Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling
Abstract
1. Introduction
2. Experimental Methods
2.1. Construction of the Primary Material Library
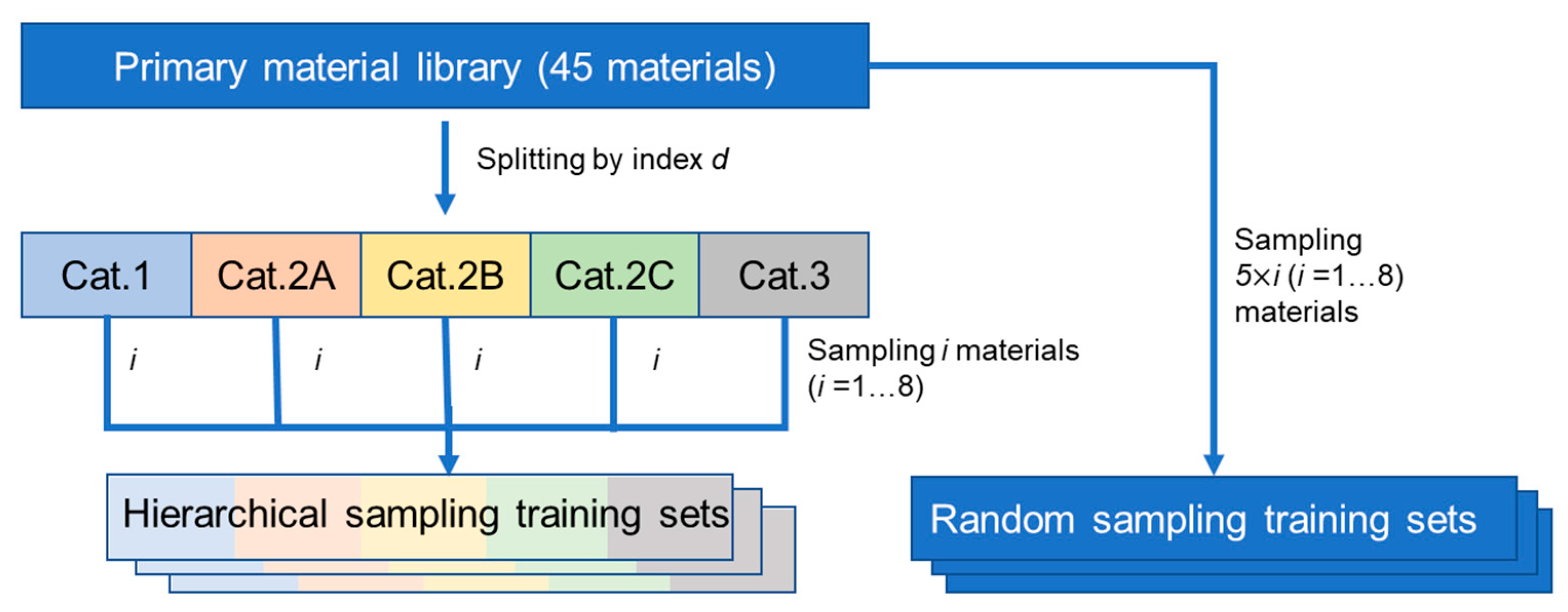
2.2. Construction of Material Libraries with Different Sample Sizes
2.3. Predictive Modeling and Model Evaluation
2.4. Establishment of the External Validation Set
3. Results and Discussion
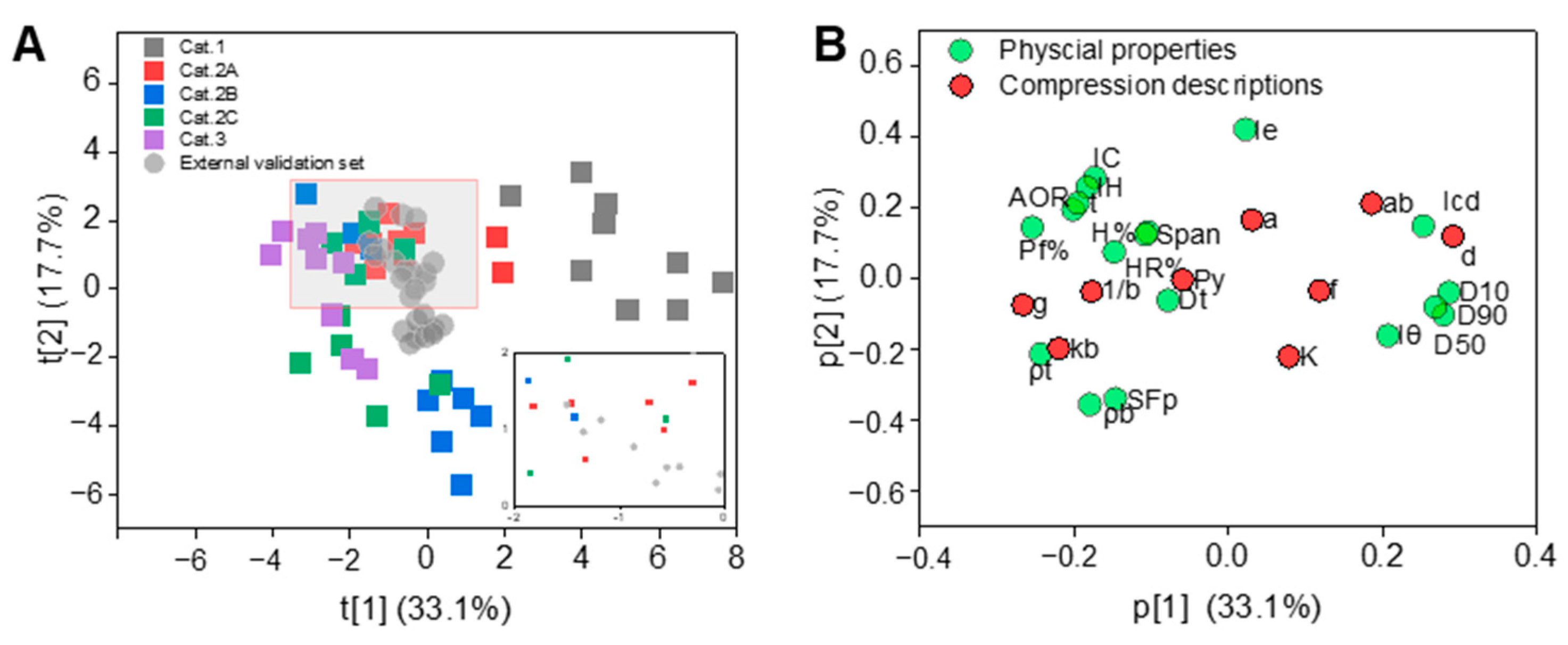
3.1. The Powder Properties
3.2. Comparison of Model Performance
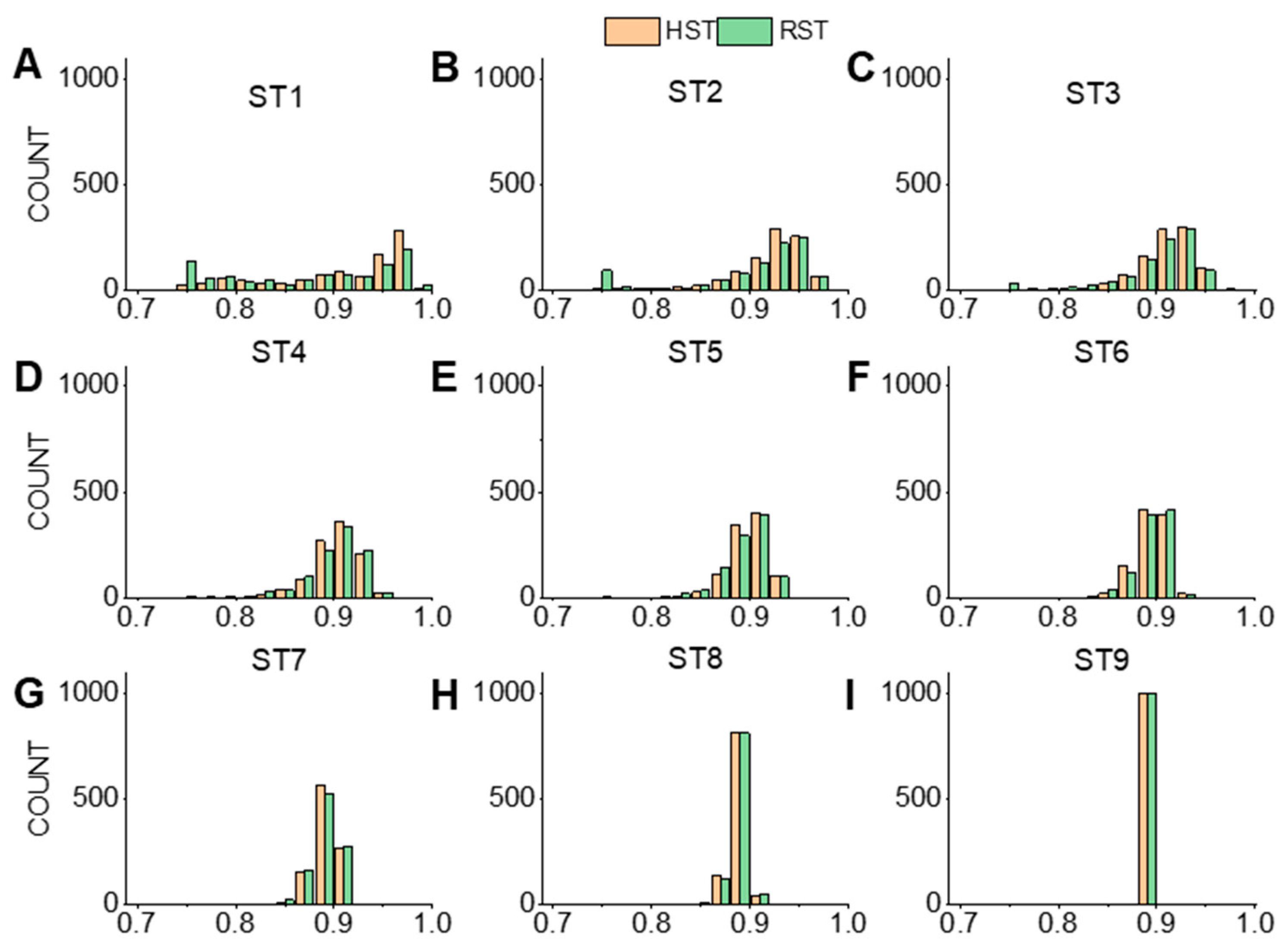
3.2.1. The Values during Cross-Validation
3.2.2. The Values during Cross-Validation
3.2.3. The Values during External Validation
3.2.4. The Values during External Validation
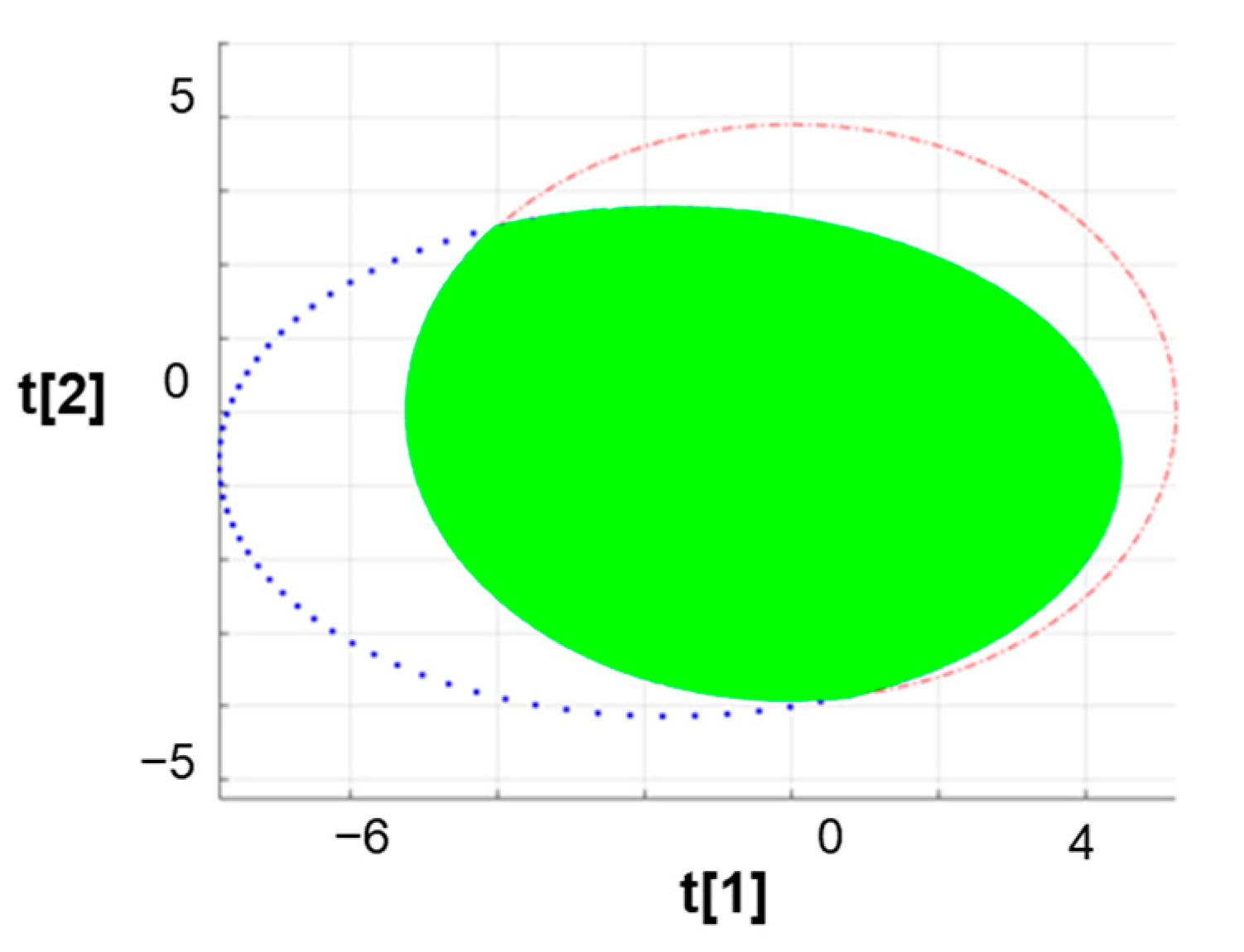
3.3. The Overlapping Rate of Confidence Area
3.4. Finding Important Materials for Model Training
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCS | Compression behavior classification system |
| iTCM | intelligent Traditional Chinese Medicine |
| API | Active pharmaceutical ingredient |
| DC | Direct compression |
| TS | Tensile strength |
| AOR | Angle of repose |
| PCA | Principal component analysis |
| PLS | Partial least squares regression |
| ST | Sampling training dataset |
| HST | Hierarchical sampling training datasets |
| RST | Random sampling training datasets |
| Max | Maximum |
| Min | Minimum |
| R2 | Correlation coefficient |
| RMSE | Root mean square error |
| MAPE | Mean absolute error percentage |
| NPP | Natural Production Powder |
| PC | Principal component |
| LV | Latent variable |
References
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Alwosheel, A.; van Cranenburgh, S.; Chorus, C.G. Is your dataset big enough? Sample size requirements when using artificial neural networks for discrete choice analysis. J. Choice Model. 2018, 28, 167–182. [Google Scholar] [CrossRef]
- Yu, J.; Xu, B.; Zhang, K.; Shi, C.; Zhang, Z.; Fu, J.; Qiao, Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics 2019, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Xu, B.; Zhang, Z.; Yu, J.; Wang, F.; Shi, X.; Qiao, Y. A compression behavior classification system of pharmaceutical powders for accelerating direct compression tablet formulation design. Int. J. Pharm. 2019, 572, 118742. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Roopwani, R.; Buckner, I.S. A material-sparing method for assessment of powder deformation characteristics using data collected during a single compression-decompression cycle. J. Pharm. Sci. 2013, 102, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Drašković, M.; Djuriš, J.; Ibrić, S.; Parojčić, J. Functionality and performance evaluation of directly compressible co-processed excipients based on dynamic compaction analysis and percolation theory. Powder Technol. 2018, 326, 292–301. [Google Scholar] [CrossRef]
