Progress of Drug Nanocrystal Self-Stabilized Pickering Emulsions: Construction, Characteristics In Vitro, and Fate In Vivo
Abstract
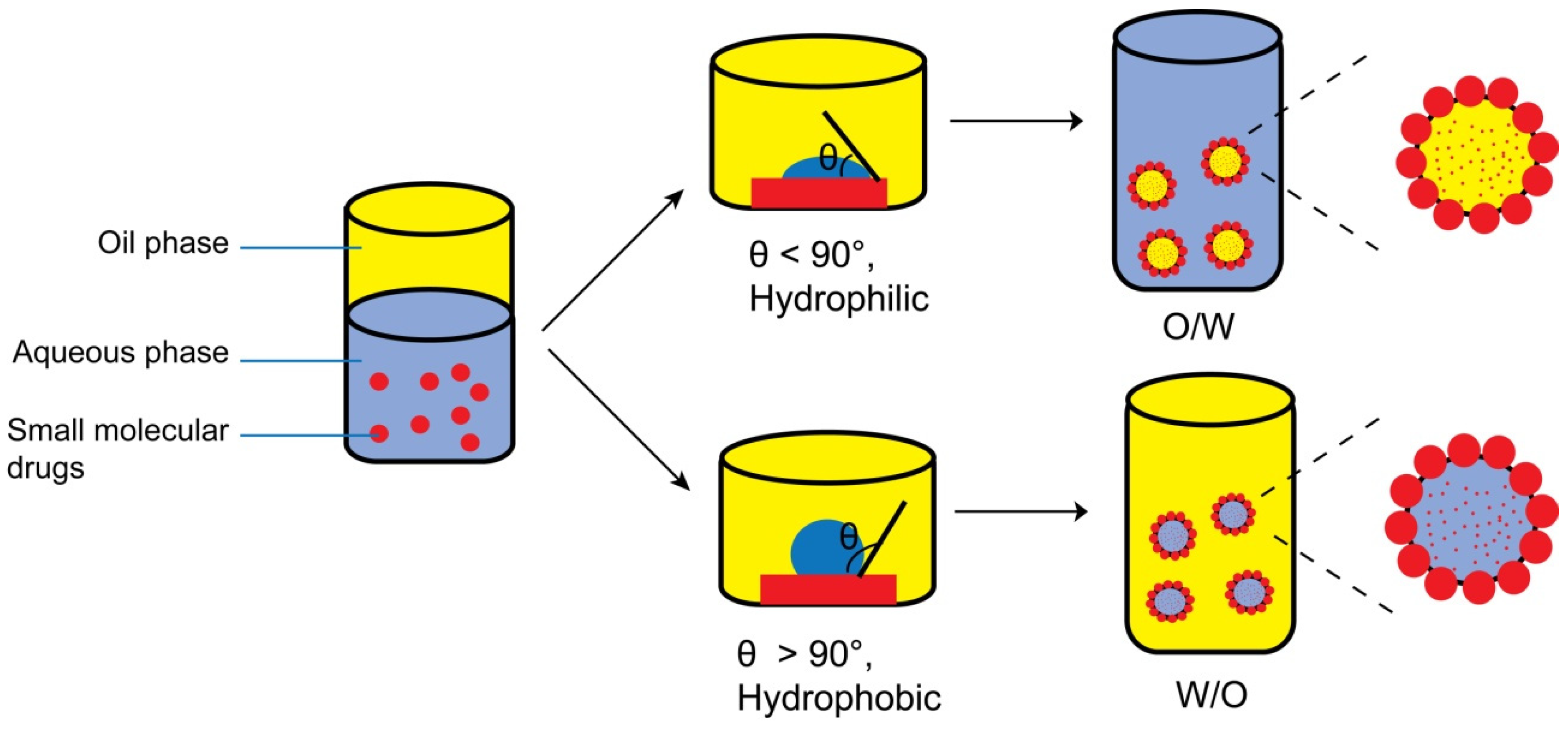
1. Introduction
| Active Ingredient | Pharmaceutical Properties | Size and Potential of Nanocrystals | Oil | Preparation Method | Emulsion Type | Size and Potential of Emulsion Droplets | Drug Delivery Properties | Reference |
|---|---|---|---|---|---|---|---|---|
| Silybin | A component mainly used for the prevention and treatment of liver diseases with poor oral bioavailability due to its low water solubility. | 232 ± 13 nm, −34.7 ± 0.3 mV | Glyceryl monocaprylate | Silybin nanosuspension was prepared first and then mixed with oil, followed by processing through a high-pressure homogenizer. | O/W | 35.8 ± 4.2 μm a, −31.4 ± 0.5 mV | The oral bioavailability of silybin was improved by DNSPE: its AUC increased by 1.6-fold and 4.0-fold compared with nanocrystals and coarse powder, respectively. | [36] |
| Curcumin | A natural polyphenolic bioactive compound with many various health benefits; low oral bioavailability due to its low water solubility and permeability. | 220 nm | High-oleic sunflower oil | Curcumin nanosuspension was prepared first and then added to the oil phase. The mixture was emulsified using ultra-probe sonication. | O/W | 1.2 μm b | Nano-sized amorphous curcumin particles fabricated using nanonization technology could be used to stabilize the Pickering emulsion. | [28] |
| Curcumin | 126.20 ± 1.16 nm, −29.50 ± 0.67 mV | Labrafil M 1944 CS:curcuma aromatic oil = 1:1 | Curcumin nanosuspension and oil phase were mixed and cut at 22,000 rpm for 10 min, followed by homogenization. | O/W | 163.66 ± 6.78 nm g −36.41 ± 0.56 mV | DNSPE improved the oral bioavailability of curcumin by improving drug release and promoting transport across Caco-2 cells, and enhanced its therapeutic effect in airway inflammation. | [37] | |
| Curcumin | Dispersed in oil: 0.2 μm, −47.6 ± 2.4 mV | Soybean oil of 95% wt % | Polyphenol was dispersed into soybean oil first. Aqueous phase was mixed with this oil phase, followed by passing through a high-pressure Leeds jet homogenizer. | W/O | 9−10 μm d | W/O emulsions could be stabilized by crystals from naturally occurring polyphenols, which may lead to various soft matter applications. | [38] | |
| Quercetin | A plant flavonoid with a variety of effects such as anti-inflammatory, antioxidant, anti-hypertensive. But it has low solubility, chemical instability, and low bioavailability. | 354.88 ± 17.35 nm | Labrafac Lipophile WL 1349 | Quercetin nanosuspension was prepared first and then oil was added and mixed by an ultrasonic cell grinder. | O/W | 10–11 μm a, −35 mV | NSSPE is a promising oral delivery system for improving the oral bioavailability of quercetin: its AUC0–t increased by 2.76 times and 1.38 times compared with coarse powder and nanocrystals. | [39] |
| Quercetin | 130 nm | Soy oil | The quercetin dispersions were mixed with soy oil at different ratios. The mixture was by homogenized at 10,000 rpm for 2 min. | O/W | 95 μm g | Natural quercetin treated with the antisolvent method had a good ability to stabilize a Pickering emulsion, and this emulsion may have good prospective application potential for the development of novel and functional emulsion foods. | [40] | |
| Ursolic acid | A typical pentacyclic triterpene carboxylic acid whose potential application as a therapeutic agent was hindered by its poor water solubility and permeability. | 2–20 µm | Rapeseed oil | Ursolic acid was dispersed in oil first and put at 80 °C for 20 min. Water was then added and mixed using a high-speed homogenizer. | W/O | 6–7 μm c | Provided a new strategy for the construction of a carrier-free delivery system for pentacyclic triterpenes. | [33] |
| Diosgenin | A natural steroidal sapogenin with favorable biological activity, such as the prevention and treatment of cancer, cardiovascular diseases, and diabetes. | Canola oil | Diosgenin was dispersed in oil at 120 °C. Then, 80 °C preheated water was added and mixed. The mixture was homogenized. | Gel-like W/O | 34.67 ± 0.58 μm c | Diosgenin was a novel Pickering emulsifier to stabilize W/O emulsions; it had great potential for application in the food, cosmetic, and pharmaceutical fields. | [30] | |
