Development of a Versatile Lipid Core for Nanostructured Lipid Carriers (NLCs) Using Design of Experiments (DoE) and Raman Mapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Excipients
2.2. Solubility Tests
2.3. Tablet Preparation
2.4. Raman Spectra
2.5. Chemical Maps
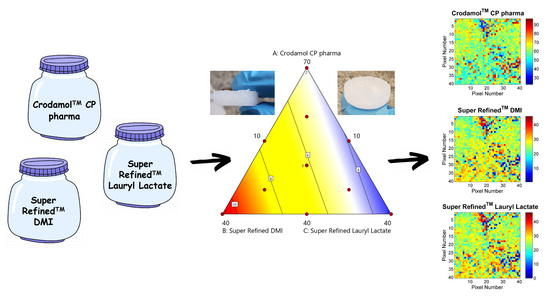
2.6. Design of Experiments (DoE)
2.7. Evaluation of the Optimized Mixture Profile Using Different Drugs
3. Results and Discussion
3.1. Solubility Tests
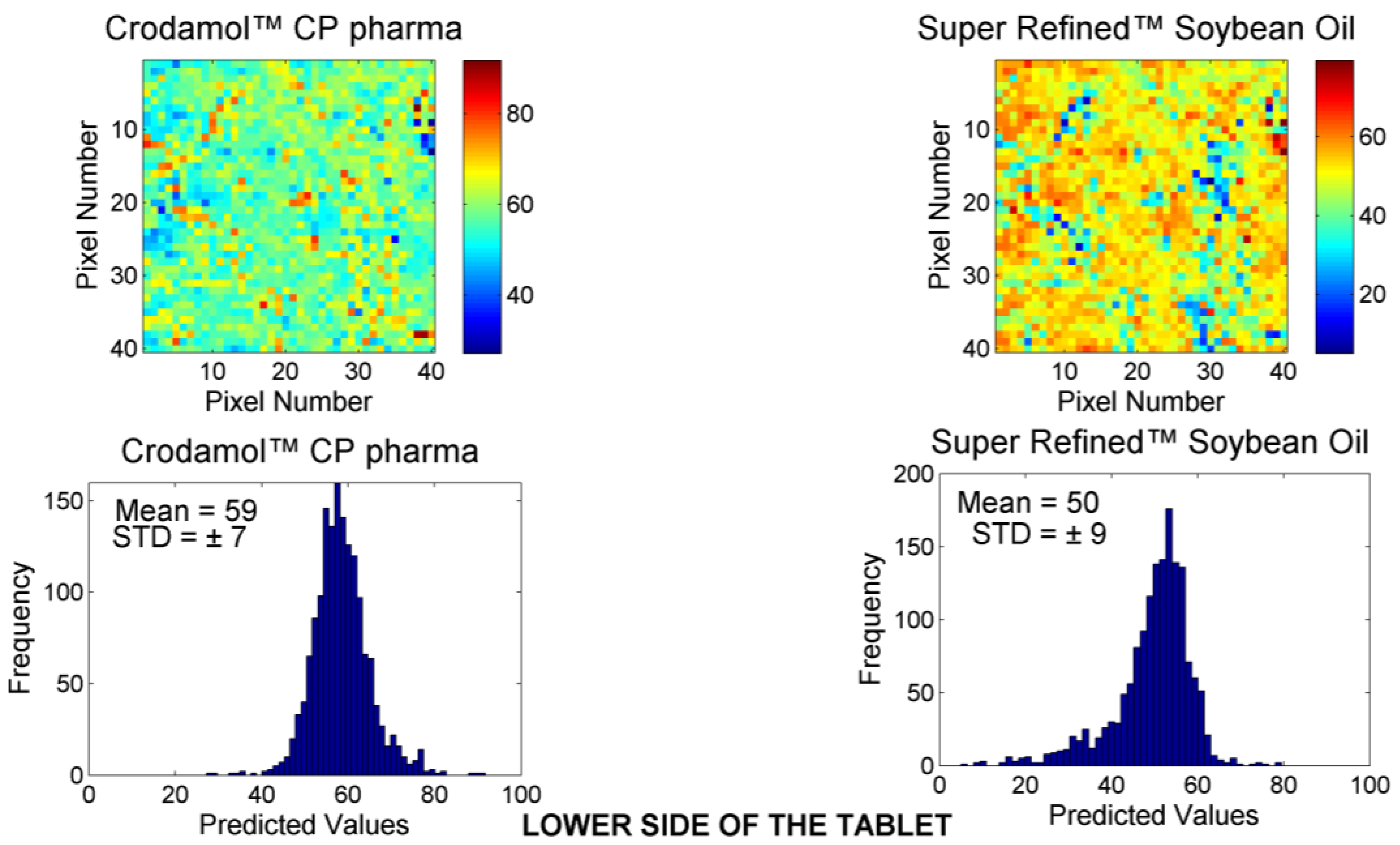
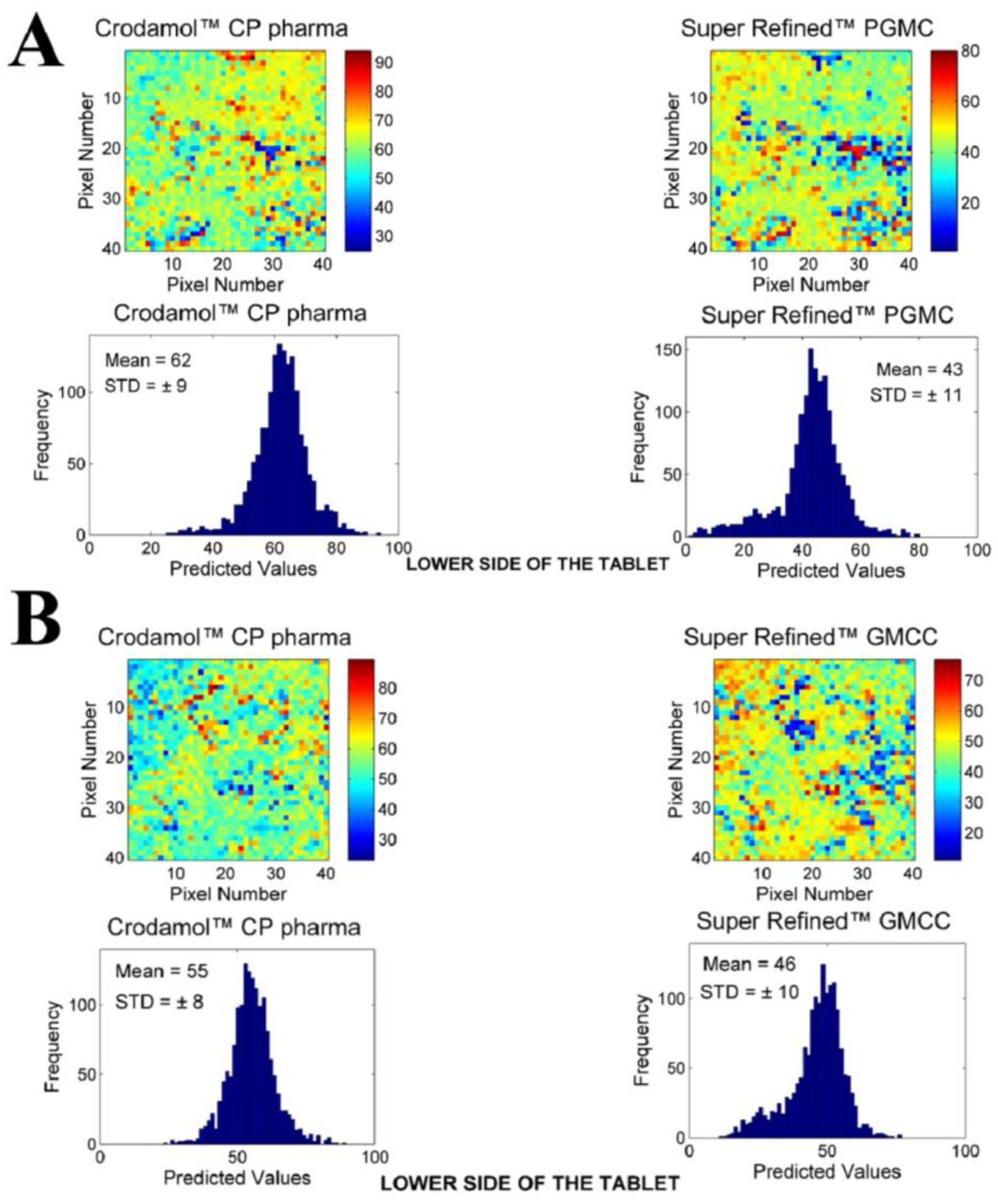
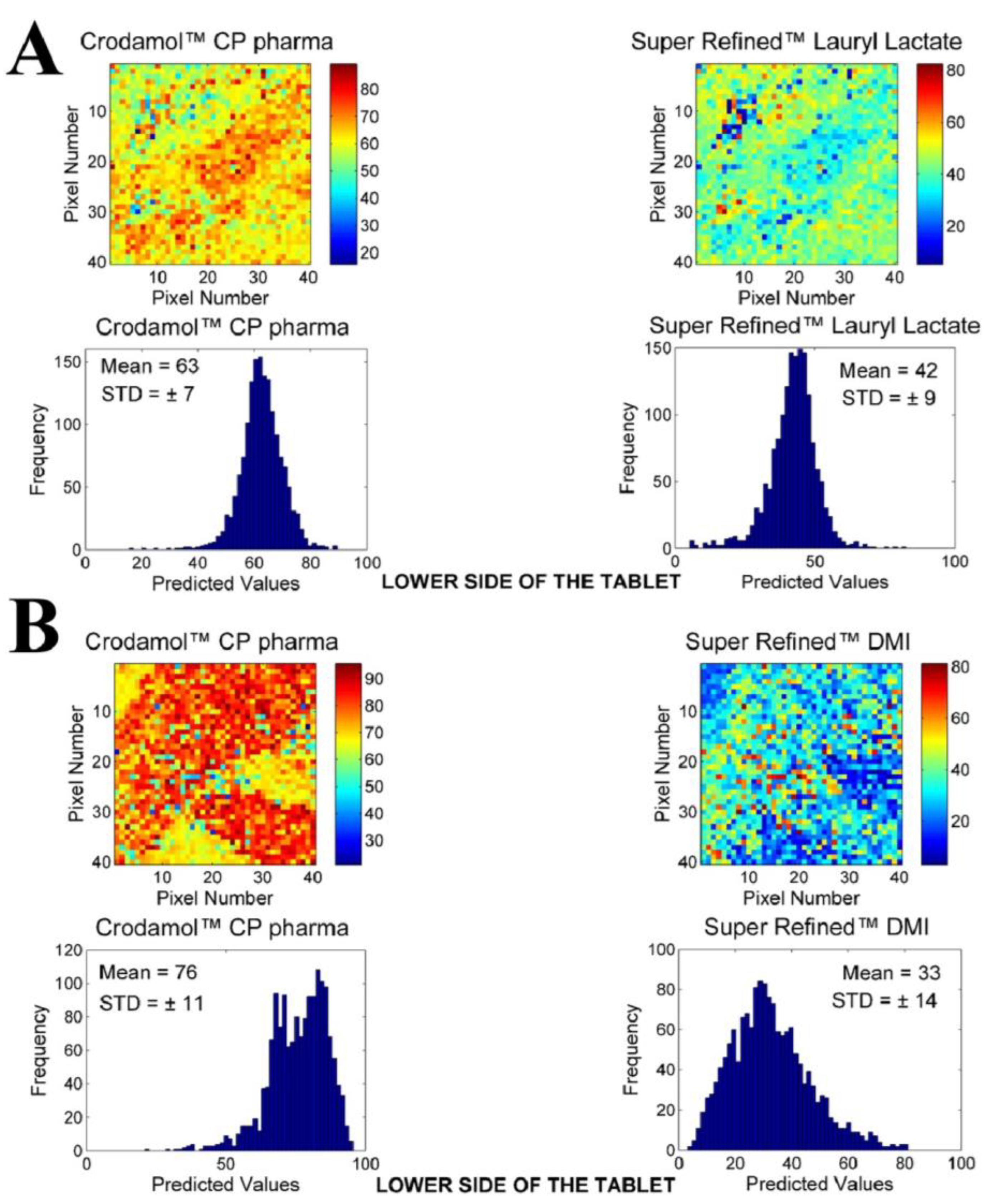
