Application of Microsponge Drug Platform to Enhance Methotrexate Administration in Rheumatoid Arthritis Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Microsponge
2.3. Loading Studies
2.4. Endotoxin Detection
2.5. Particle Size, Surface Charge Analysis, and Morphology Studies
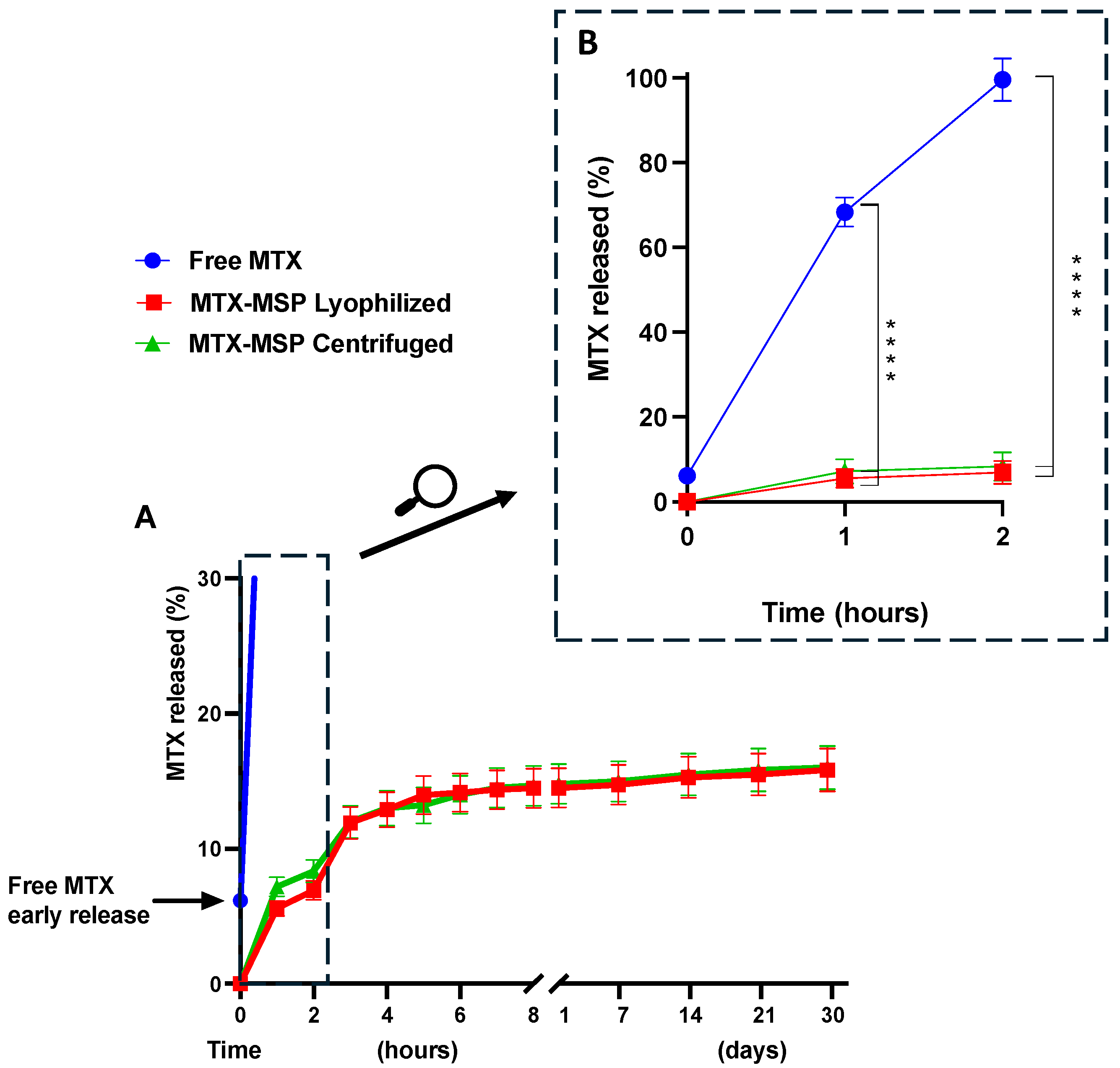
2.6. In Vitro Release Profile of MTX from the Microsponge
2.7. Ethical Approval
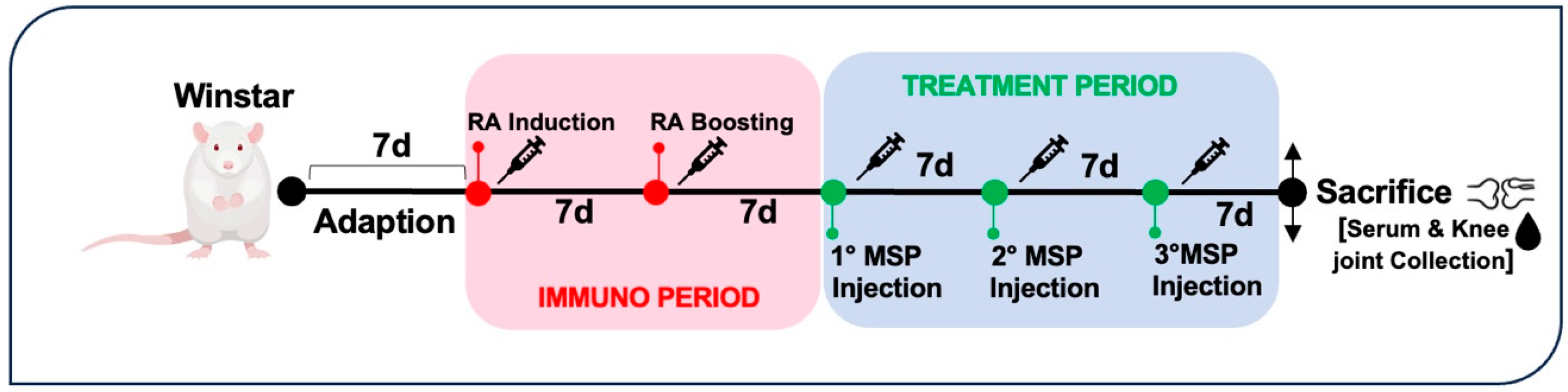
2.8. Animal Husbandry
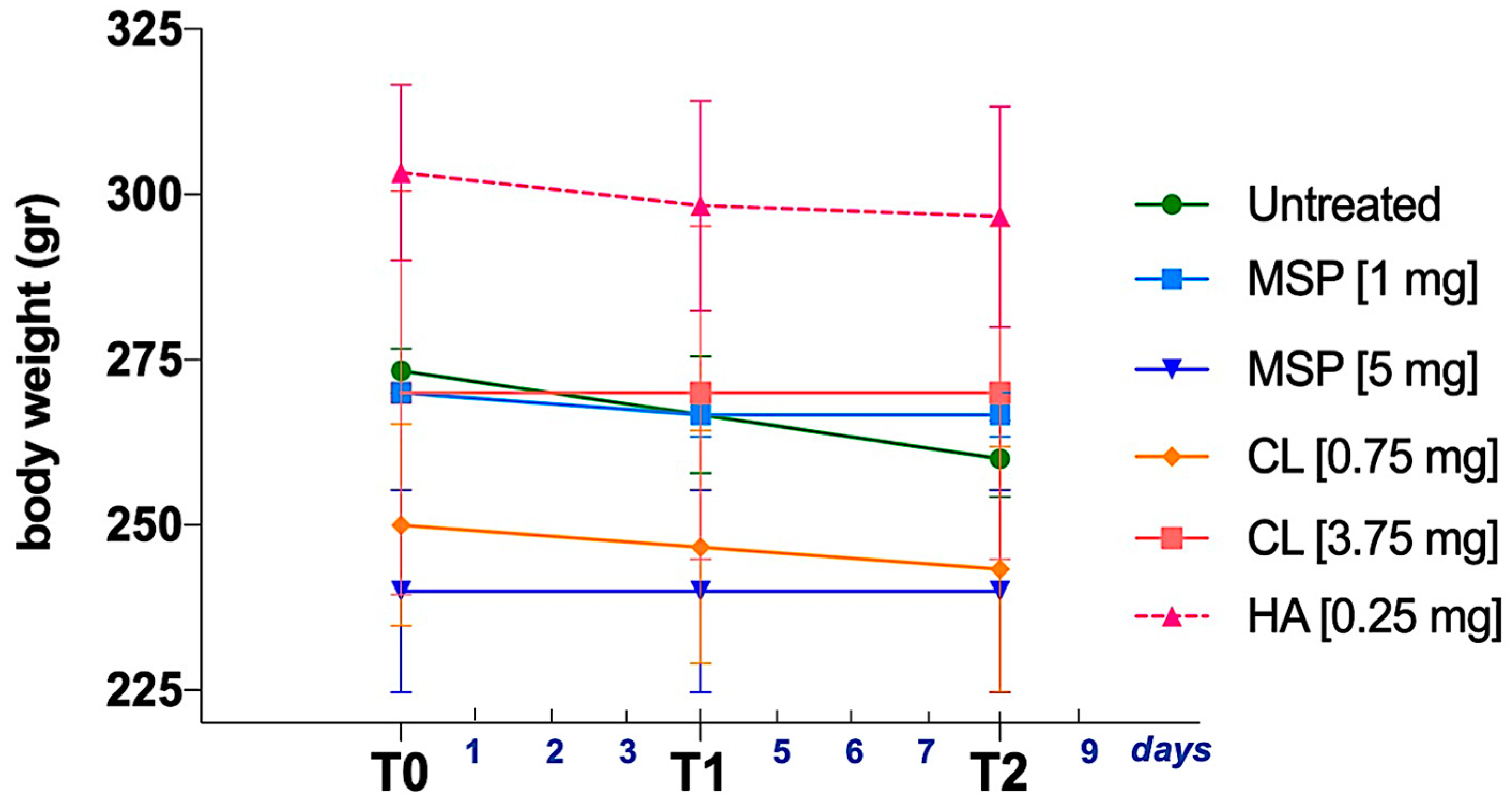
2.9. Animal Treatment for Safety Study
- (i)
- Control group 0.8 mL of normal saline (NS) as vehicle;
- (ii)
- MSP 1 mg/rat/0.8 mL in NS;
- (iii)
- MSP 5 mg/rat/0.8 mL in NS;
- (iv)
- Cross-linker 0.75 mg/rat/0.8 mL in NS;
- (v)
- Cross-linker 3.75 mg/rat/0.8 mL in NS;
- (vi)
- Hyaluronic acid 0.25 mg/rat/0.8 mL in NS.
2.10. Animal Treatment for Efficacy Study
- (i)
- Healthy non-arthritic as negative control (3 rats);
- (ii)
- Arthritic untreated as positive control (4 rats).
- (iii)
- MTX 0.125 mg/rat in 0.1 mL NS for the MTX group (3 rats);
- (iv)
- MSP 1 mg/rat in 0.1 mL NS; for the MSP group (4 rats);
- (v)
- 1 mg/rat in 0.1 mL NS; for the MTX-loaded MSP lyophilized group (3 rats).
- (i)
- Positive control group: untreated arthritic rats;
- (ii)
- MSP group: arthritic rats treated with 1.5 mg MSP/rat in 0.1 mL NS;
- (iii)
- MTX group: arthritic rats treated with 0.125 mg MTX/rat in 0.1 mL NS;
- (iv)
- MTX-MSP centrifuged group: arthritic rats treated with 1.5 mg MTX-MSP centrifuged/rat in 0.1 mL NS;
- (v)
- MTX-MPS lyophilized group: arthritic rats treated with 1.5 mg MTX-MSP lyophilized/rat in 0.1 mL NS.