| Dihydromyricetin | A bioactive component of Ampelopsis grossedentataleaves. | Up to 8 μm in length | Medium-chain triglyceride | Oil and DMY aqueous dispersions were mixed and then homogenized using an IKA Ultra-Turrax T18 disperser. | O/W | 20–30 μm a | Dihydromyricetin could spontaneously and quickly transfer to the oil–water interface, reduce the interfacial tension, and enhance the interface thickness to stabilize Pickering emulsion gels. | [32] |
| Puerarin | A potential therapeutic agent for cerebrovascular diseases with low solubility and low permeability. | 390.9 ± 78.5 nm, −44.7 ± 3.0 mV | Ligusticum chuanxiong oil and Labrafil M 1944 CS (9:1, v/v) | Puerarin nanosuspension was prepared first and mixed with oil, then processed through a high-pressure homogenizer. | O/W | 12.9 ± 2.9 μm f, −44.7 ± 2.2 mV | The oral bioavailability of puerarin was improved: the relative bioavailability of NSSPE compared to coarse powder, nanocrystals, and surfactant emulsion was 262.43%, 155.92%, and 223.65%, respectively. | [41] |
| Puerarin, ferulic acid, salvianolic acid B, and tanshinone II A | Main active components of Tongmai prescription. All had poor oral biavailability due to their low water solubility or peameability. | 0.39 ± 0.01 μm −59.70 ± 0.50 mV | Ligusticum chuanxiong essential oil and Labrafil M 1944 CS (9:1, v/v) | Drug nanosuspension was prepared first and then oil was added and mixed, followed by processing through a high-pressure homogenizer. | O/W | 1.43 ± 0.09 μm d, −54.17 ± 0.85 mV | DNSPE was a promising oral drug delivery system for complicated traditional Chinese medicine, promoting the absorption of various components in NSSPEs. | [42] |
| Sinomenine | Sinomenine has analgesic, anti-inflammatory, and immunomodulatory effects with many shortcomings, such as low oral bioavailability, short biological half-life, severe adverse reactions. | 121.49 ± 18.26 nm | Medium-chain triglyceride | Sinomenine nanosuspension was prepared first and then oil was added and emulsified. | O/W | 1086.70 ± 66.30 nm d, −25.4 ± 0.3 mV | Intra-articular injection of sinomenine DNSPE had a better therapeutic effect on rheumatoid arthritis in rats and reduced the toxic and side effects of sinomenine than sinomenine suspension. | [43] |
| Luteolin | A typical polyphenolic substance derived from natural plants with good antioxidant activity. | 379.3 ± 46.14 nm −19.36 ± 2.12 mV | Pine nut oil | The oil was poured into the luteolin micro-nanosuspension and subjected to high-speed homogenization. | O/W | 125.6 ± 24.7 nm g, −26.21 ± 3.63 mV | The dense protective layer that luteolin micro-nano particles formed stabilized the Pickering emulsion. The particles also improved the oxidation stability of pine nut oil. | [31] |
| Quercus suber bark (QSB) from Quercus suber L. | A natural lightweight material with characteristics such as being impermeable to liquids, good biodegradablility, antioxidant, anti-inflammatory, radical scavenger, and antimicrobial properties. | 47.4 ± 0.1 μm | Caprylic/capric acid triglyceride | QSB particles were dispersed in oil first. The oil and aqueous phases were then mixed. | O/W | 87.3 ± 1.0 μm e | The use of QSB particles as a multifunctional solid ingredient contributed to achieving a stable, effective, and innovative Pickering emulsion with a meaningful synergistic protection against oxidative stress. | [44] |
| Tiliroside | A natural flavonoid which is relatively insoluble in water with log10 P of 2.71. | Close to 500 nm −28 mV | n-tetradecane | Flavonoid dispersions were prepared and then mixed with oil, followed by passing through a high-pressure-jet homogenizer. | O/W | About 100 μm d | The ability of tiliroside to act as a Pickering emulsifier is sensitively dependent on pH, as a result of changes in the solubility as a function of pH. | [45] |
| Rutin hydrate | Two flavonoids which are edible, commercially attractive, and linked to particular health benefits. | In oil: 10.52 ± 0.57 μm In water: 0.18 ± 0.01 μm | Sunflower oil | Particles were sonicated in the continuous phase and heated to 45–50 °C. Then, a dispersed phase was added to them and emulsified. | O/W and W/O | O/W: about 25 μm d W/O: about 12 μm d | Provided a series of clean label food-grade emulsions in the food industry. The main particle prerequisites for successful Pickering stabilization were: particle size (200 nm–1 μm), an affinity for the emulsion continuous phase, and a sufficient particle charge. | [46] |