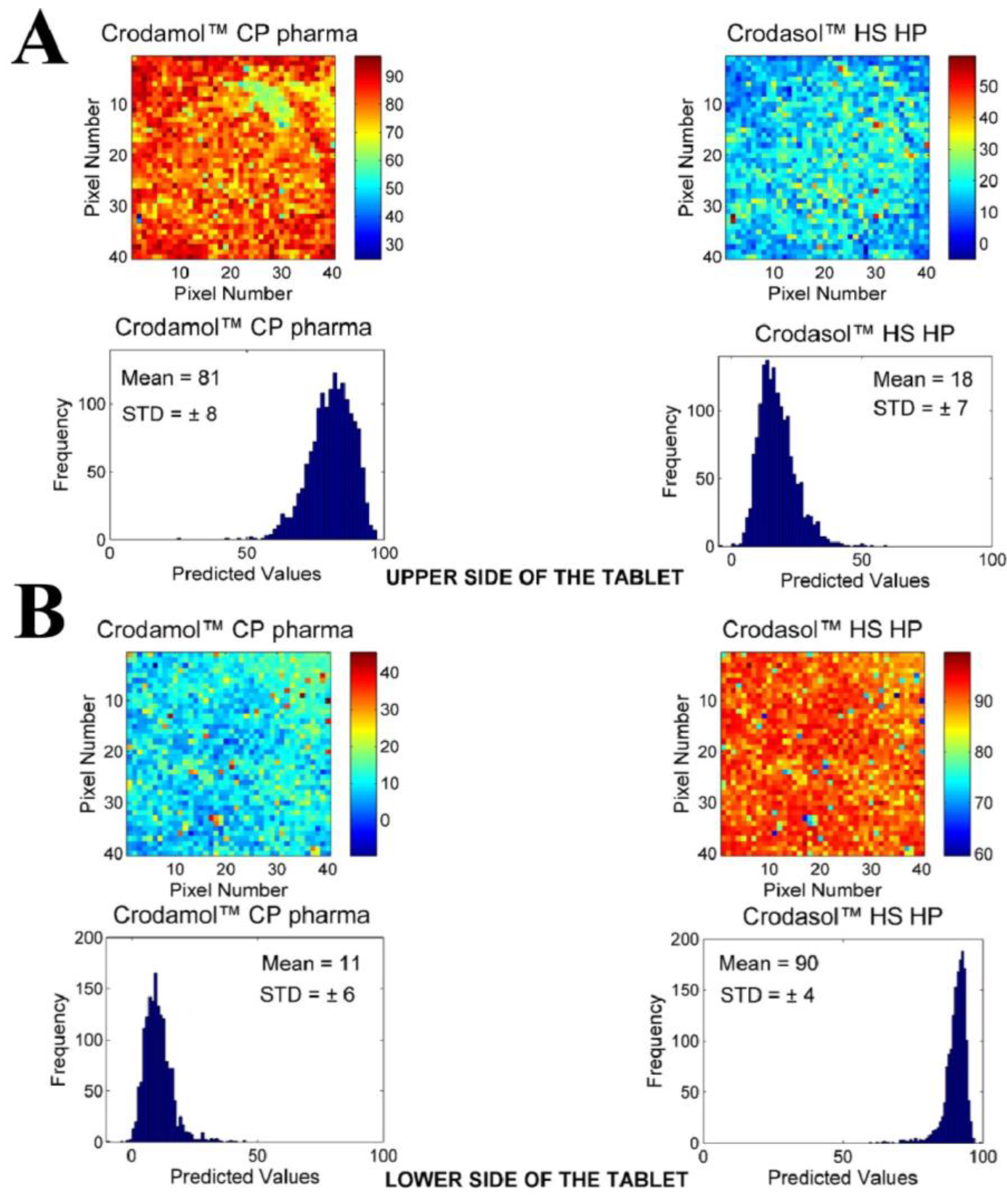
3.2. Chemical Maps of Binary Mixtures
- The excipients that exhibited a hydrophobic profile (Super Refined™ Sesame Oil, Super Refined™ Oleic Acid, Super Refined™ Soybean Oil, and Super Refined™ GTCC) were miscible in all evaluated proportions with CrodamolTM CP pharma.
