Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Humane Care Guidelines
2.2. Drug Preparation and Dosing
2.3. Virus Challenge Stock
2.4. PK Assessments
2.5. Vaginal Efficacy of Weekly Oral TAF
2.6. Rectal Efficacy of Weekly TAF
2.7. Analysis of TFV and TFV-DP Concentrations
2.8. Allele-Specific PCR for K65R
2.9. Data and Statistical Analysis
3. Results
3.1. Selection of Weekly Oral TAF Dose in Macaques
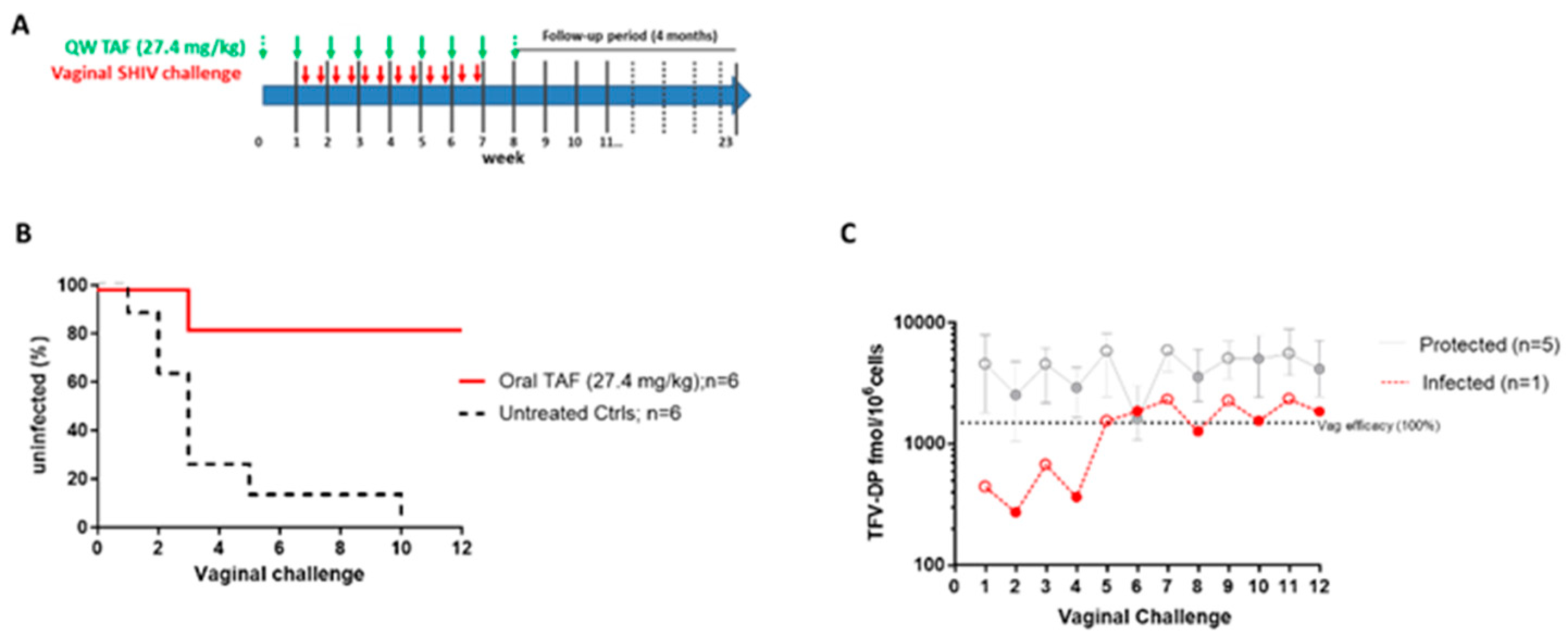
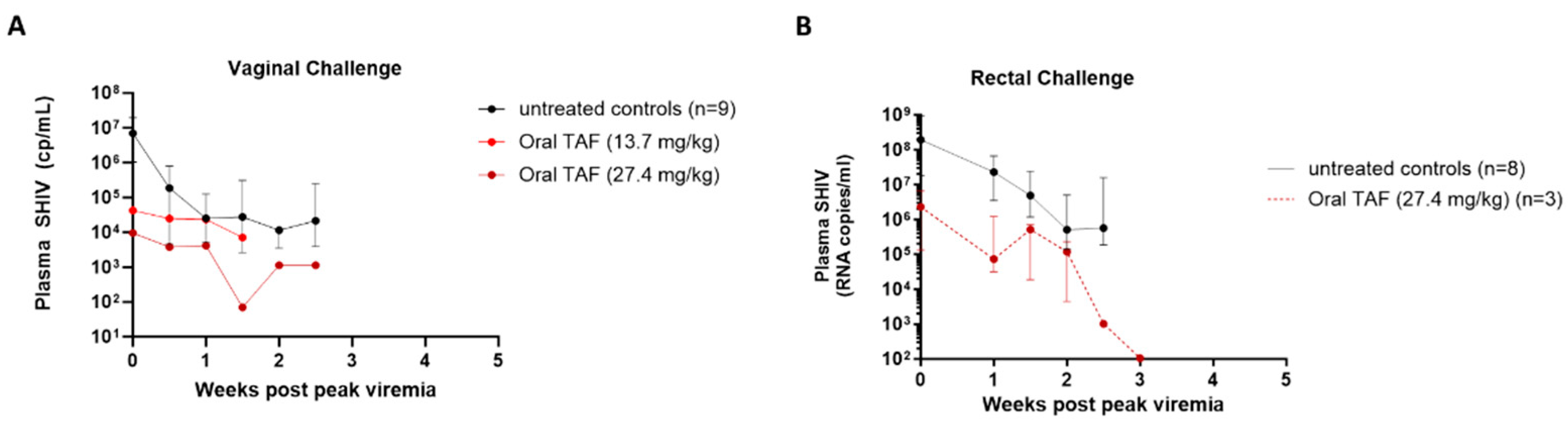
3.2. Efficacy of Weekly Oral TAF (27.4 mg/kg) against Vaginal SHIV Infection
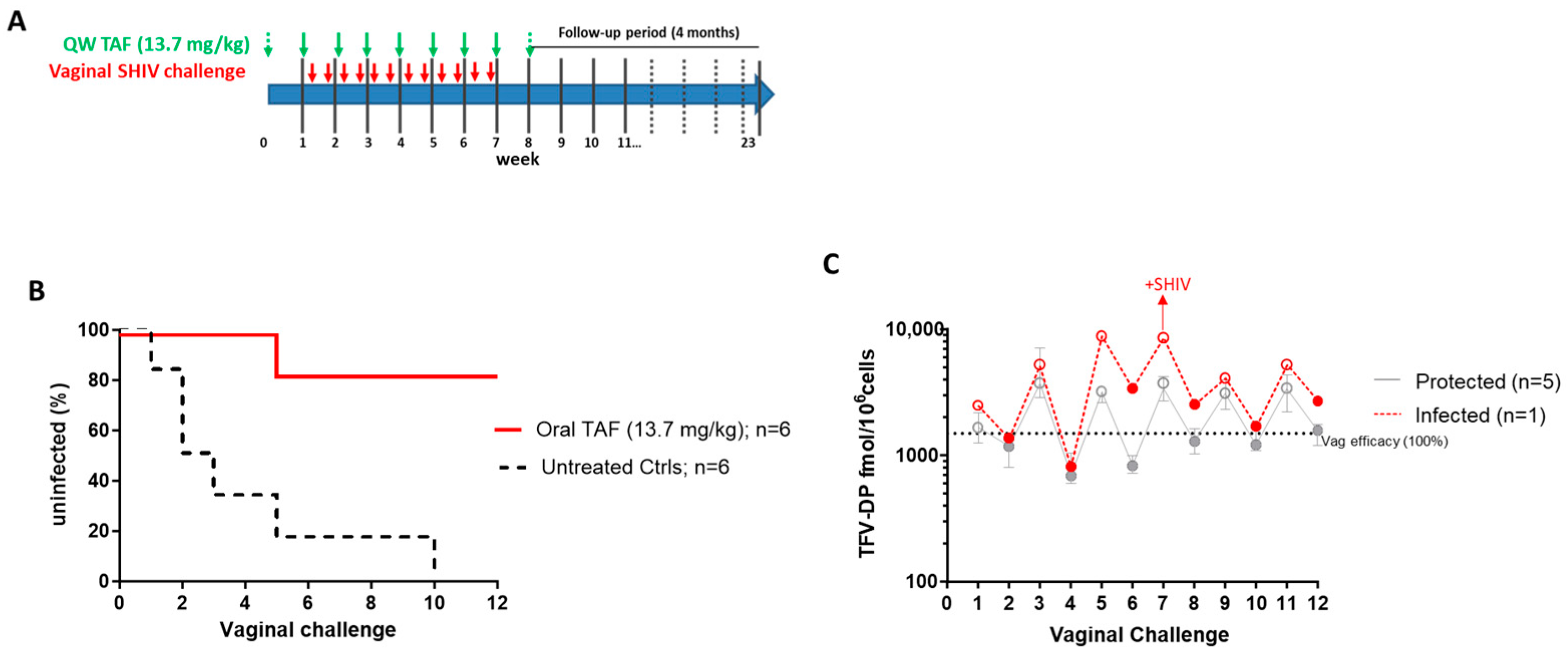
3.3. Efficacy of Weekly Oral TAF (13.7 mg/kg) against Vaginal SHIV Infection
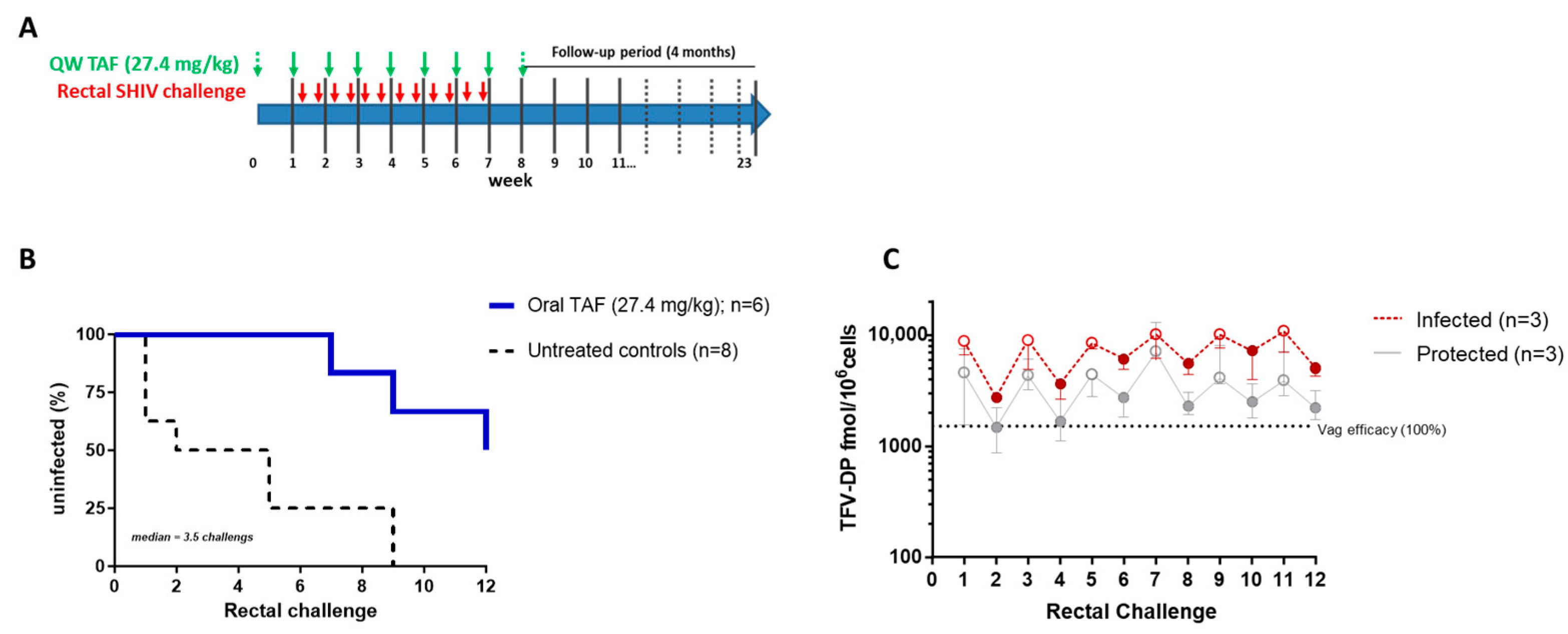
3.4. Efficacy of Weekly Oral TAF (27.4 mg/kg) against Rectal SHIV Infection
3.5. Acute Viremia and Drug Resistance Emergence in Breakthrough Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Chou, R.; Spencer, H.; Bougatsos, C.; Blazina, I.; Ahmed, A.; Selph, S. Preexposure Prophylaxis for the Prevention of HIV: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 330, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.T.; Masse, B.R.; Donnell, D. PrEP Adherence Patterns Strongly Affect Individual HIV Risk and Observed Efficacy in Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 2016, 72, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, K.; Kalichman, S.C. Barriers to HIV Medication Adherence as a Function of Regimen Simplification. Ann. Behav. Med. 2017, 51, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Cotte, F.E.; Fardellone, P.; Mercier, F.; Gaudin, A.F.; Roux, C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010, 21, 