A New Algorithm Integrating Molecular Response, Toxicity, and Plasma Level Measures for Ponatinib Dose Choice in Patients Affected by Chronic Myeloid Leukemia
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Ponatinib Plasma Concentration Measures
2.3. Ponatinib Pharmacokinetic Study
2.4. Molecular Response Evaluation and BCR-ABL1 Mutation Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population: Clinical Features and Outcome
3.2. PON Plasma Concentrations
3.3. PON Daily Dose, Plasma Concentrations, Molecular Responses, and AE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eide, C.A.; O’Hare, T. Chronic Myeloid Leukemia: Advances in Understanding Disease Biology and Mechanisms of Resistance to Tyrosine Kinase Inhibitors. Curr. Hematol. Malig. Rep. 2015, 10, 158–166. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.-L. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef]
- Pérez-Lamas, L.; Luna, A.; Boque, C.; Xicoy, B.; Giraldo, P.; Pérez López, R.; Ruiz Nuño, C.; De Las Heras, N.; Mora Casterá, E.; López Marín, J.; et al. Toxicity of Asciminib in Real Clinical Practice: Analysis of Side Effects and Cross-Toxicity with Tyrosine Kinase Inhibitors. Cancers 2023, 15, 1045. [Google Scholar] [CrossRef]
- Yoshifuji, K.; Sasaki, K. Adverse Events and Dose Modifications of Tyrosine Kinase Inhibitors in Chronic Myelogenous Leukemia. Front. Oncol. 2022, 12, 1021662. [Google Scholar] [CrossRef]
- Soverini, S.; Martelli, M.; Bavaro, L.; De Benedittis, C.; Sica, S.; Sorà, F.; Iurlo, A.; Bonifacio, M.; Pregno, P.; Galimberti, S.; et al. BCR-ABL1 Compound Mutants: Prevalence, Spectrum and Correlation with Tyrosine Kinase Inhibitor Resistance in a Consecutive Series of Philadelphia Chromosome-Positive Leukemia Patients Analyzed by NGS. Leukemia 2021, 35, 2102–2107. [Google Scholar] [CrossRef]
- Stella, S.; Tirrò, E.; Conte, E.; Stagno, F.; Di Raimondo, F.; Manzella, L.; Vigneri, P. Suppression of Survivin Induced by a BCR-ABL/JAK2/STAT3 Pathway Sensitizes Imatinib-Resistant CML Cells to Different Cytotoxic Drugs. Mol. Cancer Ther. 2013, 12, 1085–1098. [Google Scholar] [CrossRef]
- Pereira, D.; Rocha, L.S.; Gil, M.V.; Otero, M.; Silva, N.J.O.; Esteves, V.I.; Calisto, V. In Situ Functionalization of a Cellulosic-Based Activated Carbon with Magnetic Iron Oxides for the Removal of Carbamazepine from Wastewater. Environ. Sci. Pollut. Res. Int. 2021, 28, 18314–18327. [Google Scholar] [CrossRef]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.L.; Hughes, T.P.; Yong, A.S.M. CML Patients with Deep Molecular Responses to TKI Have Restored Immune Effectors and Decreased PD-1 and Immune Suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef]
- Bono, S.; Lulli, M.; D’Agostino, V.G.; Di Gesualdo, F.; Loffredo, R.; Cipolleschi, M.G.; Provenzani, A.; Rovida, E.; Dello Sbarba, P. Different BCR/Abl Protein Suppression Patterns as a Converging Trait of Chronic Myeloid Leukemia Cell Adaptation to Energy Restriction. Oncotarget 2016, 7, 84810–84825. [Google Scholar] [CrossRef]
- Poteti, M.; Menegazzi, G.; Peppicelli, S.; Tusa, I.; Cheloni, G.; Silvano, A.; Mancini, C.; Biagioni, A.; Tubita, A.; Mazure, N.M.; et al. Glutamine Availability Controls BCR/Abl Protein Expression and Functional Phenotype of Chronic Myeloid Leukemia Cells Endowed with Stem/Progenitor Cell Potential. Cancers 2021, 13, 4372. [Google Scholar] [CrossRef]
