Maltodextrin-Nanoparticles as a Delivery System for Nasal Vaccines: A Review Article
Abstract
1. Introduction
2. The Use of NPs in Nasal Vaccines
3. NPs+ as a Vaccine Delivery System
3.1. Protein Loading
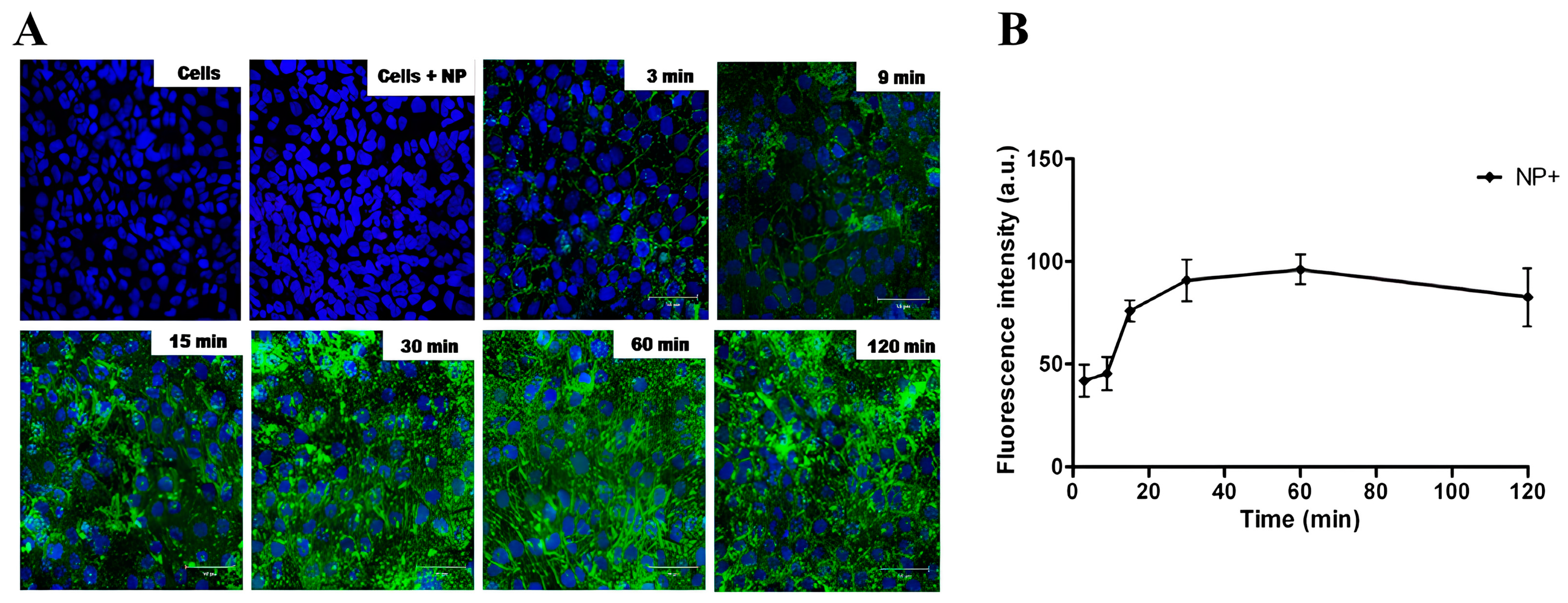
3.2. Cell Interaction
3.3. Antigen Delivery
3.4. NPs+ as a Delivery System for Mucosal Immunization
4. Lipidated Cationic Maltodextrin NPs for Antigen Delivery
4.1. SMBVs
4.1.1. Antigen Loading
4.1.2. Cell Interaction and Antigen Delivery
4.1.3. SMBVs as a Delivery System for Vaccines
- Rabies vaccine
- Meningococcal (MenC) vaccine
- Influenza clinical trials
4.2. NPL
4.2.1. Loading of Proteins and Molecules
4.2.2. Cell Interaction and Antigen Delivery
4.2.3. Antigen Delivery
4.2.4. NPLs as a Delivery System for Vaccines
- Safety
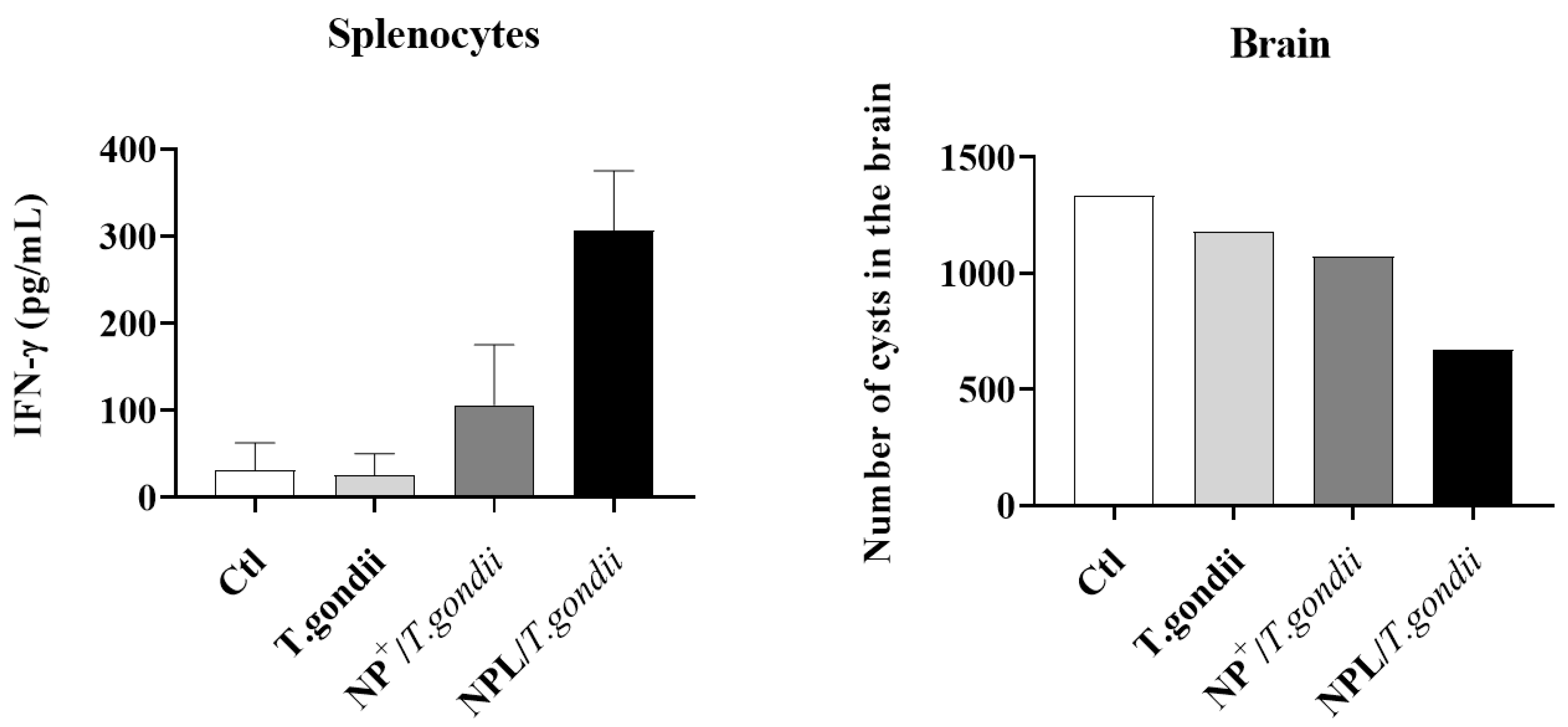
- Toxoplasma gondii vaccine
- Canine Leishmaniasis immunotreatment
- Influenza vaccine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogra, P.L.; Karzon, D.T.; Righthand, F.; MacGillivray, M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N. Engl. J. Med. 1968, 279, 893–900. [Google Scholar] [CrossRef]
- Jabbal-Gill, I. Nasal vaccine innovation. J. Drug Target. 2010, 18, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Martínez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Bertin, B.; Grenet, G.; Pizzoglio-Billaudaz, V.; Lepelley, M.; Atzenhoffer, M.; Vial, T. Vaccines and Bell’s palsy: A narrative review. Therapies 2023, 78, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Csaba, N.; Garcia-Fuentes, M.; Alonso, M.J. Nanoparticles for nasal vaccination. Adv. Drug Deliv. Rev. 2009, 61, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, S.; Kriegel, C.; Amiji, M. Nanotechnology solutions for mucosal immunization. Adv. Drug Deliv. Rev. 2010, 62, 394–407. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Heurtault, B.; Frisch, B.; Pons, F. Liposomes as delivery systems for nasal vaccination: Strategies and outcomes. Expert Opin. Drug Deliv. 2010, 7, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, T.; Zhuang, H.; Xu, Z.; Ye, R.; Sun, M. A Review on Patents of Starch Nanoparticles: Preparation, Applications, and Development. Recent Pat. Food Nutr. Agric. 2018, 9, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Skwarczynski, M.; Toth, I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017, 8, 151–167. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Bernasconi, V.; Norling, K.; Bally, M.; Höök, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 5482087. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Zhang, X.; Wang, M.; Liu, Y.; Shi, Y. Potentials of saponins-based adjuvants for nasal vaccines. Front. Immunol. 2023, 14, 1153042. [Google Scholar] [CrossRef]