145–155. [Google Scholar] [CrossRef]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Flexner, C. The future of long-acting agents for preexposure prophylaxis. Curr. Opin. HIV AIDS 2022, 17, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, D.; Dang, B.N.; Lucari, B.; Swindells, S.; Giordano, T.P. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2021, 33, 801–809. [Google Scholar] [CrossRef]
- Tolley, E.E.; Zangeneh, S.Z.; Chau, G.; Eron, J.; Grinsztejn, B.; Humphries, H.; Liu, A.; Siegel, M.; Bertha, M.; Panchia, R.; et al. Acceptability of Long-Acting Injectable Cabotegravir (CAB LA) in HIV-Uninfected Individuals: HPTN 077. AIDS Behav. 2020, 24, 2520–2531. [Google Scholar] [CrossRef]
- Wulandari, L.P.L.; He, S.Y.; Fairley, C.K.; Bavinton, B.R.; Marie-Schmidt, H.; Wiseman, V.; Guy, R.; Tang, W.; Zhang, L.; Ong, J.J. Preferences for pre-exposure prophylaxis for HIV: A systematic review of discrete choice experiments. EClinicalMedicine 2022, 51, 101507. [Google Scholar] [CrossRef]
- Giorgino, F.; Penfornis, A.; Pechtner, V.; Gentilella, R.; Corcos, A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: Clinical consequences and strategies for improvement. Patient Prefer. Adherence 2018, 12, 707–719. [Google Scholar] [CrossRef]
- Iglay, K.; Cao, X.; Mavros, P.; Joshi, K.; Yu, S.; Tunceli, K. Systematic Literature Review and Meta-analysis of Medication Adherence With Once-weekly Versus Once-daily Therapy. Clin. Ther. 2015, 37, 1813–1821.e1811. [Google Scholar] [CrossRef] [PubMed]
