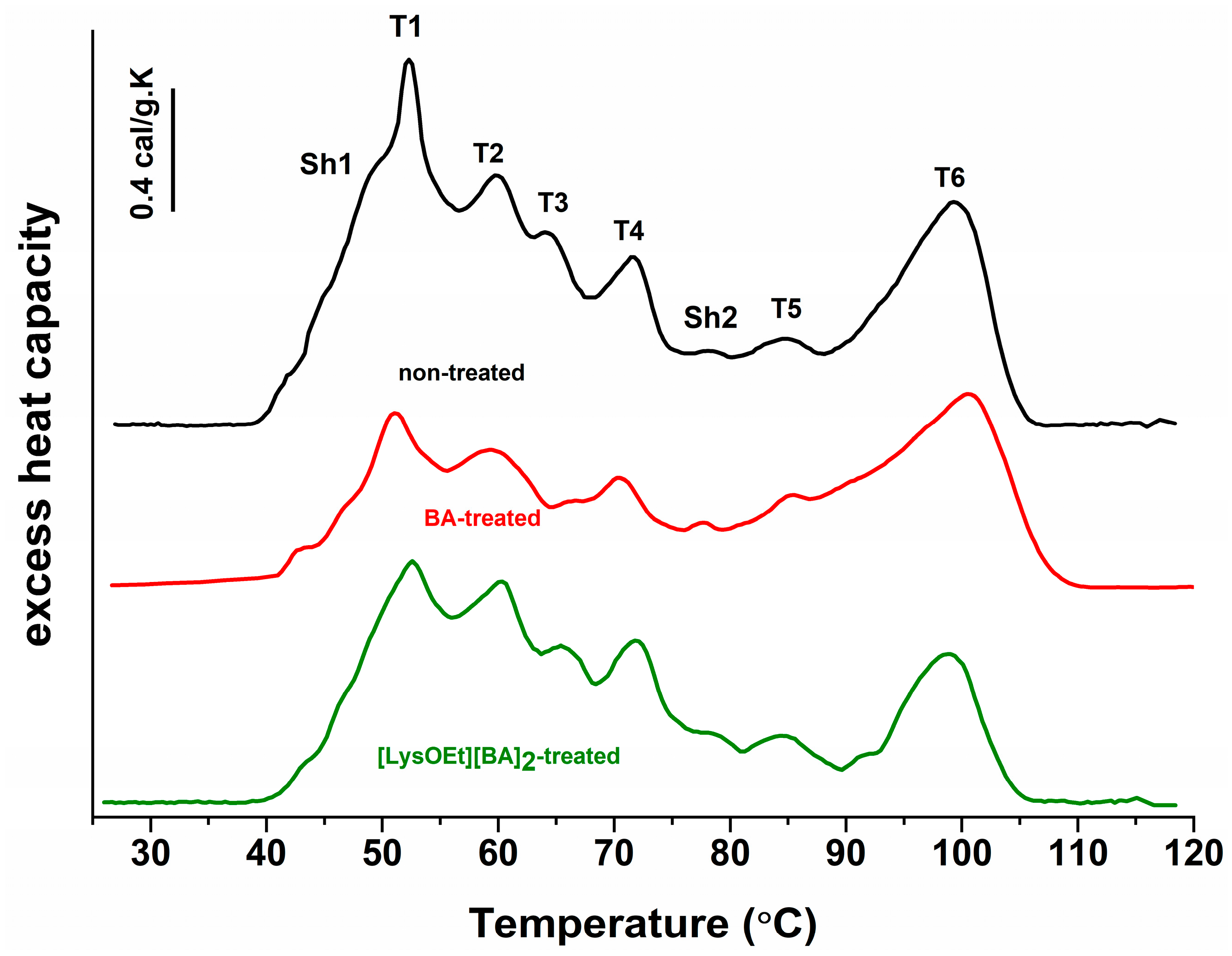
3.5. Thermodynamic Behavior of MCF-7 Cells Treated with BA or [LysOEt][BA]2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef]
- Breast Cancer Facts and Statistics. Available online: https://www.breastcancer.org/facts-statistics (accessed on 22 February 2024).