| Naringin | In oil: 18.64 ± 0.82 μm In water: 6.41 ± 0.41 μm | O/W: about 50 μm d W/O: about 55 μm d | ||||||
| Ginkgo biloba extracts (GBEs) | GBE has strong free radical scavenging activity, and antioxidant and antiatherosclerosis capacities due to the synergistic effects of flavonoids and triterpene lactones. However, the poor liposolubility and water solubility of flavonoids resulted in low oral bioavailability. | Soybean oil | FA or GBE powder was dispersed in water using an ultrasonic processor and then mixed with oil. The mixture was prehomogenized using a high-speed homogenizer and then passed through a microfluidizer. | O/W | 0.51 μm d 0.92 μm d | Potential applications of FA and GBE particles in the design of food emulsions aiming at controlling the lipid digestion rate and at increasing the bioaccessibility of foods containing flavonoids. | [34] | |
| Flavonoid glycosides fraction (FA) | O/W | 0.51 μm d 0.92 μm d | ||||||
| Ginkgolide B (GB) | A natural diterpene with notable pharmacological attributes, including neuroprotective, antioxidant, and anti-inflammatory effects. The low solubility of GB in gastric acid and poor stability in intestinal fluid significantly impede its oral absorption. | 85.4 ± 1.4 nm, −11.8 ± 0.6 mV | Ligusticum chuanxiong essential oil | GB nanocrystals were prepared by a downsized wet bead grinding technique first. Then, oil was added to the dispersion and it was ground again at 1000 rpm for 4 h. | O/W | 629.5 ± 19.3 nm, −22.7 ± 0.9 mV | GB-DNSPNE, with its intact nanoparticle slow release and absorption, was more effective in enhancing the oral bioavailability and anti-ischemic stroke efficacy of GB compared to the rapid release and absorption of GB-NCs. | [47] |
2. Materials and Methods
3. Current Application of DNSPEs in Drug Delivery
3.1. Oral Administration of DNSPEs
3.2. Injectable Drug Delivery of DNSPEs
3.3. Topical Drug Delivery of DNSPEs
4. Main Factors Influencing the Construction of DNSPEs
4.1. Wettability of Nanocrystals
4.2. Solid Particle Size
4.3. Charge of Nanocrystals
4.4. Nanocrystal Concentration
4.5. Preparation Method
5. In Vitro Characteristics of DNSPEs
5.1. Contact Angle
5.2. Particle Size and ζ-Potentials
5.3. Stability
- (1)
- Determine the changes in particle size of emulsion droplets subsequent to a specified duration of storage.
- (2)
- Determine the creaming index or coalescence index. After storing in vials for a certain period of time, unstable emulsions would develop an oil or water layer on the top or at the bottom of the vials, respectively.
- (3)
- The ratio of the measured height of the water phase or oil phase to the initial total height of the emulsion is considered the creaming index [46] or coalescence index [28], respectively. A greater creaming index or coalescence index indicates a diminished level of emulsion stability. Although this evaluation method is extensively employed, there are still some defects. It is a great challenge to precisely determine the volume of the oil layer, particularly when only a small amount of the oil phase is separated. It is also a great challenge to accurately determine the volume of the water layer in samples where the transition is gradual from the water layer to the emulsion layer without a clear demarcation.
- (4)
- Centrifugation stability. Centrifugation has been used to evaluate the stability of emulsions in many national pharmacopoeias. Due to its efficiency and simplicity of operation, this method is also frequently employed to assess the stability of DNSPEs. However, there are currently no unified and standardized centrifugation conditions. Based on the Chinese Pharmacopoeia (2020 edition) centrifugation at 1800× g for 15 min was selected for a DNSPE of puerarin [41], while centrifugation at 5000× g for 5 min and at 11,180× g for 10 min was utilized for DNSPEs of diosgenin [30] and luteolin [31], respectively. Furthermore, certain studies measured the quantity of the oil or water phase that precipitated from the DNSPE subsequent to centrifugation [30,31], while other studies examined the alteration in turbidity by measuring the absorbance of the emulsion sample at 500 nm [41]. A decrease in the absorbance value corresponded to a deterioration in the emulsion stability.
