Tobramycin Reduces Pulmonary Toxicity of Polymyxin B via Inhibiting the Megalin-Mediated Drug Uptake in the Human Lung Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cytotoxicity Assessment
2.4. Transport Study
2.5. LC-MS Analysis
Cellular Accumulation and Distribution of Polymyxin B in the Calu-3 Cell Monolayer
2.6. Distribution of Megalin Transporter in Calu-3 Cells
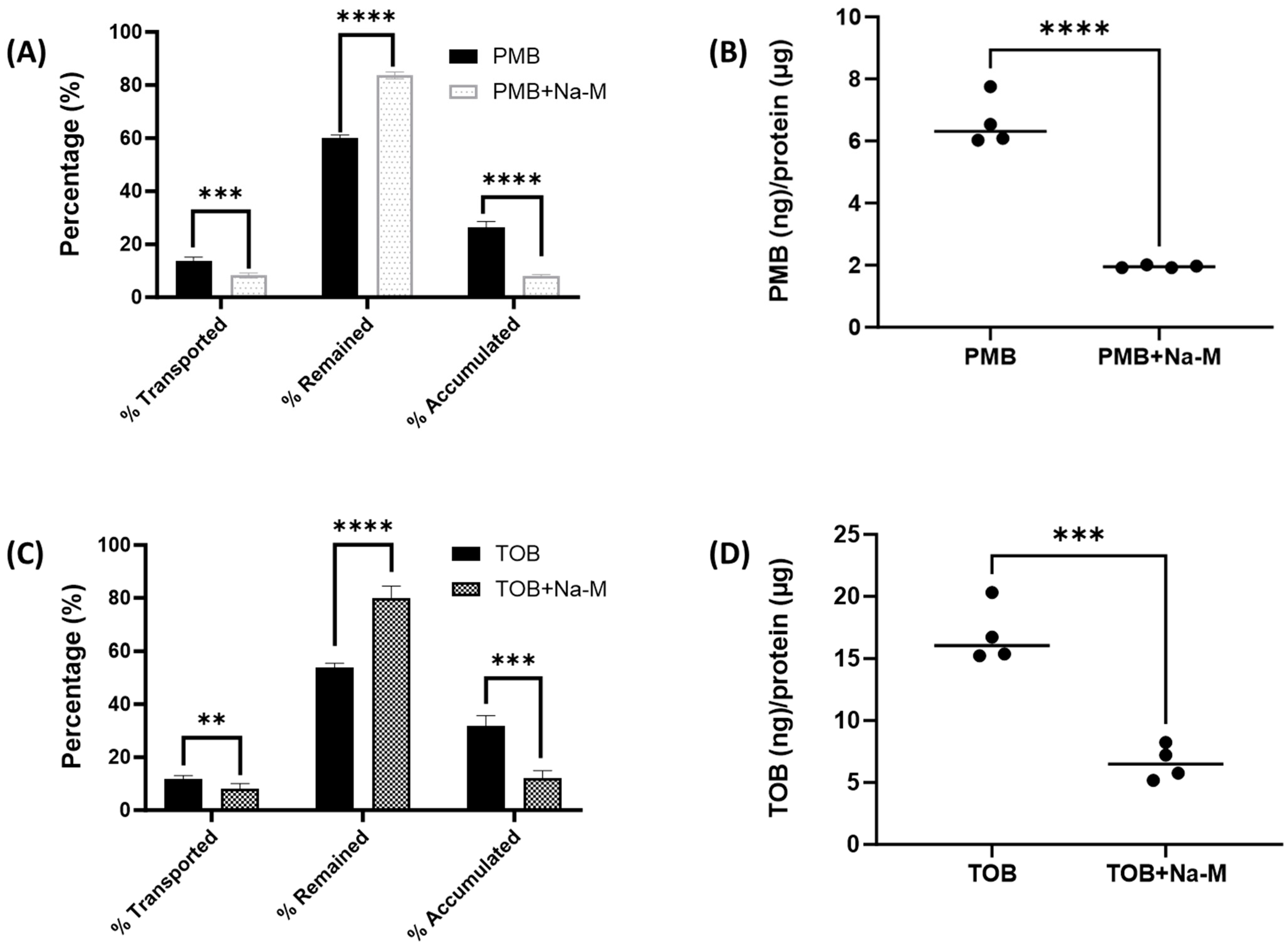
2.7. Evaluation of Megalin Substrate Uptake by Calu-3 Cells in Presence of Polymyxin B and Tobramycin
2.8. Evaluation of Polymyxin B and Tobramycin Transport Behavior in Calu-3 Cells in Presence of Megalin Inhibitor
3. Results
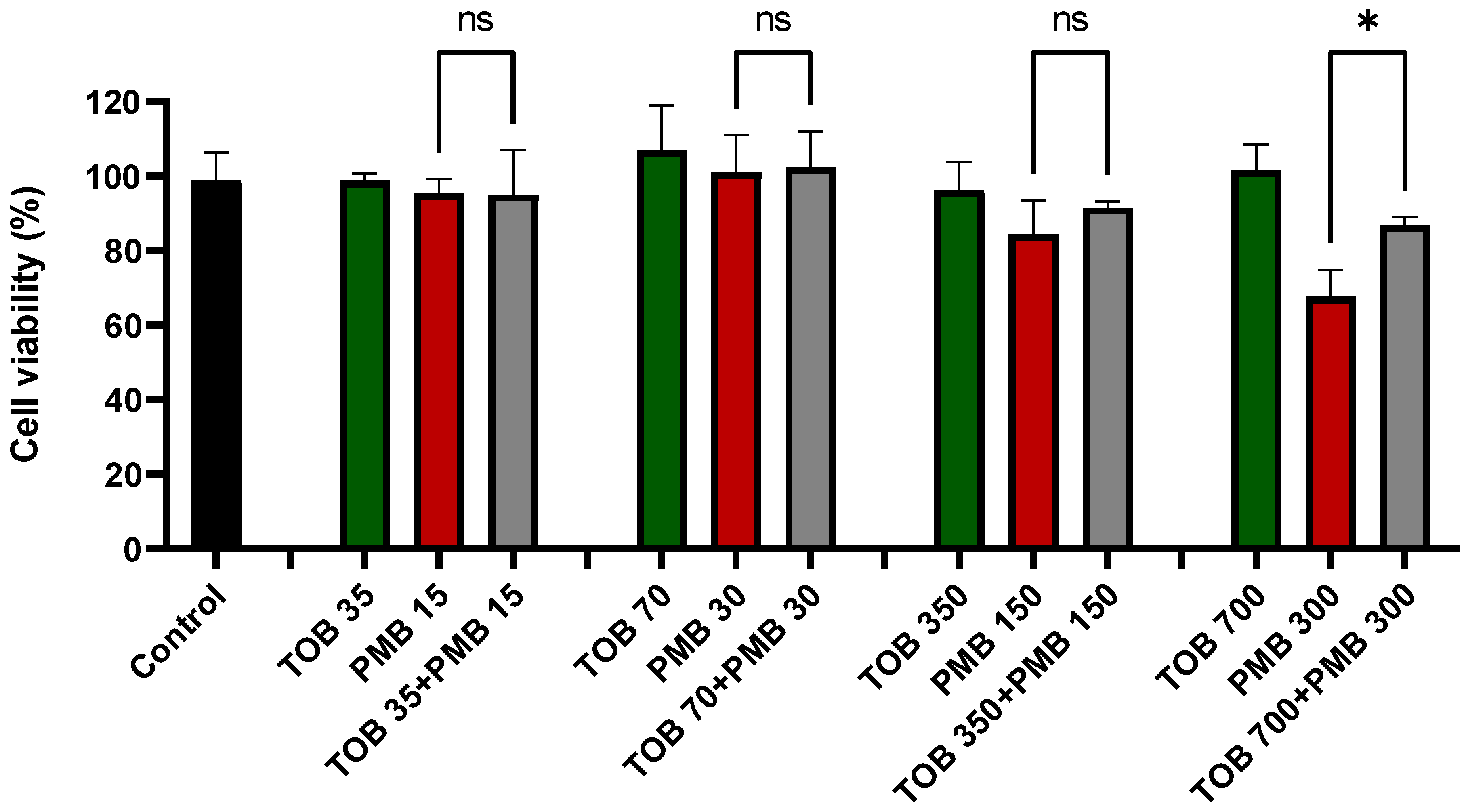
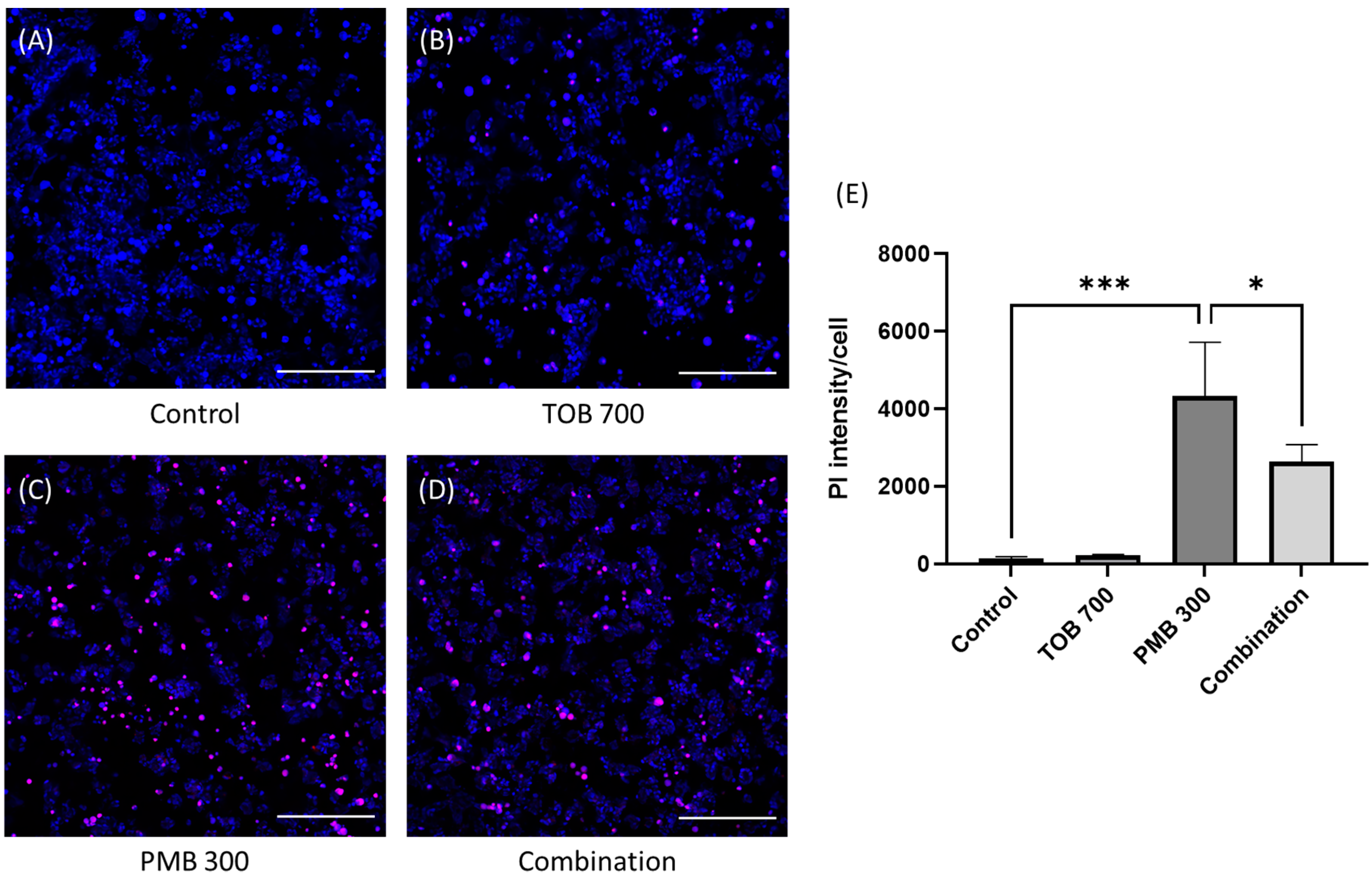
3.1. Effect of Tobramycin on the Polymyxin-Induced Cytotoxicity in Calu-3 Cells
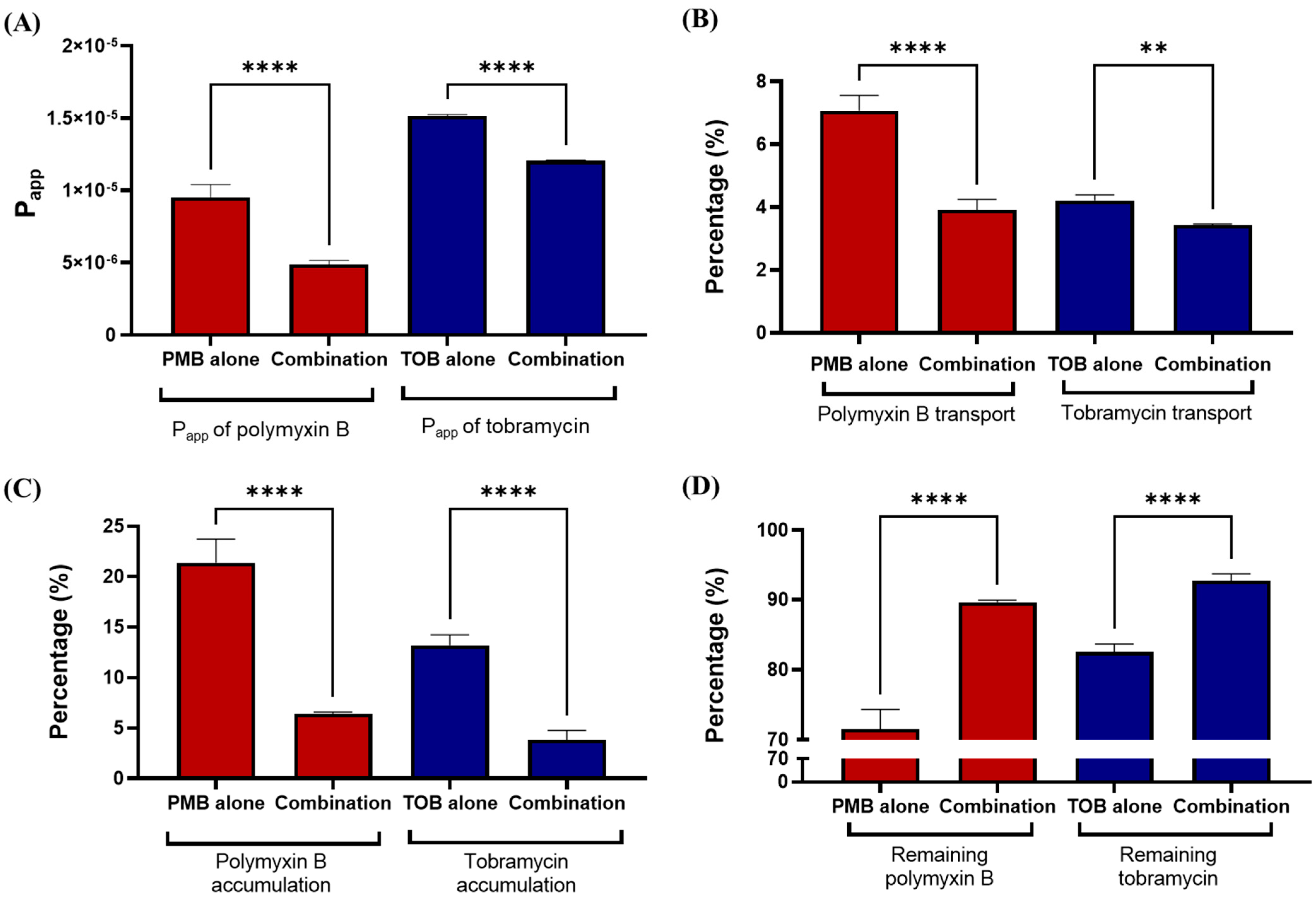
3.2. Transport of Polymyxin and Tobramycin in the Air Liquid Culture Model of Calu-3
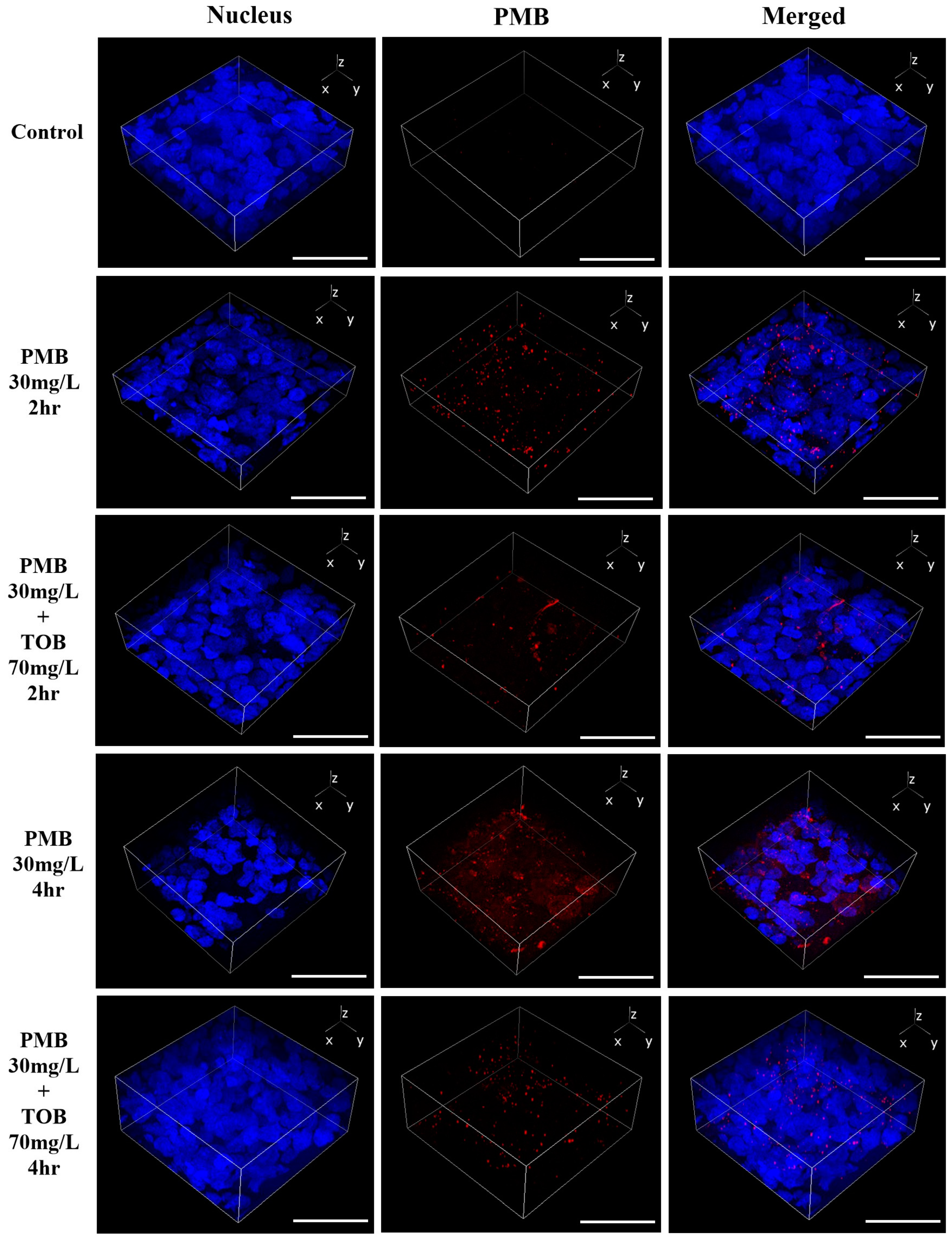
3.3. Cellular Uptake and Distribution of Polymyxin B in Calu-3 Cell Monolayer by Confocal Microscopy
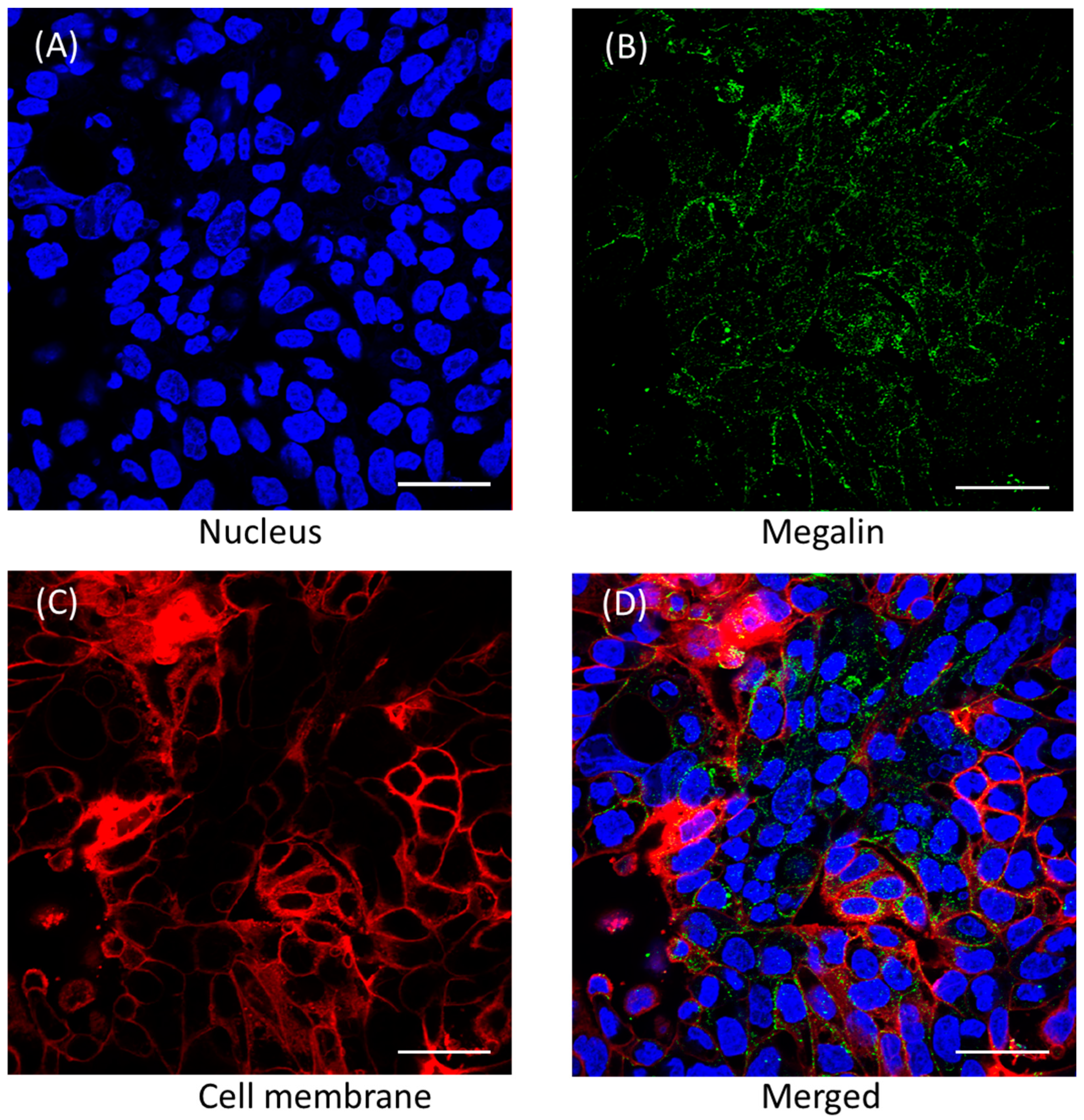
3.4. Megalin Distribution and Activity in Calu-3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheah, S.E.; Wang, J.; Nguyen, V.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population PK of CMS and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Wang, J.; Wirth, V.; Chen, K.; Kaye, K.S.; Tsuji, B.T.; Li, J.; Nation, R.L. Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J. Antimicrob. Chemother. 2018, 73, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Foweraker, J.E.; Wilkinson, P.; Kenyon, R.F.; Bilton, D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014, 189, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Haliburn, C.; Döring, G.; Goldman, M.H.; Group, F.S. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax 2013, 68, 344–350. [Google Scholar] [CrossRef]
- Kwa, A.L.; Loh, C.; Low, J.G.; Kurup, A.; Tam, V.H. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis. 2005, 41, 754–757. [Google Scholar] [CrossRef]
- Pereira, G.H.; Muller, P.R.; Levin, A.S. Salvage treatment of pneumonia and initial treatment of tracheobronchitis caused by multidrug-resistant Gram-negative bacilli with inhaled polymyxin B. Diagn. Microbiol. Infect. Dis. 2007, 58, 235–240. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systemic review and Bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef]
- Velkov, T.; Abdul Rahim, N.; Zhou, Q.T.; Chan, H.K.; Li, J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Adv. Drug Deliv. Rev. 2015, 85, 65–82. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Velkov, T.; Zhou, Q.T.; Fulcher, A.; Callaghan, J.; Zhou, F.; Chan, K.; Azad, M.A.; Li, J. Intracellular localization of polymyxins in human alveolar epithelial cells. J. Antimicrob. Chemother. 2019, 74, 48–57. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Velkov, T.; Lin, Y.-W.; Yun, B.; Nowell, C.J.; Zhou, F.; Zhou, Q.T.; Chan, K.; Azad, M.A.; Li, J. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrob. Agents Chemother. 2017, 61, e02690-16. [Google Scholar] [CrossRef]
- Azad, M.A.; Zhang, S.; Li, J.; Kim, Y.; Yu, H.H.; Fulcher, A.J.; Howard, D.L.; de Jonge, M.D.; James, S.A.; Roberts, K.D.J.A.A.; et al. Synchrotron-based X-ray fluorescence microscopy reveals accumulation of polymyxins in single human alveolar epithelial cells. Antimicrob. Agents Chemother. 2021, 65, e02314-20. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Azad, M.A.K.; Li, M.; Creek, D.J.; Han, M.; Zhou, F.; Chan, K.; Zhou, Q.T.; Velkov, T.; Li, J. Polymyxin-induced metabolic perturbations in human lung epithelial cells. Antimicrob. Agents Chemother. 2021, 65, e0083521. [Google Scholar] [CrossRef]
- Sue, C.N.; Mohammad, A.K.A.; Tony, V.; Qi, Z.; Jian, L. Rescuing the last-line polymyxins: Achievements and challenges. Pharmacol. Rev. 2021, 73, 679. [Google Scholar]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Hoiby, N.; Ulrich, M.; Molin, S.; Riethmuller, J.; Doring, G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef]