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
- Elkaeed, E.B.; Salam, H.A.A.E.; Sabt, A.; Al-Ansary, G.H.; Eldehna, W.M. Recent advancements in the development of anti-breast cancer synthetic small molecules. Molecules 2021, 26, 7611. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature (Review). Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Gordon, C.; Newman, D. Natural products as sources of anticancer agents: Current approaches and perspectives. In Natural Products as Source of Molecules with Therapeutic Potential, 1st ed.; Filho, V.C., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 309–331. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Svozil, D. An enumeration of natural products from microbial, marine and terrestrial sources. Phys. Sci. Rev. 2020, 5, 20180121. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A review on preparation of betulinic acid and its biological activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Szlasa, W.; Ślusarczyk, S.; Nawrot–Hadzik, I.; Abel, R.; Zalesińska, A.; Szewczyk, A.; Sauer, N.; Preissner, R.; Saczko, J.; Drąg, M.; et al. Betulin and its derivatives reduce inflammation and COX-2 activity in macrophages. Inflammation 2023, 46, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.C.S.; dos Santos Maia, M.; de Souza, T.A.; de Oliveira Lima, E.; dos Santos, L.E.C.G.; Silva, S.L.; da Silva, M.S.; Filho, J.M.B.; da Silva Rodrigues Junior, V.; Scotti, L.; et al. Antimicrobial potential of betulinic acid and investigation of the mechanism of action against nuclear and metabolic enzymes with molecular modeling. Pathogens 2023, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Lai, W.; Qian, K.; Ho, P.; Lee, K.H.; Chen, C.H.; Huang, L. Betulinic acid derivatives as human immunodeficiency virus type 2 (HIV-2) inhibitors. J. Med. Chem. 2009, 52, 7887–7891. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Xu, H.-G.; Wang, L.; Li, Y.-J.; Sun, P.-H.; Wu, X.-M.; Wang, G.-J.; Chen, W.-M.; Ye, W.-C. Betulinic acid and its derivatives as potential antitumour agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Alho, D.P.S.; Goncalves, B.M.F.; Valdeira, A.S.; Mendes, V.I.S.; Jing, Y. Chapter 2-Highlights of pentacyclic triterpenoids in the cancer settings. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 33–73. [Google Scholar] [CrossRef]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Rugina, D.; Diaconeasa, Z.; Socaciu, C.; Socaciu, M.A. Pentacyclic triterpenoid phytochemicals with anticancer activity: Updated studies on mechanisms and targeted delivery. Int. J. Mol. Sci. 2023, 24, 12923. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Fanga, D.; Chu, P.; Wu, H.; Zhang, Z.; Tang, Z. Multiple molecular targets in breast cancer therapy by betulinic acid. Biomed. Pharmacother. 2016, 84, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Turgambayeva, A.; Tashenova, G.; Tulebayeva, A.; Bazarbayeva, A.; Kapanova, G.; Abzaliyeva, S. Multifunctional roles of betulinic acid in cancer chemoprevention: Spotlight on JAK/STAT, VEGF, EGF/EGFR, TRAIL/TRAIL-R, AKT/mTOR and non-coding RNAs in the inhibition of carcinogenesis and metastasis. Molecules 2022, 28, 67. [Google Scholar] [CrossRef]
- Tzenov, Y.R.; Andrews, P.; Voisey, K.; Gai, L.; Carter, B.; Whelan, K.; Popadiuk, C.; Kao, K.R. Selective estrogen receptor modulators and betulinic acid act synergistically to target ERalpha and SP1 transcription factor dependent Pygopus expression in breast cancer. J. Clin. Pathol. 2016, 69, 518–526. [Google Scholar] [CrossRef]
