Abstract
Decades of pharmacogenetic research have revealed genetic biomarkers of clinical response to antipsychotics. Genetic variants in antipsychotic targets, dopamine and serotonin receptors in particular, and in metabolic enzymes have been associated with the efficacy and toxicity of antipsychotic treatments. However, genetic prediction of antipsychotic response based on these biomarkers is far from accurate. Despite the clinical validity of these findings, the clinical utility remains unclear. Nevertheless, genetic information on CYP metabolic enzymes responsible for the biotransformation of most commercially available antipsychotics has proven to be effective for the personalisation of clinical dosing, resulting in a reduction of induced side effects and in an increase in efficacy. However, pharmacogenetic information is rarely used in psychiatric settings as a prescription aid. Lack of studies on cost-effectiveness, absence of clinical guidelines based on pharmacogenetic biomarkers for several commonly used antipsychotics, the cost of genetic testing and the delay in results delivery hamper the implementation of pharmacogenetic interventions in clinical settings. This narrative review will comment on the existing pharmacogenetic information, the clinical utility of pharmacogenetic findings, and their current and future implementations.
1. Introduction
Antipsychotic drugs are the mainstay treatment for psychotic symptoms of schizophrenia and other severe mental disorders. However, about 30–50% of people receiving antipsychotics do not respond adequately and/or develop severe and long-lasting side effects. The reasons behind treatment failure and adverse reactions are difficult to discern as the precise mechanism of action of antipsychotic drugs is still unclear. Clinical and environmental factors [1] as well as genetic factors [2] have been proposed as contributors to variability in response to antipsychotic treatment.
Pharmacogenetic and pharmacogenomic research investigate the genetic contribution to response variability at individual loci and genome-wise level, respectively. The main aims of pharmacogenetic and pharmacogenomic investigations are the unravelling of the mechanism of action of antipsychotics and the identification of response predictors that could help in the improvement and personalisation of treatment. Decades of pharmacogenetic research have revealed individual genetic biomarkers of clinical response to antipsychotics [3]. However, prediction of antipsychotic response based on genetic biomarkers is far from accurate. Despite the clinical validity of several pharmacogenetic findings, the clinical utility remains unclear. Nevertheless, genetic information on metabolic enzymes involved in the biotransformation of most commercially available antipsychotics has proven to be useful for the reduction of treatment induced side effects [4,5]. At present, the implementation of pharmacogenetic interventions in psychiatric settings is very limited, and further proof of their clinical utility is required. Pharmacogenomic research conducted in the last decade has advanced our knowledge on the mechanism of action of antipsychotic drugs by confirming the therapeutic value of known targets [2,6]. Further research into genomic and epigenomic factors contributing to response may help to improve the accuracy and clinical applicability of genetic information in psychiatry.
The aim of this narrative review is to summarise the knowledge on the pharmacogenetics and pharmacogenomics of antipsychotic drugs and to give an overview of their current clinical applications.
2. Methods
Literature Review
The recommendations of the Preferred Reporting Items for Reviews and Meta-Analyses statement (PRISMA, 2021) were followed for the literature search. We searched the ISI Web of Science using the Boolean method with the following terms: (1) “antipsychotic” OR “chlorpromazine” OR “droperidol” OR “fluphenazine” OR “haloperidol” OR “loxapine” OR “perphenazine” OR “pimozide” OR “prochlorperazine” OR “thioridazine” OR “thiothixene” OR “trifluoperazine” OR “aripiprazole” OR “asenapine” OR “brespiprazole” OR “cariprazine” OR “clozapine” OR “iloperidone” OR “lurasidone” OR “olanzapine” OR “paliperidone” OR “quetiapine” OR “risperidone” OR “ziprasidone”; (2) “pharmacogenetic” OR “pharmacogenetics OR “pharmacogenomic OR pharmacogenomics”. We restricted the search from January 2010 to November 2023 (see Figure 1 for description of process). The references and discussions of all pooled articles were carefully scanned for additional publications. Before screening, we excluded studies (1) not performed in humans; (2) not published in English; and (3) not published in scientific journals. A total of 1406 studies were identified. Articles were excluded from this revision for the following reasons: (1) not drug of interest (not antipsychotics); (2) other areas of research (Alzheimer disease, depression, implementation of informatic tools, etc.); (3) not original new data (reviews of publications); (4) case-report studies; (5) studies with exclusive pharmacokinetic design and outcomes; (6) studies with not reported response phenotype. A total of 269 relevant papers were identified that reported original pharmacogenetic or pharmacogenomic results on a variety of antipsychotic response phenotypes (influence of genetic variants on treatment response, adverse reactions and/or drug clearance). Several limitations to this review need to be considered. Firstly, it is likely that the most recent publications that are not yet listed in search engines, as well as other relevant papers that did not fit our search terms, have been missed. Secondly, many important findings regarding the pharmacogenetics of antipsychotics were published before the 2010 cut-off, although most clinically relevant findings have been replicated in more recent years. Furthermore, there is publication bias in favour of studies with positive findings, and negative or contradictory findings may be underrepresented. Despite these limitations, the next sections will summarise the most significant findings in recent years and will discuss the current applicability of those findings for the personalisation of antipsychotic treatment.
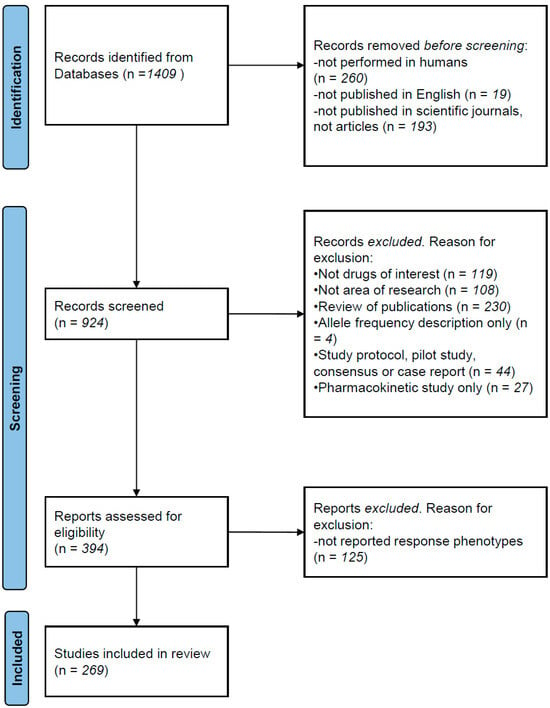
Figure 1.
PRISMA description of literature search.
3. Pharmacogenetic Studies
Pharmacogenetic or candidate-gene studies are mainly focussed on a limited number of genes selected from the kinetic or dynamic profile of antipsychotic drugs, although genetic variants determining response may also be related to disease pathology. Findings of candidate gene studies are rarely universally replicated. Limited samples sizes, differences in the duration and type of treatment, patient ethnicity, etc., may hinder the replication of results [2]. Furthermore, treatment response and clinical outcome are difficult to measure and may complicate the interpretation of results and explain discrepancies between studies. Replication of candidate-gene findings in independent studies and meta-analyses of published results are necessary to confirm and clarify the strength of the associations. A summary of candidate-gene studies reporting significant findings published in the last decade can be found in Table 1. Older papers with significant results reported or included in later publications have been excluded. While not fully comprehensive, this section will discuss the most relevant and clinically valuable findings to date.
3.1. Pharmacogenetic Associations with Treatment Response
Given the multitarget profile of most antipsychotics, it is not surprising that many genes related to neurotransmitter receptors and transporters have been associated with variability in treatment response. Dopamine and serotonin neurotransmitter pathways have been thoroughly investigated for possible pharmacogenetic markers [2]. Although the mechanism of action of antipsychotics is not fully understood, it is known that dopamine blockade is directly associated with antipsychotic efficacy [2]. Numerous studies have described associations between genetic variants in dopamine receptors and treatment response [7,8,9,10], with the DRD2 rs1799732 (−141C Ins/Del) deletion significantly associated with poorer antipsychotic drug response (OR = 0.65 for the Ins allele in meta-analyses) [2]. Although initially a non-significant trend of association of the DRD3 Ser allele with poor clozapine response was observed, a later meta-analysis [11] confirmed the association of the 9Gly allele with good response in Caucasians, but not in other ethnic groups (OR = 0.72; p = 0.002). Genetic variants in serotonin receptors have also been associated with treatment response, particularly to second-generation antipsychotics. Recent studies confirm the association, with HTR2A rs6313 T allele carriers showing generally better improvement than non-carriers [2,12]. HTR3 variants have been associated with clozapine response in Indian patients [13], although the strength of the association varied according to the response phenotype investigated, illustrating the difficulties in assessing clinical outcome. The associations of HTR2A and HTR3 variants with clozapine response have also been confirmed via meta-analyses (OR = 0.68 and 0.45, respectively) [14]. Histaminic receptors, a target of several second-generation antipsychotics (SGA) of unclear therapeutic validity, may contain genetic variants influencing response [15,16,17]. Other genes not directly targeted by antipsychotics but involved in monoamine pathways and/or mental disorders have been associated with response. The COMT rs4680 variant (Val158Met), associated with reduced cortical dopamine [18], has been associated with response to second-generation antipsychotics [19,20]. A recent meta-analysis confirmed the strength of this association (Z = 6.709, p = 9.8 × 10−12) [21]. Variants of the BDNF gene were associated with risperidone and clozapine response [22] and with treatment resistance [23].
3.2. Pharmacogenetic Associations with Antipsychotic-Induced Adverse Reactions
Antipsychotic treatment induces severe side effects such as extra-pyramidal side effects (EPS) and weight gain (WG). There is strong evidence on the contribution of genetic factors to the development of adverse reactions during antipsychotic treatment. The influence of functional polymorphisms in metabolic enzymes on the clearance of antipsychotic drugs has been proven in numerous studies [2,4,24]. Cytochrome P450 (CYP) functional variants associated with slower (poor metabolisers) or faster (rapid or ultrarapid metabolisers) metabolic rates are known to significantly contribute to antipsychotic efficacy and toxicity [2,25,26]. More robust data have been obtained for functional variants in CYP2D6 and CYP2C19 enzymes. CYP1A2 and CYP2D6 functional variants have been associated with tardive dyskinesia (TD) [27,28]. Patients with poor or intermediate CYP2D6 metaboliser phenotypes are at greater risk for risperidone and haloperidol adverse reactions [29,30]. Currently, genetic variants of CYP enzymes are the most used biomarkers to personalise psychiatric treatment, as will be discussed later. Several studies have found a relationship between oxidative stress and antipsychotic-induced TD based on genes involved in the antioxidant defence mechanism, dopamine turnover and metabolism [31]. DRD2 polymorphisms have been related to akathisia and other movement disorders [32]. Expression of the HSPG2 gene is altered by antipsychotic treatment and has been associated with movement disorders in animal studies. Numerous studies have investigated its association with EPS, and a meta-analysis found a significant association between the HSPG2 rs2445142 variant and TD [33]. Regarding weight gain, variants in HTR2C, LEP, and MC4R genes have been confirmed as genetic markers with moderate genetic effects (ORs = 1.47–1.96) [34,35]. HTR2C is involved in the regulation of appetite, and its contribution to weight gain and body mass index during antipsychotic treatment has been thoroughly investigated.