5.4. Interfacial Adsorption
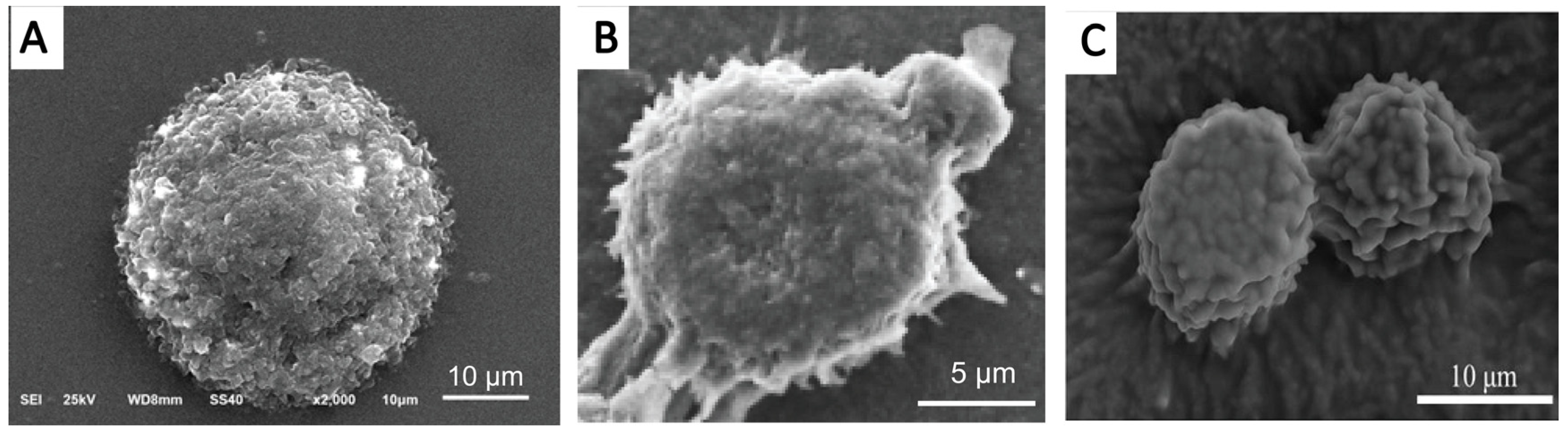
5.4.1. SEM and Cryo-Scanning Electron Microscopy (Cryo-SEM)
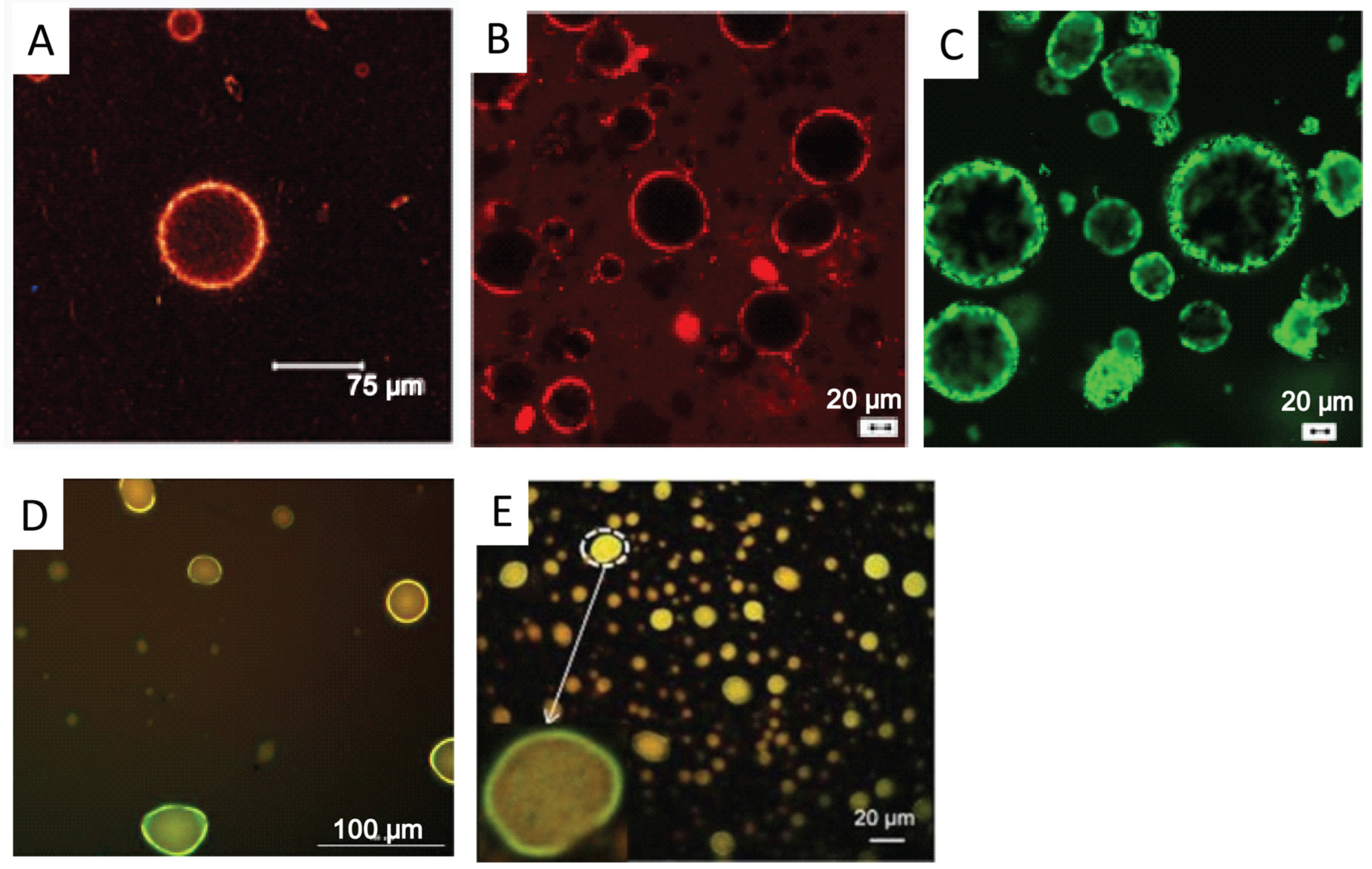
5.4.2. FM
5.4.3. CLSM
5.4.4. Drug Distribution and Existing Forms
6. In Vivo Fate of DNSPEs
7. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.; Chevalier, Y. Effects of solid particle content on properties of O/W Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Y.J.; Zou, Y. Study of Pickering emulsions stabilized by mixed particles of silica and calcite. Particuology 2010, 8, 390–393. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, L.H.; Gao, J.; Cui, Z.G.; Binks, B.P. Effect of trace impurities in triglyceride oils on phase inversion of Pickering emulsions stabilized by CaCO3 nanoparticles. Colloid. Surf. A 2013, 417, 126–132. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.G.; Qiao, X.Y.; Binks, B.P.; Sun, K. Pickering emulsions stabilized by surface-modified Fe3O4 nanoparticles. J. Colloid Interface Sci. 2012, 367, 213–224. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Ning, Y.; Wang, C.Y.; Tong, Z. Facile preparation of artemisia argyi oil-loaded antibacterial microcapsules by hydroxyapatite-stabilized Pickering emulsion templating. Colloids Surf. B 2013, 112, 96–102. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Cui, F.Z.; Zhao, S.L.; Guan, X.; McClements, D.J.; Liu, X.B.; Liu, F.G.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.A.; Perez Gonzalez, A.J.; Huang, Q.R. Kafirin nanoparticles-stabilized Pickering emulsions: Microstructure and rheological behavior. Food Hydrocoll. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, S.; Liu, J.; Pei, Y.; Tang, K.; Qiu, J.H.; Wan, J.Q.; Zheng, X.J.; Zhang, J. Preparation and application of natural protein polymer-based Pickering emulsions. e-Polymers 2023, 23, 383–391. [Google Scholar] [CrossRef]
- Ge, S.L.; Xiong, L.; Li, M.; Liu, J.; Yang, J.; Chang, R.R.; Liang, C.F.; Sun, Q.J. Characterizations of Pickering emulsions stabilized by starch nanoparticles: Influence of starch variety and particle size. Food Chem. 2017, 234, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Wang, Y.X.; Chen, L.Y. Enhanced emulsifying properties of wood-based cellulose nanocrystals as Pickering emulsion stabilizer. Carbohydr. Polym. 2017, 169, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.S.; Lim, H.P.; Tey, B.T.; Chan, E.S. Spray-dried alginate-coated Pickering emulsion stabilized by chitosan for improved oxidative stability and in vitro release profile. Carbohydr. Polym. 2021, 251, 117110. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.N.; Jiang, H.; Gong, S.J.; Yin, S.W.; Li, Y.X.; Ngai, T. Pickering Emulsions Simultaneously Stabilized by Starch Nanocrystals and Zein Nanoparticles: Fabrication, Characterization, and Application. Langmuir 2021, 37, 8577–8584. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.T.; Wu, X.L. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase Pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Z.L.; Zhu, J.Y.; Yin, S.W.; Tang, C.H.; Wu, L.Y.; Yang, X.Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Tan, L.T.; Goh, B.H.; Tey, B.T.; Ong, B.H.; Tang, S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019, 127, 76–84. [Google Scholar] [CrossRef]
- Asfour, M.H.; Elmotasem, H.; Mostafa, D.M.; Salama, A.A. Chitosan based Pickering emulsion as a promising approach for topical application of rutin in a solubilized form intended for wound healing: In vitro and in vivo study. Int. J. Pharmaceut. 2017, 534, 325–338. [Google Scholar] [CrossRef]
- Hedjazi, S.; Razavi, S.H. A comparison of Canthaxanthine Pickering emulsions, stabilized with cellulose nanocrystals of different origins. Int. J. Biol. Macromol. 2018, 106, 489–497. [Google Scholar] [CrossRef]
- Hu, J.W.; Yen, M.W.; Wang, A.J.E.; Chu, I.M. Effect of oil structure on cyclodextrin-based Pickering emulsions for bupivacaine topical application. Colloids Surf. B 2018, 161, 51–58. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Vaseneva, I.N.; Mikhaylov, V.; Martakov, I.S.; Legki, P.; Paderin, N.M.; Sitnikov, P.A. Surface, rheopexy, digestive stability and toxicity of olive oil emulsions stabilized by chitin nanocrystals for vitamin D3 delivery. Carbohydr. Polym. 2022, 284, 119162. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Formulation optimization of lecithin-enhanced Pickering emulsions stabilized by chitosan nanoparticles for hesperidin encapsulation. J. Food Eng. 2018, 229, 2–11. [Google Scholar] [CrossRef]