- US6342226.pdf. Available online: https://patents.google.com/patent/US6342226B1/en (accessed on 17 December 2023).
- Chronakis, I.S. On the Molecular Characteristics, Compositional Properties, and Structural-Functional Mechanisms of Maltodextrins: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 599–637. [Google Scholar] [CrossRef]
- Ge, S.; Xiong, L.; Li, M.; Liu, J.; Yang, J.; Chang, R.; Liang, C.; Sun, Q. Characterizations of Pickering emulsions stabilized by starch nanoparticles: Influence of starch variety and particle size. Food Chem. 2017, 234, 339–347. [Google Scholar] [CrossRef]
- Yoo, W.; Yoo, D.; Hong, E.; Jung, E.; Go, Y.; Singh, S.V.B.; Khang, G.; Lee, D. Acid-activatable oxidative stress-inducing polysaccharide nanoparticles for anticancer therapy. J. Control. Release 2018, 269, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Jeong, L.; Park, S.; Lee, M.; Song, C.; Lee, D. Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Biomaterials 2019, 192, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.; Hittinger, M.; Primavessy, D.; Zapp, A.; Groß, H.; Schneider, M. Preparation of maltodextrin nanoparticles and encapsulation of bovine serum albumin—Influence of formulation parameters. Eur. J. Pharm. Biopharm. 2019, 142, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Dikpati, A.; Mohammadi, F.; Greffard, K.; Quéant, C.; Arnaud, P.; Bastiat, G.; Rudkowska, I.; Bertrand, N. Residual Solvents in Nanomedicine and Lipid-Based Drug Delivery Systems: A Case Study to Better Understand Processes. Pharm. Res. 2020, 37, 149. [Google Scholar] [CrossRef] [PubMed]
- Merhi, M.; Dombu, C.Y.; Brient, A.; Chang, J.; Platel, A.; Le Curieux, F.; Marzin, D.; Nesslany, F.; Betbeder, D. Study of serum interaction with a cationic nanoparticle: Implications for in vitro endocytosis, cytotoxicity and genotoxicity. Int. J. Pharm. 2012, 423, 37–44. [Google Scholar] [CrossRef] [PubMed]
- De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Poly (Lactic-co-Glycolic Acid) Nanoparticles and Nanoliposomes for Protein Delivery in Targeted Therapy: A Comparative In Vitro Study. Polymers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Maurya, S.; Akhtar, M.S.; Yadav, A.B. Synthesis and Evaluation of BSA-Loaded PLGA–Chitosan Composite Nanoparticles for the Protein-Based Drug Delivery System. ACS Omega 2023, 8, 18751–18759. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Weng, J.; Wu, X.; Wang, W.; Yang, Q.; Guo, F.; Wu, D.; Song, Y.; Chen, F.; Yang, G. Characteristics, Cryoprotection Evaluation and In Vitro Release of BSA-Loaded Chitosan Nanoparticles. Mar. Drugs 2020, 18, 315. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-Charge-Dependent Cell Localization and Cytotoxicity of Cerium Oxide Nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Arslan, F.B.; Tavukcuoglu, E.; Esendagli, G.; Calis, S. Aggregation of chitosan nanoparticles in cell culture: Reasons and resolutions. Int. J. Pharm. 2020, 578, 119119. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B. The agglomeration state of nanoparticles can influence the mechanism of their cellular internalization. J. Nanobiotechnol. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Dombu, C.Y.; Kroubi, M.; Zibouche, R.; Matran, R.; Betbeder, D. Characterization of endocytosis and exocytosis of cationic nanoparticles in airway epithelium cells. Nanotechnology 2010, 21, 355102. [Google Scholar] [CrossRef]
- Zhanataev, A.K.; Anisina, E.A.; Kulakova, A.V.; Shilovskiy, I.P.; Lisitsyn, A.A.; Koloskova, O.O.; Khaitov, M.R.; Durnev, A.D. Genotoxicity of cationic lipopeptide nanoparticles. Toxicol. Lett. 2020, 328, 1–6. [Google Scholar] [CrossRef]
- Mascarell, L.; Lombardi, V.; Louise, A.; Saint-Lu, N.; Chabre, H.; Moussu, H.; Betbeder, D.; Balazuc, A.-M.; Van Overtvelt, L.; Moingeon, P. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J. Allergy Clin. Immunol. 2008, 122, 603–609.e5. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Qu, K.; Ke, Z.; Zila, V.; Anders-Össwein, M.; Glass, B.; Mücksch, F.; Müller, R.; Schultz, C.; Müller, B.; Kräusslich, H.-G.; et al. Maturation of the matrix and viral membrane of HIV-1. Science 2021, 373, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Von Hoegen, P. Synthetic biomimetic supra molecular BiovectorTM (SMBVTM) particles for nasal vaccine delivery. Adv. Drug Deliv. Rev. 2001, 51, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA 1986, 83, 2422–2426. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; Alves, A.; Coutinho, P.; Prata Ribeiro, M.; Maderuelo, C.; Sánchez Navarro, A. Lyoprotective Effects of Mannitol and Lactose Compared to Sucrose and Trehalose: Sildenafil Citrate Liposomes as a Case Study. Pharmaceutics 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Sunamoto, J. Liposomal Membranes. XII. Adsorption of Polysaccharides on Liposomal Membranes as Monitored by Fluorescence Depolarization1. J. Biochem. 1982, 91, 975–979. [Google Scholar] [CrossRef]