- El Kassem, B.; Heider, Y.; Brinz, T.; Markert, B. A multivariate statistical approach to analyze the impact of material attributes and process parameters on the quality performance of an auger dosing process. J. Drug Deliv. Sci. Technol. 2020, 60, 101950. [Google Scholar] [CrossRef]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Benedetti, A.; Khoo, J.; Sharma, S.; Facco, P.; Barolo, M.; Zomer, S. Data analytics on raw material properties to accelerate pharmaceutical drug development. Int. J. Pharm. 2019, 563, 122–134. [Google Scholar] [CrossRef]
- Bostijn, N.; Dhondt, J.; Ryckaert, A.; Szabó, E.; Dhondt, W.; Van Snick, B.; Vanhoorne, V.; Vervaet, C.; De Beer, T. A multivariate approach to predict the volumetric and gravimetric feeding behavior of a low feed rate feeder based on raw material properties. Int. J. Pharm. 2019, 557, 342–353. [Google Scholar] [CrossRef]
- Sebastian Escotet-Espinoza, M.; Moghtadernejad, S.; Oka, S.; Wang, Y.; Roman-Ospino, A.; Schäfer, E.; Cappuyns, P.; Van Assche, I.; Futran, M.; Ierapetritou, M.; et al. Effect of tracer material properties on the residence time distribution (RTD) of continuous powder blending operations. Part I of II: Experimental evaluation. Powder Technol. 2019, 342, 744–763. [Google Scholar] [CrossRef]
- Wang, Y.; O’Connor, T.; Li, T.; Ashraf, M.; Cruz, C.N. Development and applications of a material library for pharmaceutical continuous manufacturing of solid dosage forms. Int. J. Pharm. 2019, 569, 118551. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Wang, X.; Dai, S.; Shi, X.; Qiao, Y. Optimal Selection of Incoming Materials from the Inventory for Achieving the Target Drug Release Profile of High Drug Load Sustained-Release Matrix Tablet. AAPS PharmSciTech 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Penne, L.; Grymonpré, W.; Van Snick, B.; Dhondt, J.; Boeckx, J.; Vogeleer, J.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Determination of a quantitative relationship between material properties, process settings and screw feeding behavior via multivariate data-analysis. Int. J. Pharm. 2021, 602, 120603. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakano, Y.; Marumo, Y.; Kumada, S.; Okada, K.; Onuki, Y. Application of machine learning to a material library for modeling of relationships between material properties and tablet properties. Int. J. Pharm. 2021, 609, 121158. [Google Scholar] [CrossRef]
