Clinical Utility and Implementation of Pharmacogenomics for the Personalisation of Antipsychotic Treatments
Abstract
1. Introduction
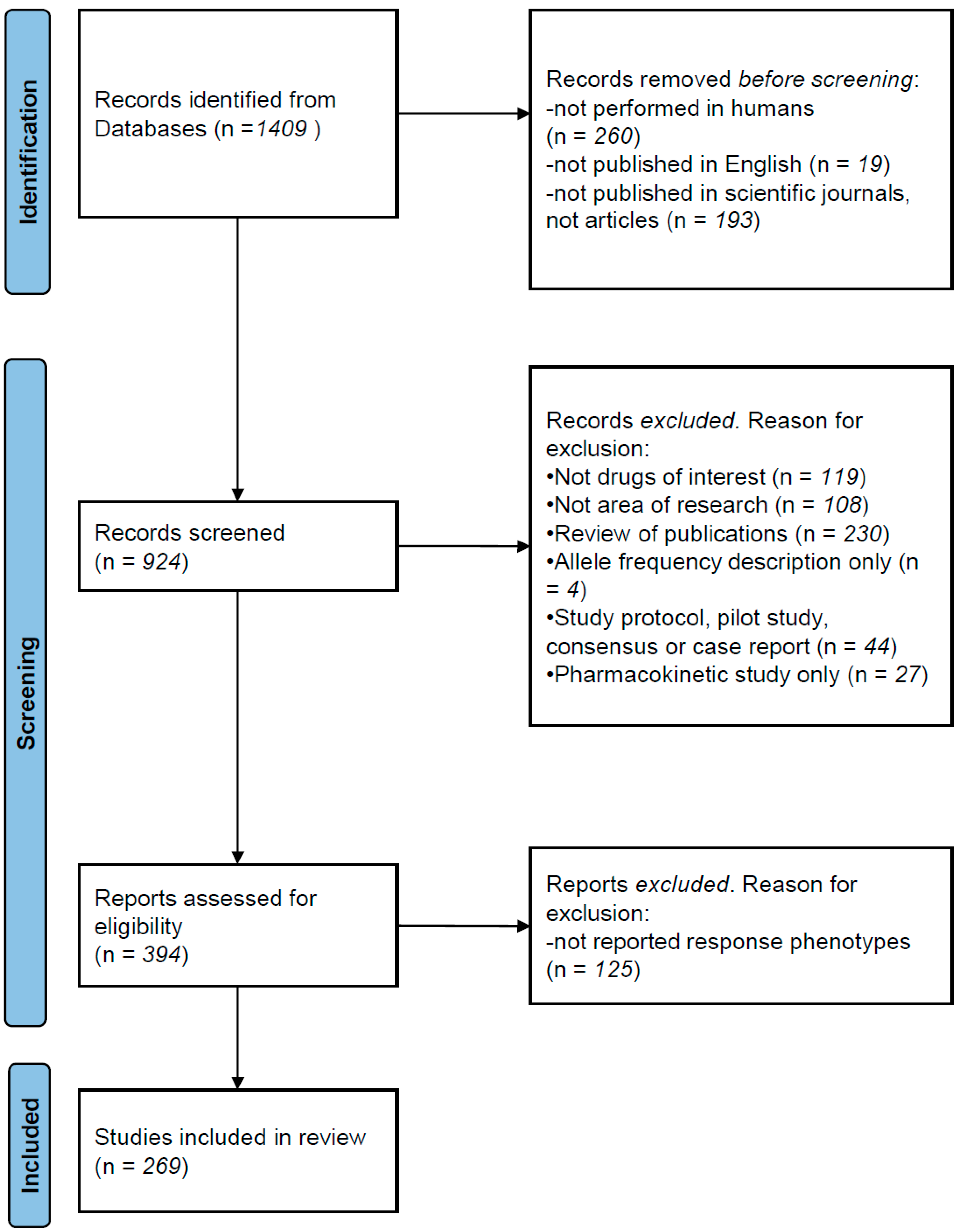
2. Methods
Literature Review
3. Pharmacogenetic Studies
3.1. Pharmacogenetic Associations with Treatment Response
3.2. Pharmacogenetic Associations with Antipsychotic-Induced Adverse Reactions
4. Pharmacogenomic Studies
5. Clinical Utility of Findings
6. Implementation of Pharmacogenetics for Antipsychotics
7. Discussion
8. Conclusions
| Gene | Variant | Drug | n | Association | Ref. |
|---|---|---|---|---|---|
| 31 genes | 202 SNPs | SGA | 113 Caucasians | Four SNPs in DRD2, SLC18A2, HTR2A and GRIK3 contributed significantly to the risk of side effects (p = 1 × 10−4). | [99] |
| 38 genes | several | SGA | 300 Caucasian | Nominally significant association between antipsychotic dosage and GFRA1 variants. | [100] |
| 380 genes | several | SGA and FGA | 240 several ethnicities | NALCN rs2152324 had most significant association with response (p = 0.004). Not significant after FDR correction. | [101] |
| 74 genes | several | several | 279 Caucasians | BDNF significantly associated with treatment resistance: rs11030104 (OR = 2.57), rs10501087 (OR = 2.19) and rs6265 (OR = 2.08) | [23] |
| ADRB2, DRD3 and SLC6A4 | several | Risperidone | 111 Caucasians | Allele 16Gly of ADRB2 significantly associated with higher risk of sexual adverse events (p = 0.002) | [102] |
| BDNF | 4 SNPs | Clozapine | 257 Caucasians | rs11030104 and Val66Met associated with response (p = 0.04; 0.007, respectively). rs1519480 associated with WG (p = 0.04). | [22] |
| C4A and C4B | several | FGA | 87 Caucasians | Number of copies of C4BL nominally associated with TD severity (p = 0.020) | [103] |
| CNR1, FTO, MC4R, LEP and FAAH | several | Risperidone | 225 Caucasians | Variants in CNR1 (p = 1 × 10−5) and LEP (p = 1.4 × 10−4) associated with AIWG | [41] |
| COMT and DRD2 | several | Risperidone | 690 Chinese | COMT rs4680, DRD2 rs6275, rs1801028 and rs6277 associated with PANSS improvement (p = 0.05) | [19] |
| COMT | rs4680 and rs4818 | SGA | 521 Caucasians | rs4680 A allele and rs4680–rs4818 C-A haplotype associated with olanzapine response, but not with response to other antipsychotics | [20] |
| CYP1A2 and CYP2D6 | several | FGA or Risperidone | 475 Caucasians | CYP1A2*1F & CY2D6*4 associated with TD in patients on antipsychotics for a long time (p = 0.03) | [27] |
| CYP1A2 and CYP2B6 | several | Aripiprazol | 19 Caucasians | CYP1A2 UM & CYP2B6*1/*1 associated with aripiprazol- induced side effects | [104] |
| CYP2C19, LEPR, CYP1A2, HTR2C and ABCB1 | several | Clozapine | 60 Caucasians | Clozapine levels in patients with metabolic syndrome were significantly higher compared to those without (p < 0.01) and were associated with CYP2C19*2 (p = 0.04) | [25] |
| CYP2D6 | several | FGA and SGA | 198 Caucasians | Individuals with either increased or no CYP2D6 activity were at higher risk of having TD | [28] |
| CYP2D6 | several | Risperidone | 257 several ethnicities | Children and adolescents with PM variants showed poorer response to risperidone treatment | [29] |
| CYP2D6 | *4 | Haloperidol | 150 Caucasians | Carriers of *4 variant presented worse safety profile (p < 0.001) | [30] |
| CYP3A4 | several | Olanzapine | CATIE sample | rs472660 significantly predicted olanzapine clearance (p = 5.9 × 10−7). | [105] |
| DISC1 | several | FGA or SGA | 193 Caucasians | Two SNPs nominally associated with TD severity (p < 0.05). | [106] |
| DRD1 | rs4532 | several | 124 Brazilians | G-allele associated with treatment resistance (p = 0.001; adjusted OR = 2.71). GG had five-fold risk compared to A (p = 0.010; OR = 5.56). | [107] |
| DRD2, DRD3, HTR2A, HTR2C, COMT, NQO1, RGS2 and MnSOD | 13 SNPs | not specified | 402 Dutch | DRD2 TaqI associated with akathisia (OR = 2.3, p = 0.001), DRD2 −141C associated with TD (OR = 0.20, p = 0.001) | [32] |
| DRD2 | rs2514218 | Haloperidol and Risperidone | 100 Americans | In the aripiprazole group, C/C homozygotes had more akathisia; in the risperidone group, male T allele carriers had greater prolactin elevations | [37] |
| DRD2 and DRD3 | rs1800497, rs6277 and rs6280 | Cariprazine | 20 Caucasians | DRD2 rs1800497 and rs6277 associated with cariprazine response | [9] |
| DRD2 and DRD3 | several | SGA | 129 Caucasians | DRD2 rs1799732, DRD3 rs6280, and HTR2A rs7997012 associated with treatment resistance. | [10] |
| DRD3, DRD2, HTR2A, HTR2C, COMT and MTHFR | several | several | 329 Caucasians | DRD3 9Gly and MTHFR 677-T had better response (p = 0.034 and p = 0.019, respectively). | [7] |
| DRD4, HTR2A, TPH1, SLC18A1 and COMT | several | Haloperidol | 198 Tartars | Several associations of DRD4, HTR2A, TPH1 and SLC18A1 polymorphisms with antipsychotic response | [8] |
| EP300 | expression levels | several | 226 Caucasians | EP300 expression levels significantly associated with increases in BMIR, cholesterol levels and triglyceride concentrations | [108] |
| FKBP5, NR3C1, BDNF and NTRK2 | several | Clozapine | 591 Caucasians | Several associations between FKBP5 rs1360780, NTRK2 rs1778929 and rs10465180 with response | [109] |
| FTO | several | SGA | 259 + 91 Caucasians | In a subpopulation without additional weight-inducing comedication (n = 178), rs7185735-G carriers gained 3.4 times more weight (1.69 ± 3.1 kg, p = 0.019) | [110] |
| GLP1R | several | SGA | 464 Caucasians | Haplotypes associated with response to olanzapine (p = 0.002), perphenazine (p = 0.01), quetiapine (p = 0.008), risperidone (p = 0.02) and ziprasidone (p = 0.007) | [111] |
| GRIN2A, DRD3, HTR2C, DRD4 and GRIN2B | 42 SNPs | FGA or SGA | 431 + 168 Caucasians | Several significant associations with TD were identified, but only GRIN2A (rs1345423) was found in both patient populations | [112] |
| GRM3 | rs1468412 | Risperidone | 61 Caucasians | GRM3 rs1468412 associated with worsening spatial working | [113] |
| HLA | several | Clozapine | 180 neutropenia/ 1396 controls | HLA-DQB1 rs113332994 associated with clozapine-induced agranulocytosis (OR = 16.31) | [45] |
| HRH1 and CHRM3 | several | Several | 430 Caucasians | HRH1 haplotype rs346074–rs346070 associated with BMI (p = 0.025) and obesity (p = 0.005) in patients using high-H1 affinity antipsychotics | [15] |
| HRH3 | several | Risperidone | 129 Han Chinese | rs3787429 (p = 0.013–0.087) and rs3787430 (p = 0.024–0.010) associated with efficacy after 4–8 weeks, respectively | [16] |
| HRH4 | 5 SNPs | Risperidone | 113 Han Chinese | rs4483927 TT genotype predicts poor therapeutic response on the positive, negative, general and total scales of PANSS scores (p = 0.017, 0.019, 0.021 and 0.002, respectively) | [17] |
