Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AP NSs and NS-Gs
2.3. Preparation of AP-Loaded MEs and ME-Gs
2.4. Physicochemical Characteristics of AP-Loaded Nanoformulations
2.4.1. Content Analysis of AP and Ascorbic Acid in Nanoformulations
2.4.2. Particle Size and Surface Charge Analysis of Nanoformulations
2.4.3. Stability Test under Stress Conditions
2.4.4. Morphological Observation of Nanoformulations
2.4.5. X-ray Diffraction (XRD) Analysis of Nanoformulations
2.4.6. Differential Scanning Calorimetry (DSC) Analysis of Nanoformulations
2.4.7. Fourier Transform Infrared (FTIR) Analysis of Nanoformulations
2.4.8. Apparent Viscosities of NS, NS-G, and ME-G
2.5. In Vitro Dissolution Profiles of AP from NS, NS-G, and ME-G
2.6. Ex Vivo Skin Absorption Profiles of AP from NS-G and ME-G
2.7. Statistical Analysis
3. Results and Discussion
3.1. Screening of Suspending Agents to Formulate AP-Loaded NSs
3.2. Effects of Formulation Variables on Particle Size and Homogeneity of NSs
3.3. Design of NS- and ME-Loaded Soft Hydrogel Formulations (NS-Gs and ME-Gs)
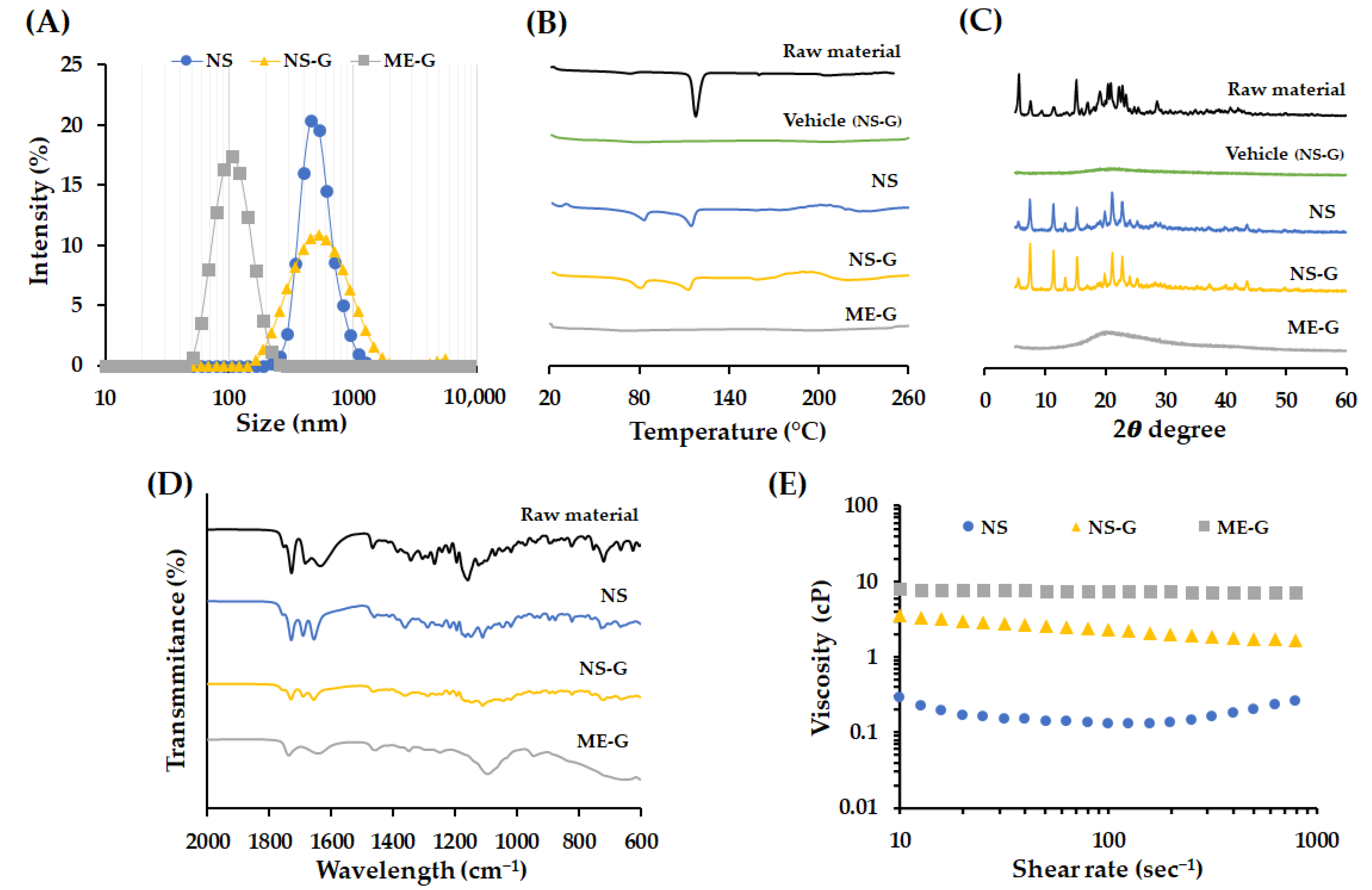
3.4. Physicochemical Characteristics of Different Nanoformulations
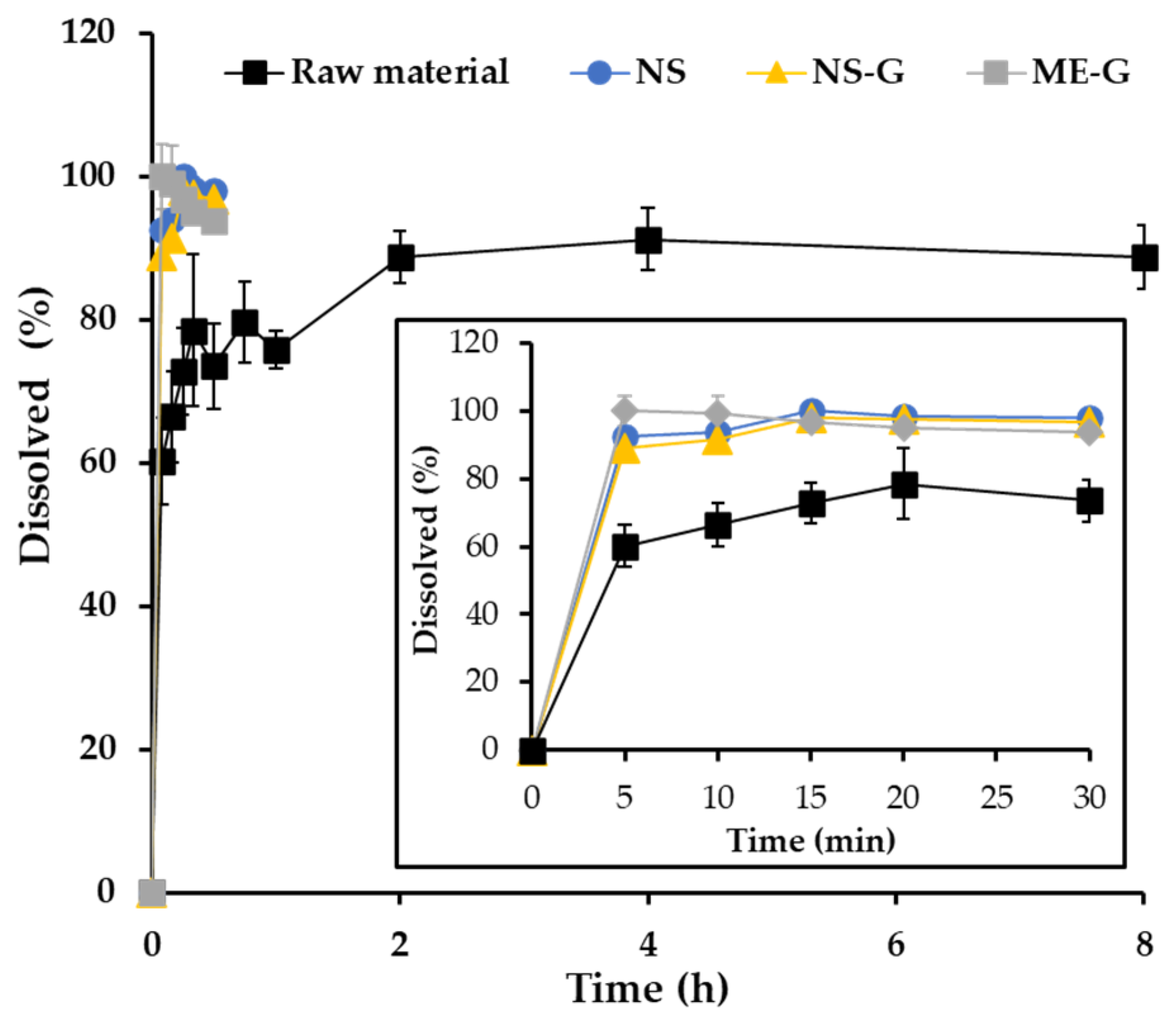
3.5. In Vitro Dissolution Profiles of AP from Different Formulations
3.6. Ex Vivo Skin Absorption Profiles of AP from NS-G and ME-G
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics 2022, 9, 85. [Google Scholar] [CrossRef]
- Enescu, C.D.; Bedford, L.M.; Potts, G.; Fahs, F. A Review of Topical Vitamin C Derivatives and Their Efficacy. J. Cosmet. Dermatol. 2022, 21, 2349–2359. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Jentzsch, A.; Habich, A.; Köpsel, C.; Ernst, A. Ascorbic Acid Salt Suspensions and Use Thereof as Antioxidants. U.S. Patent US20060078597A1, 13 April 2006. [Google Scholar]
- Gosenca, M.; Obreza, A.; Pečar, S.; Gašperlin, M. A New Approach for Increasing Ascorbyl Palmitate Stability by Addition of Non-Irritant Co-Antioxidant. AAPS PharmSciTech 2010, 11, 1485–1492. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Müller, R.H.; Junyaprasert, V.B. Encapsulation of Ascorbyl Palmitate in Nanostructured Lipid Carriers (NLC)—Effects of Formulation Parameters on Physicochemical Stability. Int. J. Pharm. 2007, 340, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Choi, Y.W. Skin Permeation Enhancement of Ascorbyl Palmitate by Liposomal Hydrogel (Lipogel) Formulation and Electrical Assistance. Biol. Pharm. Bull. 2007, 30, 393–396. [Google Scholar] [CrossRef]
- Gosenca, M.; Bešter-Rogač, M.; Gašperlin, M. Lecithin Based Lamellar Liquid Crystals as a Physiologically Acceptable Dermal Delivery System for Ascorbyl Palmitate. Eur. J. Pharm. Sci. 2013, 50, 114–122. [Google Scholar] [CrossRef]
- Date, A.A.; Patravale, V.B. Microemulsions: Applications in Transdermal and Dermal Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 547–596. [Google Scholar] [CrossRef]