- Ryckaert, A.; Van Hauwermeiren, D.; Dhondt, J.; De Man, A.; Funke, A.; Djuric, D.; Vervaet, C.; Nopens, I.; De Beer, T. TPLS as predictive platform for twin-screw wet granulation process and formulation development. Int. J. Pharm. 2021, 605, 120785. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Li, W.; Wang, Y.; Luo, G.; Qiao, Y.; Zhang, Y.; Xu, B. Using a material database and data fusion method to accelerate the process model development of high shear wet granulation. Sci. Rep. 2021, 11, 16514. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Van Snick, B.; Pandelaere, K.; Dhondt, J.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. In-depth analysis of the long-term processability of materials during continuous feeding. Int. J. Pharm. 2022, 614, 121454. [Google Scholar] [CrossRef]
- Dhondt, J.; Bertels, J.; Kumar, A.; Van Hauwermeiren, D.; Ryckaert, A.; Van Snick, B.; Klingeleers, D.; Vervaet, C.; De Beer, T. A Multivariate Formulation and Process Development Platform for Direct Compression. Int. J. Pharm. 2022, 623, 121962. [Google Scholar] [CrossRef]
- Dhondt, J.; Eeckhout, Y.; Bertels, J.; Kumar, A.; Van Snick, B.; Klingeleers, D.; Vervaet, C.; De Beer, T. A multivariate methodology for material sparing characterization and blend design in drug product development. Int. J. Pharm. 2022, 621, 120801. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Zhao, X.; Liang, Z.; Qiao, Y.; Luo, G.; Xu, B. Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation. Pharmaceutics 2022, 14, 2631. [Google Scholar] [CrossRef]
- Sousa, A.S.; Serra, J.; Estevens, C.; Costa, R.; Ribeiro, A.J. Leveraging a multivariate approach towards enhanced development of direct compression extended release tablets. Int. J. Pharm. 2023, 646, 123432. [Google Scholar] [CrossRef]
- Su, J.; Zhang, K.; Qi, F.; Cao, J.; Miao, Y.; Zhang, Z.; Qiao, Y.; Xu, B. A tabletability change classification system in supporting the tablet formulation design via the roll compaction and dry granulation process. Int. J. Pharm. X 2023, 6, 100204. [Google Scholar] [CrossRef]
- Wang, T.; Ibrahim, A.; Hoag, S.W. Understanding the impact of magnesium stearate variability on tableting performance using a multivariate modeling approach. Pharm. Dev. Technol. 2020, 25, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lamesic, D.; Planinsek, O.; Ilic, I.G. Modified equation for particle bonding area and strength with inclusion of powder fragmentation propensity. Eur. J. Pharm. Sci. 2018, 121, 218–227. [Google Scholar] [CrossRef]
- Ono, T.; Yonemochi, E. Evaluation of the physical properties of dry surface-modified ibuprofen using a powder rheometer (FT4) and analysis of the influence of pharmaceutical additives on improvement of the powder flowability. Int. J. Pharm. 2020, 579, 119165. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Gopireddy, S.R.; Fritsch, A.K.; Profitlich, T.; Scherliess, R.; Urbanetz, N.A. Evaluation and prediction of powder flowability in pharmaceutical tableting. Pharm. Dev. Technol. 2019, 24, 35–47. [Google Scholar] [CrossRef]