- Chen, J.J.; Patel, A.; Sodani, K.; Xiao, Z.J.; Tiwari, A.K.; Zhang, D.M.; Li, Y.J.; Yang, D.H.; Ye, W.C.; Chen, S.D.; et al. BBA, a synthetic derivative of 23-hydroxybutulinic acid, reverses multidrug resistance by inhibiting the efflux activity of MRP7 (ABCC10). PLoS ONE 2013, 8, e74573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.J.; Sodani, K.; Wang, J.; Bhatnagar, J.; Lan, P.; Ruan, Z.X.; Xiao, Z.J.; Ambudkar, S.V.; Chen, W.M.; et al. BBA, a derivative of 23-hydroxybetulinic acid, potently reverses ABCB1-mediated drug resistance in vitro and in vivo. Mol. Pharm. 2012, 9, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Sun, Y.; Yuan, B.; Wang, Y. Betulinic acid in the treatment of breast cancer: Application and mechanism progress. Fitoterapia 2023, 169, 105617. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Mullauer, F.B.; van Bloois, L.; Daalhuisen, J.B.; Ten Brink, M.S.; Storm, G.; Medema, J.P.; Schiffelers, R.M.; Kessler, J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer Drugs. 2011, 22, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Mioc, A.; Prodea, A.; Mioc, M.; Buzatu, R.; Ghiulai, R.; Racoviceanu, R.; Caruntu, F.; Soica, C. The optimized delivery of triterpenes by liposomal nanoformulations: Overcoming the challenges. Int. J. Mol. Sci. 2022, 23, 1140. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, A.; Mioc, M.; Balan, M.; Ghiulai, R.; Racoviceanu, R.; Avram, Ș.; Prodea, A.; Semenescu, A.; Milan, A.; et al. Novel semisynthetic betulinic acid−triazole hybrids with in vitro antiproliferative potential. Processes 2023, 11, 101. [Google Scholar] [CrossRef]
- Mosiane, K.S.; Nweke, E.E.; Balogun, M.; Fru, P.N. Polyethyleneglycol-betulinic acid (PEG-BA) polymer-drug conjugate induces apoptosis and antioxidation in a biological model of pancreatic cancer. Polymers 2023, 15, 448. [Google Scholar] [CrossRef]
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active pharmaceutical ingredients (APIs) in ionic liquids: An effective approach for API physiochemical parameter optimization. Drug Discov. Today 2022, 27, 2415–2424. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic liquids as a useful tool for tailoring active pharmaceutical ingredients. J. Control. Release 2021, 338, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzamand, M.; Goto, M. In vivo biocompatibility, pharmacokinetics, antitumour efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int. J. Pharm. 2019, 565, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Khachatrian, A.A.; Mukhametzyanov, T.A.; Salikhov, R.Z.; Klimova, A.E.; Gafurov, Z.N.; Kantyukov, A.O.; Yakhvarov, D.G.; Garifullin, B.F.; Mironova, D.A.; Voloshina, A.D.; et al. New ionic liquids based on 5-fluorouracil: Tuning of BSA binding and cytotoxicity. Int. J. Biol. Macromol. 2024, 257, 128642. [Google Scholar] [CrossRef] [PubMed]
- Hough, W.; Rogers, R.D. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Balk, A.; Holzgrabe, U.; Meinel, L. ‘Pro et contra’ ionic liquid drugs–Challenges and opportunities for pharmaceutical translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R.J. Biocatalysis in ionic liquids–advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.A.; Bairos, J.; Roque, M.; Coutinho, J.A.P.; Ventura, S.P.M.; Freire, M.G. Use of ionic liquids as co-surfactants in mixed aqueous micellar two-phase systems to Improve the simultaneous separation of immunoglobulin G and human serum albumin from expired human plasma. ACS Sustain. Chem. Eng. 2019, 7, 15102–15113. [Google Scholar] [CrossRef]