| HSD11B1 | several | SGA | 478 Caucasians | HSD11B1 rs846910-A, rs375319-A and rs4844488-G allele carriers associated with lower BMI in women | [114] |
| HTR2A | rs6313 | Olanzapine or Risperidone | 221 Caucasians | T allele carriers showed better response than non-carriers | [12] |
| HTR2C | −759T/C | SGA | 48 female Caucasians | T allele carriers gained less weight as compared to patients who did not have the allele | [36] |
| HTR2C | −759T/C | Risperidone | 108 Thai | 5-HT2C -759-T/C associated with hypertension but not with WG | [39] |
| HTR3A | rs1062613 and rs2276302 | several | 101 Indian patients | rs1062613-T and rs2276302-G alleles significantly associated with good clinical response to clozapine (p = 0.02) | [13] |
| HTR7 | several | Aripiprazol | 100 Japanese | rs12412496-rs7916403-rs1935349 A-T-A haplotype correlated with worse improvement in the cognition score (p = 0.046). | [115] |
| LEP and LEPR | several | FGA or SGA | 181 Caucasians | Significant association between a LEP haplotype (rs7799039G–rs10954173G–rs3828942G) and AIWG (p = 0.035) | [40] |
| MAOA, MAOB, DRD1, DRD2, DRD3, DRD4 and SLC6A3 | 41 SNPs | FGA or SGA | 446 Caucasians | Association between MAOB rs1799836 and HPRL in men. SLC6A3 rs40184 and rs3863145 associated with HPRL in risperidone/paliperidone subgroup | [116] |
| MC4R | rs489693 | SGA 4 weeks | 341 Caucasians | rs489693 A/A carriers showed 2.2 times higher weight increase than carriers of the C/C genotype (p = 0.039) | [43] |
| MC4R | rs17782313 | SGA 4 weeks | 51 Caucasians | rs17782313 C/C carriers higher risk of WG and BMI increase, with a dose effect of the C-allele (p = 0.002). | [117] |
| MC4R | rs489693 and rs17782313 | FGA or SGA | 1991 Chinese | Recessive effects of rs489693 on AIWG, WC and triglyceride change %, with A/A incurring more metabolic adverse effects | [44] |
| MC4R | rs17782313 | Amisulpride and Olanzapine | 212 Several | C carriers had higher WG than T homozygotes | [35] |
| NEUROD2 | several | SGA | 167 Caucasians | rs11078918 and rs12453682 associated with change in neuropsychological test results (p = 0.02–0.001). | [118] |
| NOS1AP | rs1214382 and rs10494366 | not specified | 347 Caucasian | rs12143842-CC and rs10494366-TT male carriers show positive correlation of QTc length with antipsychotic dosage | [119] |
| NPY5R | several | FGA and SGA | 99 Russians | rs11100494- C predisposes to AIWG (OR = 33.48, p< 0.001) | [120] |
| OXTR, CNR1, DDC and DRD2 | several | Clozapine or SGA | 196 Chileans | OXTR rs2228485, CNR1 rs806368 and rs1049353, and DDC rs10499696 associated with treatment resistance (p by genotype: 0.02, 0.001, 0.001 and 0.0003, respectively) | [121] |
| PLEKHA6 | rs7513240, rs4951353 | not specified | 263 Caucasians | rs7513240 and rs4951353 (A/G) associated with therapy response with different PANSS improvement after 4 weeks | [122] |
| PRKAR2B | 16 SNPs | Clozapine and Olanzapine | 99 Caucasians | rs9656135 minor allele carriers higher weight increase during treatment. | [123] |
| PTPRD | 4 SNPs | Clozapine or Olanzapine | 201 Caucasians and Africans | rs73398242 associated with AIWG in Europeans (p = 0.002) and with rs13294608 in African Americans (p = 0.003). | [124] |
| RELN | 15 SNPS | SGA | 260 Chinese | Two SNPs associated with antipsychotic treatment response (rs155333, p = 0.010 and rs6465938, p = 0.049) | [125] |
| RGS2 | several | Haloperidol | 258 Russians | RGS2*T/*T (rs2746073), *C/*C (rs4606) and *A/*A (rs2746071) associated with increased risk of antipsychotic-induced Parkinsonism | [126] |
| SLC18A2 | 9 SNPs | FGA long-term | 217 Caucasians | rs2015586 and rs363224 SNPs associated with TD and AIMS scores. | [127] |
| SLC6A5, GAD1, GRIA1, GRIA3, GRIA4, GRID2, GRIK1, GRIK2, GRIK3, GRIK4, GRIN2B, GRM1 and GRM4 | 62 SNPs | several | 101 + 71 + 118 Caucasian patients | SLC6A5 rs2298826 associated with a rapid rise of motor side effects at the beginning of the treatment (p = 0.0002) | [128] |
| SNAP25 | several | SGA and FGA | 3243 Chinese | rs6039769 significantly associated with AIWG (p < 0.001). | [129] |
| SULT4A1 | rs2285162 and rs2285167 | Olanzapine | 87 Caucasians | rs2285162 [A]-rs2285167 [G] haplotype superior olanzapine response (p = 0.004) and less AIWG per month (p = 0.04) | [130] |
| SV2C | 106 SNPs | SGA | 466 Caucasians | rs11960832-T/T significantly worse response to olanzapine treatment (p = 2.94 × 10−5; FDR = 2.18 × 10−2) | [131] |
| UGT1A4, UGT1A4 and ABCB1 | 7 SNPs | Olanzapine | 91 Japanese | Sympathetic nervous activity higher in individuals with the UGT1A4 rs2011425 G allele (p = 0.001). | [132] |
| Strategy | n | Treatment | Association | Ref. |
|---|---|---|---|---|
| GWAS | 122 + 174 Japanese | several | Association DPP6 rs6977820 with antipsychotic-induced TD (p = 0.008) | [55] |
| GWAS | 96 + 169 Caucasians | FGA or SGA | Two SNPs (rs7912580 and rs2412459) associated with response in both samples, located between ARID5B and RTKN2 genes | [48] |
| GWAS and WES | 163 Caucasians | Clozapine | HLA-DQB1 (126Q) (p = 4.7 × 10−14, OR = 0.19) and HLA-B (158T) (p = 6.4 × 10−10, OR = 3.3) associated with clozapine-induced agranulocytosis | [46] |
| Array 1995 genes | 89 Caucasians | Olanzapine or Risperidone | Significant associations between treatment response and SNPs in the chromosome 6, where the human leukocyte antigen (HLA) is located | [49] |
| GWAS | 189 + 86 Caucasians | SGA | OGFRL1 rs9346455 significantly associated with AIWG (p = 0.005) | [57] |
| WES | 11 + 103 + 87 several ethnicities | FGA or SGA | rs13025959 in MYO7B (E1647D) and rs10380 in MTRR (H622Y) associated with antipsychotic response | [51] |
| GWAS | 742 Indians | FGA or SGA | CCL2 rs4795893 (p = 7.62 × 10−4) and rs4586 (p = 1.13 × 10−3), GRIA4 rs2513265 (p = 1.44 × 10−3), ADCY2 rs1544938 (p = 7.68 × 10−4), and NRG1 rs13250975 (p = 6.81 × 10−3) and rs17716295 (p = 8.71 × 10−3) associated with response | [50] |
| GWAS | 534 + 547 Chinese | SGA | PTPRD rs10977144 (p = 9.26 × 10−9) and rs10977154 (p = 4.53 × 10−8), and GFPT2 rs12386481 (p = 1.98 × 10−7) associated with AIWG | [58] |
| GWAS | 50 + 380 Japanese | Clozapine | Variants in the human leukocyte antigen (HLA) region (rs1800625, p = 3.46 × 10−9, OR = 3.8) associated with agranulocytosis | [47] |
| WES | 316 + 1920 Chinese | FGA or SGA | Rare genetic variants in NMDA and AMPA enriched in the non-responder group | [52] |
| WES | 82 Jewish | not specified | RIMS2 showed significant enrichment of qualifying variants in TD patients (n = 39) (p = 5.32 × 10−8) | [56] |
| GWAS | 552 African ancestry | Clozapine | ACKR1 rs2814778-C/C carriers more likely to develop neutropenia and have to stop clozapine treatment (OR = 20.4, p = 3.44 × 10−7) | [60] |