- Rojas, J.; Zuluaga, C.; Cadavid, A. Effect of Reprocessing and Excipient Characteristics on Ibuprofen Tablet Properties. Trop. J. Pharm. Res. 2015, 14, 1145–1152. [Google Scholar] [CrossRef][Green Version]
- Nofrerias, I.; Nardi, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; García-Montoya, E.; Pérez-Lozano, P.; Ticó, J.R.; Miñarro, M. Comparison between Microcrystalline Celluloses of different grades made by four manufacturers using the SeDeM diagram expert system as a pharmaceutical characterization tool. Powder Technol. 2019, 342, 780–788. [Google Scholar] [CrossRef]
- Paul, S.; Chang, S.Y.; Dun, J.; Sun, W.J.; Wang, K.; Tajarobi, P.; Boissier, C.; Sun, C.C. Comparative analyses of flow and compaction properties of diverse mannitol and lactose grades. Int. J. Pharm. 2018, 546, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Castaneda Hernandez, O.; Caraballo Rodriguez, I.; Bernad Bernad, M.J.; Melgoza Contreras, L.M. Comparison of the performance of two grades of metformin hydrochloride elaboration by means of the SeDeM system, compressibility, compactability, and process capability indices. Drug Dev. Ind. Pharm. 2021, 47, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Haykin, S. Neural Networks and Learning Machines; Prentice Hall: Upper Saddle River, NJ, USA, 2009; Volume 3. [Google Scholar]
- Bongiorno, V.; Gibbon, S.; Michailidou, E.; Curioni, M. Exploring the use of machine learning for interpreting electrochemical impedance spectroscopy data: Evaluation of the training dataset size. Corros. Sci. 2022, 198, 110119. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Pan, X.; Karim, M.N. Effect of dataset size on modeling and monitoring of chemical processes. Chem. Eng. Sci. 2020, 227, 115928. [Google Scholar] [CrossRef]
- Althnian, A.; AlSaeed, D.; Al-Baity, H.; Samha, A.; Dris, A.B.; Alzakari, N.; Abou Elwafa, A.; Kurdi, H. Impact of Dataset Size on Classification Performance: An Empirical Evaluation in the Medical Domain. Appl. Sci. 2021, 11, 796. [Google Scholar] [CrossRef]
- Aroniada, M.; Bano, G.; Vueva, Y.; Christodoulou, C.; Li, F.; Litster, J.D. A novel mixing rule model to predict the flowability of directly compressed pharmaceutical blends. Int. J. Pharm. 2023, 647, 123475. [Google Scholar] [CrossRef]
- Xu, B.; Qiao, Y.J. Explore the iTCM Knowledge Base. 2018. Available online: http://info.pharm.bucm.edu.cn/xsgz/sjgxpt/48350.htm (accessed on 26 August 2019).