- Matos, M.; Marefati, A.; Barrero, P.; Rayner, M.; Gutiérrez, G. Resveratrol loaded Pickering emulsions stabilized by OSA modified rice starch granules. Food Res. Int. 2021, 139, 109837. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kratzer, R.; Murkovic, M.; Eibinger, M.; Machado Charry, E.M.; Li, S.Q.; Zhang, T.T.; Zhang, X.Y.; Zhang, M.; Chen, H.Z. Fabrication and characterization of a novel zein/pectin/pumpkin seed oil Pickering emulsion and the effects of myricetin on oxidation stability. Int. J. Biol. Macromol. 2023, 253, 127386. [Google Scholar] [CrossRef]
- Tai, Z.G.; Huang, Y.P.; Zhu, Q.G.; Wu, W.; Yi, T.; Chen, Z.J.; Lu, Y. Utility of Pickering emulsions in improved oral drug delivery. Drug Discov. 2020, 25, 2038–2045. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Aditya, N.P.; Hamilton, I.E.; Norton, I.T. Amorphous nano-curcumin stabilized oil in water emulsion: Physico chemical characterization. Food Chem. 2017, 224, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Liu, W.Q.; Sun, C.B.; Wei, X.Y.; Liu, S.Y.; Jiang, Y.B. Gastrointestinal pH-sensitive Pickering emulsions stabilized by zein nanoparticles coated with bioactive glycyrrhizic acid for improving oral bioaccessibility of Curcumin. ACS Appl. Mater. Interfaces. 2023, 15, 14678–14689. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Xia, H.P.; Guo, S.Y.; Zeng, C.X. Water-in-oil Pickering emulsions stabilized solely by a naturally occurring steroidal sapogenin: Diosgenin. Food Res. Int. 2021, 147, 110573. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, S.M.; Deng, Y.P.; Wu, W.W.; Wang, L.; Liu, Y.J.; Zu, Y.G.; Zhao, X.H. Pickering emulsions stabilized by luteolin micro-nano particles to improve the oxidative stability of pine nut oil. J. Sci. Food Agric. 2021, 101, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Jiang, Z.L.; Ma, H.L.; Pu, P.; Liu, B.G.; Liang, G.Z. Fabrication and characterization of novel edible Pickering emulsion gels stabilized by dihydromyricetin. Food Chem. 2021, 343, 128486. [Google Scholar] [CrossRef]
- Liu, Y.G.; Xia, H.P.; Guo, S.Y.; Lu, X.Y.; Zeng, C.X. Development and characterization of a novel naturally occurring pentacyclic triterpene self-stabilized Pickering emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127908. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.Y.; Ji, C.M.; Lee, K.T.; Shin, J.A.; Lee, E.S.; Hong, S.T. Influence of Ginkgo biloba extracts and of their flavonoid glycosides fraction on the in vitro digestibility of emulsion systems. Food Hydrocoll. 2014, 42, 196–203. [Google Scholar] [CrossRef]
- Yang, D.; Feng, Y.Q.; Yao, X.L.; Zhao, B.F.; Li, D.; Liu, N.; Fang, Y.P.; Midgley, A.; Liu, D.C.; Katsuyoshi, N. Recent advances in bioactive nanocrystal-stabilized Pickering emulsions: Fabrication, characterization, and biological assessment. Compr. Rev. Food Sci. Food Saf. 2023, 22, 946–970. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Liu, C.; Zhang, J.; Wang, F.; Wang, J.R.; Zhang, J.F. A new drug nanocrystal self-stabilized Pickering emulsion for oral delivery of silybin. Eur. J. Pharm. Sci. 2017, 96, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liao, Z.G.; Zhao, G.W.; Dong, W.; Huang, X.Y.; Zhou, X.; Liang, X.L. Curcumin nanocrystals self-stabilized Pickering emulsion freeze-dried powder: Development, characterization, and suppression of airway inflammation. Int. J. Biol. Macromol. 2023, 245, 125493. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil Pickering emulsions stabilized by water-insoluble polyphenol crystals. Langmuir 2018, 34, 10001–10011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, B.; Tang, X.H.; Che, Z.H.; Hu, F.; Shen, C.Y.; Wu, W.; Shen, B.D.; Yuan, H.L. Fabrication and In Vitro/Vivo Evaluation of Drug Nanocrystals Self-Stabilized Pickering Emulsion for Oral Delivery of Quercetin. Pharmaceutics 2022, 14, 897. [Google Scholar] [CrossRef]
- Lu, S.L.; Li, X.Y.; Wei, X.R.; Huang, C.H.; Zheng, J.; Ou, S.Y.; Yang, T.; Liu, F. Preparation and characterization of a novel natural quercetin self-stabilizing Pickering emulsion. Foods 2023, 12, 1415. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, J.; Wang, S.; Yi, T. Development of an oral compound Pickering emulsion composed of nanocrystals of poorly soluble ingredient and volatile oils from traditional Chinese medicine. Pharmaceutics 2018, 10, 170. [Google Scholar] [CrossRef]