- Berton, M.; Sixou, S.; Kravtzoff, R.; Dartigues, C.; Imbertie, L.; Allal, C.; Favre, G. Improved oligonucleotide uptake and stability by a new drug carrier, the SupraMolecular BioVector (SMBV). Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1997, 1355, 7–19. [Google Scholar] [CrossRef][Green Version]
- Vaz-Santiago, J.; Lulé, J.; Rohrlich, P.; Kravtzoff, R.; Le Roy, E.; Davignon, J.-L.; Betbeder, D.; Davrinche, C. IE1-pp65 recombinant protein from human CMV combined with a nanoparticulate carrier, SMBV, as a potential source for the development of anti-human CMV adoptive immunotherapy. Cytotherapy 2002, 4, 11–19. [Google Scholar] [CrossRef]
- Stephenson, I.; Zambon, M.C.; Rudin, A.; Colegate, A.; Podda, A.; Bugarini, R.; Del Giudice, G.; Minutello, A.; Bonnington, S.; Holmgren, J.; et al. Phase I Evaluation of Intranasal Trivalent Inactivated Influenza Vaccine with Nontoxigenic Escherichia coli Enterotoxin and Novel Biovector as Mucosal Adjuvants, Using Adult Volunteers. J. Virol. 2006, 80, 4962–4970. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Lamson, D.M.; St. George, K.; Walsh, T.J. Human Rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef]
- Blaas, D.; Fuchs, R. Mechanism of human rhinovirus infections. Mol. Cell. Pediatr. 2016, 3, 21. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Fasquelle, F.; Carpentier, R.; Demouveaux, B.; Desseyn, J.-L.; Betbeder, D. Importance of the Phospholipid Core for Mucin Hydrogel Penetration and Mucosal Cell Uptake of Maltodextrin Nanoparticles. ACS Appl. Bio Mater. 2020, 9, 5741–5749. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced Oral Delivery of Protein Drugs Using Zwitterion-Functionalized Nanoparticles to Overcome both the Diffusion and Absorption Barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef]
- Mansfield, E.D.H.; Sillence, K.; Hole, P.; Williams, A.C.; Khutoryanskiy, V.V. POZylation: A new approach to enhance nanoparticle diffusion through mucosal barriers. Nanoscale 2015, 7, 13671–13679. [Google Scholar] [CrossRef] [PubMed]
- Fasquelle, F.; Dubuquoy, L.; Betbeder, D. Starch-based NP act as antigen delivery systems without immunomodulating effect. PLoS ONE 2022, 17, e0272234. [Google Scholar] [CrossRef] [PubMed]
- Ducournau, C.; Moiré, N.; Carpentier, R.; Cantin, P.; Herkt, C.; Lantier, I.; Betbeder, D.; Dimier-Poisson, I. Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep. Front. Immunol. 2020, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Quan Le, M.; Ye, L.; Bernasconi, V.; Carpentier, R.; Fasquelle, F.; Lycke, N.; Staeheli, P.; Betbeder, D. Prevention of influenza virus infection and transmission by intranasal administration of a porous maltodextrin nanoparticle-formulated vaccine. Int. J. Pharm. 2020, 582, 119348. [Google Scholar] [CrossRef]