- Kawuma, R.; Nabalwanyi, Z.; Seeley, J.; Mayanja, Y. “I prefer to take pills when I plan to have sex”: Perceptions of on-demand versus daily oral pre-exposure prophylaxis among adolescents in Kampala, Uganda. Afr. J. AIDS Res. 2022, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wray, T.B.; Chan, P.A.; Klausner, J.D.; Ward, L.M.; Ocean, E.M.S. Gay, Bisexual, and Other Men Who Have Sex With Men Who Are Not on Oral PrEP may be Less Interested in Available Injectable Products than in Oral PrEP: Examining Individual-Level Determinants of Interest and Barriers Across Products. AIDS Behav. 2022, 26, 3794–3805. [Google Scholar] [CrossRef] [PubMed]
- Njenda, D.T.; Aralaguppe, S.G.; Singh, K.; Rao, R.; Sönnerborg, A.; Sarafianos, S.G.; Neogi, U. Antiretroviral potency of 4′-ethnyl-2′-fluoro-2′-deoxyadenosine, tenofovir alafenamide and second-generation NNRTIs across diverse HIV-1 subtypes. J. Antimicrob. Chemother. 2018, 73, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, D.; Rudd, D.J.; Zhang, S.; De Lepeleire, I.; Robberechts, M.; Friedman, E.; Keicher, C.; Hüser, A.; Hofmann, J.; Grobler, J.A.; et al. Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration to treatment-naive adults infected with HIV-1: An open-label, phase 1b, consecutive-panel trial. Lancet HIV 2020, 7, e164–e172. [Google Scholar] [CrossRef]
- Markowitz, M.; Gettie, A.; Bernard, L.S.; Andrews, C.D.; Mohri, H.; Horowitz, A.; Grasperge, B.F.; Blanchard, J.L.; Niu, T.; Sun, L.; et al. Once-weekly oral dosing of MK-8591 protects male rhesus Macaques from intrarectal challenge with SHIV109CP3. J. Infect. Dis. 2020, 221, 1398–1406. [Google Scholar] [CrossRef]
- Markowitz, M.; Grobler, J.A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1. Curr. Opin. HIV AIDS 2020, 15, 27–32. [Google Scholar] [CrossRef]