- Perez, P.; Sune-Negre, J.M.; Minarro, M.; Roig, M.; Fuster, R.; Garcia-Montoya, E.; Hernandez, C.; Ruhi, R.; Tico, J.R. A new expert systems (SeDeM diagram) for control batch powder formulation and preformulation drug products. Eur. J. Pharm. Biopharm. 2006, 64, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Xu, B.; Shi, G.; Liu, J.; Zhang, Z.; Shi, X.; Qiao, Y. SeDeM expert system for directly compressed tablet formulation: A review and new perspectives. Powder Technol. 2019, 342, 517–527. [Google Scholar] [CrossRef]
- Leuenberger, H. The compressibility and compactibility of powder systems. Int. J. Pharm. 1982, 12, 41–55. [Google Scholar] [CrossRef]
- Sonnergaard, J.M. Quantification of the compactibility of pharmaceutical powders. Eur. J. Pharm. Biopharm. 2006, 63, 270–277. [Google Scholar] [CrossRef]
- Šantl, M.; Ilić, I.; Vrečer, F.; Baumgartner, S. A compressibility and compactibility study of real tableting mixtures: The impact of wet and dry granulation versus a direct tableting mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef]
- Sun, C.C.; Kleinebudde, P. Mini review: Mechanisms to the loss of tabletability by dry granulation. Eur. J. Pharm. Biopharm. 2016, 106, 9–14. [Google Scholar] [CrossRef]
- Nur Azira, T.; Che Man, Y.B.; Raja Mohd Hafidz, R.N.; Aina, M.A.; Amin, I. Use of principal component analysis for differentiation of gelatine sources based on polypeptide molecular weights. Food Chem. 2014, 151, 286–292. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Chen, X. Locally Linear Embedding Orthogonal Projection to Latent Structure. In Data-Driven Fault Detection and Reasoning for Industrial Monitoring; Wang, J., Zhou, J., Chen, X., Eds.; Springer: Singapore, 2022; pp. 189–209. [Google Scholar] [CrossRef]
- Bhalode, P.; Tian, H.; Gupta, S.; Razavi, S.M.; Roman-Ospino, A.; Talebian, S.; Singh, R.; Scicolone, J.V.; Muzzio, F.J.; Ierapetritou, M. Using residence time distribution in pharmaceutical solid dose manufacturing—A critical review. Int. J. Pharm. 2021, 610, 121248. [Google Scholar] [CrossRef]
- Bratina, B.; Tovornik, B. Multivariate Statistical Methods for Industrial Process Prognostics. In Proceedings of the International Conference on Cybernetics and Informatics 2010, Baku, Azerbaijan, 6–8 September 2010; pp. 1–9. [Google Scholar]
- Paul, S.; Sun, C.C. Dependence of Friability on Tablet Mechanical Properties and a Predictive Approach for Binary Mixtures. Pharm. Res. 2017, 34, 2901–2909. [Google Scholar] [CrossRef]