- Vitore, J.G.; Pagar, S.; Singh, N.; Karunakaran, B.; Salve, S.; Hatvate, N.; Rojekar, S.; Benival, D. A Comprehensive Review of Nanosuspension Loaded Microneedles: Fabrication Methods, Applications, and Recent Developments. J. Pharm. Investig. 2023, 53, 475–504. [Google Scholar] [CrossRef]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Karakucuk, A.; Celebi, N. Screening of Stabilizing Agents to Optimize Flurbiprofen Nanosuspensions Using Experimental Design. J. Drug Deliv. Sci. Technol. 2020, 57, 101690. [Google Scholar] [CrossRef]
- Parveen, N.; Abourehab, M.A.S.; Thanikachalam, P.V.; Khar, R.K.; Kesharwani, P. Nanocrystals as an Emerging Nanocarrier for the Management of Dermatological Diseases. Colloids Surf. B Biointerfaces 2023, 225, 113231. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Shalaby, K.; Al-Sanea, M.M.; Hendawy, O.M.; Salama, A.; Ibrahim, M.F.; Ghoneim, M.M. Influence of Stabilizer on the Development of Luteolin Nanosuspension for Cutaneous Delivery: An In Vitro and In Vivo Evaluation. Pharmaceutics 2021, 13, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Park, J.S.; Kim, M.S.; Jeong, M.Y.; Park, H.J.; Choi, J.H.; Seo, J.H.; Choi, Y.S.; Kang, M.J. High-Payload Nanosuspension of Centella Asiatica Extract for Improved Skin Delivery with No Irritation. Int. J. Nanomed. 2021, 16, 7417–7432. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Smith, V.J.; Walker, R.B. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics 2020, 12, 182. [Google Scholar] [CrossRef]
- Patel, P.J.; Gajera, B.Y.; Dave, R.H. A quality-by-design study to develop Nifedipine nanosuspension: Examining the relative impact of formulation variables, wet media milling process parameters and excipient variability on drug product quality attributes. Drug Dev. Ind. Pharm. 2018, 44, 1942–1952. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; López-Romero, B.A.; Nicolás-García, M.; Nolasco-González, Y.; García-Galindo, H.S.; Montalvo-González, E. Nanosuspensions as Carriers of Active Ingredients: Chemical Composition, Development Methods, and Their Biological Activities. Food Res. Int. 2023, 174, 113583. [Google Scholar] [CrossRef]
- Kumar, P.; Verma, M. Pharmaceutical Suspensions: An Updated Patent Review on Novel Suspending Agents and Recent Advancement. Recent Adv. Drug Deliv. Formul. 2023, 17, 193–209. [Google Scholar] [CrossRef]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-Thickened Nanoemulsion System for Topical Delivery of Lipophilic Drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef]
- Singhal, M.; Baumgartner, A.; Turunen, E.; van Veen, B.; Hirvonen, J.; Peltonen, L. Nanosuspensions of a Poorly Soluble Investigational Molecule ODM-106: Impact of Milling Bead Diameter and Stabilizer Concentration. Int. J. Pharm. 2020, 587, 119636. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ho, M.J.; Joung, M.Y.; Choi, Y.S.; Kang, M.J. Effect of Dispersion Medium on Pharmacokinetic Profile of Rotigotine Crystalline Suspension Following Subcutaneous Injection. Pharmaceutics 2022, 14, 2630. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Determination of Vitamin C in Foods: Current State of Method Validation. J. Chromatogr. A 2014, 1369, 2–17. [Google Scholar] [CrossRef]