- Matthews, R.P.; Ankrom, W.; Friedman, E.; Jackson Rudd, D.; Liu, Y.; Mogg, R.; Panebianco, D.; De Lepeleire, I.; Petkova, M.; Grobler, J.A.; et al. Safety, tolerability, and pharmacokinetics of single- and multiple-dose administration of islatravir (MK-8591) in adults without HIV. Clin. Transl. Sci. 2021, 14, 1935–1944. [Google Scholar] [CrossRef]
- Matthews, R.P.; Jackson Rudd, D.; Zhang, S.; Fillgrove, K.L.; Sterling, L.M.; Grobler, J.A.; Vargo, R.C.; Stoch, S.A.; Iwamoto, M. Safety and Pharmacokinetics of Once-Daily Multiple-Dose Administration of Islatravir in Adults Without HIV. J. Acquir. Immune Defic. Syndr. 2021, 88, 314–321. [Google Scholar] [CrossRef]
- Di Perri, G. Tenofovir alafenamide (TAF) clinical pharmacology. Infez. Med. 2021, 29, 526–529. [Google Scholar] [CrossRef]
- Lee, W.A.; Cheng, A.K. Tenofovir alafenamide fumarate. Antivir. Ther. 2022, 27, 13596535211067600. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 2016, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.V.; Podany, A.T.; Thorkelson, A.; Winchester, L.C.; Mykris, T.; Anderson, J.; Jorstad, S.; Baker, J.V.; Schacker, T.W. The Lymphoid Tissue Pharmacokinetics of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide in HIV-Infected Persons. Clin. Pharmacol. Ther. 2020, 108, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Dyavar, S.R.; Kumar, S.; Gautam, N.; Podany, A.T.; Winchester, L.C.; Weinhold, J.A.; Mykris, T.M.; Nallasamy, P.; Alnouti, Y.; Fletcher, C.V. Intramuscular and subcutaneous administration of antiretroviral drugs, compared with oral, enhances delivery to lymphoid tissues in BALB/c mice. J. Antimicrob. Chemother. 2021, 76, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; He, G.