- Marzban, E.; Alavizadeh, S.H.; Ghiadi, M.; Khoshangosht, M.; Khashayarmanesh, Z.; Abbasi, A.; Jaafari, M.R. Optimizing the therapeutic efficacy of cisplatin PEGylated liposomes via incorporation of different DPPG ratios: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2015, 136, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Le, M.Q.; Carpentier, R.; Lantier, I.; Ducournau, C.; Dimier-Poisson, I.; Betbeder, D. Residence time and uptake of porous and cationic maltodextrin-based nanoparticles in the nasal mucosa: Comparison with anionic and cationic nanoparticles. Int. J. Pharm. 2018, 550, 316–324. [Google Scholar] [CrossRef]
- Dombu, C.; Carpentier, R.; Betbeder, D. Influence of surface charge and inner composition of nanoparticles on intracellular delivery of proteins in airway epithelial cells. Biomaterials 2012, 33, 9117–9126. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Kaneko, K.; Miyaji, E.N.; Gonçalves, V.M.; Ferreira, D.M.; Solórzano, C.; MacLoughlin, R.; Saleem, I. Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro. Int. J. Pharm. 2021, 599, 120407. [Google Scholar] [CrossRef] [PubMed]
- Akerele, G.; Ramadan, N.; Renu, S.; Renukaradhya, G.J.; Shanmugasundaram, R.; Selvaraj, R.K. In vitro characterization and immunogenicity of chitosan nanoparticles loaded with native and inactivated extracellular proteins from a field strain of Clostridium perfringens associated with necrotic enteritis. Vet. Immunol. Immunopathol. 2020, 224, 110059. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, R.; Platel, A.; Salah, N.; Nesslany, F.; Betbeder, D. Porous Maltodextrin-Based Nanoparticles: A Safe Delivery System for Nasal Vaccines. J. Nanomater. 2018, 2018, 9067195. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Wide Varieties of Cationic Nanoparticles Induce Defects in Supported Lipid Bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Chu, K.-B.; Quan, F.-S. Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development. Vaccines 2021, 9, 413. [Google Scholar] [CrossRef]
- Mamaghani, A.J. Toxoplasma gondii vaccine candidates: A concise review. Ir. J. Med. Sci. 2023, 192, 231–261. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.-Q. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin. Microbiol. Rev. 2020, 34, e00115-19. [Google Scholar] [CrossRef]
- Montoya, J.G.; Remington, J.S. Clinical Practice: Management of Toxoplasma gondii Infection during Pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar] [CrossRef] [PubMed]