- Ro, S.-Y.; Choi, H.-M.; Choi, S.-H.; Lee, S.-W.; Lim, S.-J. Tricaprin as a Membrane Permeability Regulator: Sustained Small Hydrophilic Substance Release from Liposomes. J. Pharm. Investig. 2023, 53, 539–548. [Google Scholar] [CrossRef]
- Jung, H.M.; Kim, C.H.; Seo, J.-E.; Goo, Y.T.; Hong, S.H.; Kang, M.J.; Lee, S.; Choi, Y.W. Development of Core–Shell Structured Nanoparticle for Sequential Release of Tariquidar and Docetaxel to Overcome Multi Drug-Resistant Cancer. J. Pharm. Investig. 2023, 54, 61–75. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of Nanofluid: A Review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Yun, T.-S.; Jung, M.; Bang, K.-H.; Lee, H.-K.; Jin, M.; Yoo, H.; Won, J.-H.; Song, B.; Hwang, Y.-R.; Baek, J.-S.; et al. An Economically Advantageous Amorphous Solid Dispersion of the Fixed Combination of Lopinavir and Ritonavir. J. Pharm. Investig. 2023, 53, 549–561. [Google Scholar] [CrossRef]
- Van Nguyen, K.; Dang, T.K.; Vu, L.T.D.; Ha, N.T.; Truong, H.D.; Tran, T.H. Orodispersible Film Incorporating Nanoparticulate Loratadine for an Enhanced Oral Bioavailability. J. Pharm. Investig. 2023, 53, 417–426. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Calabro, E.; Coppolino, S.; Magazu, S. Polymeric Systems under External Thermal Stress Studied by FTIR Technique. Curr. Metabolomics 2018, 6, 46–48. [Google Scholar] [CrossRef]
- Amatya, R.; Kim, D.; Min, K.A.; Shin, M.C. Iron Oxide Nanoparticles-Loaded Hydrogels for Effective Topical Photothermal Treatment of Skin Cancer. J. Pharm. Investig. 2022, 52, 775–785. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, H.T.; Ho, M.J.; Lee, J.E.; Kim, H.T.; Kim, D.Y.; Lee, H.C.; Choi, Y.S.; Kang, M.J. Montelukast Nanocrystals for Transdermal Delivery with Improved Chemical Stability. Pharmaceutics 2020, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.K.; Wadhawan, J.; Bansal, A.K. Pharmaceutical Nanocrystals: A Promising Approach for Improved Topical Drug Delivery. Drug Discov. Today 2021, 26, 2329–2349. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Yu, Y.; Sun, W.; Fan, R.; Shi, J.; Gu, W.; Wang, Z.; Zhang, H.; Zheng, A. Review of Nanosuspension Formulation and Process Analysis in Wet Media Milling Using Microhydrodynamic Model and Emerging Characterization Methods. Int. J. Pharm. 2022, 623, 121862. [Google Scholar] [CrossRef]
- Wang, P.; Cao, X.; Chu, Y.; Wang, P. Ginkgolides-Loaded Soybean Phospholipid-Stabilized Nanosuspension with Improved Storage Stability and In Vivo Bioavailability. Colloids Surf. B 2019, 181, 910–917. [Google Scholar] [CrossRef]
- Kolliphor® RH40. Safety Data Sheet. Available online: https://www.pharmaexcipients.com/product/kolliphor-rh-40/ (accessed on 16 January 2024).