2.11. Measurement of Hematological Parameters
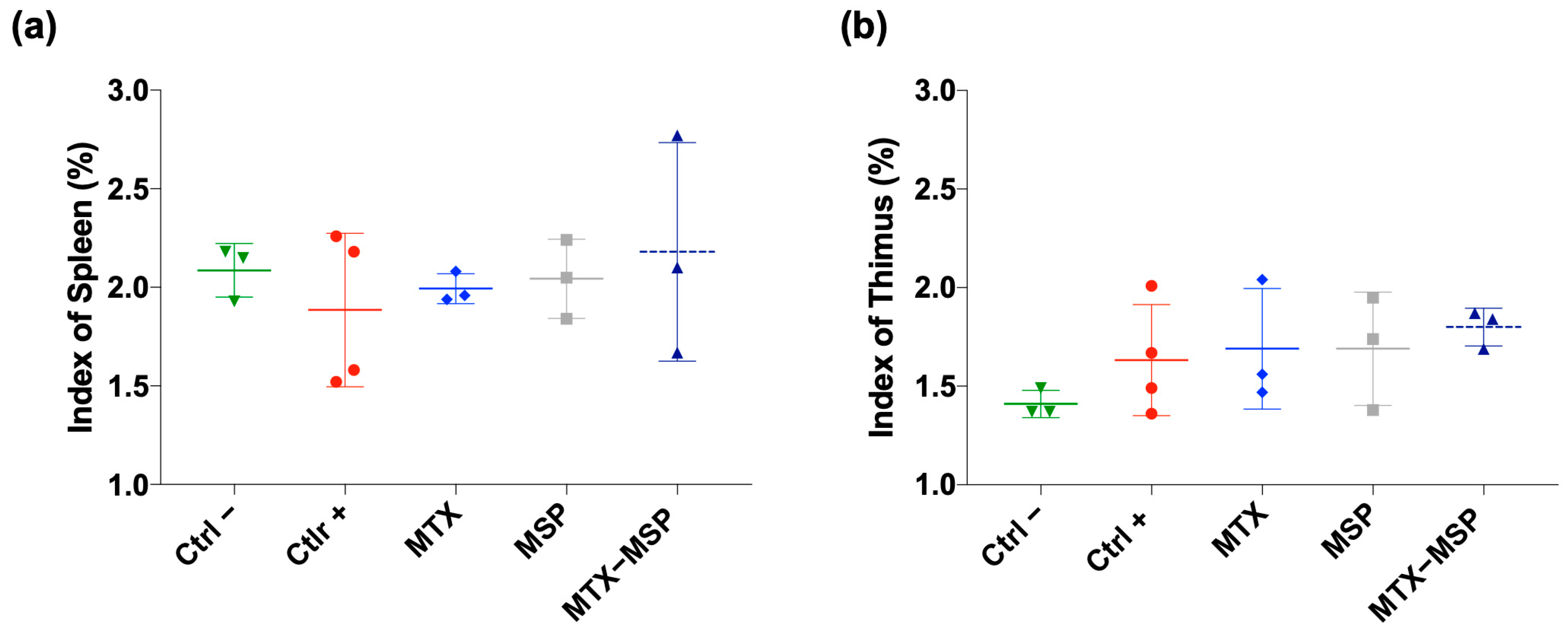
2.12. Detection of Spleen Index and Thymus Index
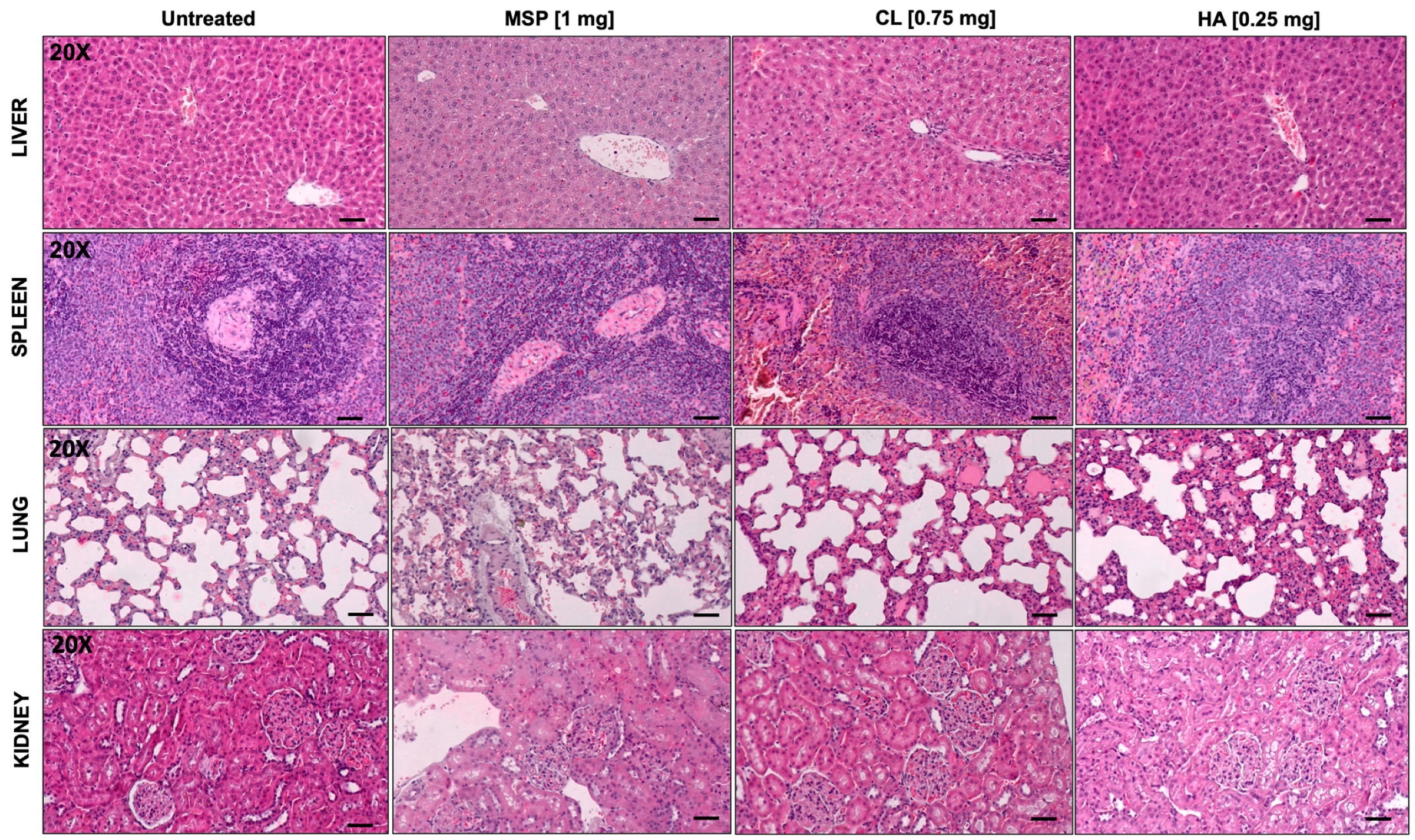
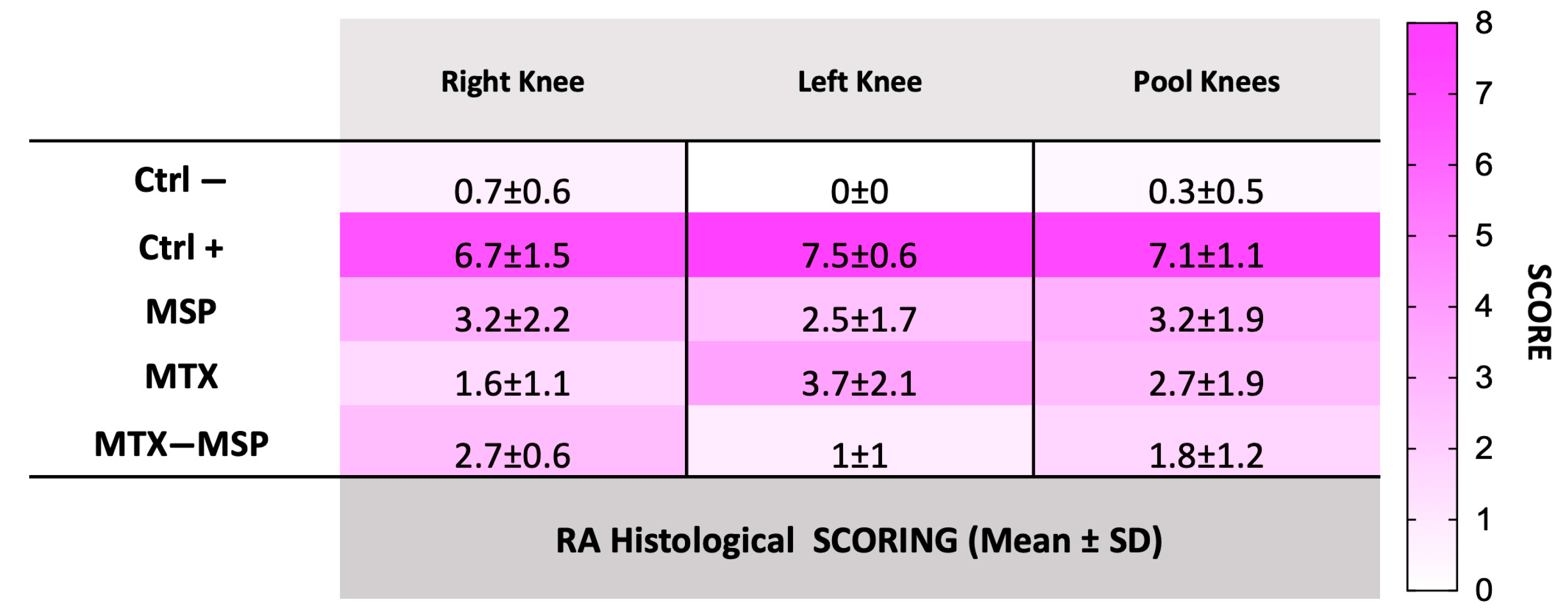
2.13. Histological Analysis
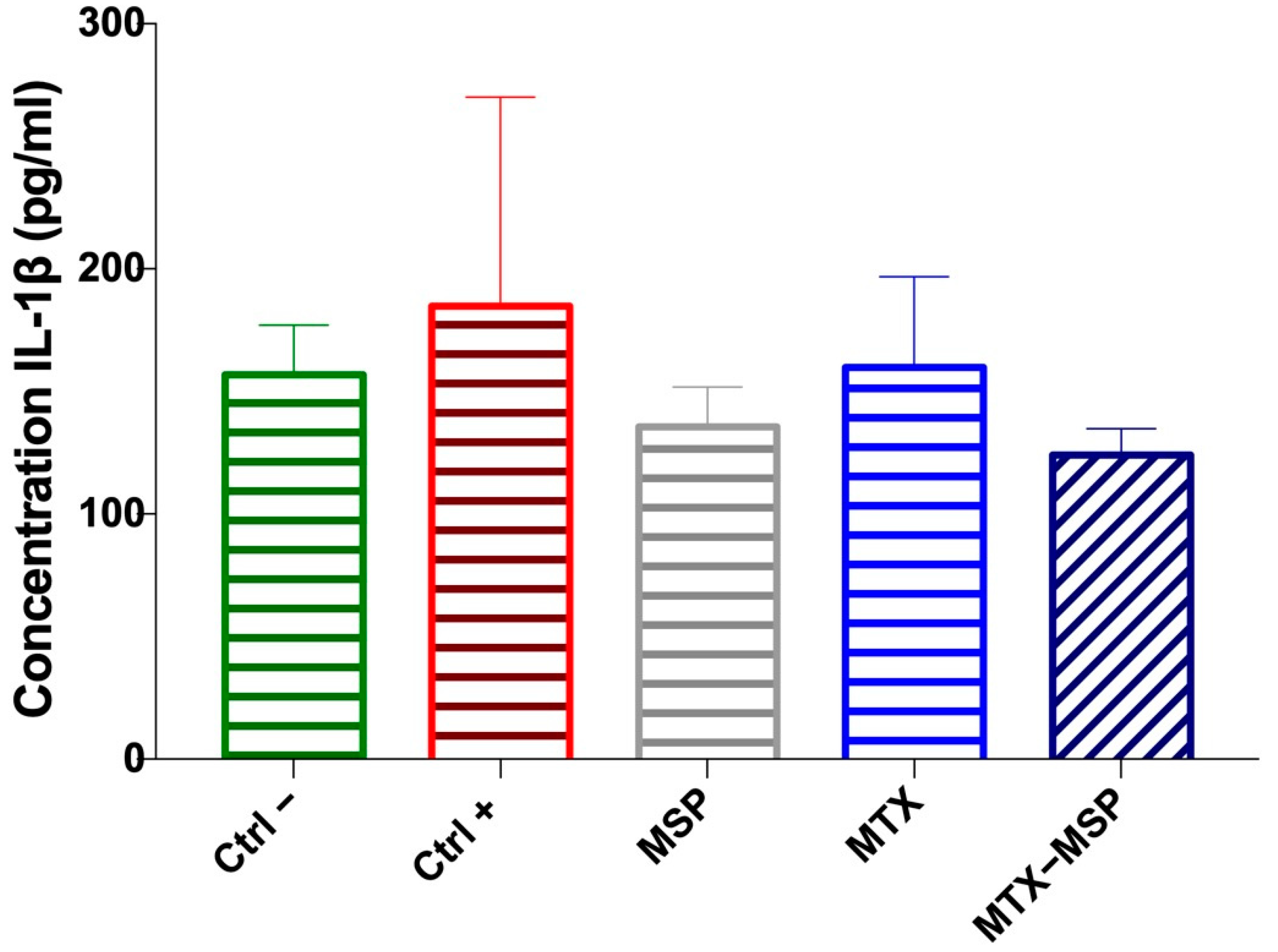
2.14. Cytokine and Antibody α-Collagen ELISA Assays
2.15. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characterization of Unloaded and Loaded Microsponge
3.2. MTX Release Studies from the Microsponge
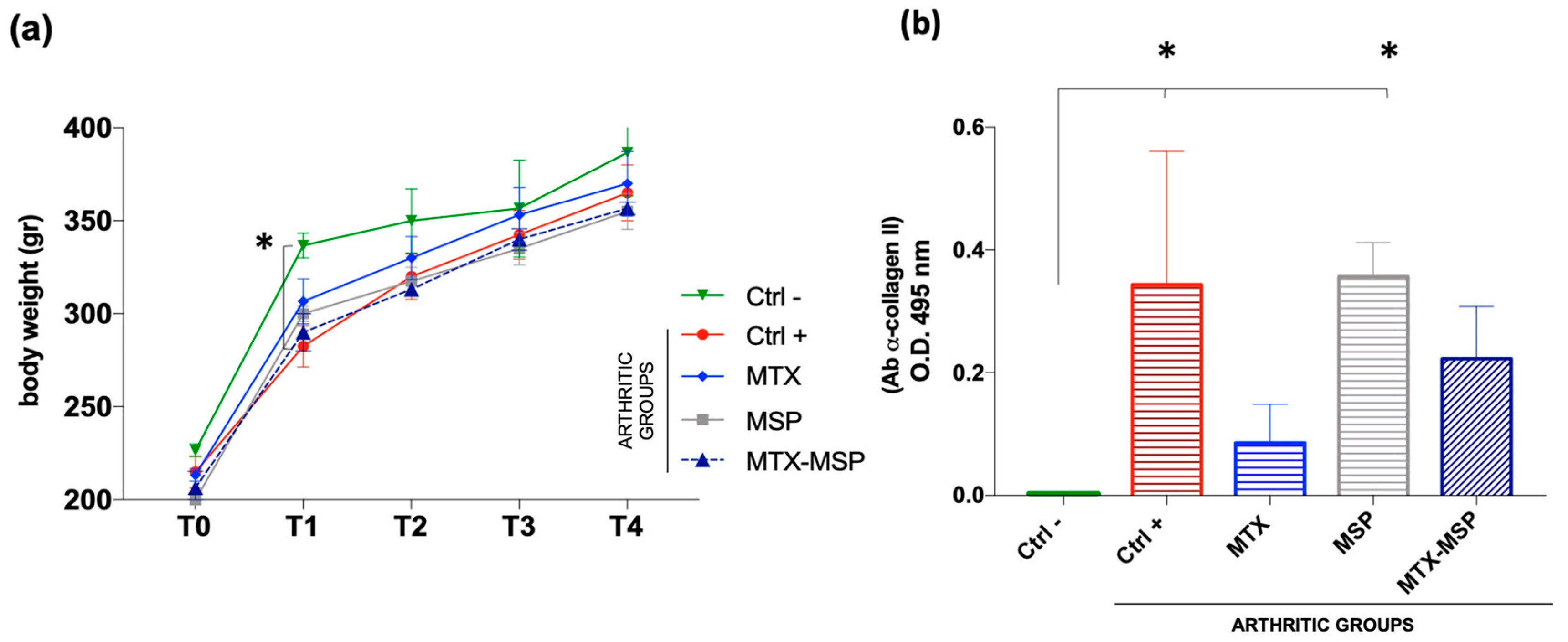
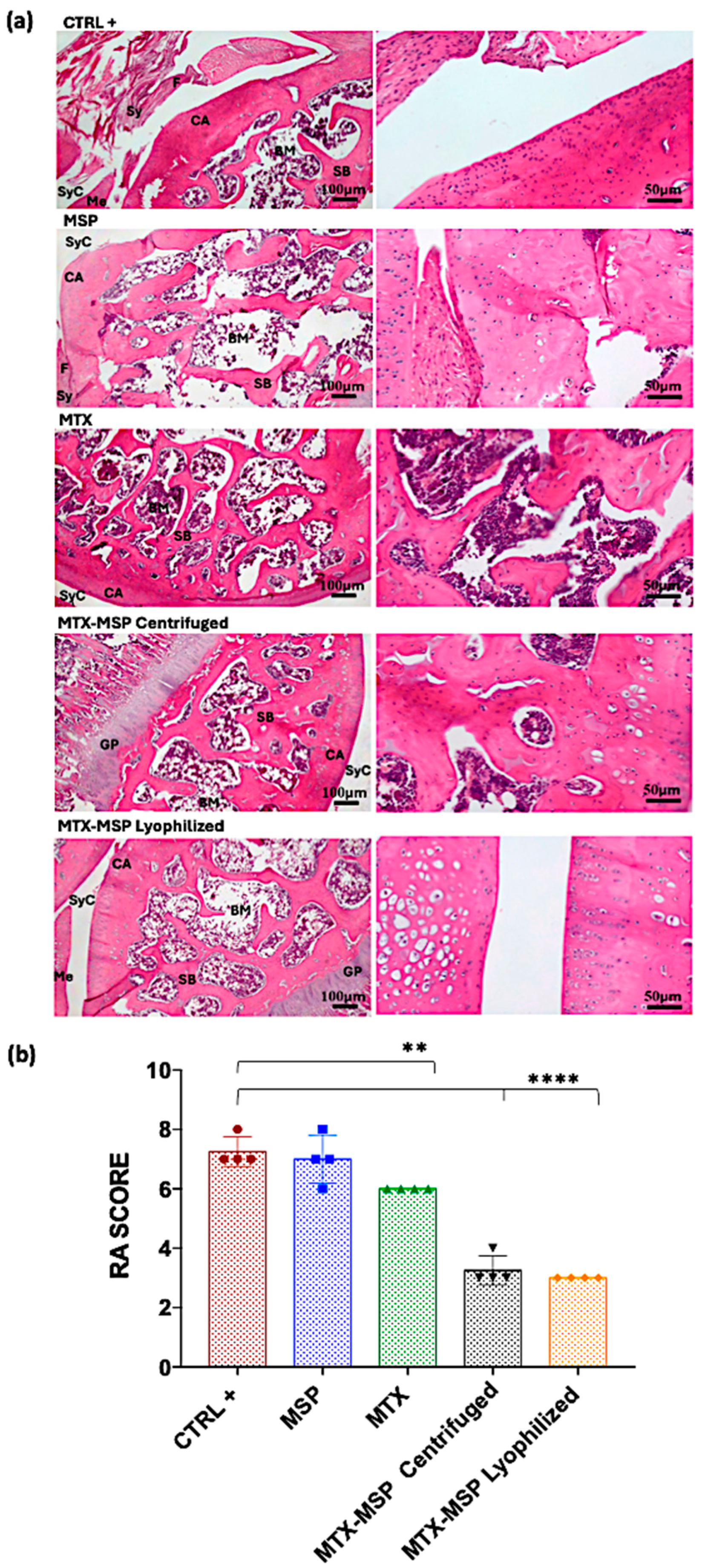
3.3. In Vivo Safety Study
3.4. In Vivo Efficacy Study
3.5. A Clinical Perspective
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, Y.; Shi, F.; Wang, J.; Ning, Q.; Zhang, Z.; Lv, H. Sulfated hyaluronic acid gel for the treatment of rheumatoid arthritis in rats. Int. J. Biol. Macromol. 2024, 256, 128537. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.E.; Kaabachi, W.; Sassi, N.; Tarhouni, L.; Rekik, S.; Jemmali, S.; Sehli, H.; Kallel-Sellami, M.; Cheour, E.; Laadhar, L. The synovial fluid fibroblast-like synoviocyte: A long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front. Immunol. 2022, 13, 942417. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liao, X.; Lin, Z.; Liu, W.; Luo, X.; Zhan, H.; Cai, X. Estimation of the global prevalence, incidence, years lived with disability of rheumatoid arthritis in 2019 and forecasted incidence in 2040: Results from the Global Burden of Disease Study 2019. Clin. Rheumatol. 2023, 42, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Bui, L.; Fedele, A.L.; Di Mario, C.; Benvenuto, R.; Federico, F.; Ferraccioli, G. Overweight/obesity affects histological features and inflammatory gene signature of synovial membrane of rheumatoid arthritis. Sci. Rep. 2019, 9, 10420. [Google Scholar] [CrossRef]
- Suresh, P.; Salem-Bekhit, M.M.; Veedu, H.P.; Alshehri, S.; Nair, S.C.; Bukhari, S.I.; Viswanad, V.; Taha, E.I.; Sahu, R.K.; Ghoneim, M.M. Development of a novel methotrexate-loaded nanoemulsion for rheumatoid arthritis treatment with site-specific targeting subcutaneous delivery. Nanomaterials 2022, 12, 1299. [Google Scholar] [CrossRef]
- Talsania, M.; Scofield, R.H. Menopause and rheumatic disease. Rheum. Dis. Clin. N. Am. 2017, 43, 287. [Google Scholar] [CrossRef]
- Brown, P.; Pratt, A.G.; Hyrich, K.L. Therapeutic advances in rheumatoid arthritis. BMJ 2024, 384, e070856. [Google Scholar] [CrossRef]
- Shinde, C.G.; Venkatesh, M.; Kumar, T.P.; Shivakumar, H. Methotrexate: A gold standard for treatment of rheumatoid arthritis. J. Pain Palliat. Care Pharmacother. 2014, 28, 351–358. [Google Scholar] [CrossRef]
- McCann, L. Efficacy and safety of oral and parenteral methotrexate therapy in children with juvenile idiopathic arthritis: An observational study with patients from the German Methotrexate Registry. Curr. Med. Lit. Rheumatol. 2013, 32, 1. [Google Scholar]
- Wilsdon, T.D.; Hill, C.L. Managing the drug treatment of rheumatoid arthritis. Aust. Prescr. 2017, 40, 51. [Google Scholar] [CrossRef]