- AU2013316781B2.pdf. Available online: https://patents.google.com/patent/AU2013316781B2/en?oq=AU2013316781B2 (accessed on 17 December 2023).
- Dubey, J.P. Clinical toxoplasmosis in zoo animals and its management. Emerg. Anim. Species 2022, 2, 100002. [Google Scholar] [CrossRef]
- Jones, J.L.; Dubey, J.P. Foodborne Toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef]
- Robert-Gangneux, F. It is not only the cat that did it: How to prevent and treat congenital toxoplasmosis. J. Infect. 2014, 68, S125–S133. [Google Scholar] [CrossRef]
- Cedillo-Peláez, C.; Rico-Torres, C.P.; Salas-Garrido, C.G.; Correa, D. Acute toxoplasmosis in squirrel monkeys (Saimiri sciureus) in Mexico. Vet. Parasitol. 2011, 180, 368–371. [Google Scholar] [CrossRef]
- Nishimura, M.; Goyama, T.; Tomikawa, S.; Fereig, R.M.; El-Alfy, E.-S.N.; Nagamune, K.; Kobayashi, Y.; Nishikawa, Y. Outbreak of toxoplasmosis in four squirrel monkeys (Saimiri sciureus) in Japan. Parasitol. Int. 2019, 68, 79–86. [Google Scholar] [CrossRef]
- Oh, H.; Eo, K.-Y.; Gumber, S.; Hong, J.J.; Kim, C.-Y.; Lee, H.-H.; Jung, Y.-M.; Kim, J.; Whang, G.-W.; Lee, J.-M.; et al. An outbreak of toxoplasmosis in squirrel monkeys (Saimiri sciureus) in South Korea. J. Med. Primatol. 2018, 47, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Pardini, L.; Dellarupe, A.; Bacigalupe, D.; Quiroga, M.A.; Moré, G.; Rambeaud, M.; Basso, W.; Unzaga, J.M.; Schares, G.; Venturini, M.C. Isolation and molecular characterization of Toxoplasma gondii in a colony of captive black-capped squirrel monkeys (Saimiri boliviensis). Parasitol. Int. 2015, 64, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Fasquelle, F.; Scuotto, A.; Vreulx, A.-C.; Petit, T.; Charpentier, T.; Betbeder, D. Nasal vaccination of six squirrel monkeys (Saimiri sciureus): Improved immunization protocol against Toxoplasma gondii with a nanoparticle-born vaccine. Int. J. Parasitol. Parasites Wildl. 2023, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Oryan, A.; Hatam, G. Immunotherapy in treatment of leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mazire, P.H.; Saha, B.; Roy, A. Immunotherapy for visceral leishmaniasis: A trapeze of balancing counteractive forces. Int. Immunopharmacol. 2022, 110, 108969. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.A.N.; Giannelli, A.; Fasquelle, F.; Scuotto, A.; Betbeder, D. Effective immuno-therapeutic treatment of Canine Leishmaniasis. PLoS Negl. Trop. Dis. 2023, 17, e0011360. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.; Day, M.J. Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci. 2019, 123, 261–272. [Google Scholar] [CrossRef]