- Rabanel, J.-M.; Hildgen, P.; Banquy, X. Assessment of PEG on Polymeric Particles Surface, a Key Step in Drug Carrier Translation. J. Control. Release. 2014, 185, 71–87. [Google Scholar] [CrossRef]
- Ali, Y.; Kimura, A.; Coffey, M.J.; Tyle, P. Pharmaceutical Development of Suspension Dosage Form. In Pharmaceutical Suspensions: From Formulation Development to Manufacturing; Kulshreshtha, A.K., Singh, O.N., Wall, G.M., Eds.; Springer: New York, NY, USA, 2010; pp. 103–126. ISBN 978-1-4419-1087-5. [Google Scholar]
- Serpen, A.; Gökmen, V. Reversible Degradation Kinetics of Ascorbic Acid under Reducing and Oxidizing Conditions. Food Chem. 2007, 104, 721–725. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chakraborty, I.; Ghosh, S. The Methods of Determination of Critical Micellar Concentrations of the Amphiphilic Systems in Aqueous Medium. Arab. J. Chem. 2011, 4, 265–270. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and Characterization of Film-Forming Voriconazole Transdermal Spray for the Treatment of Fungal Infection. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Sritharadol, R.; Nakpheng, T.; Wan Sia Heng, P.; Srichana, T. Development of a Topical Mupirocin Spray for Antibacterial and Wound-Healing Applications. Drug Dev. Ind. Pharm. 2017, 43, 1715–1728. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling Behavior of Carboxymethylcellulose Hydrogels in Relation to Cross-Linking, pH, and Charge Density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- VanGinkel, C.G.; Gayton, S. The Biodegradability and Nontoxicity of Carboxymethyl Cellulose (DS 0.7) and Intermediates. Environ. Toxicol. Chem. 1996, 15, 270–274. [Google Scholar] [CrossRef]
- Kuk, D.-H.; Ha, E.-S.; Ha, D.-H.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.; Park, H.; Choi, D.H.; et al. Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach. Pharmaceutics 2019, 11, 688. [Google Scholar] [CrossRef]
- Palmer, D.; Levina, M.; Nokhodchi, A.; Douroumis, D.; Farrell, T.; Rajabi-Siahboomi, A. The Influence of Sodium Carboxymethylcellulose on Drug Release from Polyethylene Oxide Extended Release Matrices. AAPS PharmSciTech 2011, 12, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Kolliphor RH 40. Available online: https://www.sigmaaldrich.com/KR/ko/product/sigma/07076 (accessed on 16 January 2024).
- Yadav, M.G.; Kavadia, M.R.; Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Production of 6-O-l-Ascorbyl Palmitate by Immobilized Candida Antarctica Lipase B. Appl. Biochem. Biotechnol. 2018, 184, 1168–1186. [Google Scholar] [CrossRef] [PubMed]
- Better, M. MIGLYOL® 812 N (F), Products Information. IOI Oleo GmbH. Available online: https://www.ioioleo.de/en/products/nutrition/miglyol-812-n-f/ (accessed on 5 December 2023).
- Labrasol®—Gattefossé. Available online: https://www.gattefosse.com/pharmaceuticals/product-finder/labrasol (accessed on 16 January 2024).
- Plurol® Oleique CC 497—Gattefossé. Available online: https://www.gattefosse.com/pharmaceuticals/product-finder/plurol-oleique-cc-497 (accessed on 16 January 2024).