- Amaral, M.; Pereiro, A.B.; Gaspar, M.M.; Reis, C.P. Recent advances in ionic liquids and nanotechnology for drug delivery. Nanomedicine 2021, 16, 63–80. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Licence, P.; Víllora, G. In vitro cytotoxicity assessment of monocationic and dicationic pyridinium-based ionic liquids on HeLa, MCF-7, BGM and EA.hy926 cell lines. J. Hazard. Mater. 2020, 385, 121513. [Google Scholar] [CrossRef] [PubMed]
- Guncheva, M. Ionic Liquids for Anticancer Application. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Yang, D.D.; Paterna, N.J.; Senetra, A.S.; Casey, K.R.; Trieu, P.D.; Caputo, G.A.; Vaden, T.D.; Carone, B.R. Synergistic interactions of ionic liquids and antimicrobials improve drug efficacy. iScience 2021, 24, 101853. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Pillai, V.V.S.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.; Zhao, H.; Gumbs, A.; Chetty, C.S.; Bose, H.S. New ionic derivatives of betulinic acid as highly potent anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 1734–1738. [Google Scholar] [CrossRef]
- Williams, A.; Smith, K.; Bhuiyan, Z.; Phillips, J.; Zhao, H.; Nitta, T. Betulinic acid and its ionic derivatives impaired growth of prostate cancer cells without induction of GRP78 and CHOP. Curr. Issues Pharm. Med. Sci. 2022, 35, 163–168. [Google Scholar] [CrossRef]
- Silva, A.T.; Cerqueira, M.J.; Prudêncio, C.; Fernandes, M.H.; Costa-Rodrigues, J.; Teixeira, C.; Gomes, P.; Ferraz, R. Antiproliferative organic salts derived from betulinic acid: Disclosure of an ionic liquid selective against lung and liver cancer cells. ACS Omega 2019, 4, 5682–5689. [Google Scholar] [CrossRef]
- Shimul, I.M.; Moshikur, R.M.; Minamihata, K.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Amino acid ester based phenolic ionic liquids as a potential solvent for the bioactive compound luteolin: Synthesis, characterization, and food preservation activity. J. Mol. Liq. 2022, 349, 118103. [Google Scholar] [CrossRef]
- Thiele, D.L.; Lipsky, P.E. Spectrum of toxicities of amino acid methyl esters for myeloid cells is determined by distinct metabolic pathways. Blood 1992, 79, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Watanabe, J. Inhibitory activities of aromatic amino acid esters and peptides against ovalbumin permeation through Caco-2 cell. Monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2498–2500. [Google Scholar] [CrossRef][Green Version]
- Deshmukh, M.; Chao, P.; Kutscher, H.L.; Gao, D.; Si, P. A series of α-amino Acid Ester Prodrugs of camptothecin: In vitro hydrolysis and A549 human lung carcinoma cell cytotoxicity. J. Med. Chem. 2010, 53, 1038–1047. [Google Scholar] [CrossRef]
- Vale, N.; Ferreira, A.; Matos, J.; Fresco, P.; Gouveia, M.J. Amino acids in the development of prodrugs. Molecules 2018, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Ossowicz-Rupniewska, P.; Szczepkowska, K.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Struk, Ł.; Klimowicz, A.; Czech, Z. New amino acid propyl ester ibuprofenates from synthesis to use in drug delivery systems. RSC Adv. 2022, 12, 35779–35792. [Google Scholar] [CrossRef]