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hou, J.; Song, A.; Liu, X.; Yang, X.; Xu, J.; Zhang, J.; Hu, Q.; Chen, H.; Chen, Y.; et al. Efficacy and Safety of Oral TDF-Based Pre-exposure Prophylaxis for Men Who Have Sex With Men: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Eisenberg, E.J.; He, G.X.; Lee, W.A. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1091–1098. [Google Scholar] [CrossRef]
- Ruane, P.J.; DeJesus, E.; Berger, D.; Markowitz, M.; Bredeek, U.F.; Callebaut, C.; Zhong, L.; Ramanathan, S.; Rhee, M.S.; Fordyce, M.W.; et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J. Acquir. Immune Defic. Syndr. 2013, 63, 449–455. [Google Scholar] [CrossRef]
- Thurman, A.R.; Schwartz, J.L.; Cottrell, M.L.; Brache, V.; Chen, B.A.; Cochón, L.; Ju, S.; McGowan, I.; Rooney, J.F.; McCallister, S.; et al. Safety and pharmacokinetics of a tenofovir alafenamide Fumarate-emtricitabine based oral antiretroviral regimen for prevention of HIV acquisition in women: A randomized controlled trial. EClinicalMedicine 2021, 36, 100893. [Google Scholar] [CrossRef]
- Garcia-Lerma, J.G.; McNicholl, J.M.; Heneine, W. The predictive value of macaque models of preexposure prophylaxis for HIV prevention. Curr. Opin. HIV AIDS 2022, 17, 179–185. [Google Scholar] [CrossRef]
- Massud, I.; Krovi, A.; Nishiura, K.; Ruone, S.; Li, L.; Holder, A.; Gary, J.; Mills, P.; Mitchell, J.; Khalil, G.; et al. Safety and efficacy of a biodegradable implant releasing tenofovir alafenamide for vaginal protection in a macaque model. J. Antimicrob. Chemother. 2022, 77, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Cambridge, MA, USA, 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 10 June 2012).