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Pınar, S.G.; Canpınar, H.; Tan, Ç.; Çelebi, N. A New Nanosuspension Prepared with Wet Milling Method for Oral Delivery of Highly Variable Drug Cyclosporine A: Development, Optimization and In Vivo Evaluation. Eur. J. Pharm. Sci. 2022, 171, 106123. [Google Scholar] [CrossRef]
- Hammond, S.A.; Tsonis, C.; Sellins, K.; Rushlow, K.; Scharton-Kersten, T.; Colditz, I.; Glenn, G.M. Transcutaneous Immunization of Domestic Animals: Opportunities and Challenges. Adv. Drug Deliv. Rev. 2000, 43, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Debeer, S.; Le Luduec, J.-B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.-F.; Dubois, B.; Kanitakis, J. Comparative Histology and Immunohistochemistry of Porcine versus Human Skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef]
- Meyer, W.; Schwarz, R.; Neurand, K. The Skin of Domestic Mammals as a Model for the Human Skin, with Special Reference to the Domestic Pig. Curr. Probl. Dermatol. 1978, 7, 39–52. [Google Scholar] [CrossRef]
- Vardaxis, N.J.; Brans, T.A.; Boon, M.E.; Kreis, R.W.; Marres, L.M. Confocal Laser Scanning Microscopy of Porcine Skin: Implications for Human Wound Healing Studies. J. Anat. 1997, 190, 601–611. [Google Scholar] [CrossRef]
- Müller, R.H.; Zhai, X.; Romero, G.B.; Keck, C.M. Nanocrystals for Passive Dermal Penetration Enhancement. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 283–295. ISBN 978-3-662-47862-2. [Google Scholar]
