The Role of Extracellular Vesicles in the Development and Treatment of Psoriasis: Narrative Review
Abstract
1. Introduction
2. Characterization and Analysis of Extracellular Vesicles: Subtypes, Biogenesis, Functional Roles, Isolation Techniques, and Identification Methods
2.1. Subtype and Biogenesis
2.2. Function
2.3. Isolation and Identification
3. Investigating the Role of Extracellular Vesicles in the Physiology and Pathophysiology of Skin and Its Appendages
3.1. The Involvement in Cutaneous Immunity in Sweat Exosomes
3.2. Enhanced the Cutaneous Regenerative via Extracellular Vesicle
3.3. Exploring the Application of Keratinocyte-Derived Extracellular Vesicles for the Protection of Hair Follicles
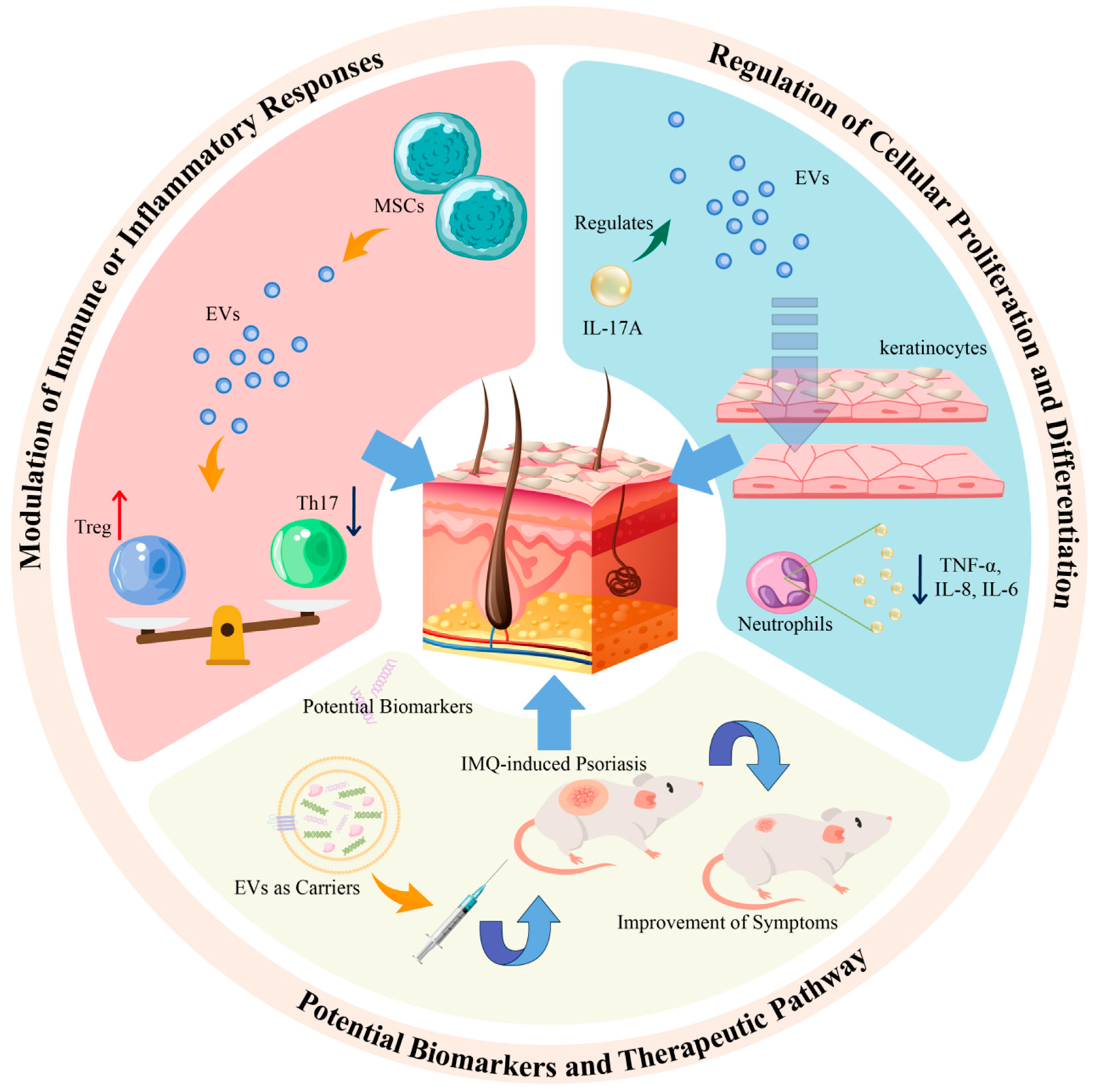
4. The Role of EVs in Psoriasis
4.1. Exploring the Role of Extracellular Vesicles from Immune Cells in the Pathogenesis of Psoriasis
4.2. Impact of Cytokine-Treated Keratinocyte-Derived Exosomes on Cell Proliferation in Psoriasis
4.3. Characterization of Extracellular Vesicle Cargo: Exploring Potential Biomarkers and Therapeutic Targets in Psoriasis
| miRNA | Source | Expression | Function | Ref. |
|---|---|---|---|---|
| hsa-miR-671-3p | Serum | ↓ | More than two-fold in PsA compare with PsV. | [21] |
| hsa-miR-151a-3p | Plasma | ↑ | Related to immune disorder and bone metabolic dysregulation. | [50] |
| hsa-miR-199a-5p | ||||
| hsa-miR-370-3p | ||||
| hsa-miR-589-5p | ||||
| hsa-miR-769-5p | ||||
| miR-1305 | Serum | ↑ | Promoted keratinocyte proliferation and the secretion of CCL20 and IL-8. | [77] |
| miR-6785-5p | ↓ | |||
| miR-381-3p | Keratinocytes | ↑ | Polarizating Th1 and Th17 in psoriasis. | [79] |
| miR-199a-3p | Serum | ↑ | Significantly up-regulating in patients with psoriasis versus healthy control. | [80] |
| let-7b-5p | Plasma | ↓ | As potential markers of PsA in patients with psoriasis. | [81] |
| miR-30e-5p | ||||
| miR-124-3p | Keratinocytes | ↓ | MiR124-3p/FGFR2 axis inhibits keratinocyte proliferation and migration. | [83] |
5. Comprehensive Profiling of Extracellular Vesicle Cargo for Advancing Psoriasis Treatment Discovery
5.1. Naive EVs
5.2. Engineering EVs
6. Implications for the Future
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Born, L.J.; Khachemoune, A. Extracellular vesicles: A comprehensive review of their roles as biomarkers and potential therapeutics in psoriasis and psoriatic arthritis. Clin. Exp. Dermatol. 2023, 48, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M.; Global Psoriasis, A. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Iskandar, I.Y.K.; Parisi, R.; Griffiths, C.E.M.; Ashcroft, D.M.; Global Psoriasis, A. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br. J. Dermatol. 2021, 184, 243–258. [Google Scholar] [CrossRef]
- Wu, J.J.; Kavanaugh, A.; Lebwohl, M.G.; Gniadecki, R.; Merola, J.F. Psoriasis and metabolic syndrome: Implications for the management and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Lee, E.B.; Tsai, T.F.; Wu, J.J. Psoriasis and Co-morbidity. Acta Derm.-Venereol. 2020, 100, adv00033. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2013, 2, e000062. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, E.; Owczarczyk-Saczonek, A.; Placek, W. Psychological Stress, Mast Cells, and Psoriasis-Is There Any Relationship? Int. J. Mol. Sci. 2021, 22, 13252. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Zhou, X.; Zhao, Z.; Yu, Q.; Chen, Z.; Wang, Y.; Xu, P.; Yu, Z.; Guo, C.; et al. Human umbilical cord-derived mesenchymal stem cells ameliorate psoriasis-like dermatitis by suppressing IL-17-producing γδ T cells. Cell Tissue Res. 2022, 388, 549–563. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef]