- Li, J.; Sha, Y. A convenient synthesis of amino acid methyl esters. Molecules 2008, 13, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Klebeko, J.; Ossowicz-Rupniewska, P.; Nowak, A.; Kucharska, E.; Kucharski, Ł.; Duchnik, W.; Struk, Ł.; Klimowicz, A.; Janus, E.J. Cations of amino acid alkyl esters conjugated with an anion from the group of NSAIDs–As tunable pharmaceutical active ionic liquids. Mol. Liq. 2023, 384, 122200. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Chai, H.-B.; Park, S.-Y.; Kim, D.S.H.L. Preparation of amino acid conjugates of betulinic acid with activity against human melanoma. Bioorg. Med. Chem. Lett. 1999, 9, 1201–1204. [Google Scholar] [CrossRef]
- Drag-Zalesinska, M.; Kulbacka, J.; Saczko, J.; Wysocka, T.; Zabel, M.; Surowiak, P.; Drag, M. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorg. Med. Chem. Lett. 2009, 19, 4814–4817. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Franken, N.; Rodermond, H.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Sambrook, J. Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Roeges, N.P.G. A Guide to the Complete Interpretation of Infrared Spectra of Organic Structures, 1 ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Vairam, S.; Premkumar, T.; Govindarajan, S. Trimellitate complexes of divalent transition metals with hydrazinium cation: Thermal and spectroscopic studies. J. Therm. Anal. Calorim. 2010, 100, 955–960. [Google Scholar] [CrossRef]
- Kolev, T.; Spiteller, M.; Koleva, B. Spectroscopic and structural elucidation of amino acid derivatives and small peptides: Experimental and theoretical tools. Amino Acids 2010, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; Arulselvan, P.; Fakurazi, S.; Hussein, M.Z. The in vitro therapeutic activity of betulinic acid nanocomposite on breast cancer cells (MCF-7) and normal fibroblast cell (3T3). J. Mater. Sci. 2014, 49, 8171–8182. [Google Scholar] [CrossRef]
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-quantitative determination of triterpenic acids of Ziziphus jujube fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression. Food Res. Int. 2015, 77, 109–117. [Google Scholar] [CrossRef]
- Benga, G.; Holmes, R.P. Interactions between components in biological membranes and their implications for membrane function. Prog. Biophys. Mol. Biol. 1984, 43, 195–257. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Skrzydlewska, E.; Figaszewski, Z.A. Changes in electric properties of human breast cancer cells. J. Membr. Biol. 2013, 246, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, J.; Shan, S.; Cao, Y. High toxicity of amino acid-based deep eutectic solvents. J. Mol. Liq. 2023, 370, 121044. [Google Scholar] [CrossRef]
- Benedetto, A.; Bodo, E.; Gontrani, L.; Ballone, P.; Caminiti, R. Amino acid anions in organic ionic compounds. An ab initio study of selected ion pairs. J. Phys. Chem. 2014, 118, 2471–2486. [Google Scholar] [CrossRef]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenko, A.V.; Ananikov, V.P. An unexpected increase of toxicity of amino acid-containing ionic liquids. Toxicol. Res. 2015, 4, 152–159. [Google Scholar] [CrossRef]
- Ranke, J.; Mölter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain length in acute Vibrio fischeri and WST-1 cell viability assays. Ecotox. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Ranke, J.; Cox, M.; Müller, A.; Schmidt, C.; Beyersmann, D. Sorption, cellular distribution, and cytotoxicity of imidazolium ionic liquids in mammalian cells—Influence of lipophilicity. Toxicol. Environ. Chem. 2006, 88, 273–285. [Google Scholar] [CrossRef]