- Poet, T.S.; McDougal, J.N. Skin Absorption and Human Risk Assessment. Chem. Biol. Interact. 2002, 140, 19–34. [Google Scholar] [CrossRef]
- Oktay, A.N.; Karakucuk, A.; Ilbasmis-Tamer, S.; Celebi, N. Dermal Flurbiprofen Nanosuspensions: Optimization with Design of Experiment Approach and In Vitro Evaluation. Eur. J. Pharm. Sci. 2018, 122, 254–263. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Lu, S.; Zeng, L.; Wang, Y.; Song, W.; Liu, J. Pramipexole Nanocrystals for Transdermal Permeation: Characterization and Its Enhancement Micro-Mechanism. Eur. J. Pharm. Sci. 2018, 124, 80–88. [Google Scholar] [CrossRef]
- Mishra, R.; Prabhavalkar, K.S.; Bhatt, L.K. Preparation, Optimization, and Evaluation of Zaltoprofen-Loaded Microemulsion and Microemulsion-Based Gel for Transdermal Delivery. J. Liposome Res. 2016, 26, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ascorbyl Palmitate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/54680660 (accessed on 16 January 2024).
- Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Pjanović, R.; Bezbradica, D. Enzymatically Derived Oil-Based L-Ascorbyl Esters: Synthesis, Antioxidant Properties and Controlled Release from Cosmetic Formulations. Sustain. Chem. Pharm. 2020, 15, 100231. [Google Scholar] [CrossRef]
- Gosenca, M.; Gašperlin, M. Dermal Delivery of Ascorbyl Palmitate: The Potential of Colloidal Delivery Systems. J. Drug Deliv. Sci. Technol. 2011, 21, 535–537. [Google Scholar] [CrossRef]