- The hydrophilic excipients, Super Refined™ Propylene Glycol and Super Refined™ PEG 400, were not miscible with CrodamolTM CP pharma (it was not possible to obtain a tablet in any proportion tested).
- Crodasol™ HS HP and Croduret™ 40 allowed the preparation of a tablet; however, chemical imaging revealed that the excipients were localized in different phases (upper/lower sides of the tablets).
- The excipient Super Refined™ DMI was the only hydrophilic excipient that formed a tablet; nevertheless, the histogram demonstrated a broad distribution of concentrations.
- Within the group of excipients classified as medium polarity, all excipients provided suitable miscibility with Crodamol™ CP pharma, with slight minor variations at different concentrations. The best overall proportion was 1:1 (Gaussian profile) in all cases.
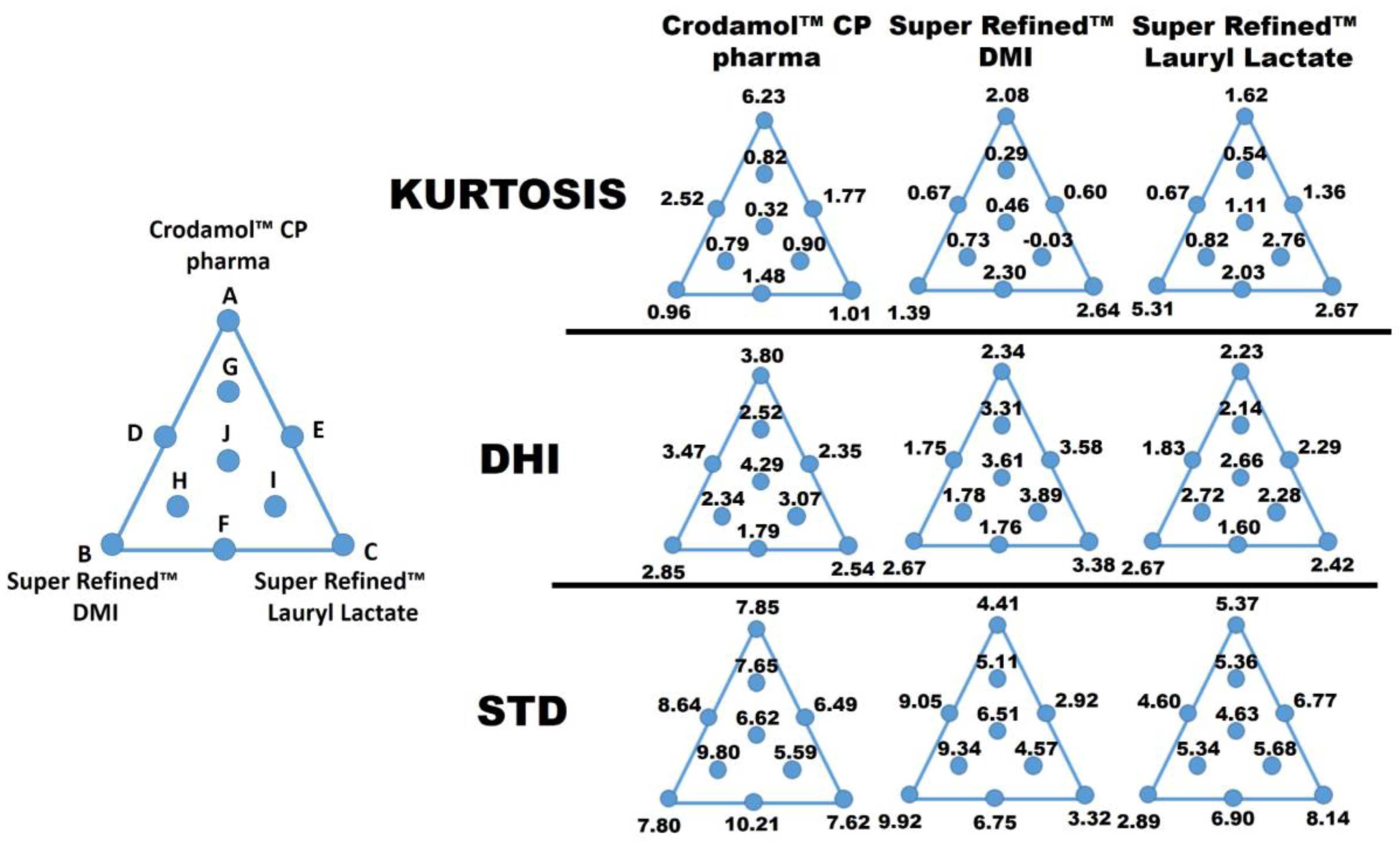
3.3. Development of an Optimized Mixture
3.3.1. Response Y1: STD Crodamol™ CP Pharma
3.3.2. Response Y2: STD Super Refined™ DMI
3.3.3. Response Y3: STD Super Refined™ Lauryl Lactate, Y3
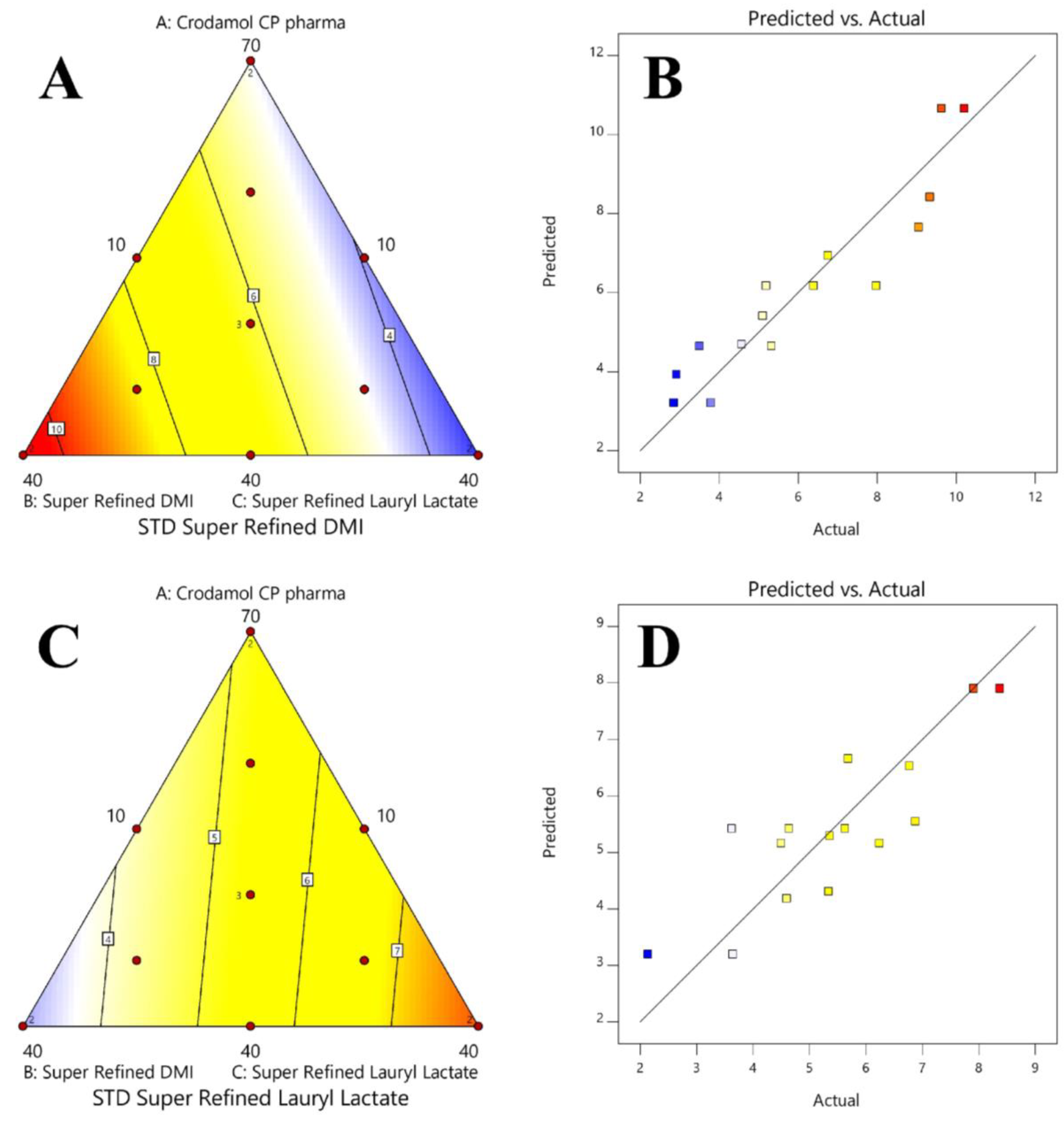
3.4. Surface and Contour Graphs
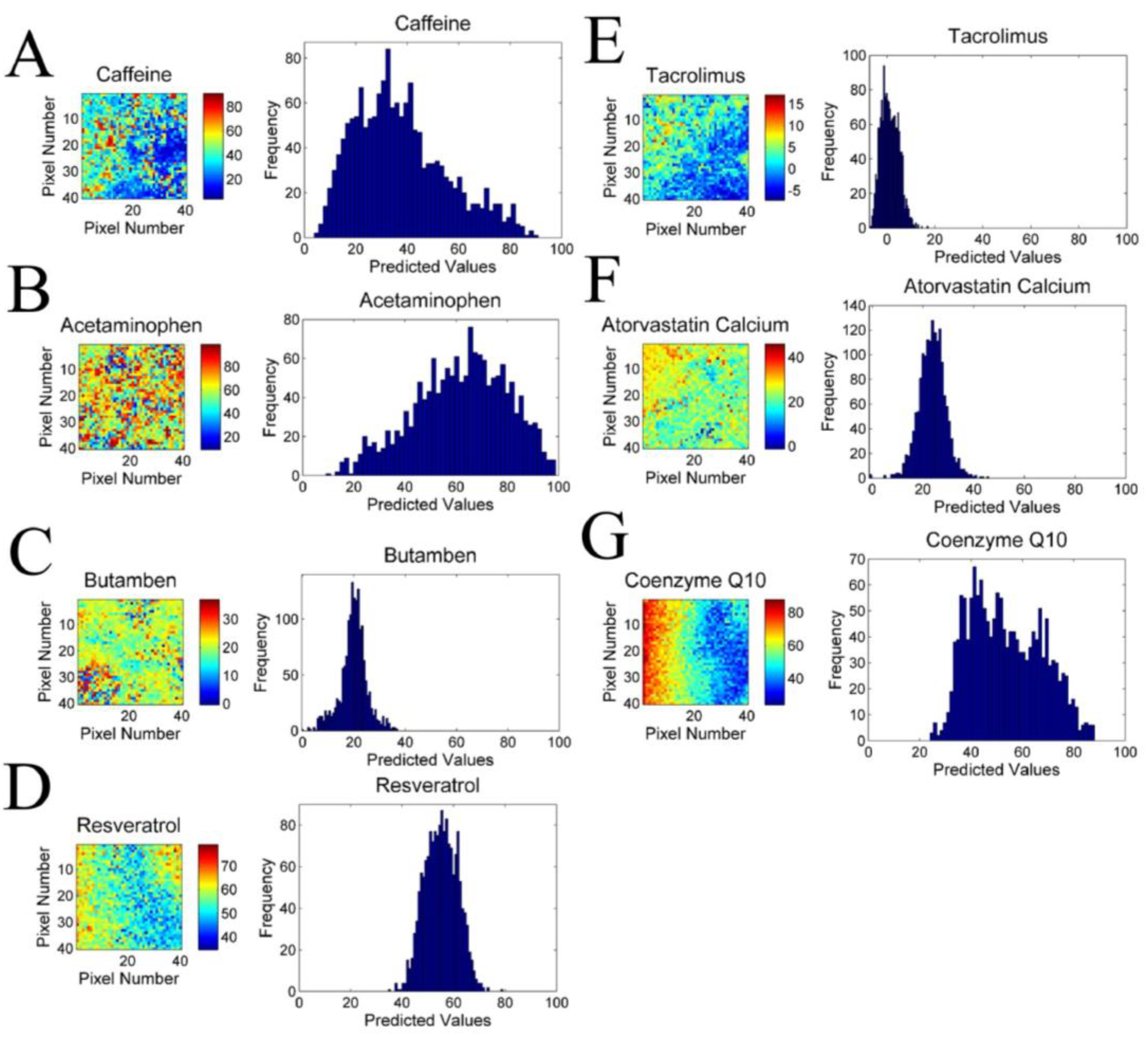
3.5. Incorporation of Different Drugs into the Lipid Core
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Smith, G.P.S.; McGoverin, C.M.; Fraser, S.J.; Gordon, K.C. Raman imaging of drug delivery systems. Adv. Drug Deliv. Rev. 2015, 89, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Mohammadreza, K. (Ed.) Current Applications of Chemometrics; Nova Science Publishers: New York, NY, USA, 2015; pp. 1–295. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenteres: Design, Innovation and Discovery, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 1–672. [Google Scholar]
- Neto, B.B.; Scarminio, I.S.; Bruns, R.E. Como Fazer Experimentos, 3rd ed.; Bookman: Campinas, Brazil, 2007; pp. 1–414. [Google Scholar]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical Basis for a Biopharmaceutic drug classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Sumimoto, Y.; Okawa, S.; Inoue, T.; Masuda, K.; Maruyama, M.; Higaki, K. Extensive improvement of oral bioavailability of mebendazole, a brick dust, by polymer-containing SNEDDS preparation: Disruption of high crystallinity by utilizing its counter ion. Eur. J. Pharm. Biopharm. 2022, 172, 213–227. [Google Scholar] [CrossRef]