| Sequencing 143 genes | 79 + 159 Han Chinese | Olanzapine | rs324026 (p = 0.023) and rs12610827 (p = 0.043) associated with response | [54] |
| GWAS | 339 several ethnicities | Amisulpride | Significant association in a locus not previously associated with AIWG (rs78310016; p = 3.66 × 10−8). Minor allale carriers had an OR of 3.98 (p = 1 × 10−3) for AIWG | [59] |
| GWAS | 2040 Chinese | FGA or SGA | ATAD3B rs20005072 and SKIL rs186507741 associated with antipsychotic-induced QTc interval change. | [61] |
| GWAS and WES | 189 + 222 Chinese | Risperidone | GWAS revealed a significant association between GRM7 SNPs (rs141134664, rs57521140 and rs73809055) and treatment response | [53] |
Summative Paragraph
- Genetic variants in genes coding for drug targets -dopamine and serotonin receptors in particular- may influence the efficacy and safety of antipsychotic medications.
- Functional variants in CYPs are associated with antipsychotic availability.
- Dose adjustment according to CYP functional variants present may help to improve adherence, efficacy and safety of antipsychotics.
- Clinical implementation of pharmacogenetic interventions for personalisation of antipsychotic treatment is limited.
- Improved clinical guidelines based on pharmacogenetic data, education and training in pharmacogenetics, reduced costs and shorter delivery times may increase implementation.
- Further research on the combined effect of pharmacogenetics, phenoconversion, and clinical and environmental factors is required.
Funding
Data Availability Statement
Conflicts of Interest
References
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [CrossRef]
- Arranz, M.J.; Salazar, J.; Hernandez, M.H. Pharmacogenetics of antipsychotics: Clinical utility and implementation. Behav. Brain Res. 2021, 401, 113058. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Muller, D.J. Pharmacogenetics of Antipsychotic Drug Treatment: Update and Clinical Implications. Mol. Neuropsychiatry 2020, 5 (Suppl. S1), 1–26. [Google Scholar] [CrossRef] [PubMed]
- Arranz, M.J.; Gonzalez-Rodriguez, A.; Perez-Blanco, J.; Penades, R.; Gutierrez, B.; Ibanez, L.; Arias, B.; Brunet, M.; Cervilla, J.; Salazar, J.; et al. A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl. Psychiatry 2019, 9, 177. [Google Scholar] [CrossRef]
- Beunk, L.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.M.; Guchelaar, H.J.; Houwink, E.J.F.; Risselada, A.; Rongen, G.; van Schaik, R.H.N.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP3A4 and CYP1A2 and antipsychotics. Eur. J. Hum. Genet. 2023, 1–8. [Google Scholar] [CrossRef]
- Vasiliu, O. The pharmacogenetics of the new-generation antipsychotics—A scoping review focused on patients with severe psychiatric disorders. Front. Psychiatry 2023, 14, 1124796. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Burger, H.; Wilffert, B.; Al Hadithy, A.; Alizadeh, B.Z.; Snieder, H.; GROUP investigators. Clinical response to antipsychotic drug treatment: Association study of polymorphisms in six candidate genes. Eur. Neuropsychopharmacol. 2012, 22, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gareeva, A.E.; Kinyasheva, K.O.; Galaktionova, D.Y.; Sabirov, E.T.; Valinourov, R.G.; Chudinov, A.V.; Zasedatelev, A.S.; Nasedkina, T.V.; Khusnutdinova, E.K. Brain neurotransmitter systems gene Polymorphism: The Search for pharmacogenetic markers of efficacy of haloperidol in Russians and Tatars. Mol. Biol. 2015, 49, 959–967. [Google Scholar] [CrossRef]
- De Pieri, M.; Ferrari, M.; Marino, F.; Traber, R.; Bolla, E.; Cosentino, M. Functional single nucleotide polymorphisms in dopaminergic receptors D2 predict clinical response to Cariprazine. Front. Pharmacol. 2023, 14, 1182393. [Google Scholar] [CrossRef]
- Del Casale, A.; Simmaco, M.; Modesti, M.N.; Zocchi, C.; Arena, J.F.; Bilotta, I.; Alcibiade, A.; Sarli, G.; Cutillo, L.; Antonelli, G.; et al. DRD2, DRD3, and HTR2A single-nucleotide polymorphisms involvement in high treatment resistance to atypical antipsychotic drugs. Biomedicines 2023, 11, 2088. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Liu, X.; Zhang, T.; Li, Y.; Yan, P. DRD3 Ser9Gly polymorphism and treatment response to antipsychotics in schizophrenia: A meta-analysis. Neurosci. Lett. 2022, 786, 136788. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Valsecchi, P.; Minelli, A.; Magri, C.; Bonvicini, C.; Barlati, S.; Sacchetti, E.; Vita, A.; Gennarelli, M. Association study between HTR2A rs6313 polymorphism and early response to risperidone and olanzapine in schizophrenia patients. Drug Dev. Res. 2020, 81, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, A.P.; Poonkuzhali, B.; Kuruvilla, A.; Jacob, M.; Jacob, K.S. Clinical predictors of serum clozapine levels in patients with treatment-resistant schizophrenia. Int. Clin. Psychopharmacol. 2013, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gressier, F.; Porcelli, S.; Calati, R.; Serretti, A. Pharmacogenetics of clozapine response and induced weight gain: A comprehensive review and meta-analysis. Eur. Neuropsychopharmacol. 2016, 26, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Risselada, A.J.; Al Hadithy, A.F.; Burger, H.; Snieder, H.; Wilffert, B.; Arends, J.; Wunderink, L.; Knegtering, H.; Wiersma, D.; et al. Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology 2011, 216, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, L.; Zhang, M.; Xuan, J.; Wang, Y.; Liu, B.; Shao, L.; Li, J.; Zeng, Z.; Li, T.; et al. A pharmacogenetic study of risperidone on histamine H3 receptor gene (HRH3) in Chinese Han schizophrenia patients. J. Psychopharmacol. 2012, 26, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, L.; Yu, T.; Wang, Y.; Sun, L.; Wang, T.; Huo, R.; Li, Y.; Wu, X.; Qin, S.; et al. Histamine H4 receptor polymorphism: A potential predictor of risperidone efficacy. J. Clin. Psychopharmacol. 2013, 33, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P. COMT val158met moderation of dopaminergic drug effects on cognitive function: A critical review. Pharmacogenom. J. 2016, 16, 430–438. [Google Scholar] [CrossRef]
- Han, C.J.; Kohen, R.; Jun, S.; Jarrett, M.E.; Cain, K.C.; Burr, R.; Heitkemper, M.M. COMT Val158Met Polymorphism and Symptom Improvement Following a Cognitively Focused Intervention for Irritable Bowel Syndrome. Nurs. Res. 2017, 66, 75–84. [Google Scholar] [CrossRef][Green Version]