- Subbarao, S.; RAOtten, R.A.; Ramos, A.; Kim, C.; Jackson, E.; Monsour, M.; Adams, D.R.; Bashirian, S.; Johnson, J.; Soriano, V.; et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J. Infect. Dis. 2006, 194, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Massud, I.; Cong, M.E.; Ruone, S.; Holder, A.; Dinh, C.; Nishiura, K.; Khalil, G.; Pan, Y.; Lipscomb, J.; Johnson, R.; et al. Efficacy of Oral Tenofovir Alafenamide/Emtricitabine Combination or Single-Agent Tenofovir Alafenamide Against Vaginal Simian Human Immunodeficiency Virus Infection in Macaques. J. Infect. Dis. 2019, 220, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Dobard, C.W.; Makarova, N.; West-Deadwyler, R.; Taylor, A.; Dinh, C.; Martin, A.; Lipscomb, J.; Mitchell, J.; Khalil, G.; Garcia-Lerma, G.; et al. Efficacy of Vaginally Administered Gel Containing Emtricitabine and Tenofovir Against Repeated Rectal Simian Human Immunodeficiency Virus Exposures in Macaques. J. Infect. Dis 2018, 218, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- García-Lerma, J.G.; Aung, W.; Cong, M.E.; Zheng, Q.; Youngpairoj, A.S.; Mitchell, J.; Holder, A.; Martin, A.; Kuklenyik, S.; Luo, W.; et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J. Virol. 2011, 85, 6610–6617. [Google Scholar] [CrossRef] [PubMed]
- Kuklenyik, Z.; Martin, A.; Pau, C.; Holder, A.; Youngpairoj, A.S.; Zheng, Q.; Cong, M.; Garcia-Lerma, J.G.; Heneine, W.; Pirkle, J.L.; et al. On-line coupling of anion exchange and ion pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.M.; Chen, X.; Meditz, A.L.; Castillo-Mancilla, J.R.; Gardner, E.M.; Predhomme, J.A.; Clayton, C.; Austin, G.; Palmer, B.E.; Zheng, J.H.; et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res. Hum. Retroviruses 2016, 32, 981–991. [Google Scholar] [CrossRef]
- Romano, J.W.; Baum, M.M.; Demkovich, Z.R.; Diana, F.; Dobard, C.; Feldman, P.L.; Garcia-Lerma, J.G.; Grattoni, A.; Gunawardana, M.; Ho, D.K.; et al. Tenofovir Alafenamide for HIV Prevention: Review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development Meeting. AIDS Res. Hum. Retroviruses 2021, 37, 409–420. [Google Scholar] [CrossRef]
- Cottrell, M.L.; Garrett, K.L.; Prince, H.M.A.; Sykes, C.; Schauer, A.; Emerson, C.W.; Peery, A.; Rooney, J.F.; McCallister, S.; Gay, C.; et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J. Antimicrob. Chemother. 2017, 72, 1731–1740. [Google Scholar] [CrossRef]
- Arts, E.J.; Marois, J.P.; Gu, Z.; Le Grice, S.F.; Wainberg, M.A. Effects of 3’-deoxynucleoside 5’-triphosphate concentrations on chain termination by nucleoside analogs during human immunodeficiency virus type 1 reverse transcription of minus-strand strong-stop DNA. J. Virol. 1996, 70, 712–720. [Google Scholar] [CrossRef]