- Zhang, J.F.; Ye, X.; Wang, Y.H.; Xu, X.Y.; Yi, T. Nanocrystals self-stabilized Pickering emulsion loaded with active components of Tongmai prescription: Preparation, characterization and evaluation by Caco-2 cell model. Acta Pharm. Sin. B 2023, 58, 208–216. [Google Scholar] [CrossRef]
- Zhang, J.H.; Liang, X.; Bai, H.T.; Li, Y.L.; Sun, S.H.; Zhang, Q.Q.; Yang, J.; Wang, R. Pharmacodynamic and pharmacodynamics of sinomenine nanocrystals self-stabilized Pickering emulsions inje cted into articular cavity. Chin. Tradit. Herb. Drugs 2022, 53, 6412–6422. [Google Scholar] [CrossRef]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and Characterization of a New Quercus suber-Based Pickering Emulsion for Topical Application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.J.; Murray, B.S.; Ross, A.; Povey, M.J.W.; Morgan, M.R.A.; Day, A.J. Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloids Surface B. 2012, 92, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Duffus, L.J.; Norton, J.E.; Smith, P.; Norton, I.T.; Spyropoulos, F. A comparative study on the capacity of a range of food-grade particles to form stable O/W and W/O Pickering emulsions. J. Colloid Interface Sci. 2016, 473, 9–21. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.G.; Cheng, L.; Wang, H.X.; Lu, M.L.; Xu, H.Y. Enhancing both oral bioavailability and anti-ischemic stroke efficacy of ginkgolide B by preparing nanocrystals self-stabilized Pickering nano-emulsion. Eur. J. Pharm. Sci. 2024, 192, 106620. [Google Scholar] [CrossRef]
- Du, Y.Q.; Song, T.T.; Wu, J.; Gao, X.D.; Ma, G.H.; Liu, Y.C.; Xia, Y.F. Engineering mannosylated Pickering emulsions for the targeted delivery of multicomponent vaccines. Biomaterials 2022, 280, 121313. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, P.F.; Jiao, L.N.; He, J.; Yu, L.; Liu, Z.G.; Yang, Y.; Hu, Y.L.; Liu, J.G.; Wang, D.Y. Chinese yam polysaccharides PLGA-stabilized Pickering emulsion as an adjuvant system for PCV- 2 vaccine to enhance immune response. Int. J. Biol. Macromol. 2022, 219, 1034–1046. [Google Scholar] [CrossRef]
- Jiao, L.N.; Liu, Z.G.; Zhang, Y.; Feng, Z.; Gu, P.F.; Huang, Y.; Liu, J.G.; Wu, Y.; Wang, D.Y. Lentinan PLGA-stabilized Pickering emulsion for the enhanced vaccination. Int. J. Pharm. 2022, 611, 121348. [Google Scholar] [CrossRef]
- Tang, J.T.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wu, J.H.; Wang, C.H.; Mu, C.D.; Ngai, T.; Lin, W. Advances in Pickering emulsions stabilized by protein particles: Toward particle fabrication, interaction and arrangement. Food Res. Int. 2022, 157, 111380. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 387–401. [Google Scholar] [CrossRef]
- Xia, T.H.; Xue, C.H.; Wei, Z.H. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Pang, B.; Liu, H.; Zhang, K. Recent progress on Pickering emulsions stabilized by polysaccharides- based micro/nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 102522. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Yi, T.; Zhang, J.F. Effects of drug-oil properties on fabrication of drug nanocrystalline self-stabilizied Pickering emulsions. China J. Chin. Mater. Med. 2017, 42, 3739–3746. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.Q.; Huang, Q.R. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef]
- Xiong, Y.T.; Chen, Y.; Yi, X.Z.; Li, Z.S.; Luo, Y.C. Effect of four plant oils on the stability of high internal phase Pickering emulsions stabilized by ovalbumin-tannic acid complex. Int. J. Biol. Macromol. 2022, 222, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.Z.; Liu, C.M.; Grossmann, L.; Weiss, J. Pickering emulsion stabilized by hydrolyzed starch: Effect of the molecular weight. J. Colloid Interface Sci. 2022, 612, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Studies on the Factors Influencing the Fabrication of Three Insoluble Components of Tongmai Formula Drug Nanocrystals Self-Stabilized Pickering Emulsions. Master’s Thesis, Southwest University, Chongqing, China, 2019. [Google Scholar]
- Ma, Y.; Gao, Y.X.; Zhao, X.; Zhu, Y.Q.; Du, F.P.; Hu, J. A natural triterpene saponin-based Pickering emulsion. Chem. A Eur. J. 2018, 24, 11703–11710. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Study on Two Novel Pickering Emulsion-Based Drug Delivery Systems of Poorly Water-Soluble Active Components from Traditional Chinese Medicines. Master’s Thesis, Southwest University, Chongqing, China, 2014. [Google Scholar]