- Maetzel, A.; Wong, A.; Strand, V.; Tugwell, P.; Wells, G.; Bombardier, C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology 2000, 39, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Breedveld, F.; Lemmel, E.; Kaltwasser, J.; Dawes, P.; Gömör, B.; Van den Bosch, F.; Nordström, D.; Bjørneboe, O.; Dahl, R. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology 2000, 39, 655–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bello, A.E.; Perkins, E.L.; Jay, R.; Efthimiou, P. Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatol. Res. Rev. 2017, 9, 67–79. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Aboelez, M.O.; Elsadek, B.E.; Sarhan, H.A.; Khaled, K.A.; Belal, A.; Khames, A.; Hassan, Y.A.; Abdel-Rheem, A.A.; Elkaeed, E.B. Tolmetin sodium fast dissolving tablets for rheumatoid arthritis treatment: Preparation and optimization using Box-Behnken design and response surface methodology. Pharmaceutics 2022, 14, 880. [Google Scholar] [CrossRef]
- Branco, J.C.; Barcelos, A.; de Araújo, F.P.; Sequeira, G.; Cunha, I.; Patto, J.V.; Oliveira, M.; Mateus, M.P.; Couto, M.; Nero, P. Utilization of subcutaneous methotrexate in rheumatoid arthritis patients after failure or intolerance to oral methotrexate: A multicenter cohort study. Adv. Ther. 2016, 33, 46–57. [Google Scholar] [CrossRef]
- Taylor, P.C.; Balsa Criado, A.; Mongey, A.-B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient?—MTX in the treat-to-target strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef]
- Vena, G.A.; Cassano, N.; Iannone, F. Update on subcutaneous methotrexate for inflammatory arthritis and psoriasis. Ther. Clin. Risk Manag. 2018, 14, 105–116. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Schiff, M.H.; Jaffe, J.S.; Freundlich, B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: Drug-exposure limitations of oral methotrexate at doses≥ 15 mg may be overcome with subcutaneous administration. Ann. Rheum. Dis. 2014, 73, 1549–1551. [Google Scholar] [CrossRef]
- Bechard, M.A.; Lemieux, J.R.; Roth, J.; Watanabe Duffy, K.; Duffy, C.M.; Aglipay, M.O.; Jurencak, R. Procedural pain and patient-reported side effects with weekly injections of subcutaneous methotrexate in children with rheumatic disorders. Pediatr. Rheumatol. 2014, 12, 54. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, N.K.; Jain, A.; Khopade, A.; Pandey, P.; Sawant, K.K. Nanocarriers mediated delivery of methotrexate is instrumental in treating auto-immune diseases and cancer. J. Drug Deliv. Sci. Technol. 2023, 88, 104969. [Google Scholar] [CrossRef]
- Gandhi, S.; Shende, P. Anti-CD64 Antibody-Conjugated PLGA Nanoparticles Containing Methotrexate and Gold for Theranostics Application in Rheumatoid Arthritis. AAPS PharmSciTech 2024, 25, 22. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Loureiro, A.; Lager, F.; Renault, G.; Cavaco-Paulo, A.; Nogueira, E. Increased encapsulation efficiency of methotrexate in liposomes for rheumatoid arthritis therapy. Biomedicines 2020, 8, 630. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Liu, R.; Zhao, Y.; Sun, L. Hierarchical Microcarriers Loaded with Peptide Dendrimer-Grafted Methotrexate for Rheumatoid Arthritis Treatment. Small Sci. 2024, 4, 2300097. [Google Scholar] [CrossRef]