- Riethmuller, J.; Herrmann, G.; Graepler-Mainka, U.; Hellwig, D.; Heuer, H.E.; Heyder, S.; Koster, H.; Kinder, B.; Kroger, K.; Paul, K.; et al. Sequential inhalational tobramycin-colistin-combination in CF-patients with chronic P. aeruginosa colonization—An observational study. Cell Physiol. Biochem. 2016, 39, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, P.; Harnan, S.; Uttley, L.; Mildred, M.; Carroll, C.; Cantrell, A. Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: Systematic review and economic model. Health Technol. Assess. 2013, 17, v–xvii. [Google Scholar] [CrossRef]
- Kashyap, S.; Kaur, S.; Sharma, P.; Capalash, N. Combination of colistin and tobramycin inhibits persistence of Acinetobacter baumannii by membrane hyperpolarization and down-regulation of efflux pumps. Microbes Infect. 2021, 23, 104795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ahmed, M.U.; Li, J.; Azad, M.A.K. Inhalation formulations of antimicrobial compounds. U.S. Patent 18/011,155, 27 July 2023. [Google Scholar]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Aoki, N.; Kuwahara, S.; Hosojima, M.; Kaseda, R.; Goto, S.; Iida, T.; De, S.; Kabasawa, H.; Kaneko, R.; et al. Megalin Blockade with cilastatin suppresses drug-induced nephrotoxicity. J. Am. Soc. Nephrol. 2017, 28, 1783. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Tanaka, H.; Nakanishi, N.; Murakami, T.; Takano, M. Role of megalin in renal handling of aminoglycosides. Am. J. Physiol. Renal Physiol. 2001, 281, F337–F344. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamaguchi, H.; Ogura, J.; Kobayashi, M.; Yamada, T.; Iseki, K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob. Agents Chemother. 2013, 57, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Manchandani, P.; Zhou, J.; Babic, J.T.; Ledesma, K.R.; Truong, L.D.; Tam, V.H. Role of renal drug exposure in polymyxin B-induced nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e02391-16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ahmed, M.U.; Zhu, C.; Yu, S.; Pan, W.; Velkov, T.; Li, J.; Tony Zhou, Q. In vitro evaluation of drug delivery behavior for inhalable amorphous nanoparticle formulations in a human lung epithelial cell model. Int. J. Pharm. 2021, 596, 120211. [Google Scholar] [CrossRef]
- Pandey, S.; Dhanani, J.; Lipman, J.; Roberts, J.A.; Wallis, S.C.; Parker, S.L. Development and validation of LC-MS/MS methods to measure tobramycin and lincomycin in plasma, microdialysis fluid and urine: Application to a pilot pharmacokinetic research study. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 274–284. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, S.; Chai, G.; Liu, J.; Zhou, Q. An LC-MS/MS method for simultaneous analysis of the cystic fibrosis therapeutic drugs colistin, ivacaftor and ciprofloxacin. J. Pharm. Anal. 2021, 11, 732–738. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Shen, M. Hydrophilic interaction chromatography combined with tandem mass spectrometry method for the quantification of tobramycin in human plasma and its application in a pharmacokinetic study. J. Chromatogr. B 2014, 973, 39–44. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Velasco, A.; Polo-Hernandez, E.; Perez-Reyes, P.L.; Tabernero, A.; Medina, J.M. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 2008, 106, 1149–1159. [Google Scholar] [CrossRef]
- Almangour, T.A.; Garcia, E.; Zhou, Q.; Forrest, A.; Kaye, K.S.; Li, J.; Velkov, T.; Rao, G.G. Polymyxins for the treatment of lower respiratory tract infections: Lessons learned from the integration of clinical pharmacokinetic studies and clinical outcomes. Int. J. Antimicrob. Agents 2021, 57, 106328. [Google Scholar] [CrossRef]
- Ehrmann, S.; Chastre, J.; Diot, P.; Lu, Q. Nebulized antibiotics in mechanically ventilated patients: A challenge for translational research from technology to clinical care. Ann. Intensive Care 2017, 7, 78. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial dysfunction in lung pathogenesis. Annu. Rev. Physiol. 