- Holm, G.; Kylberg-Hanssen, K.; Svensson, L. Use of Dodecyl Sulfate as an Esterase Inhibitor before Gas-Chromatographic Determination of Labile Beta-Adrenoceptor Blocking Drugs. Clin. Chem. 1985, 31, 868–870. [Google Scholar] [CrossRef]
- Carruthers, C.; Baumler, A. The Influence of Various Detergents on the Esterase and Glucose-6-Phosphate Activities of Mouse Liver Microsomes. Arch. Biochem. Biophys. 1962, 99, 458–465. [Google Scholar] [CrossRef]
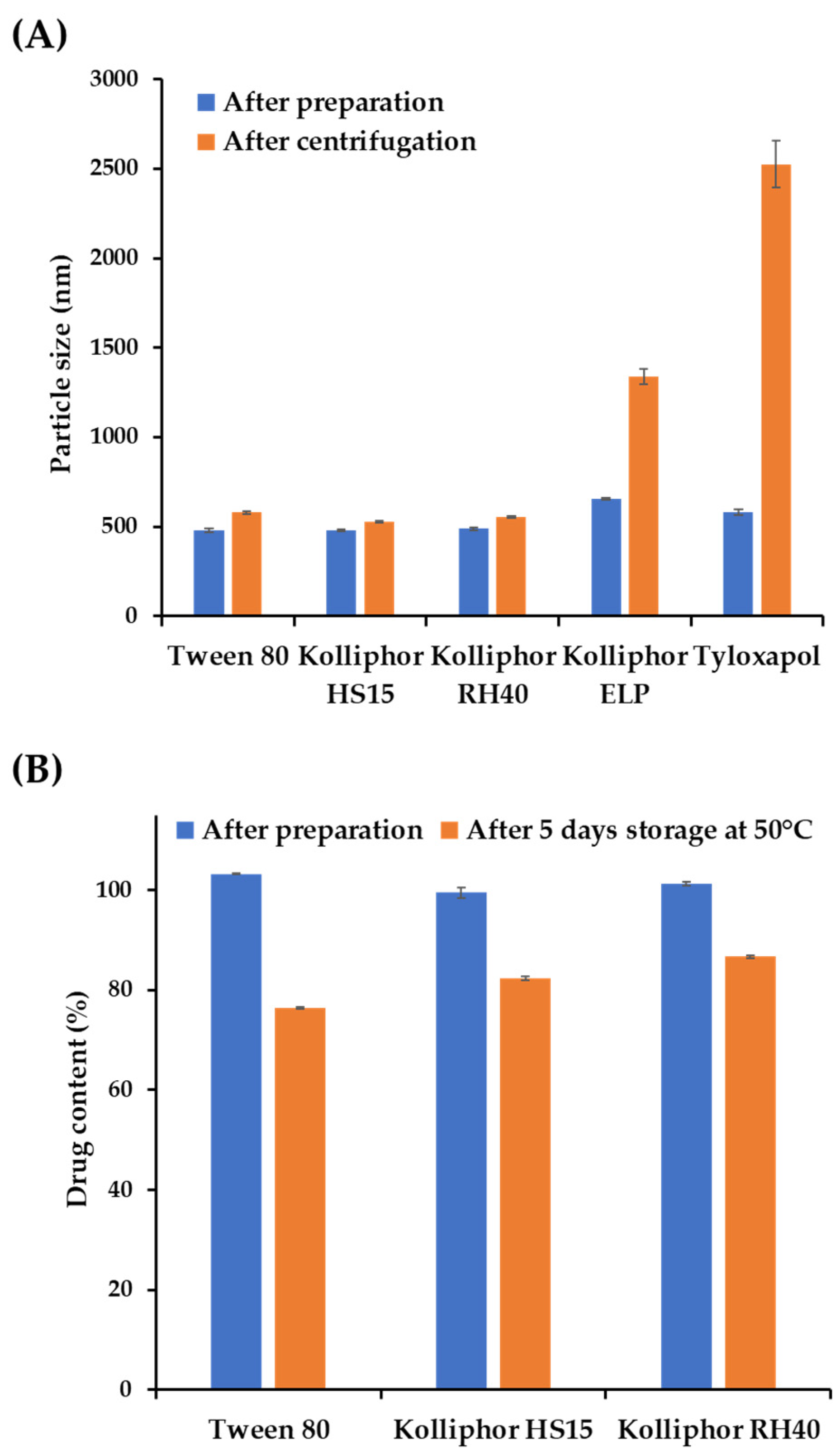
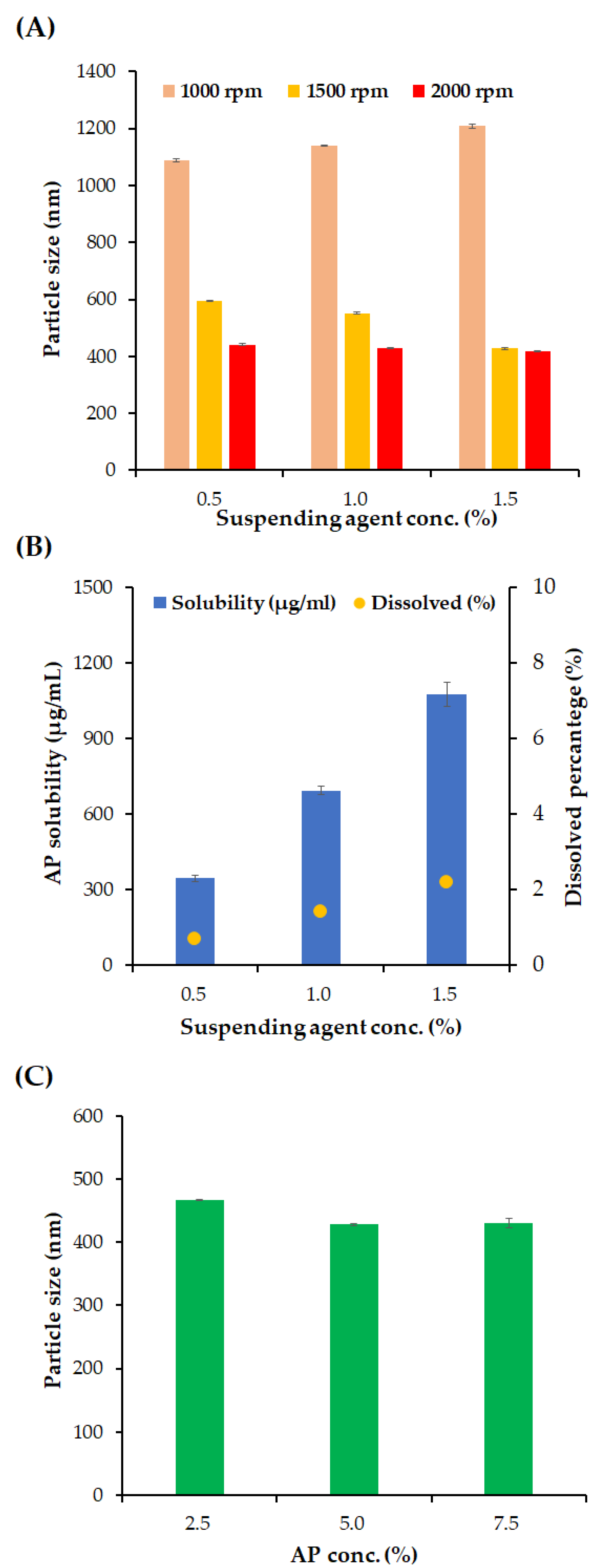
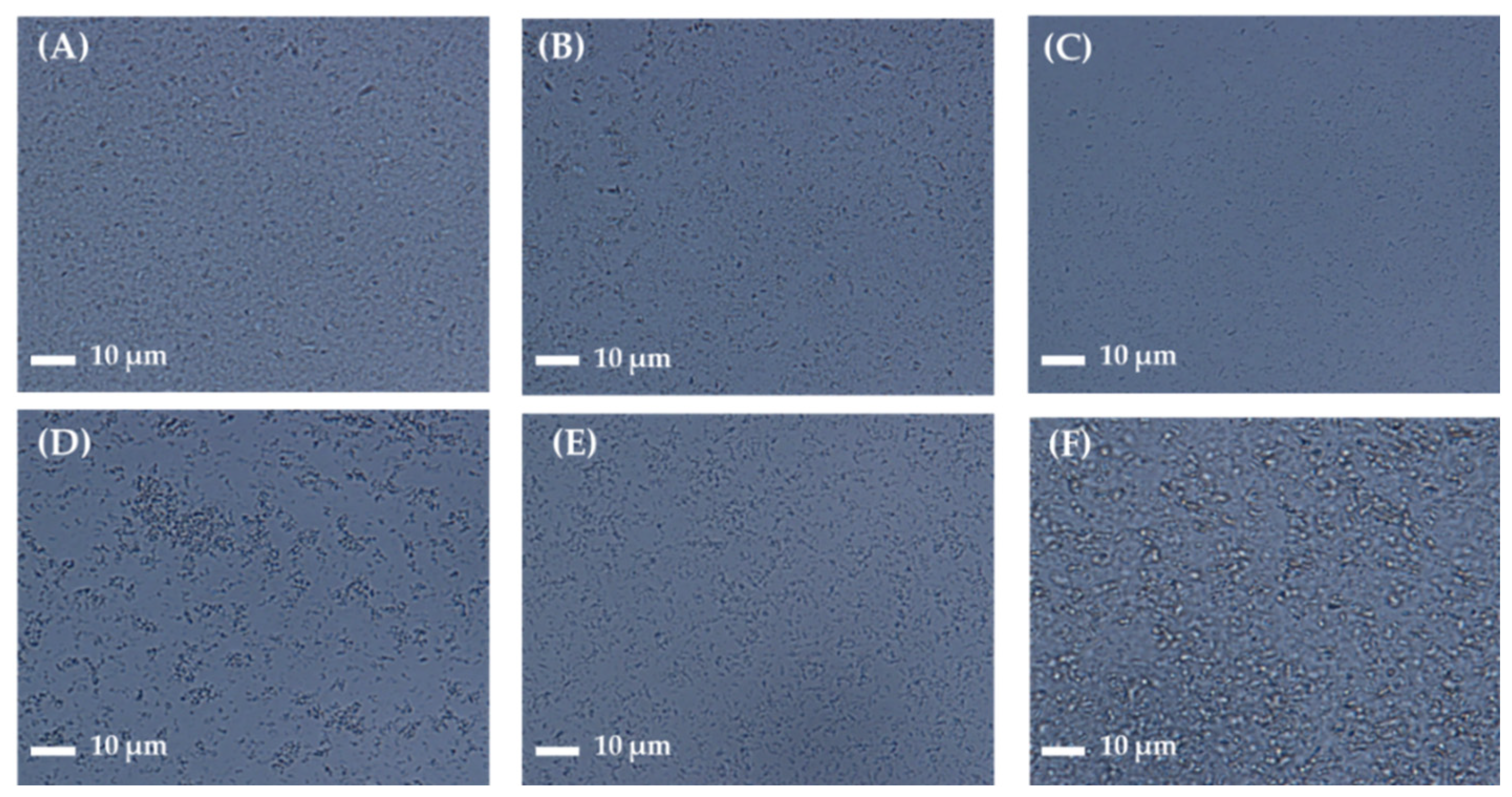
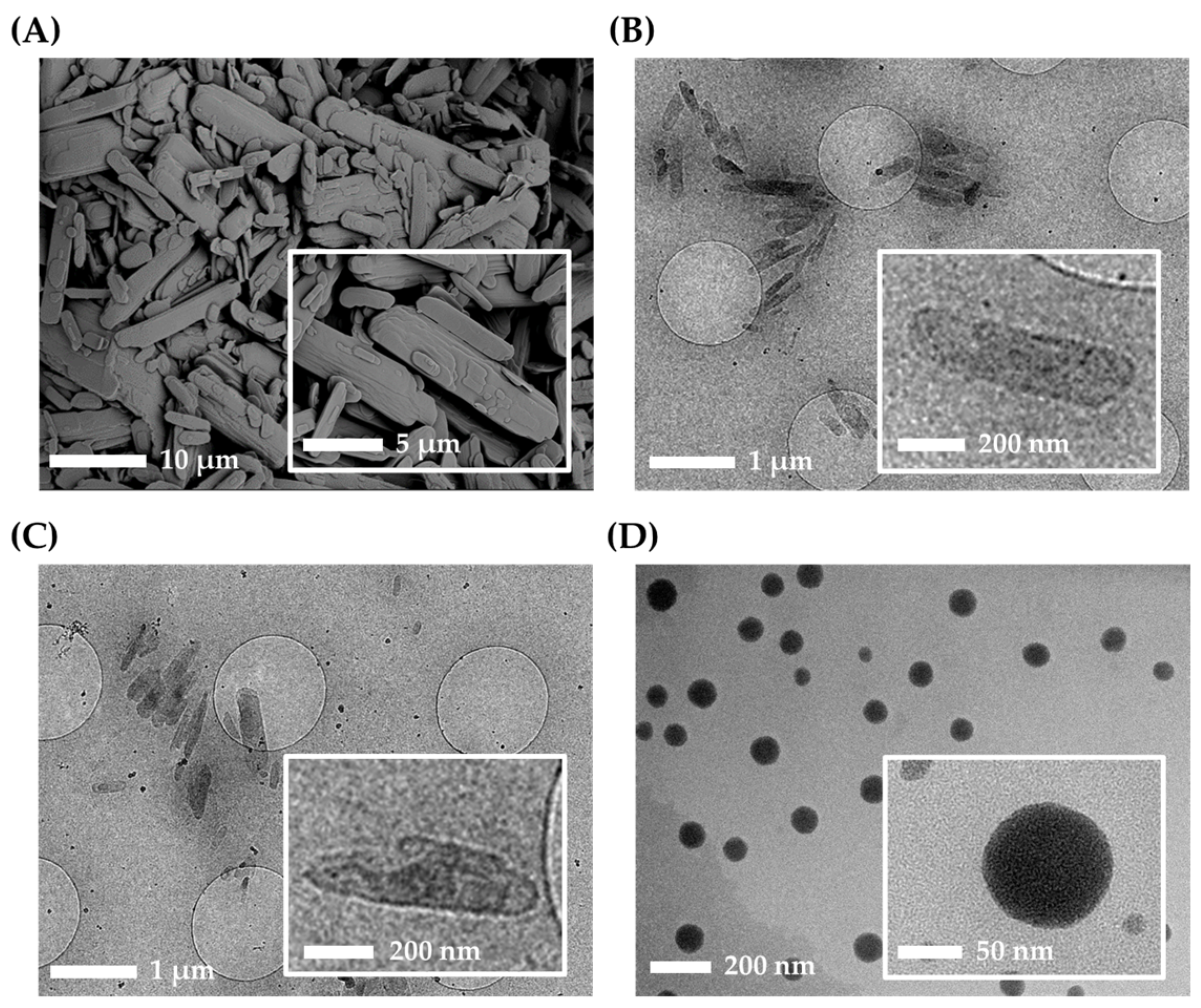

| Stabilizer a | Appearance | Particle Size (nm) b,c | Homogeneity b,d |
|---|---|---|---|
| Surfactants | |||
| Tween 80 | Flowable, homogeneous | 479.70 ± 8.50 | 0.31 ± 0.04 |
| Kolliphor® HS15 | Flowable, homogeneous | 479.47 ± 4.26 | 0.21 ± 0.02 |
| Kolliphor® RH40 | Flowable, homogeneous | 488.13 ± 7.21 | 0.26 ± 0.02 |
| Kolliphor® ELP | Flowable, homogeneous | 655.63 ± 5.42 | 0.25 ± 0.01 |
| Tyloxapol | Viscous, homogeneous | 582.07 ± 14.9 | 0.21 ± 0.05 |
| Poloxamer® 188 | Flowable, homogeneous | 534.75 ± 2.65 | 0.28 ± 0.01 |
| Hydrophilic polymers | |||
| PEG 4000 | Viscous, homogeneous | 2856.3 ± 95.6 | 0.43 ± 0.06 |
| PVP K17 | Viscous, homogeneous | 771.8 ± 11.0 | 0.22 ± 0.01 |
| Na.CMC 90 K | Viscous, homogeneous | 2326.7 ± 60.3 | 0.04 ± 0.01 |
| Parameters | NS | NS-G | ME-G |
|---|---|---|---|