- Eadie, L.N.; Hughes, T.P.; White, D.L. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin. Pharmacol. Ther. 2014, 95, 294–306. [Google Scholar] [CrossRef]
- Dalle Fratte, C.; Polesel, J.; Gagno, S.; Posocco, B.; De Mattia, E.; Roncato, R.; Orleni, M.; Puglisi, F.; Guardascione, M.; Buonadonna, A.; et al. Impact of ABCG2 and ABCB1 Polymorphisms on Imatinib Plasmatic Exposure: An Original Work and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 3303. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib Efficacy and Safety in Philadelphia Chromosome–Positive Leukemia: Final 5-Year Results of the Phase 2 PACE Trial. Blood 2018, 132, 393–404. [Google Scholar] [CrossRef]
- Haddad, F.G.; Issa, G.C.; Jabbour, E.; Yilmaz, M. Ponatinib for the Treatment of Adult Patients with Resistant or Intolerant Chronic-Phase Chronic Myeloid Leukemia. Expert Opin. Pharmacother. 2022, 23, 751–758. [Google Scholar] [CrossRef]
- Mulas, O.; Abruzzese, E.; Luciano, L.; Iurlo, A.; Attolico, I.; Castagnetti, F.; Galimberti, S.; Bonifacio, M.; Annunziata, M.; Gozzini, A.; et al. The new Systematic Coronary Risk Evaluation (SCORE2 and SCORE2-OP) estimates the risk of arterial occlusive events in chronic myeloid leukemia patients treated with nilotinib or ponatinib. Ann. Hematol. 2024, 103, 427–436. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Garasic, J.M.; Kasner, S.E.; McDonald, V.; Petrie, M.C.; Seltzer, J.; Mauro, M.; Croce, K.; Berman, E.; Deininger, M.; et al. Retrospective analysis of arterial occlusive events in the PACE trial by an independent adjudication committee. J. Hematol. Oncol. 2022, 15, 1. [Google Scholar] [CrossRef]
- Caocci, G.; Mulas, O.; Annunziata, M.; Luciano, L.; Abruzzese, E.; Bonifacio, M.; Orlandi, E.M.; Albano, F.; Galimberti, S.; Iurlo, A.; et al. Long-Term Mortality Rate for Cardiovascular Disease in 656 Chronic Myeloid Leukaemia Patients Treated with Second- and Third-Generation Tyrosine Kinase Inhibitors. Int. J. Cardiol. 2020, 301, 163–166. [Google Scholar] [CrossRef]
- Dorer, D.J.; Knickerbocker, R.K.; Baccarani, M.; Cortes, J.E.; Hochhaus, A.; Talpaz, M.; Haluska, F.G. Impact of Dose Intensity of Ponatinib on Selected Adverse Events: Multivariate Analyses from a Pooled Population of Clinical Trial Patients. Leuk. Res. 2016, 48, 84–91. [Google Scholar] [CrossRef]
- Singh, A.P.; Glennon, M.S.; Umbarkar, P.; Gupte, M.; Galindo, C.L.; Zhang, Q.; Force, T.; Becker, J.R.; Lal, H. Ponatinib-Induced Cardiotoxicity: Delineating the Signalling Mechanisms and Potential Rescue Strategies. Cardiovasc. Res. 2019, 115, 966–977. [Google Scholar] [CrossRef]
- Müller, M.C.; Cervantes, F.; Hjorth-Hansen, H.; Janssen, J.J.W.M.; Milojkovic, D.; Rea, D.; Rosti, G. Ponatinib in Chronic Myeloid Leukemia (CML): Consensus on Patient Treatment and Management from a European Expert Panel. Crit. Rev. Oncol. Hematol. 2017, 120, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Apperley, J.; Lomaia, E.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.A.; et al. Ponatinib Dose-Ranging Study in Chronic-Phase Chronic Myeloid Leukemia: A Randomized, Open-Label Phase 2 Clinical Trial. Blood 2021, 138, 2042–2050. [Google Scholar] [CrossRef]