2017, 79, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Buchackert, Y.; Rummel, S.; Vohwinkel, C.U.; Gabrielli, N.M.; Grzesik, B.A.; Mayer, K.; Herold, S.; Morty, R.E.; Seeger, W.; Vadasz, I. Megalin mediates transepithelial albumin clearance from the alveolar space of intact rabbit lungs. J. Physiol. 2012, 590, 5167–5181. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.L.; Andersen, R.K.; Hager, H.; Madsen, M. Lack of megalin expression in adult human terminal ileum suggests megalin-independent cubilin/amnionless activity during vitamin B12 absorption. Physiol. Rep. 2014, 2, e12086. [Google Scholar] [CrossRef]
- Li, M.; Balamuthusamy, S.; Simon, E.E.; Batuman, V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F82–F90. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Hilpert, J.; Jacobsen, C.; Boensch, C.; Christensen, E.I.; Luft, F.C.; Willnow, T.E. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 2002, 277, 618–622. [Google Scholar] [CrossRef]
- Akour, A.A.; Kennedy, M.J.; Gerk, P.M. The Role of megalin in the transport of gentamicin across BeWo cells, an in vitro model of the human placenta. AAPS J. 2015, 17, 1193–1199. [Google Scholar] [CrossRef]
- Tauris, J.; Christensen, E.I.; Nykjær, A.; Jacobsen, C.; Petersen, C.M.; Ovesen, T. Cubilin and megalin co-localize in the neonatal inner ear. Audiol. Neurotol. 2009, 14, 267–278. [Google Scholar] [CrossRef]