The HTR2C rs3813929 (-759-C/T) variant is the most frequently studied single-nucleotide polymorphism and one of the most strongly associated with antipsychotic-induced WG, with the rs3813929-T allele predicting less WG and body mass index (BMI) [36,37,38,39]. Gene variants in LEP and LEPR, two genes involved in the regulation of lipid metabolism, have been related to antipsychotic-induced WG and short-term dyslipidaemia [40,41]. A recent meta-analysis including 12 studies found no association between the LEP rs7799039 polymorphism and WG, although a significant association was found within a subgroup of first-episode schizophrenia patients (OR = 2.32, p = 0.0009) [42]. One of the most robust findings is the association of genetic variants in the MC4R gene with antipsychotic-induced WG. The MC4R was previously associated with obesity in the general population, adding plausibility to the finding, which has been replicated in later studies [43,44]. Finally, an HLA-DQB1 variant (6672G/C, rs113332494) was robustly associated with clozapine-induced agranulocytosis [45]. The association of HLA variants with antipsychotic-induced agranulocytosis was later corroborated in genome-wide association studies [46,47]. The strength of the association increases when patients’ ancestry is considered (OR = 16.67–53.70) [45].
Other findings reported in single studies (see Table 1) require confirmation in independent samples to determine the validity and strength of the associations. In general, the strength of the reported genetic associations is moderate (odds ratios rarely >3). This is not surprising given the multitarget profile of most currently used antipsychotics and the polygenic characteristics of treatment response. However, this suggests that the clinical utility of these findings is limited, as discussed later.
4. Pharmacogenomic Studies
As we have seen in the previous section, candidate-gene studies have succeeded in the identification of treatment response markers, some of which are currently in use for the personalisation of psychiatric medication. However, pharmacogenetic studies are based on existing knowledge on the selection of candidate genes, and their contribution to the understanding of the mechanism of action of antipsychotics is limited. Advances in technology have allowed thorough genomic investigations of disorders and treatment outcomes. No previous knowledge is required as the whole genome is investigated. However, genomic studies on antipsychotics have been marred by limited sample sizes that increase the probability of false-negative and -positive findings. Most pharmacogenomic studies on antipsychotics have been conducted with moderate sample sizes (n < 1000). Several studies included replication in independent samples to confirm their findings. Table 2 includes a summary of genome-wide and whole exome sequencing studies on antipsychotic response and their side effects published during the last decade.
A BeadChip study of 6789 SNPs conducted by Drago et al. [48] found association between response to SGA and first-generation antipsychotics (FGA) and two ARID5B SNPs, a gene involved in autoimmune disorders that are often found in schizophrenia patients. A study investigating variants in 1996 genes on patients treated with olanzapine and risperidone also found associations of SNPs in chromosome 6, a region where the HLA gene is located, with treatment response, reinforcing the evidence on the influence of genetic variant in immunologic pathways in clinical outcomes [49]. A genome-wide association study (GWAS) on Indian patients also found a variant (rs4795893) in a gene involved in pro-inflammatory response (CCL2) as well as a variant in a gene involved in mental disorders (NRG1 rs13250975), amongst others, associated with response to SGA and FGA [50]. Two genes not previously associated with treatment response or mental disorders, MYOTB and MTRR, were found to contain variants associated with antipsychotic response using whole exome sequencing (WES) [51]. This strategy also identified polymorphisms in the genes NMDA and AMPA, involved in glutamatergic pathways, associated with response [52]. Alterations in glutamatergic pathways have long been associated with the aetiology of schizophrenia, although no clear association with treatment response has been described to date. Interestingly, a recent GWAS also found association between genetic variants in the glutamatergic GRM7 gene and treatment response [53]. The same study identified novel associations of GPR12 and MAP2K3, two genes involved in glutamatergic pathways, with response using WES techniques, although no finding was replicated by both strategies. Finally, a sequencing study of 143 genes revealed statistically significant association between polymorphisms in DRD3 (rs342026) and in a neighbouring gene (PLK5 rs1261027) with olanzapine response, confirming the findings of candidate-gene studies [54].
Regarding adverse reactions, Tanaka et al. described an association between a variant (rs6977820) in the gene DPP6 and treatment-induced TD, although the biological relation of this gene with the adverse reaction is not clear [55]. Alkelai et al. [56] performed WES in patients treated chronically with antipsychotics and found a significant enrichment of RIMS2 variants associated with TD. The authors report that the expression of this gene was observed to be increased in the brains of schizophrenic patients in a previous study. One of the most robust pharmacogenetic findings to date is the association of genetic variants in the melanocortin 4 receptor (MC4R) with antipsychotic-induced WG, an association that has been confirmed in GWAS and candidate-gene studies (see Table 1). Several novel associations with antipsychotic-induced WG have been reported in subsequent genomic studies but have not yet been replicated and require further study [57,58,59]. Two GWAS studies have confirmed the previously reported association between genetic variants in the HLA complex and clozapine-induced agranulocytosis [46,47]. Furthermore, a GWAS study described a mutation in the AKCR1 gene, rs2814778, previously associated with lower neutrophil counts, associated with clozapine-induced neutropenia [60]. Finally, a relatively large GWAS study identified variants in two genes, ATAD3B and SKIL, related to antipsychotic-induced QTc interval change. The ATAD3B gene plays a role in inflammatory pathology, whereas alterations in the expression of the SKIL gene may be related to the aetiology of schizophrenia [61]. Despite their moderate power, these genomic studies have confirmed the importance of genes involved in inflammatory response and in glutamatergic pathways on treatment variability in the development of antipsychotic-induced side effects. However, some of the findings are difficult to interpret given the modest sample sizes and the difficulty in determining the interaction with other environmental and clinical factors involved in response. Independent candidate-gene studies may be required to confirm the validity of findings of underpowered GWAS and WES studies.
Aside from alterations in the DNA sequence, other genomic factors such as transcription regulation and epigenomic events may play a role in antipsychotic response variability. The study of gene expression and epigenetic changes may provide information on gene × environment interactions and yield more accurate predictions of clinical outcomes. Animal studies have shown that risperidone treatment induces gene expression changes associated with susceptibility to EPS, with the involvement of the mTOR pathway [62]. A study suggested that the basal expression of four genes can predict antipsychotic response. However, the estimation was based on a limited number of patients (n = 30) and needs replication in independent studies [63]. Several studies have provided evidence suggesting that antipsychotic medications modulate DNA methylation and that these alterations may influence treatment response [64,65]. Animal studies have shown that methylation levels of dopaminergic genes are altered by olanzapine treatment [64]. DNA methylation changes have been correlated with clinical improvement in MDD patients treated with antidepressants, and similar effects may occur in antipsychotic-treated patients [66]. Human studies have shown that global methylation levels are decreased in schizophrenia patients in comparison with controls, and antipsychotic treatment may partially explain the decrease [65]. Methylation within a recognition sequence for HES transcriptional repressors was found to correlate with clinical improvement after antipsychotic treatment [67]. Research on the interaction of genomic events and environmental factors on antipsychotic response is ongoing despite the difficulty in obtaining large, detailed cohorts for study.
5. Clinical Utility of Findings
The two previous sections have reviewed numerous studies that have identified genetic markers of variability in antipsychotic treatment. Pharmacogenetic and pharmacogenomic studies have succeeded in validating antipsychotic targets of therapeutic value as well as provided novel information highlighting new areas of putative therapeutic value. However, despite many of these findings having been confirmed and classified as true pharmacogenetic markers, their clinical utility for the personalisation of antipsychotic treatment is not clear. Even with the strong evidence confirming the association of dopaminergic and serotonergic variants and treatment response, their moderate to low genetic effects indicate that their contribution is not determinant on antipsychotic treatment outcome, and that taken individually, they could not predict response accurately. Similarly, HT2RC and MC4R associations with antipsychotic-induced WG have been confirmed in independent studies; however, they have no clear clinical utility to discriminate between different antipsychotics despite their varied pharmacological profiles. The strong association of genetic variants in the HLA complex with clozapine-induced agranulocytosis may help to predict if the patient has a high or low risk of developing severe neutropenia [68], but the relatively modest sensitivity of these markers (31–41%) [46,47] does not reach the 50% threshold for clinical applicability [69], although the addition of patient’s ancestry in the prediction algorithm may increase the clinical value [45]. Algorithm combinations of information in several genes and polygenic risk scores have been proposed to improve prediction of antipsychotic response and induced side effects. An algorithm combination of four SNPs in genes involved in mTOR regulation had a 66% accuracy to predict EPS in a sample of n = 356 antipsychotic-treated patients [70]. A panel of genetic variants in 15 genes predicted efficacy in 60% of cases and medication tolerability in 71% of the n = 352 patients included in the study [71]. Patients with higher polygenic risk scores for schizophrenia tended to have less improvement with antipsychotic drug treatment [72]. Interestingly, polygenic risk scores for schizophrenia, diabetes and BMI were also associated with AIGW [73]. Although these strategies may be useful as prognostic tools, their clinical utility for the selection of treatment remains unclear. Furthermore, most of the pharmacogenetic markers in drug targets may not help to discriminate between antipsychotic treatments given their multitarget profile and the low-to-moderate genetic effects observed in the reported associations.
While pharmacogenetic and pharmacogenomic research is still ongoing to improve response prediction and clinical utility, it is likely that the best genetic biomarkers with the greatest effect and clinical utility have already been discovered. Certainly, functional CYP polymorphisms may have both clinical utility and applicability. Functional polymorphisms in CYP enzymes have been clearly associated with the clinical outcome of antipsychotic treatments. The presence of CYP poor-metaboliser variants is strongly associated with increased adverse reactions, whereas ultrarapid-metaboliser variants may contribute to treatment failure and toxicity [2]. Guiding treatment according to CYP phenotypes has been proven useful in children [74]. Furthermore, a recent study showed that long-acting antipsychotics may still be affected by CYP genetic variants [75]. These associations are of a higher magnitude than the ones observed with genetic variants in antipsychotic targets and may offer useful information on appropriate dosing according to the functional variants carried by the patient. In the case of antidepressants, clinical dosing recommendations based on the patient’s genetic profile have already been issued by the Clinical Pharmacogenetics Implementation Consortium (CPIC) [76,77]. Several studies have proven the clinical benefits of using CYP information for the adjustment of clinical doses or selection of alternative treatments when genetic contraindications are observed. Growing evidence suggests that information on CYP1A2, CYP2C19 and CYP2D6 may be useful for the adjustment of clinical doses of antipsychotics [78,79]. In a previous study, we demonstrated that the dose adjustment of the commonly used antipsychotics haloperidol, risperidone, aripiprazole, clozapine and olanzapine according to the genetically determined phenotype of CYP2D6, CYP1A2 and CYP2C19 resulted in a decrease of side-effects [4]. A similar pharmacogenetic intervention for children and adolescents with autism spectrum disorders resistant to treatment resulted in clear improvement in 80% of cases as well as a reduction in health-associated costs [80]. CYP2D6 and CYP2C19 genotyping improved response in addition to clinician’s and patient’s satisfaction in patients given antipsychotics or antidepressants [81]. As further evidence of the clinical and economic benefits emerges, the implementation of CYP information for the improvement of antipsychotic response will surely increase in clinical settings.
Further improvement of pharmacogenetic predictions may be achieved by investigating the interaction between genes, environment, clinical and demographic factors. A recent study suggests that the consideration of pharmacogenetic and phenoconversion data that considers concomitant treatment and other dose-altering environmental factors may increase the accuracy of antipsychotic dose selection [82]. An additional area that merits further research is the possible interaction of pharmacogenetics x age. Older children are more likely to experience EPS than younger children, whereas antipsychotic adverse reactions such as sedation, weight increase and fatigue in particular are more prevalent in children than in adults [83,84]. Despite this evidence, few pharmacogenetic studies have been conducted in children. In summary, antipsychotic selection based on pharmacogenetic findings may be difficult to achieve, although information on pharmacokinetic genes has a real value for the selection of appropriate clinical doses resulting in improved efficacy and less toxicity.