- Diotallevi, F.; Di Vincenzo, M.; Martina, E.; Radi, G.; Lariccia, V.; Offidani, A.; Orciani, M.; Campanati, A. Mesenchymal Stem Cells and Psoriasis: Systematic Review. Int. J. Mol. Sci. 2022, 23, 15080. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, P.; Gegotek, A.; Zarkovic, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Guo, L.C.; Zhu, W.D.; Cai, M.H.; Chen, L.L.; Wu, L.; Chen, X.J.; Zhu, H.Y.; Wu, J. Human adipose tissue-derived MSCs improve psoriasis-like skin inflammation in mice by negatively regulating ROS. J. Dermatolog Treat. 2022, 33, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Mabuchi, T.; Iwatsuki, K.; Elmaraghy, H.; Torisu-Itakura, H.; Morisaki, Y.; Nakajo, K. Long-term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: Subgroup analyses of an open-label, phase 3 study (UNCOVER-J). J. Eur. Acad. Dermatol. Venereol. 2019, 33, 325–332. [Google Scholar] [CrossRef]
- Dogra, S.; Bishnoi, A.; Narang, T.; Handa, S. Long-term remission induced by secukinumab in a 13-year-old boy having recalcitrant chronic erythrodermic psoriasis. Dermatol. Ther. 2018, 31, e12611. [Google Scholar] [CrossRef]
- Megna, M.; Fabbrocini, G.; Ferrillo, M.; Cinelli, E. Erythrodermic psoriasis successfully and rapidly treated with brodalumab: Report of two cases. Dermatol. Ther. 2020, 33, e14351. [Google Scholar] [CrossRef]
- Blauvelt, A. Safety of secukinumab in the treatment of psoriasis. Expert Opin. Drug Saf. 2016, 15, 1413–1420. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Singh, S.K.; Sharma, D.S.; Khursheed, R.; Awasthi, A. Treatment Strategies Against Psoriasis: Principle, Perspectives and Practices. Curr. Drug Deliv. 2020, 17, 52–73. [Google Scholar] [CrossRef]
- Li, T.; Gao, S.; Han, W.; Gao, Z.; Wei, Y.; Wu, G.; Qiqiu, W.; Chen, L.; Feng, Y.; Yue, S.; et al. Potential effects and mechanisms of Chinese herbal medicine in the treatment of psoriasis. J. Ethnopharmacol. 2022, 294, 115275. [Google Scholar] [CrossRef]
- Lambert, J.L.W.; Segaert, S.; Ghislain, P.D.; Hillary, T.; Nikkels, A.; Willaert, F.; Lambert, J.; Speeckaert, R. Practical recommendations for systemic treatment in psoriasis according to age, pregnancy, metabolic syndrome, mental health, psoriasis subtype and treatment history (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 1). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1654–1665. [Google Scholar] [CrossRef]
- Lattekivi, F.; Guljavina, I.; Midekessa, G.; Viil, J.; Heath, P.R.; Baek, R.; Jorgensen, M.M.; Andronowska, A.; Kingo, K.; Fazeli, A. Profiling Blood Serum Extracellular Vesicles in Plaque Psoriasis and Psoriatic Arthritis Patients Reveals Potential Disease Biomarkers. Int. J. Mol. Sci. 2022, 23, 4005. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Song, H.; Kim, N.H.; Kim, J.H. The role of extracellular vesicles in animal reproduction and diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.; Fan, X.; Ning, X.; Yu, B.; Ou, C.; Chen, M. Targeted delivery of extracellular vesicles in heart injury. Theranostics 2021, 11, 2263–2277. [Google Scholar] [CrossRef]