| AP conc. (mg/mL) a | 49.69 ± 0.01 | 49.74 ± 0.01 | 52.75 ± 0.03 |
| Suspended (mg/mL) | 49.34 ± 0.01 | 49.41 ± 0.01 | N.A. e |
| Dissolved (mg/mL) | 0.35 ± 0.01 | 0.33 ± 0.01 | 52.75 ± 0.03 |
| Particle size (nm) a | 489.5 ± 2.6 b | 493.2 ± 2.3 b | 103.1 ± 1.7 b |
| Homogeneity a | 0.11 ± 0.03 c | 0.11 ± 0.03 c | 0.272 ± 0.03 c |
| Zeta potential (mV) a | −33.0 ± 0.7 a | −48.7 ± 0.5 a | −41.0 ± 4.9 a |
| pH | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.4 ± 0.1 |
| Viscosity (cP) d | 0.13 | 2.30 | 7.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Choi, J.-H.; Joung, M.-Y.; Yang, I.-G.; Choi, Y.-S.; Kang, M.-J.; Ho, M.-J. Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery. Pharmaceutics 2024, 16, 171. https://doi.org/10.3390/pharmaceutics16020171
Park J-S, Choi J-H, Joung M-Y, Yang I-G, Choi Y-S, Kang M-J, Ho M-J. Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery. Pharmaceutics. 2024; 16(2):171. https://doi.org/10.3390/pharmaceutics16020171
Chicago/Turabian StylePark, Jun-Soo, Jun-Hyuk Choi, Min-Yeong Joung, In-Gyu Yang, Yong-Seok Choi, Myung-Joo Kang, and Myoung-Jin Ho. 2024. "Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery" Pharmaceutics 16, no. 2: 171. https://doi.org/10.3390/pharmaceutics16020171
APA StylePark, J.-S., Choi, J.-H., Joung, M.-Y., Yang, I.-G., Choi, Y.-S., Kang, M.-J., & Ho, M.-J. (2024). Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery. Pharmaceutics, 16(2), 171. https://doi.org/10.3390/pharmaceutics16020171