- Liang, L.S.; Jackson, J.; Min, W.; Risovic, V.; Wasan, K.M.; Burt, H.M. Methotrexate loaded poly (L-lactic acid) microspheres for intra-articular delivery of methotrexate to the joint. J. Pharm. Sci. 2004, 93, 943–956. [Google Scholar] [CrossRef]
- Singh, R.; Jadhav, K.; Kamboj, R.; Malhotra, H.; Ray, E.; Jhilta, A.; Dhir, V.; Verma, R.K. Self-actuating inflammation responsive hydrogel microsphere formulation for controlled drug release in rheumatoid arthritis (RA): Animal trials and study in human fibroblast like synoviocytes (hFLS) of RA patients. Biomater. Adv. 2024, 160, 213853. [Google Scholar] [CrossRef]
- Xu, M.; Fu, T.; Zhang, C.; An, Z.; Yan, J.; Lu, Z.; Wu, H.; Liu, J.; Qiu, L.; Shi, L. Prolonged, staged, and self-regulated methotrexate release coupled with ROS scavenging in an injectable hydrogel for rheumatoid arthritis therapy. J. Control. Release 2024, 375, 60–73. [Google Scholar] [CrossRef]
- Dhanka, M.; Shetty, C.; Srivastava, R. Injectable methotrexate loaded polycaprolactone microspheres: Physicochemical characterization, biocompatibility, and hemocompatibility evaluation. Mater. Sci. Eng. C 2017, 81, 542–550. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1130. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Zhuang, L. Natural biomimetic nano-system for drug delivery in the treatment of rheumatoid arthritis: A literature review of the last 5 years. Front. Med. 2024, 11, 1385123. [Google Scholar] [CrossRef]
- Tiwari, A.; Tiwari, V.; Palaria, B.; Kumar, M.; Kaushik, D. Microsponges: A breakthrough tool in pharmaceutical research. Future J. Pharm. Sci. 2022, 8, 31. [Google Scholar] [CrossRef]
- Cavalieri, F.; Rinaldi, A. Nanoporous Microsponge Particles (nmp) of Biocompatible Polymers as Universal Carriers for Biomolecules Delivery. U.S. Patent Application 17/634,660, 13 October 2022. [Google Scholar]
- Vyas, A.; Saraf, S.; Saraf, S. Encapsulation of cyclodextrin complexed simvastatin in chitosan nanocarriers: A novel technique for oral delivery. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 251–259. [Google Scholar] [CrossRef]
- Obiedallah, M.M.; Abdel-Mageed, A.; Elfaham, T.H. Ocular administration of acetazolamide microsponges in situ gel formulations. Saudi Pharm. J. 2018, 26, 909–920. [Google Scholar] [CrossRef]
- Abdelmalak, N.S.; El-Menshawe, S.F. A new topical fluconazole microsponge loaded hydrogel: Preparation and characterization. Int. J. Pharm. Pharm. Sci. 2012, 4, 460–468. [Google Scholar]
- He, Y.; Majid, K.; Maqbool, M.; Hussain, T.; Yousaf, A.M.; Khan, I.U.; Mehmood, Y.; Aleem, A.; Arshad, M.S.; Younus, A. Formulation and characterization of lornoxicam-loaded cellulosic-microsponge gel for possible applications in arthritis. Saudi Pharm. J. 2020, 28, 994–1003. [Google Scholar] [CrossRef]