- Upadhya, R.; Shetty, A.K. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson’s Disease. Aging Dis. 2021, 12, 1438–1450. [Google Scholar] [CrossRef]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef]
- Paolino, G.; Buratta, S.; Mercuri, S.R.; Pellegrino, R.M.; Urbanelli, L.; Emiliani, C.; Bertuccini, L.; Iosi, F.; Huber, V.; Brianti, P.; et al. Lipidic Profile Changes in Exosomes and Microvesicles Derived From Plasma of Monoclonal Antibody-Treated Psoriatic Patients. Front. Cell Dev. Biol. 2022, 10, 923769. [Google Scholar] [CrossRef]
- Kim, H.; Back, J.H.; Han, G.; Lee, S.J.; Park, Y.E.; Gu, M.B.; Yang, Y.; Lee, J.E.; Kim, S.H. Extracellular vesicle-guided in situ reprogramming of synovial macrophages for the treatment of rheumatoid arthritis. Biomaterials 2022, 286, 121578. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef]
- Wong, P.M.; Yang, L.; Yang, L.; Wu, H.; Li, W.; Ma, X.; Katayama, I.; Zhang, H. New insight into the role of exosomes in vitiligo. Autoimmun. Rev. 2020, 19, 102664. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Xu, X.; Pan, C.; Lu, Y.; Liu, J. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: Current knowledge and future perspectives. Acta Biomater. 2021, 119, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ni, J.; Wang, Y.S.; Zhao, Y.; Jiang, L.Q.; Chen, C.; Zhang, R.D.; Fang, X.; Wang, P.; Pan, H.F. Exosomes as biomarkers and therapeutic delivery for autoimmune diseases: Opportunities and challenges. Autoimmun. Rev. 2023, 22, 103260. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta (BBA) Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Parcina, M.; Heyder, P.; Foermer, S.; Ostrop, J.; Leo, A.; Heeg, K.; Herrmann, M.; Lorenz, H.M.; Bekeredjian-Ding, I. Induction of type I IFN is a physiological immune reaction to apoptotic cell-derived membrane microparticles. J. Immunol. 2012, 189, 1747–1756. [Google Scholar] [CrossRef]
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis 2017, 22, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.; et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Gonzalez, E.; Berisa, A.; Gil, D.; Embade, N.; Valle, M.; Luka, Z.; Elortza, F.; Wagner, C.; et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteom. Clin. Appl. 2010, 4, 416–425. [Google Scholar] [CrossRef]
- Wang, W.M.; Wu, C.; Jin, H.Z. Exosomes in chronic inflammatory skin diseases and skin tumors. Exp. Dermatol. 2019, 28, 213–218. [Google Scholar] [CrossRef]
- Gupta, A.; Elfiky, A. Novel findings from determination of common expressed plasma exosomal microRNAs in patients with psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis, and gouty arthritis. Discov. Med. 2019, 28, 113–122. [Google Scholar]
- Delgado-Povedano, M.M.; Calderon-Santiago, M.; Priego-Capote, F.; Luque de Castro, M.D. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography-mass spectrometry in high resolution mode. Anal. Chim. Acta 2016, 905, 115–125. [Google Scholar] [CrossRef]