- Nikolac Perkovic, M.; Sagud, M.; Zivkovic, M.; Uzun, S.; Nedic Erjavec, G.; Kozumplik, O.; Svob Strac, D.; Mimica, N.; Mihaljevic Peles, A.; Pivac, N. Catechol-O-methyltransferase rs4680 and rs4818 haplotype association with treatment response to olanzapine in patients with schizophrenia. Sci. Rep. 2020, 10, 10049. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, M.; Zhou, W.; Li, M.; Huai, C.; Shen, L.; Wang, T.; Wu, H.; Zhang, N.; Zhang, Z.; et al. Association Between the COMT Val158Met Polymorphism and Antipsychotic Efficacy in Schizophrenia: An Updated Meta-Analysis. Curr. Neuropharmacol. 2021, 19, 1780–1790. [Google Scholar] [CrossRef]
- Zai, G.C.; Zai, C.C.; Chowdhury, N.I.; Tiwari, A.K.; Souza, R.P.; Lieberman, J.A.; Meltzer, H.Y.; Potkin, S.G.; Müller, D.J.; Kennedy, J.L. The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 96–101. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Geisler, S.; DeRosse, P.; Bromet, E.J.; Malhotra, A.K. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr. Res. 2013, 146, 285–288. [Google Scholar] [CrossRef]
- Kneller, L.A.; Zubiaur, P.; Koller, D.; Abad-Santos, F.; Hempel, G. Influence of CYP2D6 Phenotypes on the Pharmacokinetics of Aripiprazole and Dehydro-Aripiprazole Using a Physiologically Based Pharmacokinetic Approach. Clin. Pharmacokinet. 2021, 60, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, K.; Choi, Y.H.; Norman, R.; Kim, R.B.; Schwarz, U.I. Genetic Determinants of Clozapine-Induced Metabolic Side Effects. Can. J. Psychiatry 2017, 62, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Schneiderhan, M.E.; Butler, T.; Carpentier, R.M.; Heins, K.R.; Formea, C.M. Pharmacogenomics to support mental health medication therapy management: Clinical practice considerations and a conceptual framework to enhance patient care. J. Am. Coll. Clin. Pharm. 2023. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Toshchakova, V.A.; Filipenko, M.L.; Fedorenko, O.Y.; Boyarko, E.G.; Boiko, A.S.; Semke, A.V.; Bokhan, N.A.; Aftanas, L.I.; Loonen, A.J. Cytochrome P450 1A2 co-determines neuroleptic load and may diminish tardive dyskinesia by increased inducibility. World J. Biol. Psychiatry 2015, 16, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Tiwari, A.K.; Freeman, N.; Zai, G.C.; de Luca, V.; Muller, D.J.; Tampakeras, M.; Herbert, D.; Emmerson, H.; Cheema, S.Y.; et al. Liver enzyme CYP2D6 gene and tardive dyskinesia. Pharmacogenomics 2020, 21, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Oshikoya, K.A.; Neely, K.M.; Carroll, R.J.; Aka, I.T.; Maxwell-Horn, A.C.; Roden, D.M.; Van Driest, S.L. CYP2D6 genotype and adverse events to risperidone in children and adolescents. Pediatr. Res. 2019, 85, 602–606. [Google Scholar] [CrossRef]
- Skryabin, V.Y.; Zastrozhin, M.S.; Parkhomenko, A.A.; Pankratenko, E.P.; Pozdnyakov, S.A.; Denisenko, N.P.; Akmalova, K.A.; Bryun, E.A.; Suychev, D.A. Investigating the use of pharmacogenetic and pharmacometabolic markers to predict haloperidol efficacy and safety rates. Hosp. Pharm. 2023, 58, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Lee, H.J. Oxidative stress and tardive dyskinesia: Pharmacogenetic evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 207–213. [Google Scholar] [CrossRef]
- Koning, J.P.; Vehof, J.; Burger, H.; Wilffert, B.; Al Hadithy, A.; Alizadeh, B.; van Harten, P.N.; Snieder, H.; Genetic Risk and Outcome in Psychosis (GROUP) investigators. Association of two DRD2 gene polymorphisms with acute and tardive antipsychotic-induced movement disorders in young Caucasian patients. Psychopharmacology 2012, 219, 727–736. [Google Scholar] [CrossRef]
- Zai, C.C.; Lee, F.H.; Tiwari, A.K.; Lu, J.Y.; de Luca, V.; Maes, M.S.; Herbert, D.; Shahmirian, A.; Cheema, S.Y.; Zai, G.C.; et al. Investigation of the HSPG2 Gene in Tardive Dyskinesia—New Data and Meta-Analysis. Front. Pharmacol. 2018, 9, 974. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Zhang, R.X.; Nitta, M.; Maayan, L.; John, M.; Robinson, D.G.; Fleischhacker, W.W.; Kahn, R.S.; Ophoff, R.A.; et al. Pharmacogenetic Associations of Antipsychotic Drug-Related Weight Gain: A Systematic Review and Meta-analysis. Schizophr. Bull. 2016, 42, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, K.F.; Leucht, S.; Heres, S.; Steimer, W. Genetic association of the rs17782313 polymorphism with antipsychotic-induced weight gain. Psychopharmacology 2023, 240, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Daray, F.M.; Rodante, D.; Carosella, L.G.; Silva, M.E.; Martinez, M.; Fernandez Busch, M.V.; Faccone, D.F.; Rothlin, R.P.; Maffia, P.C. -759C>T Polymorphism of the HTR2C Gene is Associated with Second Generation Antipsychotic-Induced Weight Gain in Female Patients with Schizophrenia. Pharmacopsychiatry 2017, 50, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Robinson, D.G.; Gallego, J.A.; John, M.; Yu, J.; Addington, J.; Tohen, M.; Kane, J.M.; Malhotra, A.K.; Lencz, T. Association of a Schizophrenia Risk Variant at the DRD2 Locus With Antipsychotic Treatment Response in First-Episode Psychosis. Schizophr. Bull. 2015, 41, 1248–1255. [Google Scholar] [CrossRef]
- Luo, C.; Liu, J.; Wang, X.; Mao, X.; Zhou, H.; Liu, Z. Pharmacogenetic Correlates of Antipsychotic-Induced Weight Gain in the Chinese Population. Neurosci. Bull. 2019, 35, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Vanwong, N.; Ngamsamut, N.; Nuntamool, N.; Hongkaew, Y.; Sukprasong, R.; Puangpetch, A.; Limsila, P.; Sukasem, C. Risperidone-Induced Obesity in Children and Adolescents With Autism Spectrum Disorder: Genetic and Clinical Risk Factors. Front. Pharmacol. 2020, 11, 565074. [Google Scholar] [CrossRef] [PubMed]
- Brandl, E.J.; Frydrychowicz, C.; Tiwari, A.K.; Lett, T.A.; Kitzrow, W.; Büttner, S.; Ehrlich, S.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L.; et al. Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 134–141. [Google Scholar] [CrossRef]
- Nurmi, E.L.; Spilman, S.L.; Whelan, F.; Scahill, L.L.; Aman, M.G.; McDougle, C.J.; Arnold, L.E.; Handen, B.; Johnson, C.; Sukhodolsky, D.G.; et al. Moderation of antipsychotic-induced weight gain by energy balance gene variants in the RUPP autism network risperidone studies. Transl. Psychiatry 2013, 3, e274. [Google Scholar] [CrossRef]
- Yoshida, K.; Maciukiewicz, M.; Zai, C.C.; Goncalves, V.F.; Brandl, E.J.; Lieberman, J.A.; Meltzer, H.Y.; Tiwari, A.K.; Kennedy, J.L.; Muller, D.J. Association between the -2548G/A polymorphism of the leptin gene and antipsychotic-induced weight gain: Analysis of the CATIE sample and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109952. [Google Scholar] [CrossRef]
- Czerwensky, F.; Leucht, S.; Steimer, W. Association of the common MC4R rs17782313 polymorphism with antipsychotic-related weight gain. J. Clin. Psychopharmacol. 2013, 33, 74–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Wang, Q.; Deng, W.; Yue, W.; Yan, H.; Tan, L.; Chen, Q.; Yang, G.; Lu, T.; et al. Testing the role of genetic variation of the MC4R gene in Chinese population in antipsychotic-induced metabolic disturbance. Sci. China Life Sci. 2019, 62, 535–543. [Google Scholar] [CrossRef]