- Castagnetti, F.; Pane, F.; Rosti, G.; Saglio, G.; Breccia, M. Dosing Strategies for Improving the Risk-Benefit Profile of Ponatinib in Patients With Chronic Myeloid Leukemia in Chronic Phase. Front. Oncol. 2021, 11, 642005. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Olimpieri, P.P.; Celant, S.; Olimpieri, O.; Pane, F.; Iurlo, A.; Summa, V.; Corradini, P.; Russo, P. Management of Chronic Myeloid Leukaemia Patients Treated with Ponatinib in a Real-life Setting: A Retrospective Analysis from the Monitoring Registries of the Italian Medicines Agency (AIFA). Br. J. Haematol. 2022, 198, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Polillo, M.; Capecchi, M.; Cervetti, G.; Baratè, C.; Angelini, S.; Guerrini, F.; Fontanelli, G.; Arici, R.; Ciabatti, E.; et al. The c.480C>G Polymorphism of HOCT1 Influences Imatinib Clearance in Patients Affected by Chronic Myeloid Leukemia. Pharmacogenomics J. 2014, 14, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.J.; Diderichsen, P.M.; Narasimhan, N.; Srivastava, S.; Gupta, N.; Venkatakrishnan, K. Population Pharmacokinetics of Ponatinib in Healthy Adult Volunteers and Patients With Hematologic Malignancies and Model-Informed Dose Selection for Pediatric Development. J. Clin. Pharmacol. 2022, 62, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Savic, R.M.; Jonker, D.M.; Kerbusch, T.; Karlsson, M.O. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 2007, 34, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Abumiya, M.; Takahashi, N.; Yoshioka, T.; Kameoka, Y.; Miura, M. Evaluation of the Plasma Concentration of Ponatinib in a Chronic Myeloid Leukaemia Patient with Ponatinib Intolerance. J. Clin. Pharm. Ther. 2021, 46, 219–222. [Google Scholar] [CrossRef]
- Kawano, N.; Kimura, S.; Miura, M.; Tochigi, T.; Nakaike, T.; Yamashita, K.; Mashiba, K.; Kikuchi, I.; Takahashi, N. Serial Evaluation of the Pharmacokinetics of Ponatinib in Patients with CML and Ph + ALL. Int. J. Hematol. 2021, 114, 509–516. [Google Scholar] [CrossRef]
- Morita, T.O.; Hanada, K. Physiologically Based Pharmacokinetic Modeling of Ponatinib to Describe Drug–Drug Interactions in Patients with Cancer. Cancer Chemother. Pharmacol. 2022, 90, 315–323. [Google Scholar] [CrossRef]
- Lin, D.; Kostov, R.; Huang, J.T.J.; Henderson, C.J.; Wolf, C.R. Novel Pathways of Ponatinib Disposition Catalyzed by CYP1A1 Involving Generation of Potentially Toxic Metabolites. J. Pharmacol. Exp. Ther. 2017, 363, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kort, A.; Van Hoppe, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain Accumulation of Ponatinib and Its Active Metabolite, N-Desmethyl Ponatinib, Is Limited by P-Glycoprotein (P-GP/ABCB1) and Breast Cancer Resistance Protein (BCRP/ABCG2). Mol. Pharm. 2017, 14, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, Y.; Akamine, Y.; Abumiya, M.; Tozawa, N.; Yamashita, T.; Nara, M.; Kameoka, Y.; Takahashi, N.; Miura, M. Effects of ABCB1 Polymorphisms on the Transport of Ponatinib into the Cerebrospinal Fluid in Japanese Philadelphia Chromosome-positive Acute Lymphoblastic Leukaemia Patients. Br. J. Clin. Pharmacol. 2023, 89, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Saglio, G.; Radich, J.; Kantarjian, H. Adherence to BCR-ABL inhibitors: Issues for CML therapy. Clin. Lymphoma Myeloma Leuk. 2012, 12, 223–229. [Google Scholar] [CrossRef][Green Version]