- Cavalieri, F. Hyaluronic acid micro-sponges and method for the production thereof. Patent PCT/IB2016/052792, 17 November 2016. [Google Scholar]
- Seljak, K.B.; Kocbek, P.; Gašperlin, M. Mesoporous silica nanoparticles as delivery carriers: An overview of drug loading techniques. J. Drug Deliv. Sci. Technol. 2020, 59, 101906. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Lyophilisation improves bioactivity and stability of insulin-loaded polymeric-oligonucleotide nanoparticles for diabetes treatment. AAPS PharmSciTech 2020, 21, 108. [Google Scholar] [CrossRef]
- Di Francesco, M.; Primavera, R.; Summa, M.; Pannuzzo, M.; Di Francesco, V.; Di Mascolo, D.; Bertorelli, R.; Decuzzi, P. Engineering shape-defined PLGA microPlates for the sustained release of anti-inflammatory molecules. J. Control. Release 2020, 319, 201–212. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef]
- Pawar, A.P.; Gholap, A.P.; Kuchekar, A.B.; Bothiraja, C.; Mali, A.J. Formulation and evaluation of optimized oxybenzone microsponge gel for topical delivery. J. Drug Deliv. 2015, 2015, 261068. [Google Scholar] [CrossRef]
- Caso, M.F.; Carotenuto, F.; Di Nardo, P.; Migliore, A.; Aguilera, A.; Lopez, C.M.; Venanzi, M.; Cavalieri, F.; Rinaldi, A. Nanoporous microsponge particles (NMP) of polysaccharides as universal carriers for biomolecules delivery. Nanomaterials 2020, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, X.; Wang, K.; Yuan, Y.; Deng, K.; Chen, J.; Tan, W. Rhodamine B isothiocyanate doped silica-coated fluorescent nanoparticles (RBITC-DSFNPs)–based bioprobes conjugated to Annexin V for apoptosis detection and imaging. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, D.; Xu, P.; Li, S.; Cen, Y.; Li, Y.; Xu, Q.; Sun, Y.; Li, W.; Lin, Y. Stability of bovine serum albumin labelled by rhodamine B isothiocyanate. Biomed. Res. 2017, 28, 3851–3854. [Google Scholar]
- Chen, Y.; Qian, H.; Zheng, X.; Jiang, X.; Yu, H.; Zhang, L. Nonspherical polysaccharide vesicles and their shape and volume regulation via osmotically sensitive channels. Soft Matter 2011, 7, 5519–5523. [Google Scholar] [CrossRef]
- Andreana, I.; Bincoletto, V.; Manzoli, M.; Rodà, F.; Giarraputo, V.; Milla, P.; Arpicco, S.; Stella, B. Freeze drying of polymer nanoparticles and liposomes exploiting different saccharide-based approaches. Materials 2023, 16, 1212. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Panel, C.I.R.E.; Andersen, F.A. Final report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Li, Z.; Mu, S.; Cheng, T.; Tian, F.; Liu, Y. Effect of xianlinggubao in suppressing articular cartilage degeneration of anterior cruciate ligament transection model in rats. Chin. J. Reparative Reconstr. Surg. 2009, 23, 294–298. [Google Scholar]
- Palmieri, G.; Rinaldi, A.; Campagnolo, L.; Tortora, M.; Caso, M.F.; Mattei, M.; Notargiacomo, A.; Rosato, N.; Bottini, M.; Cavalieri, F. Hyaluronic acid nanoporous microparticles with long in vivo joint residence time and sustained release. Part. Part. Syst. Charact. 2017, 34, 1600411. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O. Wound healing promotion by hyaluronic acid: Effect of molecular weight on gene expression and in vivo wound closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Crotts, G.; Park, T.G. Preparation of porous and nonporous biodegradable polymeric hollow microspheres. J. Control. Release 1995, 35, 91–105. [Google Scholar] [CrossRef]