- Konte, B.; Walters, J.T.R.; Rujescu, D.; Legge, S.E.; Pardinas, A.F.; Cohen, D.; Pirmohamed, M.; Tiihonen, J.; Hartmann, A.M.; Bogers, J.P.; et al. HLA-DQB1 6672G>C (rs113332494) is associated with clozapine-induced neutropenia and agranulocytosis in individuals of European ancestry. Transl. Psychiatry 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Jarskog, L.F.; Hilliard, C.; Alfirevic, A.; Duncan, L.; Fourches, D.; Huang, H.; Lek, M.; Neale, B.M.; Ripke, S.; et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat. Commun. 2014, 5, 4757. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ikeda, M.; Mushiroda, T.; Ozeki, T.; Kondo, K.; Shimasaki, A.; Kawase, K.; Hashimoto, S.; Yamamori, H.; Yasuda, Y.; et al. Pharmacogenomic Study of Clozapine-Induced Agranulocytosis/Granulocytopenia in a Japanese Population. Biol. Psychiatry 2016, 80, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Drago, A.; Giegling, I.; Schafer, M.; Hartmann, A.M.; Konte, B.; Friedl, M.; Serretti, A.; Rujescu, D. Genome-wide association study supports the role of the immunological system and of the neurodevelopmental processes in response to haloperidol treatment. Pharmacogenet. Genom. 2014, 24, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Le Clerc, S.; Taing, L.; Fond, G.; Meary, A.; Llorca, P.M.; Blanc, O.; Beaune, P.; Rajagopal, K.; Jamain, S.; Tamouza, R.; et al. A double amino-acid change in the HLA-A peptide-binding groove is associated with response to psychotropic treatment in patients with schizophrenia. Transl. Psychiatry 2015, 5, e608. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Kaur, H.; Kumari, K.; Kanojia, N.; Gupta, M.; Baghel, R.; Sood, M.; Jain, S.; Chadda, R.K.; Kukreti, R. Evaluation of genetic association of neurodevelopment and neuroimmunological genes with antipsychotic treatment response in schizophrenia in Indian populations. Mol. Genet. Genom. Med. 2016, 4, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Drogemoller, B.I.; Emsley, R.; Chiliza, B.; van der Merwe, L.; Wright, G.E.; Daya, M.; Hoal, E.; Malhotra, A.K.; Lencz, T.; Robinson, D.G.; et al. The identification of novel genetic variants associated with antipsychotic treatment response outcomes in first-episode schizophrenia patients. Pharmacogenet. Genom. 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Wang, Q.; Man Wu, H.; Yue, W.; Yan, H.; Zhang, Y.; Tan, L.; Deng, W.; Chen, Q.; Yang, G.; Lu, T.; et al. Effect of Damaging Rare Mutations in Synapse-Related Gene Sets on Response to Short-term Antipsychotic Medication in Chinese Patients With Schizophrenia: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 1261–1269. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhu, W.; Zhou, W.; Shen, L.; Wu, H.; Zhang, N.; Wu, S.; Fu, C.; et al. Different responses to risperidone treatment in Schizophrenia: A multicenter genome-wide association and whole exome sequencing joint study. Transl. Psychiatry 2022, 12, 173. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, Y.; Lv, Q.; Sheng, Y.H.; Chen, L.; Li, M.; Shen, L.; Huai, C.; Yi, Z.; Cui, D.; et al. Genetic Association of Olanzapine Treatment Response in Han Chinese Schizophrenia Patients. Front. Pharmacol. 2019, 10, 177. [Google Scholar] [CrossRef]
- Tanaka, S.; Syu, A.; Ishiguro, H.; Inada, T.; Horiuchi, Y.; Ishikawa, M.; Koga, M.; Noguchi, E.; Ozaki, N.; Someya, T.; et al. DPP6 as a candidate gene for neuroleptic-induced tardive dyskinesia. Pharmacogenom. J. 2013, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Alkelai, A.; Greenbaum, L.; Heinzen, E.L.; Baugh, E.H.; Teitelbaum, A.; Zhu, X.; Strous, R.D.; Tatarskyy, P.; Zai, C.C.; Tiwari, A.K.; et al. New insights into tardive dyskinesia genetics: Implementation of whole-exome sequencing approach. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109659. [Google Scholar] [CrossRef] [PubMed]
- Brandl, E.J.; Tiwari, A.K.; Zai, C.C.; Nurmi, E.L.; Chowdhury, N.I.; Arenovich, T.; Sanches, M.; Goncalves, V.F.; Shen, J.J.; Lieberman, J.A.; et al. Genome-wide association study on antipsychotic-induced weight gain in the CATIE sample. Pharmacogenom. J. 2016, 16, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, L.; Lv, L.; Ma, C.; Du, B.; Lu, T.; Jin, C.; Yan, H.; Yang, Y.; Li, W.; et al. Genome-Wide Association Study Suggested the PTPRD Polymorphisms Were Associated With Weight Gain Effects of Atypical Antipsychotic Medications. Schizophr. Bull. 2016, 42, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Ter Hark, S.E.; Jamain, S.; Schijven, D.; Lin, B.D.; Bakker, M.K.; Boland-Auge, A.; Deleuze, J.F.; Troudet, R.; Malhotra, A.K.; Gülöksüz, S.; et al. A new genetic locus for antipsychotic-induced weight gain: A genome-wide study of first-episode psychosis patients using amisulpride (from the OPTiMiSE cohort). J. Psychopharmacol. 2020, 34, 524–531. [Google Scholar] [CrossRef]
- Legge, S.E.; Pardinas, A.F.; Helthuis, M.; Jansen, J.A.; Jollie, K.; Knapper, S.; MacCabe, J.H.; Rujescu, D.; Collier, D.A.; O’Donovan, M.C.; et al. A genome-wide association study in individuals of African ancestry reveals the importance of the Duffy-null genotype in the assessment of clozapine-related neutropenia. Mol. Psychiatry 2019, 24, 328–337. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Yan, H.; Su, Y.; Guo, L.; Liao, Y.; Lu, T.; Yu, H.; Wang, L.; Li, J.; et al. ATAD3B and SKIL polymorphisms associated with antipsychotic-induced QTc interval change in patients with schizophrenia: A genome-wide association study. Transl. Psychiatry 2022, 12, 56. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Boloc, D.; Rodriguez, N.; Marmol, F.; Sanchez, J.; Bernardo, M.; Lafuente, A. Network analysis of gene expression in mice provides new evidence of involvement of the mTOR pathway in antipsychotic-induced extrapyramidal symptoms. Pharmacogenom. J. 2016, 16, 293–300. [Google Scholar] [CrossRef]
- Sainz, J.; Prieto, C.; Ruso-Julve, F.; Crespo-Facorro, B. Blood Gene Expression Profile Predicts Response to Antipsychotics. Front. Mol. Neurosci. 2018, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, E.S.; McGregor, N.W.; Emsley, R.A.; Warnich, L. DNA methylation and antipsychotic treatment mechanisms in schizophrenia: Progress and future directions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 38–49. [Google Scholar] [CrossRef]
- Lisoway, A.J.; Chen, C.C.; Zai, C.C.; Tiwari, A.K.; Kennedy, J.L. Toward personalized medicine in schizophrenia: Genetics and epigenetics of antipsychotic treatment. Schizophr. Res. 2021, 232, 112–124. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Wang, X.; He, Y.; Xia, Y.; Sweeney, J.A.; Kopp, R.F.; Liu, C.; Chen, C. Drug Response-Related DNA Methylation Changes in Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Front. Neurosci. 2021, 15, 674273. [Google Scholar] [CrossRef]