- Cole, E.T.; Cadé, D.; Benameur, H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008, 60, 747–756. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäde, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrialapplications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.A.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, andparameters in the process offormulations. J. Nanoparticle Res. 2022, 22, 141. [Google Scholar]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Ewing, A.V.; Kazarian, S.G. Recent advances in the applications of vibrationals pectroscopic imaging and mapping to pharmaceutical formulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 10–29. [Google Scholar] [CrossRef]
- Mitsutake, H.; Silva, G.H.R.; De Paula, E.; Breitkreitz, M.C. When it is too much: Identifying butamben excess on the surface of pharmaceutical preformulation samples by Raman mapping. J. Pharm. Biomed. Anal. 2023, 235, 115644. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, H.; Neves, M.G.; Rutledge, D.N.; Poppi, R.J.; Breitkreitz, M.C. Extraction of information about structural changes in a semisolid pharmaceutical formulation from near-infrared and Raman images by multivariate curve resolution? Alternating least squares and ComDim. J. Chemom. 2020, 34, e3288. [Google Scholar] [CrossRef]
- Amigo, J.M.; Ravn, C. Direct quantification and distribution assessment of major and minor components in pharmaceutical tablets by NIR-chemical imaging. Eur. J. Pharm. Sci. 2009, 37, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, G.L.; Poppi, R.J. NIR imaging spectroscopy for quantification of constituents in polymers thin films loaded with paracetamol. Anal. Chim. Acta 2013, 765, 3–44. [Google Scholar] [CrossRef]
- Mitsutake, H.; Silva, G.H.R.; Ribeiro, L.; De Paula, E.; Poppi, R.J.; Rutledge, D.; Breitkreitz, M.C. Raman Imaging and Chemometrics Evaluation of Natural and Synthetic Beeswaxes as Matrices for Nanostructured Lipid Carriers Development. Braz. J. Anal. Chem. 2021, 8, 116–130. [Google Scholar] [CrossRef]
- Sacré, P.-Y.; Deconinck, E.; Saerens, L.; De Beer, T.; Courselle, P.; Vancauwenberghe, R.; Chiap, P.; Crommen, J.; De Beer, J.O. Detection of counterfeit Viagra by Raman microspectroscopy imaging and multivariate analysis. J. Pharm. Biomed. Anal. 2011, 56, 454–461. [Google Scholar] [CrossRef]
- Rocha, W.F.C.; Sabin, G.P.; Marco, P.H.; Poppi, R.J. Quantitative analysis of piroxicam polymorphs pharmaceutical mixtures by hyperspectral imaging and chemometrics. Chemom. Intell. Lab. Syst. 2011, 106, 198–204. [Google Scholar] [CrossRef]