6. Implementation of Pharmacogenetics for Antipsychotics
Despite the growing evidence of the clinical benefits, pharmacogenetic information is rarely used in psychiatric settings as a prescription aid. There are several reasons for this: limited evidence on the clinical benefits and cost-effectiveness of pharmacogenetic interventions, few clinical guidelines based on pharmacogenetic biomarkers, and delay in delivery of results, amongst others [2,85].
The clinical utility of pharmacogenetic-guided antidepressant treatment has been proved in several studies Genotyping for key CYP functional polymorphisms and variants in the serotonin transporter gene (SLC6A4), the main target of SSRIs, can be used to select antidepressant type and dose with significant improvement in efficacy [79]. A recent study showed that children presenting pharmacogenetic contraindications for the antidepressant treatments they were receiving showed poorer response than those without contraindications [86]. However, evidence on the clinical benefits of pharmacogenetic interventions for antipsychotics is sparse. A large clinical trial on the impact of pharmacogenetic interventions for 26 drugs of different medical areas did not find clear benefits, although a reduction in side effects on the subgroup of patients treated with antipsychotic and antidepressant medications was observed [87]. A strategy including gathering of clinical and environmental information, study of pharmacological interactions, pharmacogenetic characterisation of the patients and adjusting the pharmacological treatment according to the information obtained has given good results in patients resistant to treatment with neuroleptics [88]. Pre-emptive strategies can also be clinically beneficial. It has been estimated that pre-emptive pharmacogenetic testing could be useful to predict 95% of vulnerable patients’ exposure to inadequate drugs [89]. Routinary pharmacogenetic testing of patients that require treatment with antipsychotics may significantly decrease the prevalence of side effects, as shown in our previous study [4]. Pharmacogenetic interventions for the selection of antipsychotic treatment results in higher improvement in comparison with treatment as usual [90]. Pharmacogenetic testing increases treatment adherence, leading to greater efficacy [91]. However, further evidence in different clinical settings is required to confirm the clinical utility of genetic testing for antipsychotics.
Emerging evidence illustrates the cost-effectiveness of the implementation of pharmacogenetics in psychiatry. Pharmacogenetic-guided antidepressant treatment resulted in significant savings in medication and clinical care [92,93]. CYP2D6 genotyping is cost-effective in patients treated with neuroleptics and antidepressants [91,94,95,96]. The combination of clinical, environmental and pharmacogenetic information for the personalisation of treatment in resistant patients achieved a cost–benefit ratio of 3.31–3.59 with a reduction of direct and total costs in most patients [95]. The cost-effectiveness of pharmacogenetic testing to prevent clozapine-induced agranulocytosis has been proven but only in patients with HLA susceptibility alleles [97]. Rapid and low-cost pharmacogenetic interventions in treatment-resistant autistic patients resulted in a reduction in hospital stays and clinical visits [80]. Further evidence of the cost-effectiveness of pharmacogenetic interventions will facilitate their implementation in national health services.
A significant barrier is the existence of pharmacogenetic guidelines for the adjustment of only a limited number of antipsychotics. The Dutch Pharmacogenetics Working Group (DPWG) has recently issued guidelines for the adjustment of aripiprazole, brexpiprazole, haloperidol, pirmozide, risperidone, zuclopenthixol and quetiapine according to CYP2D6 and CYP3A4 polymorphisms [5]. However, they conclude that no useful pharmacogenetic information is available for the commonly used antipsychotics clozapine and olanzapine. In a previous study, we used local clinical guidelines for the adjustment of doses of several FGA and SGA antipsychotics [4]. Dose adjustment according to CYP2D6- predicted metabolising rates resulted in a significant reduction of the toxicity induced by the antipsychotics aripiprazole, haloperidol and risperidone, confirming the utility of the existing guidelines. Furthermore, the adjustment of clozapine and olanzapine according to CYP1A2 and CYP2C19 polymorphisms also resulted in a reduction of side effects and improved efficacy in treatment-resistant patients [80]. Further research to provide evidence and specific guidelines for clozapine and risperidone is required.
Long waits for test results can diminish the clinical utility of pharmacogenetics. Commercial pharmacogenetic tests may take a minimum of 2–3 weeks to provide results. Polygenic risk scores may predict treatment response with a higher level of accuracy [72]. However, the increase in genotyping expense and in the time until results delivery significantly diminishes clinical utility. Biochemical measures (determination of metabolite plasma levels) obtained after a few weeks from the start of the treatment can provide more accurate information on drug clearance as the effect of concomitant treatments, diet and other environmental factors are reflected. Nevertheless, although genetic prediction of antipsychotic response may not achieve 100% accuracy, pharmacogenetic interventions for dose adjustment can be highly beneficial when applied at the start of the treatment, thus allowing for the selection of the right dose or alternative treatments. Rapid pharmacogenetic interventions that provide clinical recommendations within 24–48 h of request are of significant clinical utility [2,80]. The genotyping of key polymorphisms in CYP enzymes can be easily performed in most clinical laboratories and produce information at the start of the treatment, facilitating personalisation and increasing clinical value.
7. Discussion
As described in previous sections, the information on functional variants in CYP enzymes for the adjustment of antipsychotic clinical doses has proven to be of significant clinical utility and should be incorporated as a prescription tool. Furthermore, the genotyping of genetic variants in CYP1A2, CYP2C19, CYP2D6 and CYP3A4 for the adjustment of the dose of commonly used antipsychotics can be cost-effective. However, given the variability of genotype and clinical recommendations provided by pharmacogenetic tests [98], a standardisation of the CYP markers investigated and the pharmacogenetic-guided recommendations should be performed. Currently available genetic tests interrogate a variety of CYP genes and variants, providing different information and clinical recommendations that should be standardised. In cases where sequencing technologies to detect novel or known functional variants are not available, a list of CYP pharmacogenetic markers useful to determine the metabolic status of patients of different ethnicities should be compiled. Additionally, the clinical recommendations based on pharmacogenetic information should also be standardised. Detailed information on both key CYP genetic markers and relevant dose changes for antidepressants and antipsychotics have been issued by the CPIC and DWPG consortiums [5,76,77]. Both organisations compile clinical, pharmacokinetic and pharmacogenetic information to provide precise information on required dose changes, and their recommendations should be followed to elaborate reports on pharmacogenetic-guided clinical recommendations. Alternative recommendations with insufficient confirmation should be avoided. Standardised pharmacogenetic tests and recommendations will increase their reliability and facilitate their understanding and interpretation.
Finally, it is necessary to increase the education and training of clinical staff on the use and utilisation of pharmacogenetic information as a prescription tool for antipsychotic medications, either as a help in treatment-resistant patients or as a routinary pre-emptive prescription tool to increase the efficacy and safety of treatments. Although the teaching of pharmacogenetic strategies is being introduced in faculties in several countries, further education should be provided at different stages of training and in clinical settings to facilitate the use of pharmacogenetics for the personalisation of antipsychotic treatment.
8. Conclusions
Pharmacogenetic and pharmacogenomic research have helped to unravel the mechanism of action of antipsychotics and to identify biomarkers of response. However, most biomarkers have modest clinical utility, as their capacity to discriminate between different antipsychotics is reduced. Functional biomarkers in CYP metabolic enzymes are of greater utility as they can be used to adjust clinical doses of antipsychotics, resulting in greater efficacy and safety. Nevertheless, the implementation of pharmacogenetic interventions in psychiatry is minimal. Further evidence of the clinical benefits and cost-effectiveness, rapid and low-cost pharmacogenetic tests and specific pharmacogenetic guidelines for antipsychotics are required to increase their implementation. It is envisaged that artificial intelligence combining information from different areas (genetic, epigenetic, clinical, pharmacological and demographic) will produce a much more accurate prediction of response and will further the personalisation of antipsychotic treatment. However, given the complexity of such an approach combining information on multiple factors that need to be measured before the start of the treatment and the high cost that it will imply, it may be years until it is practicable in clinical settings.

Table 1.
Summary of candidate-gene studies reporting significant associations.
Table 1.
Summary of candidate-gene studies reporting significant associations.