- Nawrocki, S.; Cha, J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Etiology and clinical work-up. J. Am. Acad. Dermatol. 2019, 81, 657–666. [Google Scholar] [CrossRef]
- Wu, C.X.; Liu, Z.F. Proteomic Profiling of Sweat Exosome Suggests its Involvement in Skin Immunity. J. Investig. Dermatol. 2018, 138, 89–97. [Google Scholar] [CrossRef]
- Csosz, E.; Kallo, G.; Markus, B.; Deak, E.; Csutak, A.; Tozser, J. Quantitative body fluid proteomics in medicine—A focus on minimal invasiveness. J. Proteom. 2017, 153, 30–43. [Google Scholar] [CrossRef]
- Karvinen, S.; Sievanen, T.; Karppinen, J.E.; Hautasaari, P.; Bart, G.; Samoylenko, A.; Vainio, S.J.; Ahtiainen, J.P.; Laakkonen, E.K.; Kujala, U.M. MicroRNAs in Extracellular Vesicles in Sweat Change in Response to Endurance Exercise. Front. Physiol. 2020, 11, 676. [Google Scholar] [CrossRef]
- Hu, J.C.; Zheng, C.X.; Sui, B.D.; Liu, W.J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef]
- Carrasco, E.; Soto-Heredero, G.; Mittelbrunn, M. The Role of Extracellular Vesicles in Cutaneous Remodeling and Hair Follicle Dynamics. Int. J. Mol. Sci. 2019, 20, 2758. [Google Scholar] [CrossRef]
- Ma, L.; Chen, C.; Liu, D.; Huang, Z.; Li, J.; Liu, H.; Kin Kwok, R.T.; Tang, B.; Sui, B.; Zhang, X.; et al. Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioact. Mater. 2023, 19, 626–641. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Wu, S.; Yuan, R.; Zhao, X.; Li, Y.; Wu, W.; Zhu, N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng. Regen. Med. 2021, 18, 685–691. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wang, Q.; Zhang, Y.; Cui, L.; Huang, X. Exosomes Secreted from Adipose-Derived Stem Cells Are a Potential Treatment Agent for Immune-Mediated Alopecia. J. Immunol. Res. 2022, 2022, 7471246. [Google Scholar] [CrossRef]
- Takano, K.; Hachiya, A.; Murase, D.; Tanabe, H.; Kasamatsu, S.; Takahashi, Y.; Moriwaki, S.; Hase, T. Quantitative changes in the secretion of exosomes from keratinocytes homeostatically regulate skin pigmentation in a paracrine manner. J. Dermatol. 2020, 47, 265–276. [Google Scholar] [CrossRef]
- Balato, N.; Di Costanzo, L.; Balato, A.; Patruno, C.; Scalvenzi, M.; Ayala, F. Psoriasis and melanocytic naevi: Does the first confer a protective role against melanocyte progression to naevi? Br. J. Dermatol. 2011, 164, 1262–1270. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guere, C.; Andre, N.; Vie, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef]
- Ogawa, M.; Udono, M.; Teruya, K.; Uehara, N.; Katakura, Y. Exosomes Derived from Fisetin-Treated Keratinocytes Mediate Hair Growth Promotion. Nutrients 2021, 13, 2087. [Google Scholar] [CrossRef]
- Mak, R.K.; Hundhausen, C.; Nestle, F.O. Progress in understanding the immunopathogenesis of psoriasis. Actas Dermosifiliogr. 2009, 100 (Suppl. S2), 2–13. [Google Scholar] [CrossRef]
- Sugiyama, H.; Gyulai, R.; Toichi, E.; Garaczi, E.; Shimada, S.; Stevens, S.R.; McCormick, T.S.; Cooper, K.D. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: Mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005, 174, 164–173. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Jacquin-Porretaz, C.; Cordonnier, M.; Nardin, C.; Boullerot, L.; Chanteloup, G.; Vautrot, V.; Adotevi, O.; Garrido, C.; Gobbo, J.; Aubin, F. Increased Levels of Interleukin-17A Exosomes in Psoriasis. Acta Derm.-Venereol. 2019, 99, 1143–1147. [Google Scholar] [CrossRef]