- Furuyama, N.; Hasegawa, S.; Hamaura, T.; Yada, S.; Nakagami, H.; Yonemochi, E.; Terada, K. Evaluation of solid dispersions on a molecular level by the Raman mapping technique. Int. J. Pharm. 2008, 361, 12–18. [Google Scholar] [CrossRef]
- Mitsutake, H.; Ribeiro, L.N.M.; Silva, G.H.R.; Castro, S.R.; Paula, E.; Poppi, R.J.; Breitkreitz, M.C. Evaluation of miscibility and polymorphism of synthetic and natural lipids for nanostructured lipid carrier (NLC) formulations by Raman mapping and multivariate curve resolution (MCR). Eur. J. Pharm. Sci. 2019, 135, 51–59. [Google Scholar] [CrossRef]
- Sacré, P.-Y.; Bleye, C.; Chavez, P.-F.; Netchacovitch, L.; Hubert, P.; Ziemons, E. Data processing of vibrational chemical imaging for Pharmaceutical applications. J. Pharm. Biomed. Anal. 2014, 101, 123–140. [Google Scholar] [CrossRef]
- Breitkreitz, M.C.; Sabin, G.P.; Polla, G.; Poppi, R.J. Characterization of semi-solid Self-Emulsifying Drug Delivery Systems (SEDDS) of atorvastatin calcium by Raman image spectroscopy and chemometrics. J. Pharm. Biomed. Anal. 2013, 73, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Amigo, J.M.; Cruz, J.; Bautista, M.; Maspoch, S.; Coello, J.; Blanco, M. Study of Pharmaceutical samples by NIR chemical-image and multivariate analysis. TrAC Trends Anal. Chem. 2008, 27, 696–713. [Google Scholar] [CrossRef]
- De Juan, A.; Tauler, R. Multivariate Curve Resolution (MCR) from 2000: Progress in concepts and applications. Crit. Rev. Anal. Chem. 2006, 36, 163–176. [Google Scholar] [CrossRef]
- Vajna, B.; Farkas, I.; Farkas, A.; Pataki, H.; Nagy, Z. Characterization of drug-cylodextrin formulations using Raman mapping and multivariate curve resolution. J. Pharm. Biomed. Anal. 2011, 56, 38–44. [Google Scholar] [CrossRef]
- Silva, V.H.; Soares-Sobrinho, J.L.; Pereira, C.F.; Rinnan, A. Evaluation of chemometric approaches for polymorphs quantification in tablets using near-infrared hyperspectral images. Eur. J. Pharm. Biopharm. 2019, 134, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sacré, P.-Y.; Lebrun, P.; Chavez, P.F.; Bleye, C.; Netchacovitch, L.; Rozet, E.; Klinkenberg, R.; Streel, B.; Hubert, P.; Ziemons, E. A new criterion to assess distributional homogeneity in hyperspectral images of solid pharmaceutical dosage forms. Anal. Chim. Acta 2014, 818, 7–14. [Google Scholar] [CrossRef]
- Hamad, M.L.; Ellison, C.D.; Khan, M.A.; Lyon, R.C. Drug product characterization by macropixel analysis of chemical images. J. Pharm. Sci. 2007, 96, 3390–3401. [Google Scholar] [CrossRef]