- Rojas, J.; Ciro, Y.; Correa, L. Functionality of chitin as a direct compression excipient: An acetaminophen comparative study. Carbohydr. Polym. 2014, 103, 134–139. [Google Scholar] [CrossRef]
- Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression Modulus and Apparent Density of Polymeric Excipients during Compression-Impact on Tabletability. Pharmaceutics 2022, 14, 913. [Google Scholar] [CrossRef]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Goodall, R.; Nofrerias, I.; Nardi, A.; Suñé-Pou, M.; Boeckmans, J.; Suñé-Negre, J.M.; García-Montoya, E.; Pérez-Lozano, P.; Ticó-Grau, J.R.; Miñarro-Carmona, M. Optimization of the Cohesion Index in the SeDeM Diagram Expert System and application of SeDeM Diagram: An improved methodology to determine the Cohesion Index. PLoS ONE 2018, 13, e203846. [Google Scholar] [CrossRef]
- Yost, E.; Mazel, V.; Sluga, K.K.; Nagapudi, K.; Muliadi, A.R. Beyond Brittle/Ductile Classification: Applying Proper Constitutive Mechanical Metrics to Understand the Compression Characteristics of Pharmaceutical Materials. J. Pharm. Sci. 2022, 111, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Persaud, D.; Choudhary, K.; DeCost, B.; Greenwood, M.; Hattrick-Simpers, J. Exploiting redundancy in large materials datasets for efficient machine learning with less data. Nat. Commun. 2023, 14, 7283. [Google Scholar] [CrossRef] [PubMed]
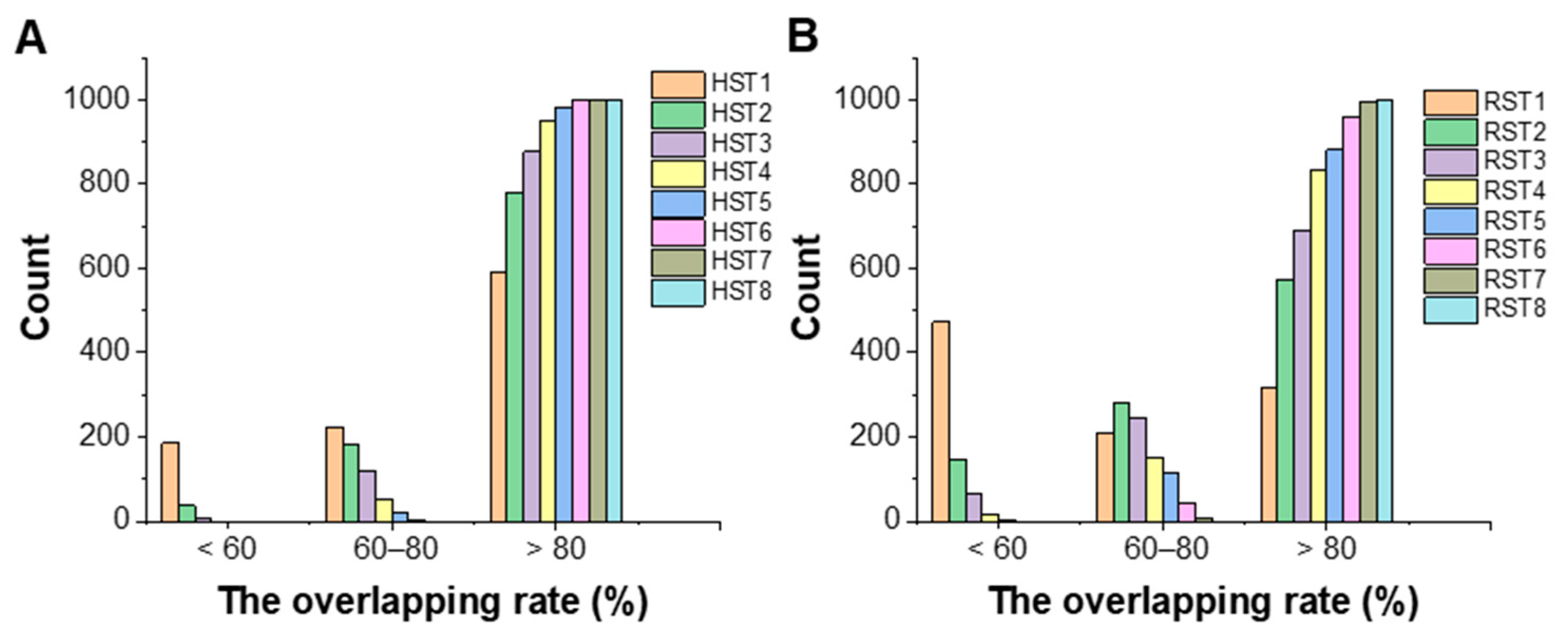
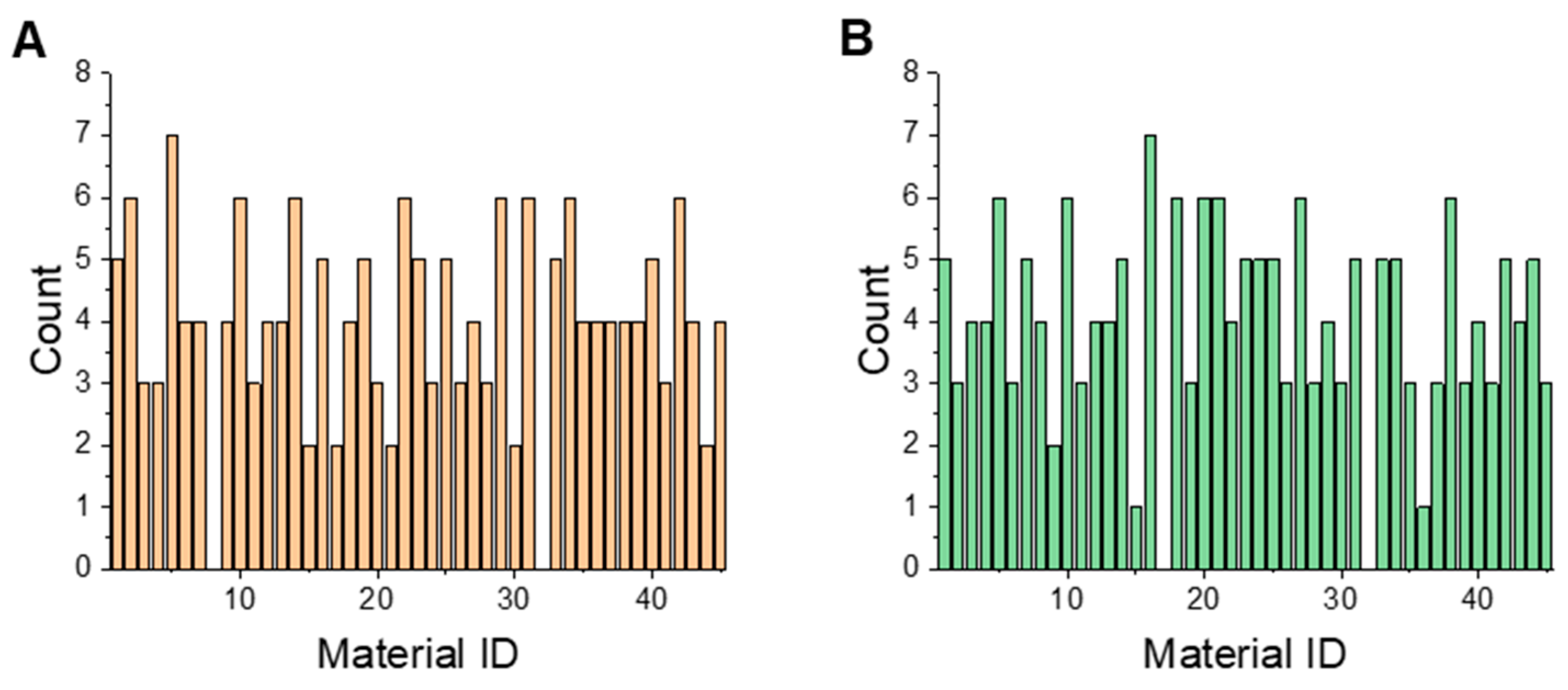
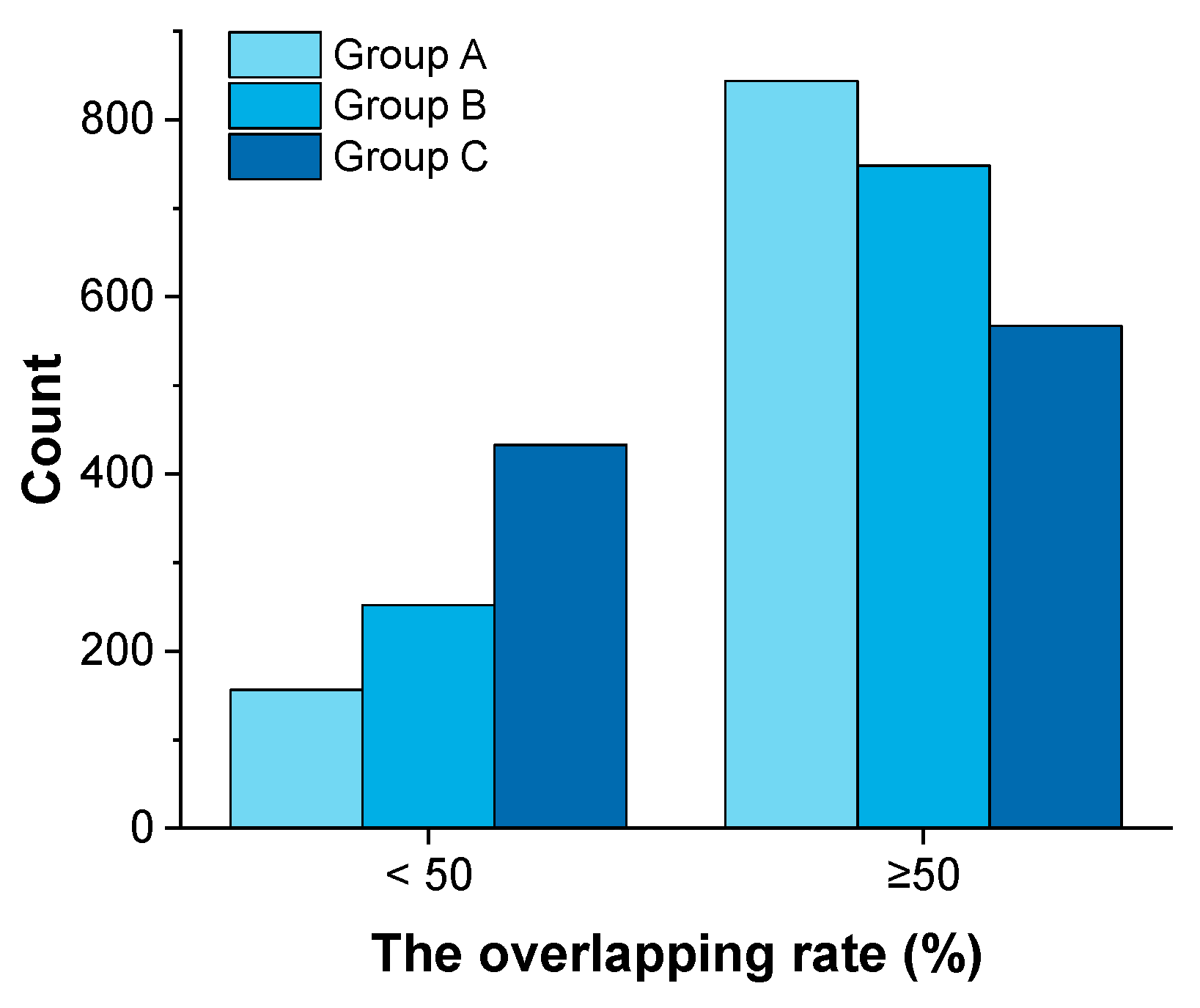
| No. | Number of Samples | Number of Material Attributes | Year | Application Area | Reference |
|---|---|---|---|---|---|
| 1 | 20 | 30 | 2018 | Find surrogate materials for pharmaceutical process development. | [1] |
| 2 | 55 | Over 100 | 2018 | Build predictive models for in silico process. | [8] |
| 3 | 41 | 8 | 2019 | Develop a direct compression decision-making tool to accelerate materials’ screening. | [9] |
| 4 | 15 | 25 | 2019 | Predict the volumetric and gravimetric feeding behavior of a low-feed-rate feeder. | [10] |
| 5 | 130 | 18 | 2019 | Develop a compression behavior classification system for direct compression. | [4] |
| 6 | 20 | 32 | 2019 | Study the effect of tracer material properties on the residence time distribution of continuous powder-blending operations. | [11] |
| 7 | 20 | 44 | 2019 | Evaluate material performance on a loss-in-weight feeder. | [12] |
| 8 | 111 | 22 | 2019 | Develop a compression behavior classification system for roll compaction. | [3] |
| 9 | 12 | 18 | 2019 | Analyze the effect of material attributes on the dissolution profile of the matrix tablet. | [13] |