- Lackner, A.A.; Mohan, M.; Veazey, R.S. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology 2009, 136, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Elliott, J.; Fuerst, M.; Taing, P.; Boscardin, J.; Poles, M.; Anton, P. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J. Acquir. Immune Defic. Syndr. 2004, 37, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Xu, R.; Wen, L.; Deng, Y.; He, L.; Zhong, H.; Wang, Y. Efficacy and safety profiles of dolutegravir plus lamivudine vs. bictegravir/emtricitabine/tenofovir alafenamide in therapy-naive adults with HIV-1. Chin. Med. J. 2023, 136, 2677–2685. [Google Scholar] [CrossRef]
- Otten, R.A.; Adams, D.R.; Kim, C.N.; Jackson, E.; Pullium, J.K.; Lee, K.; Grohskopf, L.A.; Monsour, M.; Butera, S.; Folks, T.M. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: Strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 2005, 19, 164–173. [Google Scholar] [CrossRef]
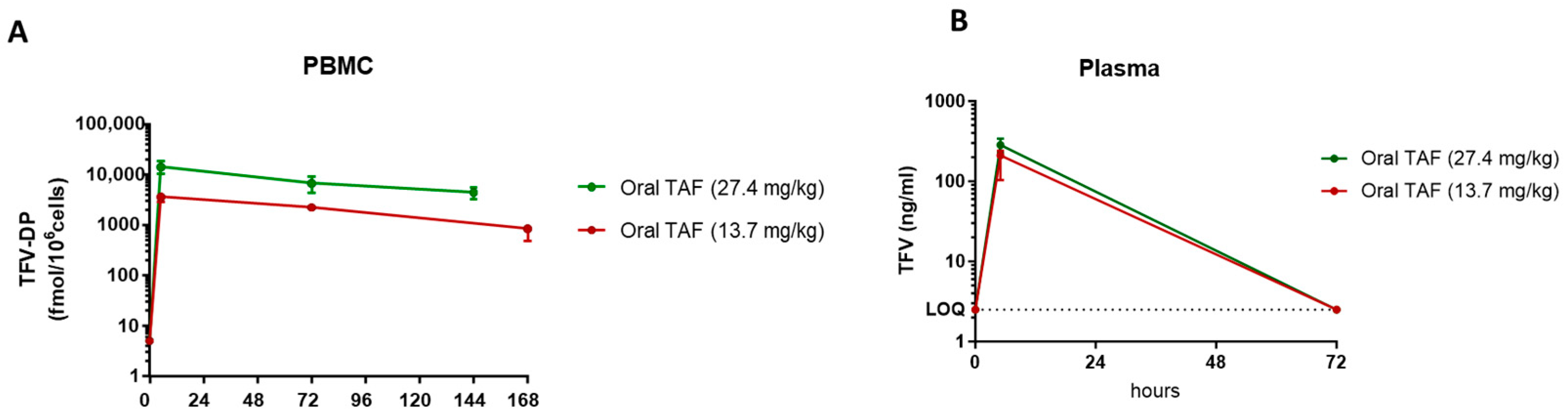
| Plasma | PBMC | |||||
|---|---|---|---|---|---|---|
| Oral TAF Dose | TFV [Tmax] Hours | TFV [Cmax] ng/mL | TFV [AUC0–72h] ng∗h/mL | TFV-DP [Tmax] Hours | TFV-DP [Cmax] fmol/106 Cells | TFV-DP [AUC0–72h] fmol∗h/106 Cells |
| 13.7 mg/kg | 5 | 188.2 ± 54.9 | 6865 ± 1844 | 5 | 3552 ± 550.4 | 203,293 ± 25,735 |
| 27.4 mg/kg | 5 | 284.1 ± 139.0 | 10,319 ± 2714 | 5 | 14,090 ± 7700 | 697,805 ± 391,389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massud, I.; Nishiura, K.; Ruone, S.; Holder, A.; Dinh, C.; Lipscomb, J.; Mitchell, J.; Khalil, G.M.; Heneine, W.; Garcίa-Lerma, J.G.; et al. Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection. Pharmaceutics 2024, 16, 384. https://doi.org/10.3390/pharmaceutics16030384
Massud I, Nishiura K, Ruone S, Holder A, Dinh C, Lipscomb J, Mitchell J, Khalil GM, Heneine W, Garcίa-Lerma JG, et al. Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection. Pharmaceutics. 2024; 16(3):384. https://doi.org/10.3390/pharmaceutics16030384
Chicago/Turabian StyleMassud, Ivana, Kenji Nishiura, Susan Ruone, Angela Holder, Chuong Dinh, Jonathan Lipscomb, James Mitchell, George M. Khalil, Walid Heneine, J. Gerardo Garcίa-Lerma, and et al. 2024. "Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection" Pharmaceutics 16, no. 3: 384. https://doi.org/10.3390/pharmaceutics16030384
APA StyleMassud, I., Nishiura, K., Ruone, S., Holder, A., Dinh, C., Lipscomb, J., Mitchell, J., Khalil, G. M., Heneine, W., Garcίa-Lerma, J. G., & Dobard, C. W. (2024). Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection. Pharmaceutics, 16(3), 384. https://doi.org/10.3390/pharmaceutics16030384