- Cheng, F.; Cui, Z.; Li, Q.; Wang, L.; Li, W. Adherence to tyrosine kinase inhibitor and clinical outcomes in patients with chronic myeloid leukemia. Int. Immunopharmacol. 2023, 124, 110847. [Google Scholar] [CrossRef]
- Fegers-Wustrow, I.; Gianos, E.; Halle, M.; Yang, E. Comparison of American and European Guidelines for Primary Prevention of Cardiovascular Disease: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2022, 79, 1304–1313. [Google Scholar] [CrossRef]

| Number | Percentage | ||
|---|---|---|---|
| Number of pts | 32 | / | |
| Sex | Male | 16 | 50% |
| Female | 16 | 50% | |
| Age (years) | Median | 56.5 | / |
| Range | 22–71 | / | |
| Risk score at diagnosis (Sokal) | Low | 11 | 34.4% |
| Intermediate | 14 | 43.7% | |
| High | 7 | 21.9% | |
| Cause of switch to PON | Resistance | 18 | 56.2% |
| Toxicity | 14 | 43.8% | |
| Previous lines of treatment | 1 | 17 | 53.1% |
| 2 | 11 | 34.4% | |
| >2 | 4 | 12.5% | |
| PON daily dose (mg) | 45 | 9 | 23.7% |
| 30 | 17 | 44.7% | |
| 15 | 12 | 31.6% | |
| Best molecular response (38 assessments) | <MR3 1 | 13 | 34.2% |
| MR3 | 25 | 65.7% | |
| DMR | 20 | 52.6% | |
| Adverse Events (grade 3–4) | Hematological | 5 | 15.6% |
| Extra-hematological | 8 | 25% |
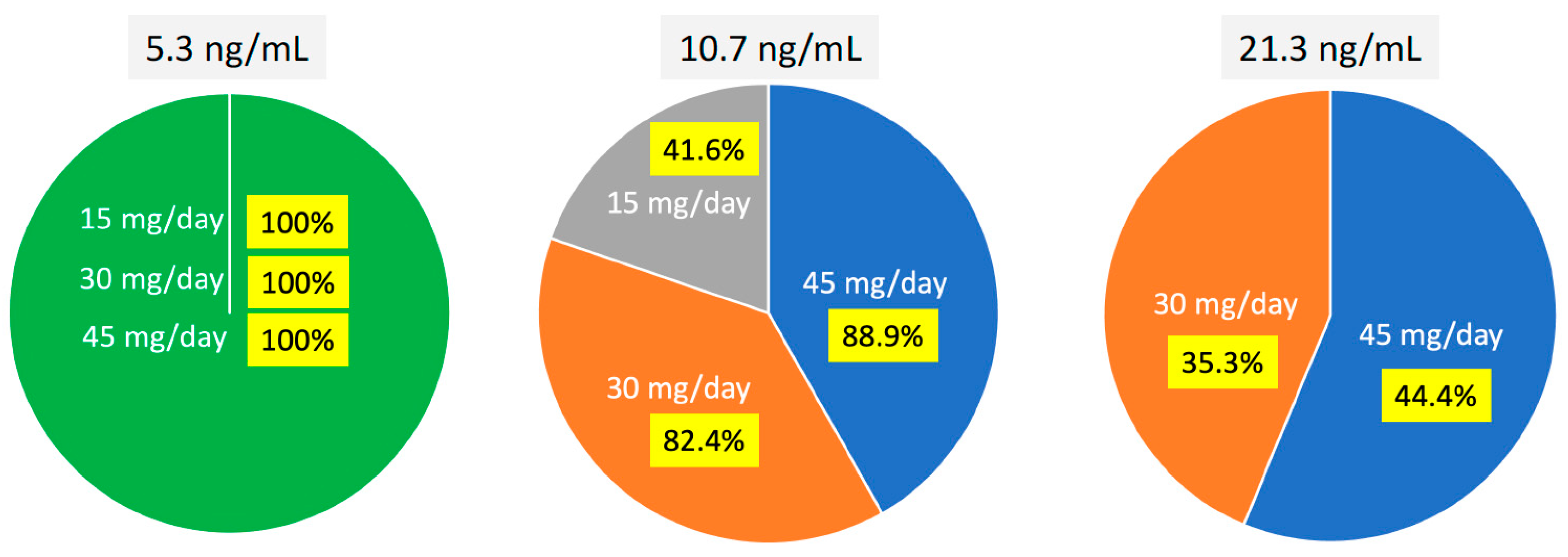
| Threshold of PON Plasma Concentration | Cmin 1 | MR3 | DMR |
|---|---|---|---|
| 10.7 ng/mL | Higher than | 9/11 (81.8%) | 10/27 (37.0%) |
| Lower than | 16/27 (81.8%) | 5/11 (45.5%) | |
| 21.3 ng/mL | Higher than | 6/25 (24.0%) | 5/15 (33.3%) |
| Lower than | 19/25 (76%) | 10/15 (66.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galimberti, S.; Abruzzese, E.; Luci, G.; Baratè, C.; Luciano, L.; Iurlo, A.; Caocci, G.; Morganti, R.; Stefanelli, F.; Di Paolo, A. A New Algorithm Integrating Molecular Response, Toxicity, and Plasma Level Measures for Ponatinib Dose Choice in Patients Affected by Chronic Myeloid Leukemia. Pharmaceutics 2024, 16, 383. https://doi.org/10.3390/pharmaceutics16030383
Galimberti S, Abruzzese E, Luci G, Baratè C, Luciano L, Iurlo A, Caocci G, Morganti R, Stefanelli F, Di Paolo A. A New Algorithm Integrating Molecular Response, Toxicity, and Plasma Level Measures for Ponatinib Dose Choice in Patients Affected by Chronic Myeloid Leukemia. Pharmaceutics. 2024; 16(3):383. https://doi.org/10.3390/pharmaceutics16030383
Chicago/Turabian StyleGalimberti, Sara, Elisabetta Abruzzese, Giacomo Luci, Claudia Baratè, Luigia Luciano, Alessandra Iurlo, Giovanni Caocci, Riccardo Morganti, Fabio Stefanelli, and Antonello Di Paolo. 2024. "A New Algorithm Integrating Molecular Response, Toxicity, and Plasma Level Measures for Ponatinib Dose Choice in Patients Affected by Chronic Myeloid Leukemia" Pharmaceutics 16, no. 3: 383. https://doi.org/10.3390/pharmaceutics16030383
APA StyleGalimberti, S., Abruzzese, E., Luci, G., Baratè, C., Luciano, L., Iurlo, A., Caocci, G., Morganti, R., Stefanelli, F., & Di Paolo, A. (2024). A New Algorithm Integrating Molecular Response, Toxicity, and Plasma Level Measures for Ponatinib Dose Choice in Patients Affected by Chronic Myeloid Leukemia. Pharmaceutics, 16(3), 383. https://doi.org/10.3390/pharmaceutics16030383