- Tang, H.; Dalton, C.F.; Srisawat, U.; Zhang, Z.J.; Reynolds, G.P. Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 645–649. [Google Scholar] [CrossRef]
- Athanasiou, M.C.; Dettling, M.; Cascorbi, I.; Mosyagin, I.; Salisbury, B.A.; Pierz, K.A.; Zou, W.; Whalen, H.; Malhotra, A.K.; Lencz, T.; et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J. Clin. Psychiatry 2011, 72, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Umehara, H.; Ohmori, T.; Hashimoto, R. Clozapine Pharmacogenetic Studies in Schizophrenia: Efficacy and Agranulocytosis. Front. Pharmacol. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Boloc, D.; Gortat, A.; Cheng-Zhang, J.Q.; Garcia-Cerro, S.; Rodriguez, N.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Gasso, P.; Lafuente, A.; et al. Improving pharmacogenetic prediction of extrapyramidal symptoms induced by antipsychotics. Transl. Psychiatry 2018, 8, 276. [Google Scholar] [CrossRef]
- Tonozzi, T.R.; Braunstein, G.D.; Kammesheidt, A.; Curran, C.; Golshan, S.; Kelsoe, J. Pharmacogenetic profile and major depressive and/or bipolar disorder treatment: A retrospective, cross-sectional study. Pharmacogenomics 2018, 19, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Robinson, D.; Yu, J.; Gallego, J.; Fleischhacker, W.W.; Kahn, R.S.; Crespo-Facorro, B.; Vazquez-Bourgon, J.; Kane, J.M.; Malhotra, A.K.; et al. Schizophrenia Polygenic Risk Score as a Predictor of Antipsychotic Efficacy in First-Episode Psychosis. Am. J. Psychiatry 2019, 176, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Marshe, V.S.; Elsheikh, S.S.M.; Maciukiewicz, M.; Tiwari, A.; Brandl, E.J.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Muller, D.J. Polygenic risk scores analyses of psychiatric and metabolic traits with antipsychotic-induced weight gain in schizophrenia: An exploratory study. Pharmacogenom. J. 2023, 23, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rodieux, F.; Daali, Y.; Rollason, V.; Samer, C.F.; Lorenzini, K.I. Practice of CYP450 genotyping and phenotyping in children in a real-life setting. Front. Pharmacol. 2023, 14, 1130100. [Google Scholar] [CrossRef] [PubMed]
- Toja-Camba, F.J.; Gesto-Antelo, N.; Maronas, O.; Echarri Arrieta, E.; Zarra-Ferro, I.; Gonzalez-Barcia, M.; Bandin-Vilar, E.; Mangas Sanjuan, V.; Facal, F.; Arrojo Romero, M.; et al. Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics. Pharmaceutics 2021, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruano, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef]
- Altar, C.A.; Carhart, J.; Allen, J.D.; Hall-Flavin, D.; Winner, J.; Dechairo, B. Clinical Utility of Combinatorial Pharmacogenomics-Guided Antidepressant Therapy: Evidence from Three Clinical Studies. Mol. Neuropsychiatry 2015, 1, 145–155. [Google Scholar] [CrossRef]
- Winner, J.G.; Carhart, J.M.; Altar, C.A.; Goldfarb, S.; Allen, J.D.; Lavezzari, G.; Parsons, K.K.; Marshak, A.G.; Garavaglia, S.; Dechairo, B.M. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr. Med. Res. Opin. 2015, 31, 1633–1643. [Google Scholar] [CrossRef]
- Arranz, M.J.; Salazar, J.; Bote, V.; Artigas-Baleri, A.; Serra-LLovich, A.; Triviño, E.; Roige, J.; Lombardia, C.; Cancino, M.; Hernandez, M.; et al. Pharmacogenetic Interventions Improve the Clinical Outcome of Treatment-Resistant Autistic Spectrum Disorder Sufferers. Pharmaceutics 2022, 14, 999. [Google Scholar] [CrossRef]
- Walden, L.M.; Brandl, E.J.; Tiwari, A.K.; Cheema, S.; Freeman, N.; Braganza, N.; Kennedy, J.L.; Müller, D.J. Genetic testing for CYP2D6 and CYP2C19 suggests improved outcome for antidepressant and antipsychotic medication. Psychiatry Res. 2019, 279, 111–115. [Google Scholar] [CrossRef]
- Scherf-Clavel, M.; Frantz, A.; Eckert, A.; Weber, H.; Unterecker, S.; Deckert, J.; Reif, A.; Hahn, M. Effect of CYP2D6 pharmacogenetic phenotype and phenoconversion on serum concentrations of antidepressants and antipsychotics: A retrospective cohort study. Int. J. Clin. Pharm. 2023, 45, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Campo-Soria, C.; Bishop, J.R. Current strategies for predicting side effects from second generation antipsychotics in youth. Expert Opin. Drug Metab. Toxicol. 2021, 17, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.I.; Schuette, P.; Burckart, G.J.; Green, D.J.; La, J.; Burnham, J.M.; Rakhmanina, N.; Robb, A.; Huang, S.M.; van den Anker, J.N. A Comparison of Pediatric and Adult Safety Studies for Antipsychotic and Antidepressant Drugs Submitted to the United States Food and Drug Administration. J. Pediatr. 2019, 208, 236–242.e3. [Google Scholar] [CrossRef] [PubMed]
- Jameson, A.; Fylan, B.; Bristow, G.C.; Sagoo, G.S.; Dalton, C.; Cardno, A.; Sohal, J.; McLean, S.L. What Are the Barriers and Enablers to the Implementation of Pharmacogenetic Testing in Mental Health Care Settings? Front. Genet. 2021, 12, 740216. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Martinez, J.; Shah, N.; Kenan, W.; Fowler, A.; Limdi, N.; Burns, L.; Cogan, E.S.; Gardiner, A.; Hain, D.; et al. Pharmacogenomic profiling of pediatric patients on psychotropic medications in an emergency department. Pediatr. Emerg. Care 2023, 39, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Eadon, M.T.; Rosenman, M.B.; Zhang, P.; Fulton, C.R.; Callaghan, J.T.; Holmes, A.M.; Levy, K.D.; Gupta, S.K.; Haas, D.M.; Vuppalanchi, R.; et al. The INGENIOUS trial: Impact of pharmacogenetic testing on adverse events in a pragmatic clinical trial. Pharmacogenom. J. 2023, 23, 169–177. [Google Scholar] [CrossRef]
- Carrascal-Laso, L.; Franco-Martin, M.A.; Garcia-Berrocal, M.B.; Marcos-Vadillo, E.; Sanchez-Iglesias, S.; Lorenzo, C.; Sanchez-Martin, A.; Ramos-Gallego, I.; Garcia-Salgado, M.J.; Isidoro-Garcia, M. Application of a Pharmacogenetics-Based Precision Medicine Model (5SPM) to Psychotic Patients That Presented Poor Response to Neuroleptic Therapy. J. Pers. Med. 2020, 10, 289. [Google Scholar] [CrossRef]
- Alshabeeb, M.A.; Deneer, V.H.M.; Khan, A.; Asselbergs, F.W. Use of Pharmacogenetic Drugs by the Dutch Population. Front. Genet. 2019, 10, 567. [Google Scholar] [CrossRef]
- Kang, Z.; Qin, Y.; Sun, Y.; Lu, Z.; Sun, Y.; Chen, H.; Feng, X.; Zhang, Y.; Guo, H.; Yan, H.; et al. Multigenetic pharmacogenomics-guided treatment vs treatment as usual among hospitalised men with schizophrenia. JAMA Netw. Open 2023, 6, e2335518. [Google Scholar] [CrossRef]