- Bernasconi, V.; Bernocchi, B.; Ye, L.; Lê, M.Q.; Omokanye, A.; Carpentier, R.; Schön, K.; Saelens, X.; Staeheli, P.; Betbeder, D.; et al. Porous Nanoparticles with Self-Adjuvanting M2e-Fusion Protein and Recombinant Hemagglutinin Provide Strong and Broadly Protective Immunity Against Influenza Virus Infections. Front. Immunol. 2018, 9, 2060. [Google Scholar] [CrossRef]
- Yasamineh, S.; Kalajahi, H.G.; Yasamineh, P.; Yazdani, Y.; Gholizadeh, O.; Tabatabaie, R.; Afkhami, H.; Davodabadi, F.; Farkhad, A.K.; Pahlevan, D.; et al. An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19. J Nanobiotechnol. 2022, 20, 440. [Google Scholar] [CrossRef]
- Topol, E.J.; Iwasaki, A. Operation Nasal Vaccine—Lightning speed to counter COVID-19. Sci. Immunol. 2022, 7, eadd9947. [Google Scholar] [CrossRef] [PubMed]


| NPs+ | SMBVs | NPLs | |
|---|---|---|---|
| Structure | Cationic maltodextrin scaffold | Cationic maltodextrin scaffold covered by cationic phospholipids | Cationic maltodextrin scaffold loaded with anionic phospholipid |
| Size | 60–100 nm | 50–90 nm | 60–100 nm |
| Surface charge | Cationic | Neutral | Cationic |
| Ability to load protein | Yes | Yes | Yes |
| Ability to load hydrophobic drugs | No | Yes | Yes |
| Uptake 1 | Fast | Slow | Fast |
| Main endocytosis pathway 1 | Clathrin | Caveolae | Clathrin/Caveolae |
| Exocytosis 1 | Yes | N.A. | Yes |
| Cytoplasmic protein delivery 1 | No | Yes | Yes |
| Mucus interaction | Mucoadherence | Mucopenetration | Mucopenetration |
| Immune response after mucosal administration | Th2 > Th1 | Th1 ≈ Th2 | Th1/Th17 > Th2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasquelle, F.; Scuotto, A.; Howsam, M.; Betbeder, D. Maltodextrin-Nanoparticles as a Delivery System for Nasal Vaccines: A Review Article. Pharmaceutics 2024, 16, 247. https://doi.org/10.3390/pharmaceutics16020247
Fasquelle F, Scuotto A, Howsam M, Betbeder D. Maltodextrin-Nanoparticles as a Delivery System for Nasal Vaccines: A Review Article. Pharmaceutics. 2024; 16(2):247. https://doi.org/10.3390/pharmaceutics16020247
Chicago/Turabian StyleFasquelle, François, Angelo Scuotto, Michael Howsam, and Didier Betbeder. 2024. "Maltodextrin-Nanoparticles as a Delivery System for Nasal Vaccines: A Review Article" Pharmaceutics 16, no. 2: 247. https://doi.org/10.3390/pharmaceutics16020247
APA StyleFasquelle, F., Scuotto, A., Howsam, M., & Betbeder, D. (2024). Maltodextrin-Nanoparticles as a Delivery System for Nasal Vaccines: A Review Article. Pharmaceutics, 16(2), 247. https://doi.org/10.3390/pharmaceutics16020247