- Dhir, V.; Singla, M.; Gupta, N.; Goyal, P.; Sagar, V.; Sharma, A.; Khanna, S.; Singh, S. Randomized controlled trial comparing 2 different starting doses of methotrexate in rheumatoid arthritis. Clin. Ther. 2014, 36, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Abd El-Mageed, H.; Anwar, M.J.; Mahmood, D. Adsorption of methotrexate on hyaluronic acid: A comparative DFT and molecular dynamics simulation insights. Chem. Phys. Impact 2024, 8, 100573. [Google Scholar] [CrossRef]
- Wang, S.; Tavakoli, S.; Parvathaneni, R.P.; Nawale, G.N.; Oommen, O.P.; Hilborn, J.; Varghese, O.P. Dynamic covalent crosslinked hyaluronic acid hydrogels and nanomaterials for biomedical applications. Biomater. Sci. 2022, 10, 6399–6412. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, S.-H.; Thambi, T.; You, D.G.; Jeon, J.; Lee, J.O.; Chung, B.Y.; Jo, D.-G.; Park, J.H. A hyaluronic acid–methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem. Commun. 2014, 50, 7632–7635. [Google Scholar] [CrossRef]
- Alva-Ensastegui, J.; Morales-Avila, E.; de la Luz, A.P.; Bernad-Bernad, M. Determination of pKa values and deprotonation order of methotrexate using a combined experimental-theoretical study and binding constants of the methotrexate-Laponite complex at different pH values. J. Photochem. Photobiol. A Chem. 2024, 449, 115406. [Google Scholar] [CrossRef]
- Abla, K.K.; Mehanna, M.M. Freeze-drying: A flourishing strategy to fabricate stable pharmaceutical and biological products. Int. J. Pharm. 2022, 628, 122233. [Google Scholar] [CrossRef]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of particle size on drug loading and release kinetics of gefitinib-loaded PLGA microspheres. Mol. Pharm. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Özdemir, S.; Üner, B.; Baranauskaite, J.; Sümer, E.; Yıldırım, E.; Yaba, A. Design and characterization of dexamethasone loaded microsponges for the management of ulcerative colitis. Eur. J. Pharm. Biopharm. 2023, 187, 34–45. [Google Scholar] [CrossRef]
- Yoshioka, K.; Katayama, M.; Nishiyama, T.; Harada, K.; Takeshita, S.; Kawamata, Y. Biocompatibility study of different hyaluronan products for intra-articular treatment of knee osteoarthritis. BMC Musculoskelet. Disord. 2019, 20, 424. [Google Scholar] [CrossRef]
- Gooch, N.; Burr, R.M.; Holt, D.J.; Gale, B.; Ambati, B. Design and in vitro biocompatibility of a novel ocular drug delivery device. J. Funct. Biomater. 2013, 4, 14–26. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.Z.; American Pharmaceutical Review. Calculating Endotoxin Limits for Drug Products. 2018. Available online: https://www.americanpharmaceuticalreview.com/featured-articles/353977-calculating-endotoxin-limits-for-drug-products/ (accessed on 20 November 2023).