- Ravn, C.; Skibsted, E.; Rasmus Bro, R. Near-infrared chemical imaging (NIR-CI) on pharmaceutical solid dosage forms-comparing common calibration approaches. J. Pharm. Biomed. Anal. 2008, 48, 554–561. [Google Scholar] [CrossRef]
- Eilers, P.H.C. Parametric Time Warping. Anal. Chem. 2004, 76, 404–411. [Google Scholar] [CrossRef]
- Sabin, G.P.; Souza, A.M.; Breitkreitz, M.C.; Poppi, R.J. Desenvolvimento de um algoritmo para identificacão e correcão de spikes em espectroscopia Raman de imagem. Quim. Nova 2012, 35, 612–615. [Google Scholar] [CrossRef][Green Version]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]

| Independent Variables | Range (% w/w) | |
|---|---|---|
| Minimum | Maximum | |
| X1: Lipid solid (Crodamol™ CP pharma) | 40 | 70 |
| X2: Hydrophilic excipient (Super Refined™ DMI) | 10 | 40 |
| X3: Liquid lipid (Super Refined™ Lauryl Lactate) | 10 | 40 |
| Dependent variables: CLS standard deviation | Target | |
| Y1: STD Crodamol™ CP pharma | Minimize | |
| Y2: STD Super Refined™ DMI | Minimize | |
| Y3: STD Super Refined™ Lauryl Lactate | Minimize | |
| Independent Variables | |||
|---|---|---|---|
| Mixture | Crodamol™ CP Pharma (X1, % w/w) | Super Refined™ DMI (X2, % w/w) | Super Refined™ Lauryl Lactate (X3, % w/w) |
| 1 | 70 | 10 | 10 |
| 2 | 40 | 40 | 10 |
| 3 | 40 | 10 | 40 |
| 4 | 55 | 25 | 10 |
| 5 | 55 | 10 | 25 |
| 6 | 40 | 25 | 25 |
| 7 | 60 | 15 | 15 |
| 8 | 45 | 30 | 15 |
| 9 | 45 | 15 | 30 |
| 10 | 50 | 20 | 20 |
| 11 | 70 | 10 | 10 |
| 12 | 40 | 40 | 10 |
| 13 | 40 | 10 | 40 |
| 14 | 50 | 20 | 20 |
| 15 | 50 | 20 | 20 |
| Independent Variables | Dependent Variables (Responses) | ||||||
|---|---|---|---|---|---|---|---|
| Point | Ord. | Crodamol™ CP Pharma (X1, % w/w) | Super Refined™ DMI (X2, % w/w) | Super Refined™ Lauryl Lactate (X3, % w/w) | STD Crodamol™ CP Pharma (Y1) | STD Super Refined™ DMI (Y2) | STD Super Refined™ Lauryl Lactate (Y3) |
| A (REP. 1) | 3 | 70 | 10 | 10 | 10.2399 | 5.3213 | 6.2387 |
| B (REP. 1) | 15 | 40 | 40 | 10 | 9.7734 | 10.2075 | 3.6419 |
| C (REP. 1) | 10 | 40 | 10 | 40 | 8.2503 | 2.8535 | 8.3707 |
| D | 14 | 55 | 25 | 10 | 8.6425 | 9.049 | 4.5951 |
| E | 8 | 55 | 10 | 25 | 6.4922 | 2.9182 | 6.7683 |
| F | 2 | 40 | 25 | 25 | 10.2123 | 6.7501 | 6.8751 |
| G | 6 | 60 | 15 | 15 | 7.6515 | 5.106 | 5.3594 |
| H | 11 | 45 | 30 | 15 | 9.8017 | 9.3369 | 5.3381 |
| I | 1 | 45 | 15 | 30 | 5.5927 | 4.5703 | 5.6872 |
| J (REP. 1) | 5 | 50 | 20 | 20 | 5.2051 | 5.1883 | 3.6223 |
| A (REP. 2) | 4 | 70 | 10 | 10 | 5.4646 | 3.5034 | 4.4966 |
| B (REP. 2) | 7 | 40 | 40 | 10 | 5.8229 | 9.6252 | 2.1367 |