- Capriotti, L.; Iuliano, M.; Lande, R.; Frasca, L.; Falchi, M.; Rosa, P.; Mangino, G.; Romeo, G. Potential Pathogenetic Role of Antimicrobial Peptides Carried by Extracellular Vesicles in an in vitro Psoriatic Model. J. Inflamm. Res. 2022, 15, 5387–5399. [Google Scholar] [CrossRef]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef]
- DePaolo, L.V. Attenuation of preovulatory gonadotrophin surges by epostane: A new inhibitor of 3β-hydroxysteroid dehydrogenase. J. Endocrinol. 1988, 118, 59–68. [Google Scholar] [CrossRef]
- Tavasolian, F.; Moghaddam, A.S.; Rohani, F.; Abdollahi, E.; Janzamin, E.; Momtazi-Borojeni, A.A.; Moallem, S.A.; Jamialahmadi, T.; Sahebkar, A. Exosomes: Effectual players in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102511. [Google Scholar] [CrossRef]
- Torri, A.; Carpi, D.; Bulgheroni, E.; Crosti, M.C.; Moro, M.; Gruarin, P.; Rossi, R.L.; Rossetti, G.; Di Vizio, D.; Hoxha, M.; et al. Extracellular MicroRNA Signature of Human Helper T Cell Subsets in Health and Autoimmunity. J. Biol. Chem. 2017, 292, 2903–2915. [Google Scholar] [CrossRef]
- Yan, J.; Zhen, Y.; Wang, R.; Li, X.; Huang, S.; Zhong, H.; Wen, H.; Sun, Q. Downregulation of miRNA miR-1305 and upregulation of miRNA miR-6785-5p may be associated with psoriasis. Front. Genet. 2022, 13, 891465. [Google Scholar] [CrossRef]
- Wu, M.H.; Tsai, C.H.; Huang, Y.L.; Fong, Y.C.; Tang, C.H. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018, 18, 190. [Google Scholar] [CrossRef]
- Jiang, M.; Fang, H.; Dang, E.; Zhang, J.; Qiao, P.; Yu, C.; Yang, A.; Wang, G. Small Extracellular Vesicles Containing miR-381-3p from Keratinocytes Promote T Helper Type 1 and T Helper Type 17 Polarization in Psoriasis. J. Investig. Dermatol. 2021, 141, 563–574. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yan, B.X.; Zhou, Y.; Chen, X.Y.; Zhang, J.; Cai, S.Q.; Zheng, M.; Man, X.Y. miRNA Profiling of Extracellular Vesicles Reveals Biomarkers for Psoriasis. J. Investig. Dermatol. 2021, 141, 185–189.e4. [Google Scholar] [CrossRef]
- Pasquali, L.; Svedbom, A.; Srivastava, A.; Rosen, E.; Lindqvist, U.; Stahle, M.; Pivarcsi, A.; Sonkoly, E. Circulating microRNAs in extracellular vesicles as potential biomarkers for psoriatic arthritis in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1248–1256. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Bian, C.; Diao, Q.; Nisar, M.F.; Jiang, X.; Bartsch, J.W.; Zhong, M.; Hu, X.; Zhong, J.L. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Commun. Signal. 2018, 16, 58. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, C.; Zeng, B.; Tang, X.; Zhang, Y.; Xiang, L.; Mi, L.; Pan, Y.; Wang, H.; Yang, Z. miR124-3p/FGFR2 axis inhibits human keratinocyte proliferation and migration and improve the inflammatory microenvironment in psoriasis. Mol. Immunol. 2020, 122, 89–98. [Google Scholar] [CrossRef]
- Chen, X.M.; Yao, D.N.; Wang, M.J.; Wu, X.D.; Deng, J.W.; Deng, H.; Huang, R.Y.; Lu, C.J. Deep Sequencing of Plasma Exosomal microRNA Level in Psoriasis Vulgaris Patients. Front. Med. 2022, 9, 895564. [Google Scholar] [CrossRef]