- Zhang, J.; Wang, F.; Wang, J.R.; Yi, T.; Zhang, J.F. Study on feasibility of puerarin nanocrystalline self-stabilizied Pickering emulsion. Chin. Tradit. Herb. Drugs 2017, 48, 75–84. [Google Scholar]
- Parajuli, S.; Alazzam, O.; Wang, M.; Mota, L.C.; Adhikari, S.; Wicks, D.; Ureña-Benavides, E.E. Surface properties of cellulose nanocrystal stabilized crude oil emulsions and their effect on petroleum biodegradation. Colloids Surface A. 2020, 596, 124705. [Google Scholar] [CrossRef]
- Li, Z.S.; Wang, Y.; Luo, Y.C. High internal phase Pickering emulsions stabilized by egg yolk low density lipoprotein for delivery of curcumin. Colloids Surface B 2022, 211, 112334. [Google Scholar] [CrossRef]
- Ben Cheikh, F.; Mabrouk, A.B.; Magnin, A.; Putaux, J.L.; Boufi, S. Chitin nanocrystals as Pickering stabilizer for O/W emulsions: Effect of the oil chemical structure on the emulsion properties. Colloids Surface B 2021, 200, 111604. [Google Scholar] [CrossRef]
- Jiang, Y.K.; Jin, W.P.; Huang, Q.R. Fabrication and in vitro digestion behavior of Pickering emulsions stabilized by chitosan-caseinophosphopeptides nanocomplexes. Int. J. Biol. Macromol. 2021, 193, 619–628. [Google Scholar] [CrossRef]
- Luo, Z.J.; Murray, B.S.; Yusoff, A.; Morgan, M.R.A.; Povey, M.J.W.; Day, A.J. Particle-stabilizing effects of flavonoids at the oil-water interface. J. Agric. Food Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef]
- Li, R.R.; Yuan, G.B.; Li, D.H.; Xu, C.; Du, M.Y.; Tan, S.Y.; Liu, Z.F.; He, Q.; Rong, L.Y.; Li, J.R. Enhancing the bioaccessibility of puerarin through the collaboration of high internal phase Pickering emulsions with β-carotene. Food Funct. 2022, 13, 2534–2544. [Google Scholar] [CrossRef]
- Tao, S.N.; Guan, X.; Li, Y.X.; Jiang, H.; Gong, S.L.; Ngai, T. All-natural oil-in-water high internal phase Pickering emulsions featuring interfacial bilayer stabilization. J. Colloid Interface Sci. 2022, 607, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.K.; Fan, L.X.; Yang, Y.Y.; Jiang, Q.X.; Xu, Y.S.; Xia, W.S. Characterization of surimi particles stabilized novel Pickering emulsions: Effect of particles concentration, pH and NaCl levels. Food Hydrocoll. 2021, 117, 106731. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Chen, C.; Wang, B.J.; Mao, Z.P.; Xu, H.; Zhong, Y.; Zhang, L.P.; Sui, X.F.; Qu, S. In Vitro digestion of oil-in-water emulsions stabilized by regenerated chitin. J. Agric. Food Chem. 2018, 66, 12344–12352. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Huang, G.T.; Ma, Y.Q.; Liu, Y.; Huang, X.Y.; Zheng, Q.; Yue, P.F.; Yang, M. Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int. J. Biol. Macromol. 2021, 170, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.X.; Wu, Y.H.; He, K.H.; Li, Y.; Luo, X.G.; Li, B.; Wang, C.T.; Liu, S.L. Flexible cellulose nanofibrils as novel Pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Chevallier, M.; Riaublanc, A.; Lopez, C.; Hamon, P.; Rousseau, F.; Thevenot, J.; Croguennec, T. Increasing the heat stability of whey protein-rich emulsions by combining the functional role of WPM and caseins. Food Hydrocoll. 2018, 76, 164–172. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.N.; Holmes, M.; Ettelaie, R. Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics. Adv. Colloid Interface Sci. 2019, 263, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Oral Absorption Mechanism of Puerarin Nanocrystals Self-Stabilized Pickering Emulsion. Master’s Thesis, Southwest University, Chongqing, China, 2019. [Google Scholar]
- Wang, S.; Li, Q.Q.; Wang, F.; Yi, T.; Zhang, J.F. Intestinal absorption of puerarin nanocrystalline self-stabilizing Pickering emulsion in rats. China J. Chin. Mater. Med. 2018, 43, 2162–2167. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ye, X.; Meng, F.J.; Yi, T.; Zhang, J.F. Study on oral absorption mechanisms of puerarin in nanocrystals self-stabilized Pickering emulsion. China J. Chin. Mater. Med. 2021, 46, 2051–2060. [Google Scholar] [CrossRef]
- Li, K.Y.; Zhou, Y.; Huang, G.Q.; Li, X.D.; Xiao, J.X. Preparation of powdered oil by spray drying the Pickering emulsion stabilized by ovalbumin—Gum Arabic polyelectrolyte complex. Food Chem. 2022, 391, 133223. [Google Scholar] [CrossRef]