| 10 | 10 | 30 | 2020 | Analyze the impact of material attributes on the performance of an auger dosing process. | [7] |
| 11 | 13 | 44 | 2021 | Predict feeding performance based on material properties. | [14] |
| 12 | 81 | 28 | 2021 | Develop machine learning models by linking material properties and direct compression tablet properties. | [15] |
| 13 | 27 | 48 | 2021 | Develop a TPLS model for the twin-screw wet granulation process and formulation development. | [16] |
| 14 | 56 | 18 | 2021 | Develop a formulation process quality model for high-shear wet granulation. | [17] |
| 15 | 12 | 44 | 2022 | Analyze the impact of material attributes on the gravimetric feeding process. | [18] |
| 16 | 14 | 55 | 2022 | Develop a TPLS model for the direct compression process and formulation design. | [19] |
| 17 | 32 | 19 | 2022 | Develop a PCA model to recognize the highest amount of variability in physical powder properties. | [20] |
| 18 | 30 | 19 | 2022 | Develop a tabletability change classification system for high-shear wet granulation and tableting. | [21] |
| 19 | 15 | 14 | 2023 | Analyze the impact of material attributes on direct compression extended-release formulations. | [22] |
| 20 | 31 | 18 | 2023 | Develop a tabletability change classification system for roll compaction, dry granulation, and tableting. | [23] |
| Property | Characterization Technique | Descriptor | Abbreviation |
|---|---|---|---|
| Powder properties | Powder density test | Bulk and tapped density | ρb and ρt |
| Ture density and porosity | Dt and ε | ||
| Carr’s index | IC | ||
| Inter-particle porosity | Ie | ||
| Hausner ratio | IH | ||
| Diametrical crushing test under maximum compression pressure | Cohesion index | Icd | |
| Flow through an orifice | Angle of repose | AOR/α | |
| Flow time | t″ | ||
| Rapid moisture test | Moisture content | HR% | |
| Moisture sorption | Water uptake at 76% (±2%) of the relative humidity | H% | |
| Laser diffraction | 10, 50, and 90% cumulative undersize of volumetric particle size distribution | D10, D50, D90 | |
| Width and span of volumetric particle size distribution | Span | ||
| Percentage of particles measuring <50 μm | %Pf | ||
| Homogeneity index | Iθ | ||
| Compression descriptor | The Kawakita model | a, ab, b−1 | |
| The Heckel model | Py | ||
| The Gurnham model | K | ||
| The Ryshkewitch–Duckworth model | kb | ||
| The Shapiro model | f | ||
| The Power model | d, g | ||
| Tablet Mechanical property | Diametrical crushing test | Tensile strength | TS |
| Factors | Levels | |||