- Osmani, R.A.M.; Aloorkar, N.H.; Thaware, B.U.; Kulkarni, P.K.; Moin, A.; Hani, U.; Srivastava, A.; Bhosale, R.R. Microsponge based drug delivery system for augmented gastroparesis therapy: Formulation development and evaluation. Asian J. Pharm. Sci. 2015, 10, 442–451. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Dychter, S.S.; Gold, D.A.; Haller, M.F. Subcutaneous drug delivery: A route to increased safety, patient satisfaction, and reduced costs. J. Infus. Nurs. 2012, 35, 154–160. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Luciani, P. Injectable lipid-based depot formulations: Where do we stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
- Ho, M.J.; Jeong, M.Y.; Jeong, H.T.; Kim, M.S.; Park, H.J.; Kim, D.Y.; Lee, H.C.; Song, W.H.; Kim, C.H.; Lee, C.H. Effect of particle size on in vivo performances of long-acting injectable drug suspension. J. Control. Release 2022, 341, 533–547. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gorain, B.; Choudhury, H.; Srivastava, A.; Kesharwani, P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon 2019, 5, e01343. [Google Scholar] [CrossRef]





| Histological Scoring | |||
|---|---|---|---|
| Architectural Damage | Inflammatory Infiltrate | ||
| No alterations | 0 | No inflammatory cells | 0 |
| Slight alterations | 1 | Rare isolated inflammatory cells | 1 |
| Moderate alterations | 2 | Focal infiltration of inflammatory cells | 2 |
| Severe alterations | 3 | Widespread infiltration of inflammatory cells | 3 |
| Histological Rheumatoid Arthritis Scoring | |||||||
|---|---|---|---|---|---|---|---|
| Structure | Cells | Matrix Staining | Tidemark Integrity | ||||
| Normal | 0 | Normal | 0 | Normal | 0 | Intact | 1 |
| Surface irregularities | 1 | Diffuse hypercelluarity | 1 | Slight reduction | 1 | ||
| Pannus and surface irregularities | 2 | Clusters | 2 | Moderate reduction | 2 | ||
| Clefts to transitional zone | 3 | Hypocellularity | 3 | Severe reduction | 3 | Destroyed | 2 |
| Clefts to calcified zone | 4 | No staining | 4 | ||||
| Complete disorganization | 5 | ||||||
| Formulation | Centrifuged | Lyophilized |
|---|---|---|
| Dimensions (mm ± SD) | Dimensions (mm ± SD) | |
| MSP | 8.34 ± 0.35 | 7.44 ± 0.64 |
| MTX-MSP | 7.25 ± 0.55 | 6.87 ± 0.73 |
| Formulation | Mean Particle Size (μm) ± SD | ζ-Potential (mV) ± SD | Endotoxin (EU/mL) |
|---|---|---|---|
| MSP | 34.5 ± 0.2 | −36 ± 2 | <0.01 |
| MTX-MSP | 60.8 ± 0.5 | −43 ± 2 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiaschini, N.; Hanieh, P.N.; Ariaudo, D.; Cimino, R.; Abbate, C.; Romano, E.; Cavalieri, F.; Venanzi, M.; Palumbo, V.; Scimeca, M.; et al. Application of Microsponge Drug Platform to Enhance Methotrexate Administration in Rheumatoid Arthritis Therapy. Pharmaceutics 2024, 16, 1593. https://doi.org/10.3390/pharmaceutics16121593
Fiaschini N, Hanieh PN, Ariaudo D, Cimino R, Abbate C, Romano E, Cavalieri F, Venanzi M, Palumbo V, Scimeca M, et al. Application of Microsponge Drug Platform to Enhance Methotrexate Administration in Rheumatoid Arthritis Therapy. Pharmaceutics. 2024; 16(12):1593. https://doi.org/10.3390/pharmaceutics16121593
Chicago/Turabian StyleFiaschini, Noemi, Patrizia Nadia Hanieh, Daniela Ariaudo, Rita Cimino, Carlo Abbate, Elena Romano, Francesca Cavalieri, Mariano Venanzi, Valeria Palumbo, Manuel Scimeca, and et al. 2024. "Application of Microsponge Drug Platform to Enhance Methotrexate Administration in Rheumatoid Arthritis Therapy" Pharmaceutics 16, no. 12: 1593. https://doi.org/10.3390/pharmaceutics16121593
APA StyleFiaschini, N., Hanieh, P. N., Ariaudo, D., Cimino, R., Abbate, C., Romano, E., Cavalieri, F., Venanzi, M., Palumbo, V., Scimeca, M., Bernardini, R., Mattei, M., Migliore, A., & Rinaldi, A. (2024). Application of Microsponge Drug Platform to Enhance Methotrexate Administration in Rheumatoid Arthritis Therapy. Pharmaceutics, 16(12), 1593. https://doi.org/10.3390/pharmaceutics16121593