| C (REP. 2) | 9 | 40 | 10 | 40 | 6.9807 | 3.791 | 7.9058 |
| J (REP. 2) | 12 | 50 | 20 | 20 | 7.209 | 6.3858 | 4.6373 |
| J (REP. 3) | 13 | 50 | 20 | 20 | 7.4431 | 7.9701 | 5.6311 |
| Response | Model | Sequential p-Value | SD | R2 | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|---|---|
| Y1 | Mean | <0.0001 | |||||
| Y2 | Linear | <0.0001 | 0.988 | 0.870 | 0.848 | 0.799 | |
| Y3 | Linear | 0.000518 | 0.959 | 0.717 | 0.669 | 0.567 | |
| Response | Source | Sum of Squares | df | Mean square | F-value | p-value, prob > F | Conclusion |
| Y1 | Model | 0 | 0 | ||||
| Residual | 44.1 | 14 | 3.15 | ||||
| Lack of Fit | 21.1 | 9 | 2.34 | 0.508 | 0.822 | Not significant | |
| Pure Error | 23 | 5 | 4.61 | ||||
| Corrected Total | 44.1 | 14 | |||||
| Y2 | Model | 78.2 | 2 | 39.1 | 40.1 | <0.0001 | Significant |
| Residual | 11.7 | 12 | 0.975 | ||||
| Lack of Fit | 5.55 | 7 | 0.793 | 0.644 | 0.712 | Not significant | |
| Pure Error | 6.16 | 5 | 1.23 | ||||
| Corrected Total | 89.9 | 14 | |||||
| Y3 | Model | 27.9 | 2 | 14 | 15.2 | 0.000518 | Significant |
| Residual | 11 | 12 | 0.919 | ||||
| Lack of Fit | 6.26 | 7 | 0.894 | 0.936 | 0.549 | Not significant | |
| Pure Error | 4.78 | 5 | 0.955 | ||||
| Corrected Total | 38.9 | 14 |
| STD | Lower | Upper | Criteria |
|---|---|---|---|
| Y1 | 5.2051 | 10.2399 | None |
| Y2 | 2.8535 | 10.2075 | Minimize |
| Y3 | 2.1367 | 8.3707 | Minimize |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios, C.A.; Ondei, R.; Breitkreitz, M.C. Development of a Versatile Lipid Core for Nanostructured Lipid Carriers (NLCs) Using Design of Experiments (DoE) and Raman Mapping. Pharmaceutics 2024, 16, 250. https://doi.org/10.3390/pharmaceutics16020250
Rios CA, Ondei R, Breitkreitz MC. Development of a Versatile Lipid Core for Nanostructured Lipid Carriers (NLCs) Using Design of Experiments (DoE) and Raman Mapping. Pharmaceutics. 2024; 16(2):250. https://doi.org/10.3390/pharmaceutics16020250
Chicago/Turabian StyleRios, Carlos Alberto, Roberta Ondei, and Márcia Cristina Breitkreitz. 2024. "Development of a Versatile Lipid Core for Nanostructured Lipid Carriers (NLCs) Using Design of Experiments (DoE) and Raman Mapping" Pharmaceutics 16, no. 2: 250. https://doi.org/10.3390/pharmaceutics16020250
APA StyleRios, C. A., Ondei, R., & Breitkreitz, M. C. (2024). Development of a Versatile Lipid Core for Nanostructured Lipid Carriers (NLCs) Using Design of Experiments (DoE) and Raman Mapping. Pharmaceutics, 16(2), 250. https://doi.org/10.3390/pharmaceutics16020250