- Garcia-Perez, L.; Linertova, R.; Serrano-Perez, P.; Trujillo-Martin, M.; Rodriguez-Rodriguez, L.; Valcarcel-Nazco, C.; Del Pino-Sedeno, T. Interventions to improve medication adherence in mental health: The update of a systematic review of cost-effectiveness. Int. J. Psychiatry Clin. Pract. 2020, 24, 416–427. [Google Scholar] [CrossRef]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; Mrazek, D.A. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef]
- Pérez, V.; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-García, E.; Vieta, E.; Olivares, J.M.; et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: Results of a randomized, double-blind clinical trial. BMC Psychiatry 2017, 17, 250. [Google Scholar] [CrossRef]
- Laika, B.; Leucht, S.; Heres, S.; Steimer, W. Intermediate metabolizer: Increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenom. J. 2009, 9, 395–403. [Google Scholar] [CrossRef]
- Carrascal-Laso, L.; Franco-Martin, M.A.; Marcos-Vadillo, E.; Ramos-Gallego, I.; Garcia-Berrocal, B.; Mayor-Toranzo, E.; Sanchez-Iglesias, S.; Lorenzo, C.; Sevillano-Jimenez, A.; Sanchez-Martin, A.; et al. Economic Impact of the Application of a Precision Medicine Model (5SPM) on Psychotic Patients. Pharmacogenom. Pers. Med. 2021, 14, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Karamperis, K.; Koromina, M.; Papantoniou, P.; Skokou, M.; Kanellakis, F.; Mitropoulos, K.; Vozikis, A.; Muller, D.J.; Patrinos, G.P.; Mitropoulou, C. Economic evaluation in psychiatric pharmacogenomics: A systematic review. Pharmacogenom. J. 2021, 21, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Girardin, F.R.; Poncet, A.; Perrier, A.; Vernaz, N.; Pletscher, M.; Samer, C.F.; Lieberman, J.A.; Villard, J. Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. Pharmacogenom. J. 2019, 19, 211–218. [Google Scholar] [CrossRef]
- Bousman, C.A.; Hopwood, M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry 2016, 3, 585–590. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Lafuente, A.; Bioque, M.; Lobo, A.; Gonzalez-Pinto, A.; Olmeda, M.S.; Corripio, I.; Llerena, A.; Cabrera, B.; et al. Pharmacogenetic study of antipsychotic induced acute extrapyramidal symptoms in a first episode psychosis cohort: Role of dopamine, serotonin and glutamate candidate genes. Pharmacogenom. J. 2016, 16, 439–445. [Google Scholar] [CrossRef]
- Hettige, N.C.; Zai, C.; Hazra, M.; Borlido, C.; Kennedy, J.L.; Strauss, J.; Le Foll, B.; Wong, A.; Remington, G.; De Luca, V. Use of candidate gene markers to guide antipsychotic dosage adjustment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 315–320. [Google Scholar] [CrossRef]
- Teo, C.; Zai, C.; Borlido, C.; Tomasetti, C.; Strauss, J.; Shinkai, T.; Le Foll, B.; Wong, A.; Kennedy, J.L.; De Luca, V. Analysis of treatment-resistant schizophrenia and 384 markers from candidate genes. Pharmacogenet. Genom. 2012, 22, 807–811. [Google Scholar] [CrossRef]
- Almoguera, B.; Riveiro-Alvarez, R.; Lopez-Castroman, J.; Dorado, P.; Vaquero-Lorenzo, C.; Fernandez-Piqueras, J.; Llerena, A.; Abad-Santos, F.; Baca-García, E.; Dal-Ré, R.; et al. Association of common genetic variants with risperidone adverse events in a Spanish schizophrenic population. Pharmacogenom. J. 2013, 13, 197–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zai, C.C.; Tiwari, A.K.; Zai, G.C.; Freeman, N.; Pouget, J.G.; Greco, J.; Tampakeras, M.; Shaikh, S.A.; Herbert, D.; Emmerson, H.; et al. Association Study of the Complement Component C4 Gene in Tardive Dyskinesia. Front. Pharmacol. 2019, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Guzek, K.; Stelmach, A.; Roznowska, A.; Najbar, I.; Cichocki, L.; Sadakierska-Chudy, A. A preliminary study of genetic polymorphisms potentially related to the adverse effects of aripiprazole. Arch. Pharm. Pract. 2023, 14, 13–18. [Google Scholar] [CrossRef]
- Bigos, K.L.; Bies, R.R.; Pollock, B.G.; Lowy, J.J.; Zhang, F.; Weinberger, D.R. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol. Psychiatry 2011, 16, 620–625. [Google Scholar] [CrossRef]
- Lu, J.Y.; Tiwari, A.K.; Zai, G.C.; Rastogi, A.; Shaikh, S.A.; Müller, D.J.; Voineskos, A.N.; Potkin, S.G.; Lieberman, J.A.; Meltzer, H.Y.; et al. Association study of Disrupted-In-Schizophrenia-1 gene variants and tardive dyskinesia. Neurosci. Lett. 2018, 686, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ota, V.K.; Spíndola, L.N.; Gadelha, A.; dos Santos Filho, A.F.; Santoro, M.L.; Christofolini, D.M.; Bellucco, F.T.; Ribeiro-dos-Santos, Â.; Santos, S.; Mari, J.D.J.; et al. DRD1 rs4532 polymorphism: A potential pharmacogenomic marker for treatment response to antipsychotic drugs. Schizophr. Res. 2012, 142, 206–208. [Google Scholar] [CrossRef]
- Martinez-Pinteno, A.; Gasso, P.; Prohens, L.; Segura, A.G.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Bernardo, M.; Lafuente, A.; Mas, S.; et al. Identification of EP300 as a Key Gene Involved in Antipsychotic-Induced Metabolic Dysregulation Based on Integrative Bioinformatics Analysis of Multi-Tissue Gene Expression Data. Front. Pharmacol. 2021, 12, 729474. [Google Scholar] [CrossRef] [PubMed]
- Mitjans, M.; Catalán, R.; Vázquez, M.; González-Rodríguez, A.; Penadés, R.; Pons, A.; Massana, G.; Munro, J.; Arranz, M.J.; Arias, B. Hypothalamic–pituitary–adrenal system, neurotrophic factors and clozapine response: Association with FKBP5 and NTRK2 genes. Pharmacogenet. Genom. 2015, 25, 274–277. [Google Scholar] [CrossRef]
- Schröder, C.; Czerwensky, F.; Leucht, S.; Steimer, W. Fat Mass and Obesity-Related Gene Variants rs9939609 and rs7185735 are Associated with Second-Generation Antipsychotic-Induced Weight Gain. Pharmacopsychiatry 2019, 52, 16–23. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Brennan, M.D. Glucagon-like peptide 1 receptor (GLP1R) haplotypes correlate with altered response to multiple antipsychotics in the CATIE trial. Schizophr. Res. 2014, 160, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.R.; Al Hadithy, A.F.; Amin, N.; van Duijn, C.M.; van Os, J.; van Harten, P.N. Antipsychotic-induced movement disorders in long-stay psychiatric patients and 45 tag SNPs in 7 candidate genes: A prospective study. PLoS ONE 2012, 7, e50970. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Reilly, J.L.; Harris, M.S.; Patel, S.R.; Kittles, R.; Badner, J.A.; Prasad, K.M.; Nimgaonkar, V.L.; Keshavan, M.S.; Sweeney, J.A. Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology 2015, 232, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Quteineh, L.; Vandenberghe, F.; Saigi Morgui, N.; Delacretaz, A.; Choong, E.; Gholam-Rezaee, M.; Magistretti, P.; Bondolfi, G.; Von Gunten, A.; Preisig, M.; et al. Impact of HSD11B1 polymorphisms on BMI and components of the metabolic syndrome in patients receiving psychotropic treatments. Pharmacogenet. Genom. 2015, 25, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Takekita, Y.; Fabbri, C.; Kato, M.; Nonen, S.; Sakai, S.; Sunada, N.; Koshikawa, Y.; Wakeno, M.; Okugawa, G.; Kinoshita, T.; et al. Serotonin 7 Receptor Variants Are Not Associated with Response to Second-Generation Antipsychotics in Japanese Schizophrenia Patients. Neuropsychobiology 2015, 72, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Osmanova, D.Z.; Freidin, M.B.; Fedorenko, O.Y.; Pozhidaev, I.V.; Boiko, A.S.; Vyalova, N.M.; Tiguntsev, V.V.; Kornetova, E.G.; Loonen, A.J.M.; Semke, A.V.; et al. A pharmacogenetic study of patients with schizophrenia from West Siberia gets insight into dopaminergic mechanisms of antipsychotic-induced hyperprolactinemia. BMC Med. Genet. 2019, 20 (Suppl. S1), 47. [Google Scholar] [CrossRef]