- Ranke, J.; Müller, A.; Bottin-Weber, U.; Stock, F.; Stolte, S.; Arning, J.; Stormanna, R.; Jastorff, B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol. Environ. Saf. 2007, 67, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Vraneš, M.; Tot, A.; Ćosić, J.; Papović, S.; Panić, J.; Gadžurić, S.; Janković, N.; Vrandečić, K. Correlation between lipophilicity of newly synthesized ionic liquids and selected Fusariumgenus growth rate. RSC Adv. 2019, 9, 19189–19196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lv, S.J.; Wu, Z.; Qian, M.; Xu, Y.; Gao, X.; Wang, T.; Guo, W.; Hou, T.; Li, X.; et al. Role of betulinic acid derivative SH-479 in triple negative breast cancer and bone microenvironment. Oncol. Lett. 2021, 22, 605. [Google Scholar] [CrossRef] [PubMed]
- Lepcock, J.R.; Frey, H.E.; Rodahl, A.M.; Kruuv, J. Thermal analysis of CHL V79 cells using differential scanning calorimetry: Implications for hyperthermic cell killing and the heat shock response. J. Cell. Physiol. 1988, 137, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lepcock, J.R. Measurement of protein stability and protein denaturation in cells using differential scanning calorimetry. Methods 2005, 35, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Todinova, S.; Nikolova, B.; Iliev, I.; Semkova, S.; Krumova, S.; Taneva, S.G. Thermodynamic behavior of breast cancer cell lines after miltefosine and cisplatin treatment. J. Therm. Anal. Calorim. 2022, 147, 7819–7828. [Google Scholar] [CrossRef]
- Egorova, K.; Seitkalieva, M.; Kashin, A.; Gordeev, E.; Vavina, A.; Posvyatenko, A.; Ananikov, V. Biological activity, solvation properties and microstructuring of protic imidazolium ionic liquids. J. Mol. Liq. 2022, 367, 12045. [Google Scholar] [CrossRef]
- Almagor, M.; Cole, R.D. Differential scanning calorimetry of nuclei as a test for the effects of anticancer drugs on human chromatin. Cancer Res. 1989, 49, 5561–5566. [Google Scholar]
- Goswami, P.; Paul, S.; Banerjee, R.; Kundu, R.; Mukherjee, A. Betulinic acid induces DNA damage and apoptosis in SiHa cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Król, S.K.; KieBbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. BioMed. Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef]
- McElhaney, R.N. The use of differential scanning calorimetry and differential thermal analysis in studies of model and biological membranes. Chem. Phys. Lipids 1982, 30, 229–259. [Google Scholar] [CrossRef]
- Lewis, R.N.; Mannock, D.A.; McElhaney, R.N. Differential scanning calorimetry in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Mol. Biol. 2007, 400, 171–195. [Google Scholar] [CrossRef]
- Mitra, S.; Sharma, V.K.; Ghosh, S.K. Effects of ionic liquids on biomembranes: A review on recent biophysical studies. Chem. Phys. Lipids 2023, 256, 105336. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.S.; Rodriguez, B.J.; Prencipe, M.; Benedetto, A. Sub-toxic concentrations of ionic liquids enhance cell migration by reducing the elasticity of the cellular lipid membrane. J. Phys. Chem. Lett. 2020, 11, 7327–7333. [Google Scholar] [CrossRef]
- Galluzzi, M.; Schulte, C.; Milani, P.; Podestà, A. Imidazolium-based ionic liquids affect morphology and rigidity of living cells: An atomic force microscopy study. Langmuir 2018, 34, 12452–12462. [Google Scholar] [CrossRef]
| Compound | Tm (°C) | TTGonset (°C) | TDTGmax (°C) | Water Solubility | ||
|---|---|---|---|---|---|---|