| Gene | Variant | Drug | n | Association | Ref. |
|---|---|---|---|---|---|
| 31 genes | 202 SNPs | SGA | 113 Caucasians | Four SNPs in DRD2, SLC18A2, HTR2A and GRIK3 contributed significantly to the risk of side effects (p = 1 × 10−4). | [99] |
| 38 genes | several | SGA | 300 Caucasian | Nominally significant association between antipsychotic dosage and GFRA1 variants. | [100] |
| 380 genes | several | SGA and FGA | 240 several ethnicities | NALCN rs2152324 had most significant association with response (p = 0.004). Not significant after FDR correction. | [101] |
| 74 genes | several | several | 279 Caucasians | BDNF significantly associated with treatment resistance: rs11030104 (OR = 2.57), rs10501087 (OR = 2.19) and rs6265 (OR = 2.08) | [23] |
| ADRB2, DRD3 and SLC6A4 | several | Risperidone | 111 Caucasians | Allele 16Gly of ADRB2 significantly associated with higher risk of sexual adverse events (p = 0.002) | [102] |
| BDNF | 4 SNPs | Clozapine | 257 Caucasians | rs11030104 and Val66Met associated with response (p = 0.04; 0.007, respectively). rs1519480 associated with WG (p = 0.04). | [22] |
| C4A and C4B | several | FGA | 87 Caucasians | Number of copies of C4BL nominally associated with TD severity (p = 0.020) | [103] |
| CNR1, FTO, MC4R, LEP and FAAH | several | Risperidone | 225 Caucasians | Variants in CNR1 (p = 1 × 10−5) and LEP (p = 1.4 × 10−4) associated with AIWG | [41] |
| COMT and DRD2 | several | Risperidone | 690 Chinese | COMT rs4680, DRD2 rs6275, rs1801028 and rs6277 associated with PANSS improvement (p = 0.05) | [19] |
| COMT | rs4680 and rs4818 | SGA | 521 Caucasians | rs4680 A allele and rs4680–rs4818 C-A haplotype associated with olanzapine response, but not with response to other antipsychotics | [20] |
| CYP1A2 and CYP2D6 | several | FGA or Risperidone | 475 Caucasians | CYP1A2*1F & CY2D6*4 associated with TD in patients on antipsychotics for a long time (p = 0.03) | [27] |
| CYP1A2 and CYP2B6 | several | Aripiprazol | 19 Caucasians | CYP1A2 UM & CYP2B6*1/*1 associated with aripiprazol- induced side effects | [104] |
| CYP2C19, LEPR, CYP1A2, HTR2C and ABCB1 | several | Clozapine | 60 Caucasians | Clozapine levels in patients with metabolic syndrome were significantly higher compared to those without (p < 0.01) and were associated with CYP2C19*2 (p = 0.04) | [25] |
| CYP2D6 | several | FGA and SGA | 198 Caucasians | Individuals with either increased or no CYP2D6 activity were at higher risk of having TD | [28] |
| CYP2D6 | several | Risperidone | 257 several ethnicities | Children and adolescents with PM variants showed poorer response to risperidone treatment | [29] |
| CYP2D6 | *4 | Haloperidol | 150 Caucasians | Carriers of *4 variant presented worse safety profile (p < 0.001) | [30] |
| CYP3A4 | several | Olanzapine | CATIE sample | rs472660 significantly predicted olanzapine clearance (p = 5.9 × 10−7). | [105] |
| DISC1 | several | FGA or SGA | 193 Caucasians | Two SNPs nominally associated with TD severity (p < 0.05). | [106] |
| DRD1 | rs4532 | several | 124 Brazilians | G-allele associated with treatment resistance (p = 0.001; adjusted OR = 2.71). GG had five-fold risk compared to A (p = 0.010; OR = 5.56). | [107] |
| DRD2, DRD3, HTR2A, HTR2C, COMT, NQO1, RGS2 and MnSOD | 13 SNPs | not specified | 402 Dutch | DRD2 TaqI associated with akathisia (OR = 2.3, p = 0.001), DRD2 −141C associated with TD (OR = 0.20, p = 0.001) | [32] |
| DRD2 | rs2514218 | Haloperidol and Risperidone | 100 Americans | In the aripiprazole group, C/C homozygotes had more akathisia; in the risperidone group, male T allele carriers had greater prolactin elevations | [37] |
| DRD2 and DRD3 | rs1800497, rs6277 and rs6280 | Cariprazine | 20 Caucasians | DRD2 rs1800497 and rs6277 associated with cariprazine response | [9] |
| DRD2 and DRD3 | several | SGA | 129 Caucasians | DRD2 rs1799732, DRD3 rs6280, and HTR2A rs7997012 associated with treatment resistance. | [10] |
| DRD3, DRD2, HTR2A, HTR2C, COMT and MTHFR | several | several | 329 Caucasians | DRD3 9Gly and MTHFR 677-T had better response (p = 0.034 and p = 0.019, respectively). | [7] |
| DRD4, HTR2A, TPH1, SLC18A1 and COMT | several | Haloperidol | 198 Tartars | Several associations of DRD4, HTR2A, TPH1 and SLC18A1 polymorphisms with antipsychotic response | [8] |
| EP300 | expression levels | several | 226 Caucasians | EP300 expression levels significantly associated with increases in BMIR, cholesterol levels and triglyceride concentrations | [108] |
| FKBP5, NR3C1, BDNF and NTRK2 | several | Clozapine | 591 Caucasians | Several associations between FKBP5 rs1360780, NTRK2 rs1778929 and rs10465180 with response | [109] |
| FTO | several | SGA | 259 + 91 Caucasians | In a subpopulation without additional weight-inducing comedication (n = 178), rs7185735-G carriers gained 3.4 times more weight (1.69 ± 3.1 kg, p = 0.019) | [110] |
| GLP1R | several | SGA | 464 Caucasians | Haplotypes associated with response to olanzapine (p = 0.002), perphenazine (p = 0.01), quetiapine (p = 0.008), risperidone (p = 0.02) and ziprasidone (p = 0.007) | [111] |
| GRIN2A, DRD3, HTR2C, DRD4 and GRIN2B | 42 SNPs | FGA or SGA | 431 + 168 Caucasians | Several significant associations with TD were identified, but only GRIN2A (rs1345423) was found in both patient populations | [112] |
| GRM3 | rs1468412 | Risperidone | 61 Caucasians | GRM3 rs1468412 associated with worsening spatial working | [113] |
| HLA | several | Clozapine | 180 neutropenia/ 1396 controls | HLA-DQB1 rs113332994 associated with clozapine-induced agranulocytosis (OR = 16.31) | [45] |
| HRH1 and CHRM3 | several | Several | 430 Caucasians | HRH1 haplotype rs346074–rs346070 associated with BMI (p = 0.025) and obesity (p = 0.005) in patients using high-H1 affinity antipsychotics | [15] |
| HRH3 | several | Risperidone | 129 Han Chinese | rs3787429 (p = 0.013–0.087) and rs3787430 (p = 0.024–0.010) associated with efficacy after 4–8 weeks, respectively | [16] |
| HRH4 | 5 SNPs | Risperidone | 113 Han Chinese | rs4483927 TT genotype predicts poor therapeutic response on the positive, negative, general and total scales of PANSS scores (p = 0.017, 0.019, 0.021 and 0.002, respectively) | [17] |
| HSD11B1 | several | SGA | 478 Caucasians | HSD11B1 rs846910-A, rs375319-A and rs4844488-G allele carriers associated with lower BMI in women | [114] |
| HTR2A | rs6313 | Olanzapine or Risperidone | 221 Caucasians | T allele carriers showed better response than non-carriers | [12] |
| HTR2C | −759T/C | SGA | 48 female Caucasians | T allele carriers gained less weight as compared to patients who did not have the allele | [36] |
| HTR2C | −759T/C | Risperidone | 108 Thai | 5-HT2C -759-T/C associated with hypertension but not with WG | [39] |
| HTR3A | rs1062613 and rs2276302 | several | 101 Indian patients | rs1062613-T and rs2276302-G alleles significantly associated with good clinical response to clozapine (p = 0.02) | [13] |
| HTR7 | several | Aripiprazol | 100 Japanese | rs12412496-rs7916403-rs1935349 A-T-A haplotype correlated with worse improvement in the cognition score (p = 0.046). | [115] |
| LEP and LEPR | several | FGA or SGA | 181 Caucasians | Significant association between a LEP haplotype (rs7799039G–rs10954173G–rs3828942G) and AIWG (p = 0.035) | [40] |
| MAOA, MAOB, DRD1, DRD2, DRD3, DRD4 and SLC6A3 | 41 SNPs | FGA or SGA | 446 Caucasians | Association between MAOB rs1799836 and HPRL in men. SLC6A3 rs40184 and rs3863145 associated with HPRL in risperidone/paliperidone subgroup | [116] |
| MC4R | rs489693 | SGA 4 weeks | 341 Caucasians | rs489693 A/A carriers showed 2.2 times higher weight increase than carriers of the C/C genotype (p = 0.039) | [43] |
| MC4R | rs17782313 | SGA 4 weeks | 51 Caucasians | rs17782313 C/C carriers higher risk of WG and BMI increase, with a dose effect of the C-allele (p = 0.002). | [117] |
| MC4R | rs489693 and rs17782313 | FGA or SGA | 1991 Chinese | Recessive effects of rs489693 on AIWG, WC and triglyceride change %, with A/A incurring more metabolic adverse effects | [44] |
| MC4R | rs17782313 | Amisulpride and Olanzapine | 212 Several | C carriers had higher WG than T homozygotes | [35] |
| NEUROD2 | several | SGA | 167 Caucasians | rs11078918 and rs12453682 associated with change in neuropsychological test results (p = 0.02–0.001). | [118] |
| NOS1AP | rs1214382 and rs10494366 | not specified | 347 Caucasian | rs12143842-CC and rs10494366-TT male carriers show positive correlation of QTc length with antipsychotic dosage | [119] |
| NPY5R | several | FGA and SGA | 99 Russians | rs11100494- C predisposes to AIWG (OR = 33.48, p< 0.001) | [120] |
| OXTR, CNR1, DDC and DRD2 | several | Clozapine or SGA | 196 Chileans | OXTR rs2228485, CNR1 rs806368 and rs1049353, and DDC rs10499696 associated with treatment resistance (p by genotype: 0.02, 0.001, 0.001 and 0.0003, respectively) | [121] |
| PLEKHA6 | rs7513240, rs4951353 | not specified | 263 Caucasians | rs7513240 and rs4951353 (A/G) associated with therapy response with different PANSS improvement after 4 weeks | [122] |
| PRKAR2B | 16 SNPs | Clozapine and Olanzapine | 99 Caucasians | rs9656135 minor allele carriers higher weight increase during treatment. | [123] |
| PTPRD | 4 SNPs | Clozapine or Olanzapine | 201 Caucasians and Africans | rs73398242 associated with AIWG in Europeans (p = 0.002) and with rs13294608 in African Americans (p = 0.003). | [124] |
| RELN | 15 SNPS | SGA | 260 Chinese | Two SNPs associated with antipsychotic treatment response (rs155333, p = 0.010 and rs6465938, p = 0.049) | [125] |
| RGS2 | several | Haloperidol | 258 Russians | RGS2*T/*T (rs2746073), *C/*C (rs4606) and *A/*A (rs2746071) associated with increased risk of antipsychotic-induced Parkinsonism | [126] |
| SLC18A2 | 9 SNPs | FGA long-term | 217 Caucasians | rs2015586 and rs363224 SNPs associated with TD and AIMS scores. | [127] |
| SLC6A5, GAD1, GRIA1, GRIA3, GRIA4, GRID2, GRIK1, GRIK2, GRIK3, GRIK4, GRIN2B, GRM1 and GRM4 | 62 SNPs | several | 101 + 71 + 118 Caucasian patients | SLC6A5 rs2298826 associated with a rapid rise of motor side effects at the beginning of the treatment (p = 0.0002) | [128] |
| SNAP25 | several | SGA and FGA | 3243 Chinese | rs6039769 significantly associated with AIWG (p < 0.001). | [129] |
| SULT4A1 | rs2285162 and rs2285167 | Olanzapine | 87 Caucasians | rs2285162 [A]-rs2285167 [G] haplotype superior olanzapine response (p = 0.004) and less AIWG per month (p = 0.04) | [130] |
| SV2C | 106 SNPs | SGA | 466 Caucasians | rs11960832-T/T significantly worse response to olanzapine treatment (p = 2.94 × 10−5; FDR = 2.18 × 10−2) | [131] |
| UGT1A4, UGT1A4 and ABCB1 | 7 SNPs | Olanzapine | 91 Japanese | Sympathetic nervous activity higher in individuals with the UGT1A4 rs2011425 G allele (p = 0.001). | [132] |
Abbreviations: AIWG: antipsychotic-induced weight -gain; BMI: body mass index; FGA: first-generation antipsychotics; SGA: second-generation antipsychotics; SNP: single nucleotide polymorphism; TD: tardive dyskinesia; WC: waist circumference; WG: weight gain.

Table 2.
Summary of genomic studies on antipsychotic medications.
Table 2.
Summary of genomic studies on antipsychotic medications.