- Czerwensky, F.; Leucht, S.; Steimer, W. MC4R rs489693: A clinical risk factor for second generation antipsychotic-related weight gain? Int. J. Neuropsychopharmacol. 2013, 16, 2103–2109. [Google Scholar] [CrossRef][Green Version]
- Spellmann, I.; Riedel, M.; Städtler, J.; Zill, P.; Obermeier, M.; Cerovecki, A.; Dehning, S.; Schennach, R.; Epple, M.; Opgen-Rhein, M.; et al. Associations of NEUROD2 polymorphisms and change of cognitive dysfunctions in schizophrenia and schizoaffective disorder after eight weeks of antipsychotic treatment. Cogn. Neuropsychiatry 2017, 22, 280–297. [Google Scholar] [CrossRef]
- Esen-Sehir, D.; Kopf, J.; Hagele, S.; Plichta, M.M.; Reif, A.; Freudenberg, F. Influence of NOS1AP Risk Variants on the Corrected QT (QTc) Interval in the Pharmacotherapy of Schizophrenia. Pharmacopsychiatry 2022, 55, 266–273. [Google Scholar] [CrossRef]
- Dobrodeeva, V.S.; Shnayder, N.A.; Novitsky, M.A.; Asadullin, A.R.; Vaiman, E.E.; Petrova, M.M.; Limankin, O.V.; Neznanov, N.G.; Garganeeva, N.P.; Nasyrova, R.F. Association of a Single-Nucleotide Variant rs11100494 of the NPY5R Gene with Antipsychotic-Induced Metabolic Disorders. Pharmaceutics 2022, 14, 222. [Google Scholar] [CrossRef]
- Zazueta, A.; Castillo, T.; Cavieres, A.; Gonzalez, R.; Abarca, M.; Nieto, R.R.; Deneken, J.; Araneda, C.; Moya, P.R.; Bustamante, M.L. Polymorphisms in Schizophrenia-Related Genes Are Potential Predictors of Antipsychotic Treatment Resistance and Refractoriness. Int. J. Neuropsychopharmacol. 2022, 25, 701–708. [Google Scholar] [CrossRef]
- Spellmann, I.; Rujescu, D.; Musil, R.; Meyerwas, S.; Giegling, I.; Genius, J.; Zill, P.; Dehning, S.; Cerovecki, A.; Seemuller, F.; et al. Pleckstrin homology domain containing 6 protein (PLEKHA6) polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 51, 190–195. [Google Scholar] [CrossRef]
- Gagliano, S.A.; Tiwari, A.K.; Freeman, N.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Knight, J.; Muller, D.J. Protein kinase cAMP-dependent regulatory type II beta (PRKAR2B) gene variants in antipsychotic-induced weight gain. Hum. Psychopharmacol. 2014, 29, 330–335. [Google Scholar] [CrossRef]
- Maciukiewicz, M.; Gorbovskaya, I.; Tiwari, A.K.; Zai, C.C.; Freeman, N.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. Genetic validation study of protein tyrosine phosphatase receptor type D (PTPRD) gene variants and risk for antipsychotic-induced weight gain. J. Neural Transm. 2019, 126, 27–33. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Qin, S.; Li, Y.; Ning, A.; Fu, Y.; Wang, D.; Zeng, D.; Li, H.; Yu, W.; et al. Two Novel Loci of RELN Associated With Antipsychotics Response in Chinese Han Population. Front. Pharmacol. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Gareeva, A.E.; Zakirov, D.F.; Valinurov, R.G.; Khusnutdinova, E.K. Polymorphism of RGS2 gene: Genetic markers of risk for schizophrenia and pharmacogenetic markers of typical neuroleptics efficiency. Mol. Biol. 2013, 47, 934–941. [Google Scholar] [CrossRef]
- Zai, C.C.; Tiwari, A.K.; Mazzoco, M.; de Luca, V.; Muller, D.J.; Shaikh, S.A.; Lohoff, F.W.; Freeman, N.; Voineskos, A.N.; Potkin, S.G.; et al. Association study of the vesicular monoamine transporter gene SLC18A2 with tardive dyskinesia. J. Psychiatr. Res. 2013, 47, 1760–1765. [Google Scholar] [CrossRef]
- Giegling, I.; Drago, A.; Dolzan, V.; Plesnicar, B.K.; Schafer, M.; Hartmann, A.M.; Sander, T.; Toliat, M.R.; Moller, H.J.; Stassen, H.H.; et al. Glutamatergic gene variants impact the clinical profile of efficacy and side effects of haloperidol. Pharmacogenet. Genom. 2011, 21, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhang, T.; Han, W.; Zhu, L.; Ni, T.; Lin, H.; Liu, D.; Chen, G.; Xiao, J.; Li, T. Relationship of SNAP25 variants with schizophrenia and antipsychotic-induced weight change in large-scale schizophrenia patients. Schizophr. Res. 2020, 215, 250–255. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Liu, Q.; Brennan, M.D. Replication of SULT4A1-1 as a pharmacogenetic marker of olanzapine response and evidence of lower weight gain in the high response group. Pharmacogenomics 2014, 15, 933–939. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Liu, Q.; Massey, B.W.; Brennan, M.D. Genotypic variation in the SV2C gene impacts response to atypical antipsychotics the CATIE study. Schizophr. Res. 2013, 149, 21–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hattori, S.; Suda, A.; Miyauchi, M.; Shiraishi, Y.; Saeki, T.; Fukushima, T.; Fujibayashi, M.; Tsujita, N.; Ishii, C.; Ishii, N.; et al. The association of genetic polymorphisms in CYP1A2, UGT1A4, and ABCB1 with autonomic nervous system dysfunction in schizophrenia patients treated with olanzapine. BMC Psychiatry 2020, 20, 72. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, M.; Cullell, N.; Cendros, M.; Serra-Llovich, A.; Arranz, M.J. Clinical Utility and Implementation of Pharmacogenomics for the Personalisation of Antipsychotic Treatments. Pharmaceutics 2024, 16, 244. https://doi.org/10.3390/pharmaceutics16020244
Hernandez M, Cullell N, Cendros M, Serra-Llovich A, Arranz MJ. Clinical Utility and Implementation of Pharmacogenomics for the Personalisation of Antipsychotic Treatments. Pharmaceutics. 2024; 16(2):244. https://doi.org/10.3390/pharmaceutics16020244
Chicago/Turabian StyleHernandez, Marta, Natalia Cullell, Marc Cendros, Alexandre Serra-Llovich, and Maria J. Arranz. 2024. "Clinical Utility and Implementation of Pharmacogenomics for the Personalisation of Antipsychotic Treatments" Pharmaceutics 16, no. 2: 244. https://doi.org/10.3390/pharmaceutics16020244
APA StyleHernandez, M., Cullell, N., Cendros, M., Serra-Llovich, A., & Arranz, M. J. (2024). Clinical Utility and Implementation of Pharmacogenomics for the Personalisation of Antipsychotic Treatments. Pharmaceutics, 16(2), 244. https://doi.org/10.3390/pharmaceutics16020244