| Compound Name | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (V) |
|---|---|---|---|
| Colistin A | 585.3 | 241.1 | 15 |
| Colistin B | 578.3 | 227.0 | 15 |
| Polymyxin B1 | 602.3 | 241.1 | 15 |
| Polymyxin B2 | 595.3 | 227.1 | 15 |
| Tobramycin | 468.2 | 163.1 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.U.; Li, J.; Zhou, Q. Tobramycin Reduces Pulmonary Toxicity of Polymyxin B via Inhibiting the Megalin-Mediated Drug Uptake in the Human Lung Epithelial Cells. Pharmaceutics 2024, 16, 389. https://doi.org/10.3390/pharmaceutics16030389
Ahmed MU, Li J, Zhou Q. Tobramycin Reduces Pulmonary Toxicity of Polymyxin B via Inhibiting the Megalin-Mediated Drug Uptake in the Human Lung Epithelial Cells. Pharmaceutics. 2024; 16(3):389. https://doi.org/10.3390/pharmaceutics16030389
Chicago/Turabian StyleAhmed, Maizbha Uddin, Jian Li, and Qi (Tony) Zhou. 2024. "Tobramycin Reduces Pulmonary Toxicity of Polymyxin B via Inhibiting the Megalin-Mediated Drug Uptake in the Human Lung Epithelial Cells" Pharmaceutics 16, no. 3: 389. https://doi.org/10.3390/pharmaceutics16030389
APA StyleAhmed, M. U., Li, J., & Zhou, Q. (2024). Tobramycin Reduces Pulmonary Toxicity of Polymyxin B via Inhibiting the Megalin-Mediated Drug Uptake in the Human Lung Epithelial Cells. Pharmaceutics, 16(3), 389. https://doi.org/10.3390/pharmaceutics16030389