| Strategy | n | Treatment | Association | Ref. |
|---|---|---|---|---|
| GWAS | 122 + 174 Japanese | several | Association DPP6 rs6977820 with antipsychotic-induced TD (p = 0.008) | [55] |
| GWAS | 96 + 169 Caucasians | FGA or SGA | Two SNPs (rs7912580 and rs2412459) associated with response in both samples, located between ARID5B and RTKN2 genes | [48] |
| GWAS and WES | 163 Caucasians | Clozapine | HLA-DQB1 (126Q) (p = 4.7 × 10−14, OR = 0.19) and HLA-B (158T) (p = 6.4 × 10−10, OR = 3.3) associated with clozapine-induced agranulocytosis | [46] |
| Array 1995 genes | 89 Caucasians | Olanzapine or Risperidone | Significant associations between treatment response and SNPs in the chromosome 6, where the human leukocyte antigen (HLA) is located | [49] |
| GWAS | 189 + 86 Caucasians | SGA | OGFRL1 rs9346455 significantly associated with AIWG (p = 0.005) | [57] |
| WES | 11 + 103 + 87 several ethnicities | FGA or SGA | rs13025959 in MYO7B (E1647D) and rs10380 in MTRR (H622Y) associated with antipsychotic response | [51] |
| GWAS | 742 Indians | FGA or SGA | CCL2 rs4795893 (p = 7.62 × 10−4) and rs4586 (p = 1.13 × 10−3), GRIA4 rs2513265 (p = 1.44 × 10−3), ADCY2 rs1544938 (p = 7.68 × 10−4), and NRG1 rs13250975 (p = 6.81 × 10−3) and rs17716295 (p = 8.71 × 10−3) associated with response | [50] |
| GWAS | 534 + 547 Chinese | SGA | PTPRD rs10977144 (p = 9.26 × 10−9) and rs10977154 (p = 4.53 × 10−8), and GFPT2 rs12386481 (p = 1.98 × 10−7) associated with AIWG | [58] |
| GWAS | 50 + 380 Japanese | Clozapine | Variants in the human leukocyte antigen (HLA) region (rs1800625, p = 3.46 × 10−9, OR = 3.8) associated with agranulocytosis | [47] |
| WES | 316 + 1920 Chinese | FGA or SGA | Rare genetic variants in NMDA and AMPA enriched in the non-responder group | [52] |
| WES | 82 Jewish | not specified | RIMS2 showed significant enrichment of qualifying variants in TD patients (n = 39) (p = 5.32 × 10−8) | [56] |
| GWAS | 552 African ancestry | Clozapine | ACKR1 rs2814778-C/C carriers more likely to develop neutropenia and have to stop clozapine treatment (OR = 20.4, p = 3.44 × 10−7) | [60] |
| Sequencing 143 genes | 79 + 159 Han Chinese | Olanzapine | rs324026 (p = 0.023) and rs12610827 (p = 0.043) associated with response | [54] |
| GWAS | 339 several ethnicities | Amisulpride | Significant association in a locus not previously associated with AIWG (rs78310016; p = 3.66 × 10−8). Minor allale carriers had an OR of 3.98 (p = 1 × 10−3) for AIWG | [59] |
| GWAS | 2040 Chinese | FGA or SGA | ATAD3B rs20005072 and SKIL rs186507741 associated with antipsychotic-induced QTc interval change. | [61] |
| GWAS and WES | 189 + 222 Chinese | Risperidone | GWAS revealed a significant association between GRM7 SNPs (rs141134664, rs57521140 and rs73809055) and treatment response | [53] |
Abbreviations: AIWG: antipsychotic-induced weight -gain; FGA: first-generation antipsychotics; GWAS: genome-wide association study; SGA: second-generation antipsychotics; SNP: single nucleotide polymorphism; TD: tardive dyskinesia; WES: whole exome sequencing; WG: weight gain.
Summative Paragraph
- Genetic variants in genes coding for drug targets -dopamine and serotonin receptors in particular- may influence the efficacy and safety of antipsychotic medications.
- Functional variants in CYPs are associated with antipsychotic availability.
- Dose adjustment according to CYP functional variants present may help to improve adherence, efficacy and safety of antipsychotics.
- Clinical implementation of pharmacogenetic interventions for personalisation of antipsychotic treatment is limited.
- Improved clinical guidelines based on pharmacogenetic data, education and training in pharmacogenetics, reduced costs and shorter delivery times may increase implementation.
- Further research on the combined effect of pharmacogenetics, phenoconversion, and clinical and environmental factors is required.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [CrossRef]
- Arranz, M.J.; Salazar, J.; Hernandez, M.H. Pharmacogenetics of antipsychotics: Clinical utility and implementation. Behav. Brain Res. 2021, 401, 113058. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Muller, D.J. Pharmacogenetics of Antipsychotic Drug Treatment: Update and Clinical Implications. Mol. Neuropsychiatry 2020, 5 (Suppl. S1), 1–26. [Google Scholar] [CrossRef] [PubMed]
- Arranz, M.J.; Gonzalez-Rodriguez, A.; Perez-Blanco, J.; Penades, R.; Gutierrez, B.; Ibanez, L.; Arias, B.; Brunet, M.; Cervilla, J.; Salazar, J.; et al. A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl. Psychiatry 2019, 9, 177. [Google Scholar] [CrossRef]
- Beunk, L.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.M.; Guchelaar, H.J.; Houwink, E.J.F.; Risselada, A.; Rongen, G.; van Schaik, R.H.N.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP3A4 and CYP1A2 and antipsychotics. Eur. J. Hum. Genet. 2023, 1–8. [Google Scholar] [CrossRef]
- Vasiliu, O. The pharmacogenetics of the new-generation antipsychotics—A scoping review focused on patients with severe psychiatric disorders. Front. Psychiatry 2023, 14, 1124796. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Burger, H.; Wilffert, B.; Al Hadithy, A.; Alizadeh, B.Z.; Snieder, H.; GROUP investigators. Clinical response to antipsychotic drug treatment: Association study of polymorphisms in six candidate genes. Eur. Neuropsychopharmacol. 2012, 22, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Gareeva, A.E.; Kinyasheva, K.O.; Galaktionova, D.Y.; Sabirov, E.T.; Valinourov, R.G.; Chudinov, A.V.; Zasedatelev, A.S.; Nasedkina, T.V.; Khusnutdinova, E.K. Brain neurotransmitter systems gene Polymorphism: The Search for pharmacogenetic markers of efficacy of haloperidol in Russians and Tatars. Mol. Biol. 2015, 49, 959–967. [Google Scholar] [CrossRef]
- De Pieri, M.; Ferrari, M.; Marino, F.; Traber, R.; Bolla, E.; Cosentino, M. Functional single nucleotide polymorphisms in dopaminergic receptors D2 predict clinical response to Cariprazine. Front. Pharmacol. 2023, 14, 1182393. [Google Scholar] [CrossRef]
- Del Casale, A.; Simmaco, M.; Modesti, M.N.; Zocchi, C.; Arena, J.F.; Bilotta, I.; Alcibiade, A.; Sarli, G.; Cutillo, L.; Antonelli, G.; et al. DRD2, DRD3, and HTR2A single-nucleotide polymorphisms involvement in high treatment resistance to atypical antipsychotic drugs. Biomedicines 2023, 11, 2088. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Liu, X.; Zhang, T.; Li, Y.; Yan, P. DRD3 Ser9Gly polymorphism and treatment response to antipsychotics in schizophrenia: A meta-analysis. Neurosci. Lett. 2022, 786, 136788. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Valsecchi, P.; Minelli, A.; Magri, C.; Bonvicini, C.; Barlati, S.; Sacchetti, E.; Vita, A.; Gennarelli, M. Association study between HTR2A rs6313 polymorphism and early response to risperidone and olanzapine in schizophrenia patients. Drug Dev. Res. 2020, 81, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, A.P.; Poonkuzhali, B.; Kuruvilla, A.; Jacob, M.; Jacob, K.S. Clinical predictors of serum clozapine levels in patients with treatment-resistant schizophrenia. Int. Clin. Psychopharmacol. 2013, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gressier, F.; Porcelli, S.; Calati, R.; Serretti, A. Pharmacogenetics of clozapine response and induced weight gain: A comprehensive review and meta-analysis. Eur. Neuropsychopharmacol. 2016, 26, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Risselada, A.J.; Al Hadithy, A.F.; Burger, H.; Snieder, H.; Wilffert, B.; Arends, J.; Wunderink, L.; Knegtering, H.; Wiersma, D.; et al. Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology 2011, 216, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, L.; Zhang, M.; Xuan, J.; Wang, Y.; Liu, B.; Shao, L.; Li, J.; Zeng, Z.; Li, T.; et al. A pharmacogenetic study of risperidone on histamine H3 receptor gene (HRH3) in Chinese Han schizophrenia patients. J. Psychopharmacol. 2012, 26, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, L.; Yu, T.; Wang, Y.; Sun, L.; Wang, T.; Huo, R.; Li, Y.; Wu, X.; Qin, S.; et al. Histamine H4 receptor polymorphism: A potential predictor of risperidone efficacy. J. Clin. Psychopharmacol. 2013, 33, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Schacht, J.P. COMT val158met moderation of dopaminergic drug effects on cognitive function: A critical review. Pharmacogenom. J. 2016, 16, 430–438. [Google Scholar] [CrossRef]
- Han, C.J.; Kohen, R.; Jun, S.; Jarrett, M.E.; Cain, K.C.; Burr, R.; Heitkemper, M.M. COMT Val158Met Polymorphism and Symptom Improvement Following a Cognitively Focused Intervention for Irritable Bowel Syndrome. Nurs. Res. 2017, 66, 75–84. [Google Scholar] [CrossRef][Green Version]
- Nikolac Perkovic, M.; Sagud, M.; Zivkovic, M.; Uzun, S.; Nedic Erjavec, G.; Kozumplik, O.; Svob Strac, D.; Mimica, N.; Mihaljevic Peles, A.; Pivac, N. Catechol-O-methyltransferase rs4680 and rs4818 haplotype association with treatment response to olanzapine in patients with schizophrenia. Sci. Rep. 2020, 10, 10049. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, M.; Zhou, W.; Li, M.; Huai, C.; Shen, L.; Wang, T.; Wu, H.; Zhang, N.; Zhang, Z.; et al. Association Between the COMT Val158Met Polymorphism and Antipsychotic Efficacy in Schizophrenia: An Updated Meta-Analysis. Curr. Neuropharmacol. 2021, 19, 1780–1790. [Google Scholar] [CrossRef]
- Zai, G.C.; Zai, C.C.; Chowdhury, N.I.; Tiwari, A.K.; Souza, R.P.; Lieberman, J.A.; Meltzer, H.Y.; Potkin, S.G.; Müller, D.J.; Kennedy, J.L. The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 96–101. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Geisler, S.; DeRosse, P.; Bromet, E.J.; Malhotra, A.K. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr. Res. 2013, 146, 285–288. [Google Scholar] [CrossRef]