- Franco Ribeiro, E.F.; Carregari Polachini, T.C.; Dutra Alvim, I.D.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Microencapsulation of roasted coffee oil Pickering emulsions using spray and freeze-drying. physical, structural, and in vitro bioaccessibility studies. Int. J. Food Sci. Technol. 2022, 57, 145–153. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, Y.H.; Wang, J.R.; Yi, T. A novel solid nanocrystals self-stabilized Pickering emulsion prepared by spray-drying with hydroxypropyl-β-cyclodextrin as carriers. Molecules 2021, 26, 1809. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.P.; Dong, X.C.; Zhao, W.L.; Wu, W. The in vivo fate of nanocrystals. Drug Discov. Today 2017, 22, 744–750. [Google Scholar] [CrossRef]
- Shen, B.D.; Shen, C.Y.; Zhu, W.F.; Yuan, H.L. The contribution of absorption of integral nanocrystals to enhancement of oral bioavailability of quercetin. Acta Pharm. Sin. B 2021, 11, 978–988. [Google Scholar] [CrossRef]
- Wang, T.; Qi, J.P.; Ding, N.; Dong, X.C.; Zhao, W.L.; Lu, Y.; Wang, C.H.; Wu, W. Tracking translocation of self-discriminating curcumin hybrid nanocrystals following intravenous delivery. Int. J. Pharm. 2018, 546, 10–19. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.M.; Wang, D.L.; Du, J.L.; Guo, H.Y.; Chen, H.N.; Zhao, S.J.; Liu, Z.P.; Liu, Y. Synergistic antitumor effects on drug-resistant breast cancer of paclitaxel/lapatinib composite nanocrystals. Molecules 2020, 25, 604. [Google Scholar] [CrossRef]
- Zhang, J.F.; Corpstein, C.D.; Li, T.L. Intracellular uptake of nanocrystals: Probing with aggregation-induced emission of fluorescence and kinetic modeling. Acta Pharm. Sin. B 2021, 11, 1021–1029. [Google Scholar] [CrossRef]

| Active Ingredient | Oil | Determination Operation | Results | Reference |
|---|---|---|---|---|
| Curcumin | High-oleic sunflower oil | Nanosuspensions were lyophilized and pressed into a tablet. Then, 5 μL of water was placed on the flat surface. | θw/a: 59° | [28] |
| Curcumin | Soybean oil | A volume of 3 μL of water or oil was added to the compressed disc surfaces of drug crystals. | θw/a: 73.4 ± 1.2° θo/a: 11.9 ± 2.0° | [38] |
| Quercetin | Soy oil | Thick slices of lyophilized quercetin nanocrystals were placed into soybean oil, and 5 μL of water droplets were released onto the surface. | θw/o: 65° | [40] |
| Ursolic acid | Rapeseed oil | Glass substrates coated with ursolic acid films were placed on top of an oil subphase. Then, a water droplet (~5 μL) was released from the tip of a high-precision syringe onto the surface of the UA film, allowing it to be absorbed on the UA film. | θw/o: 156.4 ± 0.1° | [33] |
| Puerarin | Ligusticum chuanxiong oil and Labrafil M 1944 CS (9:1, v/v) | A tablet of lyophilized puerarin crystal was immersed in oil for 30 min. Then, 2 μL of water was dropped on the interface. | θw/o: 81 ± 4° | [41] |
| Dihydromyricetin | Medium-chain triglyceride | DMY was compressed into a film and placed into MCT. A water drop was added on the film surface. | θw/o: 118.8° | [32] |
| Diosgenin | Canola oil | Diosgenin was compressed into films. Then a water drop around 3 μL was placed on the film surface using a micro syringe. | θw/a: 139.6° | [30] |
| Rutin hydrate | Sunflower oil | A volume of 7.5 μL of water or oil droplets was spotted onto compressed particle dick/pellet surfaces. | θo/a: 77° θw/a: 34° | [46] |
| Naringin | θo/a: 34° θw/a: 25° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Dong, F.; Liu, C.; Nie, J.; Feng, S.; Yi, T. Progress of Drug Nanocrystal Self-Stabilized Pickering Emulsions: Construction, Characteristics In Vitro, and Fate In Vivo. Pharmaceutics 2024, 16, 293. https://doi.org/10.3390/pharmaceutics16020293
Zhang J, Dong F, Liu C, Nie J, Feng S, Yi T. Progress of Drug Nanocrystal Self-Stabilized Pickering Emulsions: Construction, Characteristics In Vitro, and Fate In Vivo. Pharmaceutics. 2024; 16(2):293. https://doi.org/10.3390/pharmaceutics16020293
Chicago/Turabian StyleZhang, Jifen, Fangming Dong, Chuan Liu, Jinyu Nie, Shan Feng, and Tao Yi. 2024. "Progress of Drug Nanocrystal Self-Stabilized Pickering Emulsions: Construction, Characteristics In Vitro, and Fate In Vivo" Pharmaceutics 16, no. 2: 293. https://doi.org/10.3390/pharmaceutics16020293
APA StyleZhang, J., Dong, F., Liu, C., Nie, J., Feng, S., & Yi, T. (2024). Progress of Drug Nanocrystal Self-Stabilized Pickering Emulsions: Construction, Characteristics In Vitro, and Fate In Vivo. Pharmaceutics, 16(2), 293. https://doi.org/10.3390/pharmaceutics16020293