- Chang, C.J.; Zhang, J.; Tsai, Y.L.; Chen, C.B.; Lu, C.W.; Huo, Y.P.; Liou, H.M.; Ji, C.; Chung, W.H. Compositional Features of Distinct Microbiota Base on Serum Extracellular Vesicle Metagenomics Analysis in Moderate to Severe Psoriasis Patients. Cells 2021, 10, 2349. [Google Scholar] [CrossRef]
- Khosrotehrani, K. Mesenchymal stem cell therapy in skin: Why and what for? Exp. Dermatol. 2013, 22, 307–310. [Google Scholar] [CrossRef]
- Sarsenova, M.; Kim, Y.; Raziyeva, K.; Kazybay, B.; Ogay, V.; Saparov, A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front. Immunol. 2022, 13, 1010399. [Google Scholar] [CrossRef]
- Zhang, B.; Yeo, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Xu, F.; Fei, Z.; Dai, H.; Xu, J.; Fan, Q.; Shen, S.; Zhang, Y.; Ma, Q.; Chu, J.; Peng, F.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles with High PD-L1 Expression for Autoimmune Diseases Treatment. Adv. Mater. 2022, 34, e2106265. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Shi, P.; Su, D.; Cheng, X.; Yi, W.; Yan, J.; Chen, H.; Cheng, F. Small Extracellular Vesicles Derived From MSCs Have Immunomodulatory Effects to Enhance Delivery of ASO-210 for Psoriasis Treatment. Front. Cell Dev. Biol. 2022, 10, 842813. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Z.; Zheng, J.; Sun, Q. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Psoriasis-like Skin Inflammation. J. Interferon Cytokine Res. 2022, 42, 8–18. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Cardoso, R.M.S.; Freire, P.C.; Gomes, C.F.; Duarte, F.V.; Neves, R.P.D.; Simoes-Correia, J. Immunomodulatory Properties of Umbilical Cord Blood-Derived Small Extracellular Vesicles and Their Therapeutic Potential for Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 9797. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, Z.; Huang, S.; Mao, P.; Zhang, L.; Wang, M.; Ye, J.; Sun, L.; Sun, M.; Lu, R.; et al. Ultraviolet B radiation-induced JPH203-loaded keratinocyte extracellular vesicles exert etiological interventions for psoriasis therapy. J. Control. Release 2023, 362, 468–478. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandao, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Bi, Y.; Zheng, X.; Yang, Y.; Huang, X.; Yang, K.; Zhong, H.; Han, L.; Lu, C.; Chen, H. The Role of Extracellular Vesicles in the Development and Treatment of Psoriasis: Narrative Review. Pharmaceutics 2024, 16, 1586. https://doi.org/10.3390/pharmaceutics16121586
Tang B, Bi Y, Zheng X, Yang Y, Huang X, Yang K, Zhong H, Han L, Lu C, Chen H. The Role of Extracellular Vesicles in the Development and Treatment of Psoriasis: Narrative Review. Pharmaceutics. 2024; 16(12):1586. https://doi.org/10.3390/pharmaceutics16121586
Chicago/Turabian StyleTang, Bin, Yang Bi, Xuwei Zheng, Yujie Yang, Xiaobing Huang, Kexin Yang, Haixin Zhong, Ling Han, Chuanjian Lu, and Haiming Chen. 2024. "The Role of Extracellular Vesicles in the Development and Treatment of Psoriasis: Narrative Review" Pharmaceutics 16, no. 12: 1586. https://doi.org/10.3390/pharmaceutics16121586
APA StyleTang, B., Bi, Y., Zheng, X., Yang, Y., Huang, X., Yang, K., Zhong, H., Han, L., Lu, C., & Chen, H. (2024). The Role of Extracellular Vesicles in the Development and Treatment of Psoriasis: Narrative Review. Pharmaceutics, 16(12), 1586. https://doi.org/10.3390/pharmaceutics16121586