- Kneller, L.A.; Zubiaur, P.; Koller, D.; Abad-Santos, F.; Hempel, G. Influence of CYP2D6 Phenotypes on the Pharmacokinetics of Aripiprazole and Dehydro-Aripiprazole Using a Physiologically Based Pharmacokinetic Approach. Clin. Pharmacokinet. 2021, 60, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, K.; Choi, Y.H.; Norman, R.; Kim, R.B.; Schwarz, U.I. Genetic Determinants of Clozapine-Induced Metabolic Side Effects. Can. J. Psychiatry 2017, 62, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Schneiderhan, M.E.; Butler, T.; Carpentier, R.M.; Heins, K.R.; Formea, C.M. Pharmacogenomics to support mental health medication therapy management: Clinical practice considerations and a conceptual framework to enhance patient care. J. Am. Coll. Clin. Pharm. 2023. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Toshchakova, V.A.; Filipenko, M.L.; Fedorenko, O.Y.; Boyarko, E.G.; Boiko, A.S.; Semke, A.V.; Bokhan, N.A.; Aftanas, L.I.; Loonen, A.J. Cytochrome P450 1A2 co-determines neuroleptic load and may diminish tardive dyskinesia by increased inducibility. World J. Biol. Psychiatry 2015, 16, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Tiwari, A.K.; Freeman, N.; Zai, G.C.; de Luca, V.; Muller, D.J.; Tampakeras, M.; Herbert, D.; Emmerson, H.; Cheema, S.Y.; et al. Liver enzyme CYP2D6 gene and tardive dyskinesia. Pharmacogenomics 2020, 21, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Oshikoya, K.A.; Neely, K.M.; Carroll, R.J.; Aka, I.T.; Maxwell-Horn, A.C.; Roden, D.M.; Van Driest, S.L. CYP2D6 genotype and adverse events to risperidone in children and adolescents. Pediatr. Res. 2019, 85, 602–606. [Google Scholar] [CrossRef]
- Skryabin, V.Y.; Zastrozhin, M.S.; Parkhomenko, A.A.; Pankratenko, E.P.; Pozdnyakov, S.A.; Denisenko, N.P.; Akmalova, K.A.; Bryun, E.A.; Suychev, D.A. Investigating the use of pharmacogenetic and pharmacometabolic markers to predict haloperidol efficacy and safety rates. Hosp. Pharm. 2023, 58, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Lee, H.J. Oxidative stress and tardive dyskinesia: Pharmacogenetic evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 207–213. [Google Scholar] [CrossRef]
- Koning, J.P.; Vehof, J.; Burger, H.; Wilffert, B.; Al Hadithy, A.; Alizadeh, B.; van Harten, P.N.; Snieder, H.; Genetic Risk and Outcome in Psychosis (GROUP) investigators. Association of two DRD2 gene polymorphisms with acute and tardive antipsychotic-induced movement disorders in young Caucasian patients. Psychopharmacology 2012, 219, 727–736. [Google Scholar] [CrossRef]
- Zai, C.C.; Lee, F.H.; Tiwari, A.K.; Lu, J.Y.; de Luca, V.; Maes, M.S.; Herbert, D.; Shahmirian, A.; Cheema, S.Y.; Zai, G.C.; et al. Investigation of the HSPG2 Gene in Tardive Dyskinesia—New Data and Meta-Analysis. Front. Pharmacol. 2018, 9, 974. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Zhang, R.X.; Nitta, M.; Maayan, L.; John, M.; Robinson, D.G.; Fleischhacker, W.W.; Kahn, R.S.; Ophoff, R.A.; et al. Pharmacogenetic Associations of Antipsychotic Drug-Related Weight Gain: A Systematic Review and Meta-analysis. Schizophr. Bull. 2016, 42, 1418–1437. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, K.F.; Leucht, S.; Heres, S.; Steimer, W. Genetic association of the rs17782313 polymorphism with antipsychotic-induced weight gain. Psychopharmacology 2023, 240, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Daray, F.M.; Rodante, D.; Carosella, L.G.; Silva, M.E.; Martinez, M.; Fernandez Busch, M.V.; Faccone, D.F.; Rothlin, R.P.; Maffia, P.C. -759C>T Polymorphism of the HTR2C Gene is Associated with Second Generation Antipsychotic-Induced Weight Gain in Female Patients with Schizophrenia. Pharmacopsychiatry 2017, 50, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Robinson, D.G.; Gallego, J.A.; John, M.; Yu, J.; Addington, J.; Tohen, M.; Kane, J.M.; Malhotra, A.K.; Lencz, T. Association of a Schizophrenia Risk Variant at the DRD2 Locus With Antipsychotic Treatment Response in First-Episode Psychosis. Schizophr. Bull. 2015, 41, 1248–1255. [Google Scholar] [CrossRef]
- Luo, C.; Liu, J.; Wang, X.; Mao, X.; Zhou, H.; Liu, Z. Pharmacogenetic Correlates of Antipsychotic-Induced Weight Gain in the Chinese Population. Neurosci. Bull. 2019, 35, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Vanwong, N.; Ngamsamut, N.; Nuntamool, N.; Hongkaew, Y.; Sukprasong, R.; Puangpetch, A.; Limsila, P.; Sukasem, C. Risperidone-Induced Obesity in Children and Adolescents With Autism Spectrum Disorder: Genetic and Clinical Risk Factors. Front. Pharmacol. 2020, 11, 565074. [Google Scholar] [CrossRef] [PubMed]
- Brandl, E.J.; Frydrychowicz, C.; Tiwari, A.K.; Lett, T.A.; Kitzrow, W.; Büttner, S.; Ehrlich, S.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L.; et al. Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 134–141. [Google Scholar] [CrossRef]
- Nurmi, E.L.; Spilman, S.L.; Whelan, F.; Scahill, L.L.; Aman, M.G.; McDougle, C.J.; Arnold, L.E.; Handen, B.; Johnson, C.; Sukhodolsky, D.G.; et al. Moderation of antipsychotic-induced weight gain by energy balance gene variants in the RUPP autism network risperidone studies. Transl. Psychiatry 2013, 3, e274. [Google Scholar] [CrossRef]
- Yoshida, K.; Maciukiewicz, M.; Zai, C.C.; Goncalves, V.F.; Brandl, E.J.; Lieberman, J.A.; Meltzer, H.Y.; Tiwari, A.K.; Kennedy, J.L.; Muller, D.J. Association between the -2548G/A polymorphism of the leptin gene and antipsychotic-induced weight gain: Analysis of the CATIE sample and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109952. [Google Scholar] [CrossRef]
- Czerwensky, F.; Leucht, S.; Steimer, W. Association of the common MC4R rs17782313 polymorphism with antipsychotic-related weight gain. J. Clin. Psychopharmacol. 2013, 33, 74–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Wang, Q.; Deng, W.; Yue, W.; Yan, H.; Tan, L.; Chen, Q.; Yang, G.; Lu, T.; et al. Testing the role of genetic variation of the MC4R gene in Chinese population in antipsychotic-induced metabolic disturbance. Sci. China Life Sci. 2019, 62, 535–543. [Google Scholar] [CrossRef]
- Konte, B.; Walters, J.T.R.; Rujescu, D.; Legge, S.E.; Pardinas, A.F.; Cohen, D.; Pirmohamed, M.; Tiihonen, J.; Hartmann, A.M.; Bogers, J.P.; et al. HLA-DQB1 6672G>C (rs113332494) is associated with clozapine-induced neutropenia and agranulocytosis in individuals of European ancestry. Transl. Psychiatry 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Jarskog, L.F.; Hilliard, C.; Alfirevic, A.; Duncan, L.; Fourches, D.; Huang, H.; Lek, M.; Neale, B.M.; Ripke, S.; et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat. Commun. 2014, 5, 4757. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ikeda, M.; Mushiroda, T.; Ozeki, T.; Kondo, K.; Shimasaki, A.; Kawase, K.; Hashimoto, S.; Yamamori, H.; Yasuda, Y.; et al. Pharmacogenomic Study of Clozapine-Induced Agranulocytosis/Granulocytopenia in a Japanese Population. Biol. Psychiatry 2016, 80, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Drago, A.; Giegling, I.; Schafer, M.; Hartmann, A.M.; Konte, B.; Friedl, M.; Serretti, A.; Rujescu, D. Genome-wide association study supports the role of the immunological system and of the neurodevelopmental processes in response to haloperidol treatment. Pharmacogenet. Genom. 2014, 24, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Le Clerc, S.; Taing, L.; Fond, G.; Meary, A.; Llorca, P.M.; Blanc, O.; Beaune, P.; Rajagopal, K.; Jamain, S.; Tamouza, R.; et al. A double amino-acid change in the HLA-A peptide-binding groove is associated with response to psychotropic treatment in patients with schizophrenia. Transl. Psychiatry 2015, 5, e608. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Kaur, H.; Kumari, K.; Kanojia, N.; Gupta, M.; Baghel, R.; Sood, M.; Jain, S.; Chadda, R.K.; Kukreti, R. Evaluation of genetic association of neurodevelopment and neuroimmunological genes with antipsychotic treatment response in schizophrenia in Indian populations. Mol. Genet. Genom. Med. 2016, 4, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Drogemoller, B.I.; Emsley, R.; Chiliza, B.; van der Merwe, L.; Wright, G.E.; Daya, M.; Hoal, E.; Malhotra, A.K.; Lencz, T.; Robinson, D.G.; et al. The identification of novel genetic variants associated with antipsychotic treatment response outcomes in first-episode schizophrenia patients. Pharmacogenet. Genom. 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Wang, Q.; Man Wu, H.; Yue, W.; Yan, H.; Zhang, Y.; Tan, L.; Deng, W.; Chen, Q.; Yang, G.; Lu, T.; et al. Effect of Damaging Rare Mutations in Synapse-Related Gene Sets on Response to Short-term Antipsychotic Medication in Chinese Patients With Schizophrenia: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 1261–1269. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhu, W.; Zhou, W.; Shen, L.; Wu, H.; Zhang, N.; Wu, S.; Fu, C.; et al. Different responses to risperidone treatment in Schizophrenia: A multicenter genome-wide association and whole exome sequencing joint study. Transl. Psychiatry 2022, 12, 173. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, Y.; Lv, Q.; Sheng, Y.H.; Chen, L.; Li, M.; Shen, L.; Huai, C.; Yi, Z.; Cui, D.; et al. Genetic Association of Olanzapine Treatment Response in Han Chinese Schizophrenia Patients. Front. Pharmacol. 2019, 10, 177. [Google Scholar] [CrossRef]
- Tanaka, S.; Syu, A.; Ishiguro, H.; Inada, T.; Horiuchi, Y.; Ishikawa, M.; Koga, M.; Noguchi, E.; Ozaki, N.; Someya, T.; et al. DPP6 as a candidate gene for neuroleptic-induced tardive dyskinesia. Pharmacogenom. J. 2013, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Alkelai, A.; Greenbaum, L.; Heinzen, E.L.; Baugh, E.H.; Teitelbaum, A.; Zhu, X.; Strous, R.D.; Tatarskyy, P.; Zai, C.C.; Tiwari, A.K.; et al. New insights into tardive dyskinesia genetics: Implementation of whole-exome sequencing approach. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109659. [Google Scholar] [CrossRef] [PubMed]
- Brandl, E.J.; Tiwari, A.K.; Zai, C.C.; Nurmi, E.L.; Chowdhury, N.I.; Arenovich, T.; Sanches, M.; Goncalves, V.F.; Shen, J.J.; Lieberman, J.A.; et al. Genome-wide association study on antipsychotic-induced weight gain in the CATIE sample. Pharmacogenom. J. 2016, 16, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, L.; Lv, L.; Ma, C.; Du, B.; Lu, T.; Jin, C.; Yan, H.; Yang, Y.; Li, W.; et al. Genome-Wide Association Study Suggested the PTPRD Polymorphisms Were Associated With Weight Gain Effects of Atypical Antipsychotic Medications. Schizophr. Bull. 2016, 42, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Ter Hark, S.E.; Jamain, S.; Schijven, D.; Lin, B.D.; Bakker, M.K.; Boland-Auge, A.; Deleuze, J.F.; Troudet, R.; Malhotra, A.K.; Gülöksüz, S.; et al. A new genetic locus for antipsychotic-induced weight gain: A genome-wide study of first-episode psychosis patients using amisulpride (from the OPTiMiSE cohort). J. Psychopharmacol. 2020, 34, 524–531. [Google Scholar] [CrossRef]