|---|---|---|---|---|
| Composition A | MCC PH102 | Lactose Tablettose® 80 | ||
| Composition B | Stellariae Radix extract | Radix Rehmanniae Preparata extract | Rhizoma Alismatis extract | Flos Farfarae extract |
| Ratio of A to B (w/w) | 1:3 | 1:2 | 1:1 | |
| Max | Min | Max | Min | ||
|---|---|---|---|---|---|
| HST1 | 1.31 | 0.41 | RST1 | 1.33 | 0.19 |
| HST2 | 1.21 | 0.52 | RST2 | 1.30 | 0.28 |
| HST3 | 1.19 | 0.60 | RST3 | 1.15 | 0.48 |
| HST4 | 1.15 | 0.71 | RST4 | 1.20 | 0.55 |
| HST5 | 1.14 | 0.77 | RST5 | 1.20 | 0.71 |
| HST6 | 1.14 | 0.80 | RST6 | 1.14 | 0.76 |
| HST7 | 1.10 | 0.88 | RST7 | 1.12 | 0.83 |
| HST8 | 1.08 | 0.93 | RST8 | 1.09 | 0.91 |
| HST9 | 1.04 | 1.03 | RST9 | 1.04 | 1.03 |
| Max | Min | Max | Min | ||
|---|---|---|---|---|---|
| HST1 | 0.94 | 0.00 | RST1 | 0.94 | 0.00 |
| HST2 | 0.95 | 0.05 | RST2 | 0.95 | 0.01 |
| HST3 | 0.95 | 0.33 | RST3 | 0.95 | 0.16 |
| HST4 | 0.95 | 0.50 | RST4 | 0.95 | 0.45 |
| HST5 | 0.95 | 0.79 | RST5 | 0.95 | 0.72 |
| HST6 | 0.95 | 0.82 | RST6 | 0.95 | 0.74 |
| HST7 | 0.95 | 0.85 | RST7 | 0.95 | 0.84 |
| HST8 | 0.94 | 0.91 | RST8 | 0.94 | 0.88 |
| HST9 | 0.93 | 0.93 | RST9 | 0.93 | 0.93 |
| Max | Min | Max | Min | ||
|---|---|---|---|---|---|
| HST1 | 9.70 | 0.49 | RST1 | 41.56 | 0.52 |
| HST2 | 4.09 | 0.41 | RST2 | 4.30 | 0.48 |
| HST3 | 2.88 | 0.45 | RST3 | 4.12 | 0.45 |
| HST4 | 2.30 | 0.49 | RST4 | 2.55 | 0.45 |
| HST5 | 1.68 | 0.52 | RST5 | 2.39 | 0.47 |
| HST6 | 1.44 | 0.46 | RST6 | 1.74 | 0.51 |
| HST7 | 1.30 | 0.56 | RST7 | 1.30 | 0.58 |
| HST8 | 1.20 | 0.65 | RST8 | 1.23 | 0.66 |
| HST9 | 0.97 | 0.97 | RST9 | 0.97 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Shen, H.; Zhao, S.; Ma, X.; Chen, L.; Dai, S.; Xu, B.; Qiao, Y. Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling. Pharmaceutics 2024, 16, 242. https://doi.org/10.3390/pharmaceutics16020242
Cao J, Shen H, Zhao S, Ma X, Chen L, Dai S, Xu B, Qiao Y. Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling. Pharmaceutics. 2024; 16(2):242. https://doi.org/10.3390/pharmaceutics16020242
Chicago/Turabian StyleCao, Junjie, Haoran Shen, Shuying Zhao, Xiao Ma, Liping Chen, Shengyun Dai, Bing Xu, and Yanjiang Qiao. 2024. "Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling" Pharmaceutics 16, no. 2: 242. https://doi.org/10.3390/pharmaceutics16020242
APA StyleCao, J., Shen, H., Zhao, S., Ma, X., Chen, L., Dai, S., Xu, B., & Qiao, Y. (2024). Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling. Pharmaceutics, 16(2), 242. https://doi.org/10.3390/pharmaceutics16020242