| BA | - | 305.7 | 358.7 | +8.841 | +41.173 | weak |
| [AlaOEt][BA] | - | 92.0 | 370.7 | +8.478 | +49.412 | weak |
| [ValOEt][BA] | - | 116.8 | 354.0 | +15.101 | +92.252 | weak |
| [LeuOEt][BA] | - | 114.9 | 359.4 | +9.654 | +60.331 | weak |
| [IleOEt][BA] | 88.8 | 127.5 | 369.0 | +6.229 | +38.927 | weak |
| [ThrOEt][BA] | 51.5 | 109.0 | 353.5 | +7.512 | +46.038 | middle |
| [SerOEt][BA] | - | 118.2 | 364.1 | +7.020 | +42.040 | middle |
| [CysOEt][BA] | - | 150.1 | 359.2 | +21.818 | +134.165 | middle |
| [MetOEt][BA] | 86.5 | 125.7 | 358.6 | +7.420 | +47.708 | weak |
| [LysOEt][BA] | 84.9 | 144.0 | 327.3 | +11.091 | +70.976 | very good |
| [LysOEt][BA]2 | - | 157.7 | 368.8 | +10.413 | +236.367 | good |
| [PheOEt][BA] | 82.8 | 133.9 | 360.1 | +15.332 | +101.032 | middle |
| [ProOEt][BA] | 91.2 | 121.6 | 372.1 | +2.235 | +13.607 | weak |
| [TyrOEt][BA] | 70.7/103.1 | 202.3 | 354.4 | +15.810 | +106.712 | weak |
| [TrpOEt][BA] | - | 231.3 | 359.8 | +15.436 | +107.743 | middle |
| [Asp(OEt)2][BA] | 76.5 | 119.8 | 357.4 | +4.057 | +26.569 | middle |
| Compound | IC50, µM |
|---|---|
| BA | 11.5 ± 1.8 |
| [AlaOEt][BA] | 12.7 ± 4.5 |
| [ValOEt][BA] | 25.7 ± 0.8 * |
| [LeuOEt][BA] | 7.5 ± 1.2 |
| [IleOEt][BA] | 8.5 ± 1.3 |
| [ThrOEt][BA] | 8.2 ± 2.4 |
| [SerOEt][BA] | 12.2 ± 2.6 |
| [CysOEt][BA] | 9.6 ± 3.8 |
| [MetOEt][BA] | 7.5 ± 0.3 |
| [LysOEt][BA] | 12.9 ± 0.3 |
| [LysOEt][BA]2 | 4.8 ± 1.3 *** (4.0 ± 0.2 *** for MCF-10A) |
| [PheOEt][BA] | 12.3 ± 1.7 |
| [ProOEt][BA] | 7.6 ± 1.8 |
| [TyrOEt][BA] | 14.2 ± 2.4 |
| [TrpOEt][BA] | 13.1 ± 0.4 |
| [Asp(OEt)2][BA] | 8.9 ± 2.1 |
| Thermodynamic Parameters | MCF-7 | MCF-7-BA | MCF-7-[LysOEt][BA]2 |
|---|---|---|---|
| Shm1 (°C) | 48.9 | 46.80 | 43.4 |
| cPSh1 (cal/g·K) | 0.83 | 0.13/ | 0.14 |
| Tm1 (°C) | 52.4 | 51.2 | 52.7 |
| cPT1 (cal/g·K) | 1.21 | 0.58 | 0.80 |
| Tm2 (°C) | 59.8 | 59.2 | 60.2 |
| cPT2 (cal/g·K) | 0.83 | 0.45 | 0.73 |
| Tm3 (°C) | 64.2 | 66.3 (Sh) | 65.5 |
| cPT3 (cal/g·K) | 0.64 | 0.29 | 0.52 |
| Tm4 (°C) | 71.5 | 70.5 | 71.8 |
| cPT4 (cal/g·K) | 0.55 | 0.36 | 0.54 |
| Shm2 (°C) | 78.3 | 77.6 | 78.4 |
| cPSh2 (cal/g·K) | 0.24 | 0.22 | 0.23 |
| Tm5 (°C) | 84.8 | 85.5 | 84.4 |
| cPT5 (cal/g·K) | 0.28 | 0.31 | 0.22 |
| Tm6 (°C) | 99.2 | 100.7 | 98.9 |
| cPT6 (cal/g·K) | 0.73 | 0.64 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowicz-Rupniewska, P.; Klebeko, J.; Georgieva, I.; Apostolova, S.; Struk, Ł.; Todinova, S.; Tzoneva, R.D.; Guncheva, M. Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids. Pharmaceutics 2024, 16, 496. https://doi.org/10.3390/pharmaceutics16040496
Ossowicz-Rupniewska P, Klebeko J, Georgieva I, Apostolova S, Struk Ł, Todinova S, Tzoneva RD, Guncheva M. Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids. Pharmaceutics. 2024; 16(4):496. https://doi.org/10.3390/pharmaceutics16040496
Chicago/Turabian StyleOssowicz-Rupniewska, Paula, Joanna Klebeko, Irina Georgieva, Sonia Apostolova, Łukasz Struk, Svetla Todinova, Rumiana Dimitrova Tzoneva, and Maya Guncheva. 2024. "Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids" Pharmaceutics 16, no. 4: 496. https://doi.org/10.3390/pharmaceutics16040496
APA StyleOssowicz-Rupniewska, P., Klebeko, J., Georgieva, I., Apostolova, S., Struk, Ł., Todinova, S., Tzoneva, R. D., & Guncheva, M. (2024). Tuning of the Anti-Breast Cancer Activity of Betulinic Acid via Its Conversion to Ionic Liquids. Pharmaceutics, 16(4), 496. https://doi.org/10.3390/pharmaceutics16040496