- Legge, S.E.; Pardinas, A.F.; Helthuis, M.; Jansen, J.A.; Jollie, K.; Knapper, S.; MacCabe, J.H.; Rujescu, D.; Collier, D.A.; O’Donovan, M.C.; et al. A genome-wide association study in individuals of African ancestry reveals the importance of the Duffy-null genotype in the assessment of clozapine-related neutropenia. Mol. Psychiatry 2019, 24, 328–337. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Yan, H.; Su, Y.; Guo, L.; Liao, Y.; Lu, T.; Yu, H.; Wang, L.; Li, J.; et al. ATAD3B and SKIL polymorphisms associated with antipsychotic-induced QTc interval change in patients with schizophrenia: A genome-wide association study. Transl. Psychiatry 2022, 12, 56. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Boloc, D.; Rodriguez, N.; Marmol, F.; Sanchez, J.; Bernardo, M.; Lafuente, A. Network analysis of gene expression in mice provides new evidence of involvement of the mTOR pathway in antipsychotic-induced extrapyramidal symptoms. Pharmacogenom. J. 2016, 16, 293–300. [Google Scholar] [CrossRef]
- Sainz, J.; Prieto, C.; Ruso-Julve, F.; Crespo-Facorro, B. Blood Gene Expression Profile Predicts Response to Antipsychotics. Front. Mol. Neurosci. 2018, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, E.S.; McGregor, N.W.; Emsley, R.A.; Warnich, L. DNA methylation and antipsychotic treatment mechanisms in schizophrenia: Progress and future directions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 38–49. [Google Scholar] [CrossRef]
- Lisoway, A.J.; Chen, C.C.; Zai, C.C.; Tiwari, A.K.; Kennedy, J.L. Toward personalized medicine in schizophrenia: Genetics and epigenetics of antipsychotic treatment. Schizophr. Res. 2021, 232, 112–124. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Wang, X.; He, Y.; Xia, Y.; Sweeney, J.A.; Kopp, R.F.; Liu, C.; Chen, C. Drug Response-Related DNA Methylation Changes in Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Front. Neurosci. 2021, 15, 674273. [Google Scholar] [CrossRef]
- Tang, H.; Dalton, C.F.; Srisawat, U.; Zhang, Z.J.; Reynolds, G.P. Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 645–649. [Google Scholar] [CrossRef]
- Athanasiou, M.C.; Dettling, M.; Cascorbi, I.; Mosyagin, I.; Salisbury, B.A.; Pierz, K.A.; Zou, W.; Whalen, H.; Malhotra, A.K.; Lencz, T.; et al. Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J. Clin. Psychiatry 2011, 72, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Umehara, H.; Ohmori, T.; Hashimoto, R. Clozapine Pharmacogenetic Studies in Schizophrenia: Efficacy and Agranulocytosis. Front. Pharmacol. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Boloc, D.; Gortat, A.; Cheng-Zhang, J.Q.; Garcia-Cerro, S.; Rodriguez, N.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Gasso, P.; Lafuente, A.; et al. Improving pharmacogenetic prediction of extrapyramidal symptoms induced by antipsychotics. Transl. Psychiatry 2018, 8, 276. [Google Scholar] [CrossRef]
- Tonozzi, T.R.; Braunstein, G.D.; Kammesheidt, A.; Curran, C.; Golshan, S.; Kelsoe, J. Pharmacogenetic profile and major depressive and/or bipolar disorder treatment: A retrospective, cross-sectional study. Pharmacogenomics 2018, 19, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Robinson, D.; Yu, J.; Gallego, J.; Fleischhacker, W.W.; Kahn, R.S.; Crespo-Facorro, B.; Vazquez-Bourgon, J.; Kane, J.M.; Malhotra, A.K.; et al. Schizophrenia Polygenic Risk Score as a Predictor of Antipsychotic Efficacy in First-Episode Psychosis. Am. J. Psychiatry 2019, 176, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Marshe, V.S.; Elsheikh, S.S.M.; Maciukiewicz, M.; Tiwari, A.; Brandl, E.J.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Muller, D.J. Polygenic risk scores analyses of psychiatric and metabolic traits with antipsychotic-induced weight gain in schizophrenia: An exploratory study. Pharmacogenom. J. 2023, 23, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rodieux, F.; Daali, Y.; Rollason, V.; Samer, C.F.; Lorenzini, K.I. Practice of CYP450 genotyping and phenotyping in children in a real-life setting. Front. Pharmacol. 2023, 14, 1130100. [Google Scholar] [CrossRef] [PubMed]
- Toja-Camba, F.J.; Gesto-Antelo, N.; Maronas, O.; Echarri Arrieta, E.; Zarra-Ferro, I.; Gonzalez-Barcia, M.; Bandin-Vilar, E.; Mangas Sanjuan, V.; Facal, F.; Arrojo Romero, M.; et al. Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics. Pharmaceutics 2021, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruano, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef]
- Altar, C.A.; Carhart, J.; Allen, J.D.; Hall-Flavin, D.; Winner, J.; Dechairo, B. Clinical Utility of Combinatorial Pharmacogenomics-Guided Antidepressant Therapy: Evidence from Three Clinical Studies. Mol. Neuropsychiatry 2015, 1, 145–155. [Google Scholar] [CrossRef]
- Winner, J.G.; Carhart, J.M.; Altar, C.A.; Goldfarb, S.; Allen, J.D.; Lavezzari, G.; Parsons, K.K.; Marshak, A.G.; Garavaglia, S.; Dechairo, B.M. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr. Med. Res. Opin. 2015, 31, 1633–1643. [Google Scholar] [CrossRef]
- Arranz, M.J.; Salazar, J.; Bote, V.; Artigas-Baleri, A.; Serra-LLovich, A.; Triviño, E.; Roige, J.; Lombardia, C.; Cancino, M.; Hernandez, M.; et al. Pharmacogenetic Interventions Improve the Clinical Outcome of Treatment-Resistant Autistic Spectrum Disorder Sufferers. Pharmaceutics 2022, 14, 999. [Google Scholar] [CrossRef]
- Walden, L.M.; Brandl, E.J.; Tiwari, A.K.; Cheema, S.; Freeman, N.; Braganza, N.; Kennedy, J.L.; Müller, D.J. Genetic testing for CYP2D6 and CYP2C19 suggests improved outcome for antidepressant and antipsychotic medication. Psychiatry Res. 2019, 279, 111–115. [Google Scholar] [CrossRef]
- Scherf-Clavel, M.; Frantz, A.; Eckert, A.; Weber, H.; Unterecker, S.; Deckert, J.; Reif, A.; Hahn, M. Effect of CYP2D6 pharmacogenetic phenotype and phenoconversion on serum concentrations of antidepressants and antipsychotics: A retrospective cohort study. Int. J. Clin. Pharm. 2023, 45, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Campo-Soria, C.; Bishop, J.R. Current strategies for predicting side effects from second generation antipsychotics in youth. Expert Opin. Drug Metab. Toxicol. 2021, 17, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.I.; Schuette, P.; Burckart, G.J.; Green, D.J.; La, J.; Burnham, J.M.; Rakhmanina, N.; Robb, A.; Huang, S.M.; van den Anker, J.N. A Comparison of Pediatric and Adult Safety Studies for Antipsychotic and Antidepressant Drugs Submitted to the United States Food and Drug Administration. J. Pediatr. 2019, 208, 236–242.e3. [Google Scholar] [CrossRef] [PubMed]
- Jameson, A.; Fylan, B.; Bristow, G.C.; Sagoo, G.S.; Dalton, C.; Cardno, A.; Sohal, J.; McLean, S.L. What Are the Barriers and Enablers to the Implementation of Pharmacogenetic Testing in Mental Health Care Settings? Front. Genet. 2021, 12, 740216. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Martinez, J.; Shah, N.; Kenan, W.; Fowler, A.; Limdi, N.; Burns, L.; Cogan, E.S.; Gardiner, A.; Hain, D.; et al. Pharmacogenomic profiling of pediatric patients on psychotropic medications in an emergency department. Pediatr. Emerg. Care 2023, 39, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Eadon, M.T.; Rosenman, M.B.; Zhang, P.; Fulton, C.R.; Callaghan, J.T.; Holmes, A.M.; Levy, K.D.; Gupta, S.K.; Haas, D.M.; Vuppalanchi, R.; et al. The INGENIOUS trial: Impact of pharmacogenetic testing on adverse events in a pragmatic clinical trial. Pharmacogenom. J. 2023, 23, 169–177. [Google Scholar] [CrossRef]
- Carrascal-Laso, L.; Franco-Martin, M.A.; Garcia-Berrocal, M.B.; Marcos-Vadillo, E.; Sanchez-Iglesias, S.; Lorenzo, C.; Sanchez-Martin, A.; Ramos-Gallego, I.; Garcia-Salgado, M.J.; Isidoro-Garcia, M. Application of a Pharmacogenetics-Based Precision Medicine Model (5SPM) to Psychotic Patients That Presented Poor Response to Neuroleptic Therapy. J. Pers. Med. 2020, 10, 289. [Google Scholar] [CrossRef]
- Alshabeeb, M.A.; Deneer, V.H.M.; Khan, A.; Asselbergs, F.W. Use of Pharmacogenetic Drugs by the Dutch Population. Front. Genet. 2019, 10, 567. [Google Scholar] [CrossRef]
- Kang, Z.; Qin, Y.; Sun, Y.; Lu, Z.; Sun, Y.; Chen, H.; Feng, X.; Zhang, Y.; Guo, H.; Yan, H.; et al. Multigenetic pharmacogenomics-guided treatment vs treatment as usual among hospitalised men with schizophrenia. JAMA Netw. Open 2023, 6, e2335518. [Google Scholar] [CrossRef]
- Garcia-Perez, L.; Linertova, R.; Serrano-Perez, P.; Trujillo-Martin, M.; Rodriguez-Rodriguez, L.; Valcarcel-Nazco, C.; Del Pino-Sedeno, T. Interventions to improve medication adherence in mental health: The update of a systematic review of cost-effectiveness. Int. J. Psychiatry Clin. Pract. 2020, 24, 416–427. [Google Scholar] [CrossRef]
- Hall-Flavin, D.K.; Winner, J.G.; Allen, J.D.; Jordan, J.J.; Nesheim, R.S.; Snyder, K.A.; Drews, M.S.; Eisterhold, L.L.; Biernacka, J.M.; Mrazek, D.A. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl. Psychiatry 2012, 2, e172. [Google Scholar] [CrossRef]
- Pérez, V.; Salavert, A.; Espadaler, J.; Tuson, M.; Saiz-Ruiz, J.; Sáez-Navarro, C.; Bobes, J.; Baca-García, E.; Vieta, E.; Olivares, J.M.; et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: Results of a randomized, double-blind clinical trial. BMC Psychiatry 2017, 17, 250. [Google Scholar] [CrossRef]
- Laika, B.; Leucht, S.; Heres, S.; Steimer, W. Intermediate metabolizer: Increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenom. J. 2009, 9, 395–403. [Google Scholar] [CrossRef]
- Carrascal-Laso, L.; Franco-Martin, M.A.; Marcos-Vadillo, E.; Ramos-Gallego, I.; Garcia-Berrocal, B.; Mayor-Toranzo, E.; Sanchez-Iglesias, S.; Lorenzo, C.; Sevillano-Jimenez, A.; Sanchez-Martin, A.; et al. Economic Impact of the Application of a Precision Medicine Model (5SPM) on Psychotic Patients. Pharmacogenom. Pers. Med. 2021, 14, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Karamperis, K.; Koromina, M.; Papantoniou, P.; Skokou, M.; Kanellakis, F.; Mitropoulos, K.; Vozikis, A.; Muller, D.J.; Patrinos, G.P.; Mitropoulou, C. Economic evaluation in psychiatric pharmacogenomics: A systematic review. Pharmacogenom. J. 2021, 21, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Girardin, F.R.; Poncet, A.; Perrier, A.; Vernaz, N.; Pletscher, M.; Samer, C.F.; Lieberman, J.A.; Villard, J. Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. Pharmacogenom. J. 2019, 19, 211–218. [Google Scholar] [CrossRef]
- Bousman, C.A.; Hopwood, M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry 2016, 3, 585–590. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Lafuente, A.; Bioque, M.; Lobo, A.; Gonzalez-Pinto, A.; Olmeda, M.S.; Corripio, I.; Llerena, A.; Cabrera, B.; et al. Pharmacogenetic study of antipsychotic induced acute extrapyramidal symptoms in a first episode psychosis cohort: Role of dopamine, serotonin and glutamate candidate genes. Pharmacogenom. J. 2016, 16, 439–445. [Google Scholar] [CrossRef]
- Hettige, N.C.; Zai, C.; Hazra, M.; Borlido, C.; Kennedy, J.L.; Strauss, J.; Le Foll, B.; Wong, A.; Remington, G.; De Luca, V. Use of candidate gene markers to guide antipsychotic dosage adjustment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 315–320. [Google Scholar] [CrossRef]
- Teo, C.; Zai, C.; Borlido, C.; Tomasetti, C.; Strauss, J.; Shinkai, T.; Le Foll, B.; Wong, A.; Kennedy, J.L.; De Luca, V. Analysis of treatment-resistant schizophrenia and 384 markers from candidate genes. Pharmacogenet. Genom. 2012, 22, 807–811. [Google Scholar] [CrossRef]
- Almoguera, B.; Riveiro-Alvarez, R.; Lopez-Castroman, J.; Dorado, P.; Vaquero-Lorenzo, C.; Fernandez-Piqueras, J.; Llerena, A.; Abad-Santos, F.; Baca-García, E.; Dal-Ré, R.; et al. Association of common genetic variants with risperidone adverse events in a Spanish schizophrenic population. Pharmacogenom. J. 2013, 13, 197–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zai, C.C.; Tiwari, A.K.; Zai, G.C.; Freeman, N.; Pouget, J.G.; Greco, J.; Tampakeras, M.; Shaikh, S.A.; Herbert, D.; Emmerson, H.; et al. Association Study of the Complement Component C4 Gene in Tardive Dyskinesia. Front. Pharmacol. 2019, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Guzek, K.; Stelmach, A.; Roznowska, A.; Najbar, I.; Cichocki, L.; Sadakierska-Chudy, A. A preliminary study of genetic polymorphisms potentially related to the adverse effects of aripiprazole. Arch. Pharm. Pract. 2023, 14, 13–18. [Google Scholar] [CrossRef]
- Bigos, K.L.; Bies, R.R.; Pollock, B.G.; Lowy, J.J.; Zhang, F.; Weinberger, D.R. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol. Psychiatry 2011, 16, 620–625. [Google Scholar] [CrossRef]
- Lu, J.Y.; Tiwari, A.K.; Zai, G.C.; Rastogi, A.; Shaikh, S.A.; Müller, D.J.; Voineskos, A.N.; Potkin, S.G.; Lieberman, J.A.; Meltzer, H.Y.; et al. Association study of Disrupted-In-Schizophrenia-1 gene variants and tardive dyskinesia. Neurosci. Lett. 2018, 686, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ota, V.K.; Spíndola, L.N.; Gadelha, A.; dos Santos Filho, A.F.; Santoro, M.L.; Christofolini, D.M.; Bellucco, F.T.; Ribeiro-dos-Santos, Â.; Santos, S.; Mari, J.D.J.; et al. DRD1 rs4532 polymorphism: A potential pharmacogenomic marker for treatment response to antipsychotic drugs. Schizophr. Res. 2012, 142, 206–208. [Google Scholar] [CrossRef]
- Martinez-Pinteno, A.; Gasso, P.; Prohens, L.; Segura, A.G.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Bernardo, M.; Lafuente, A.; Mas, S.; et al. Identification of EP300 as a Key Gene Involved in Antipsychotic-Induced Metabolic Dysregulation Based on Integrative Bioinformatics Analysis of Multi-Tissue Gene Expression Data. Front. Pharmacol. 2021, 12, 729474. [Google Scholar] [CrossRef] [PubMed]
- Mitjans, M.; Catalán, R.; Vázquez, M.; González-Rodríguez, A.; Penadés, R.; Pons, A.; Massana, G.; Munro, J.; Arranz, M.J.; Arias, B. Hypothalamic–pituitary–adrenal system, neurotrophic factors and clozapine response: Association with FKBP5 and NTRK2 genes. Pharmacogenet. Genom. 2015, 25, 274–277. [Google Scholar] [CrossRef]
- Schröder, C.; Czerwensky, F.; Leucht, S.; Steimer, W. Fat Mass and Obesity-Related Gene Variants rs9939609 and rs7185735 are Associated with Second-Generation Antipsychotic-Induced Weight Gain. Pharmacopsychiatry 2019, 52, 16–23. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Brennan, M.D. Glucagon-like peptide 1 receptor (GLP1R) haplotypes correlate with altered response to multiple antipsychotics in the CATIE trial. Schizophr. Res. 2014, 160, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.R.; Al Hadithy, A.F.; Amin, N.; van Duijn, C.M.; van Os, J.; van Harten, P.N. Antipsychotic-induced movement disorders in long-stay psychiatric patients and 45 tag SNPs in 7 candidate genes: A prospective study. PLoS ONE 2012, 7, e50970. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Reilly, J.L.; Harris, M.S.; Patel, S.R.; Kittles, R.; Badner, J.A.; Prasad, K.M.; Nimgaonkar, V.L.; Keshavan, M.S.; Sweeney, J.A. Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology 2015, 232, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Quteineh, L.; Vandenberghe, F.; Saigi Morgui, N.; Delacretaz, A.; Choong, E.; Gholam-Rezaee, M.; Magistretti, P.; Bondolfi, G.; Von Gunten, A.; Preisig, M.; et al. Impact of HSD11B1 polymorphisms on BMI and components of the metabolic syndrome in patients receiving psychotropic treatments. Pharmacogenet. Genom. 2015, 25, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Takekita, Y.; Fabbri, C.; Kato, M.; Nonen, S.; Sakai, S.; Sunada, N.; Koshikawa, Y.; Wakeno, M.; Okugawa, G.; Kinoshita, T.; et al. Serotonin 7 Receptor Variants Are Not Associated with Response to Second-Generation Antipsychotics in Japanese Schizophrenia Patients. Neuropsychobiology 2015, 72, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Osmanova, D.Z.; Freidin, M.B.; Fedorenko, O.Y.; Pozhidaev, I.V.; Boiko, A.S.; Vyalova, N.M.; Tiguntsev, V.V.; Kornetova, E.G.; Loonen, A.J.M.; Semke, A.V.; et al. A pharmacogenetic study of patients with schizophrenia from West Siberia gets insight into dopaminergic mechanisms of antipsychotic-induced hyperprolactinemia. BMC Med. Genet. 2019, 20 (Suppl. S1), 47. [Google Scholar] [CrossRef]
- Czerwensky, F.; Leucht, S.; Steimer, W. MC4R rs489693: A clinical risk factor for second generation antipsychotic-related weight gain? Int. J. Neuropsychopharmacol. 2013, 16, 2103–2109. [Google Scholar] [CrossRef][Green Version]
- Spellmann, I.; Riedel, M.; Städtler, J.; Zill, P.; Obermeier, M.; Cerovecki, A.; Dehning, S.; Schennach, R.; Epple, M.; Opgen-Rhein, M.; et al. Associations of NEUROD2 polymorphisms and change of cognitive dysfunctions in schizophrenia and schizoaffective disorder after eight weeks of antipsychotic treatment. Cogn. Neuropsychiatry 2017, 22, 280–297. [Google Scholar] [CrossRef]
- Esen-Sehir, D.; Kopf, J.; Hagele, S.; Plichta, M.M.; Reif, A.; Freudenberg, F. Influence of NOS1AP Risk Variants on the Corrected QT (QTc) Interval in the Pharmacotherapy of Schizophrenia. Pharmacopsychiatry 2022, 55, 266–273. [Google Scholar] [CrossRef]
- Dobrodeeva, V.S.; Shnayder, N.A.; Novitsky, M.A.; Asadullin, A.R.; Vaiman, E.E.; Petrova, M.M.; Limankin, O.V.; Neznanov, N.G.; Garganeeva, N.P.; Nasyrova, R.F. Association of a Single-Nucleotide Variant rs11100494 of the NPY5R Gene with Antipsychotic-Induced Metabolic Disorders. Pharmaceutics 2022, 14, 222. [Google Scholar] [CrossRef]
- Zazueta, A.; Castillo, T.; Cavieres, A.; Gonzalez, R.; Abarca, M.; Nieto, R.R.; Deneken, J.; Araneda, C.; Moya, P.R.; Bustamante, M.L. Polymorphisms in Schizophrenia-Related Genes Are Potential Predictors of Antipsychotic Treatment Resistance and Refractoriness. Int. J. Neuropsychopharmacol. 2022, 25, 701–708. [Google Scholar] [CrossRef]
- Spellmann, I.; Rujescu, D.; Musil, R.; Meyerwas, S.; Giegling, I.; Genius, J.; Zill, P.; Dehning, S.; Cerovecki, A.; Seemuller, F.; et al. Pleckstrin homology domain containing 6 protein (PLEKHA6) polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 51, 190–195. [Google Scholar] [CrossRef]
- Gagliano, S.A.; Tiwari, A.K.; Freeman, N.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Knight, J.; Muller, D.J. Protein kinase cAMP-dependent regulatory type II beta (PRKAR2B) gene variants in antipsychotic-induced weight gain. Hum. Psychopharmacol. 2014, 29, 330–335. [Google Scholar] [CrossRef]
- Maciukiewicz, M.; Gorbovskaya, I.; Tiwari, A.K.; Zai, C.C.; Freeman, N.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. Genetic validation study of protein tyrosine phosphatase receptor type D (PTPRD) gene variants and risk for antipsychotic-induced weight gain. J. Neural Transm. 2019, 126, 27–33. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Qin, S.; Li, Y.; Ning, A.; Fu, Y.; Wang, D.; Zeng, D.; Li, H.; Yu, W.; et al. Two Novel Loci of RELN Associated With Antipsychotics Response in Chinese Han Population. Front. Pharmacol. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Gareeva, A.E.; Zakirov, D.F.; Valinurov, R.G.; Khusnutdinova, E.K. Polymorphism of RGS2 gene: Genetic markers of risk for schizophrenia and pharmacogenetic markers of typical neuroleptics efficiency. Mol. Biol. 2013, 47, 934–941. [Google Scholar] [CrossRef]
- Zai, C.C.; Tiwari, A.K.; Mazzoco, M.; de Luca, V.; Muller, D.J.; Shaikh, S.A.; Lohoff, F.W.; Freeman, N.; Voineskos, A.N.; Potkin, S.G.; et al. Association study of the vesicular monoamine transporter gene SLC18A2 with tardive dyskinesia. J. Psychiatr. Res. 2013, 47, 1760–1765. [Google Scholar] [CrossRef]
- Giegling, I.; Drago, A.; Dolzan, V.; Plesnicar, B.K.; Schafer, M.; Hartmann, A.M.; Sander, T.; Toliat, M.R.; Moller, H.J.; Stassen, H.H.; et al. Glutamatergic gene variants impact the clinical profile of efficacy and side effects of haloperidol. Pharmacogenet. Genom. 2011, 21, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhang, T.; Han, W.; Zhu, L.; Ni, T.; Lin, H.; Liu, D.; Chen, G.; Xiao, J.; Li, T. Relationship of SNAP25 variants with schizophrenia and antipsychotic-induced weight change in large-scale schizophrenia patients. Schizophr. Res. 2020, 215, 250–255. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Liu, Q.; Brennan, M.D. Replication of SULT4A1-1 as a pharmacogenetic marker of olanzapine response and evidence of lower weight gain in the high response group. Pharmacogenomics 2014, 15, 933–939. [Google Scholar] [CrossRef]
- Ramsey, T.L.; Liu, Q.; Massey, B.W.; Brennan, M.D. Genotypic variation in the SV2C gene impacts response to atypical antipsychotics the CATIE study. Schizophr. Res. 2013, 149, 21–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hattori, S.; Suda, A.; Miyauchi, M.; Shiraishi, Y.; Saeki, T.; Fukushima, T.; Fujibayashi, M.; Tsujita, N.; Ishii, C.; Ishii, N.; et al. The association of genetic polymorphisms in CYP1A2, UGT1A4, and ABCB1 with autonomic nervous system dysfunction in schizophrenia patients treated with olanzapine. BMC Psychiatry 2020, 20, 72. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).