Assessing Protein Content and Dimer Formation in the Bevacizumab Reference Product and Biosimilar Versions Marketed in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Size Exclusion Chromatography System
2.3. Analytical Method Validation
2.4. Statistical Model
3. Results and Discussion
3.1. Manufacturing Process Control
3.1.1. Reference Product
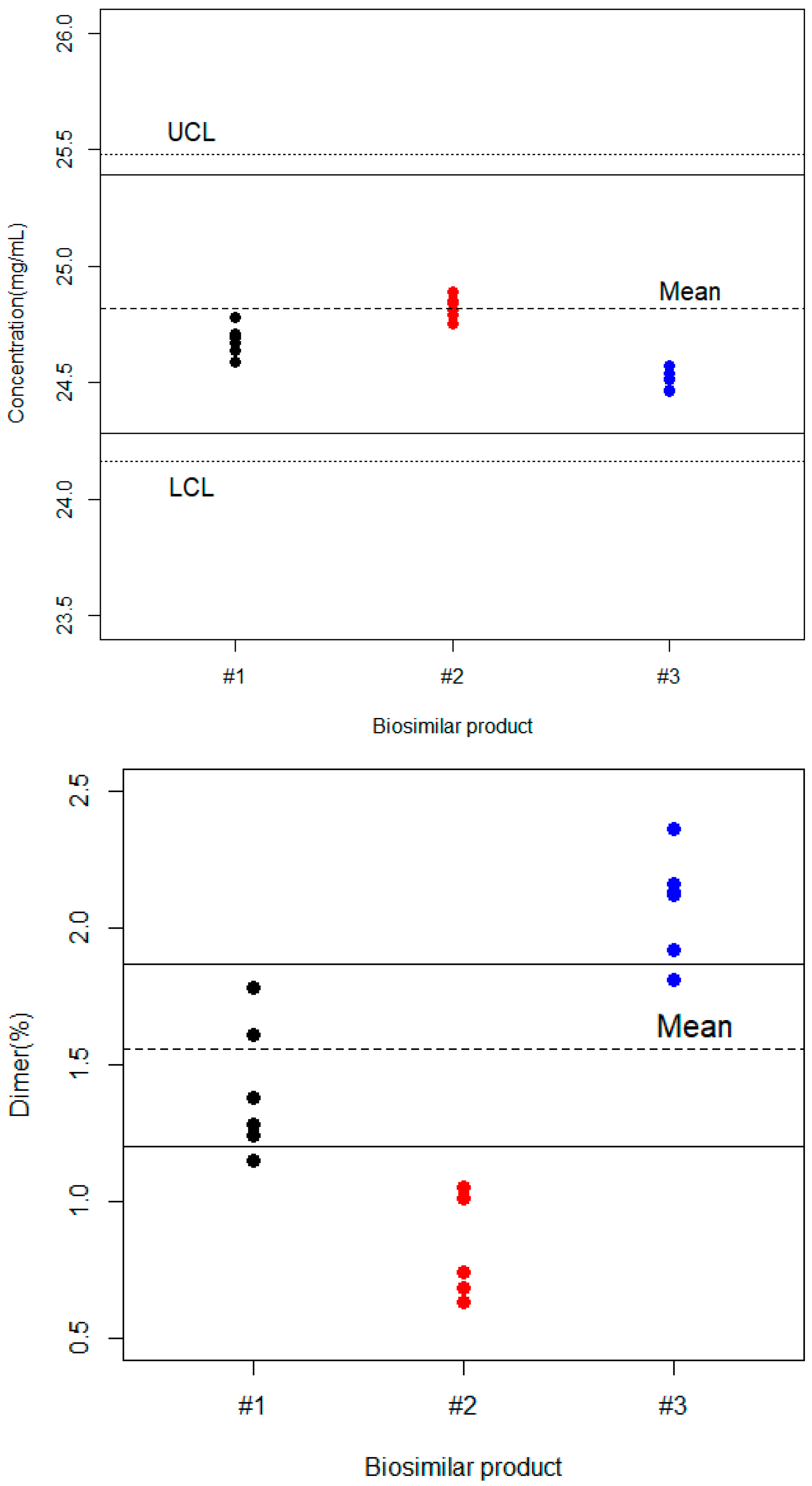
3.1.2. Biosimilar Products
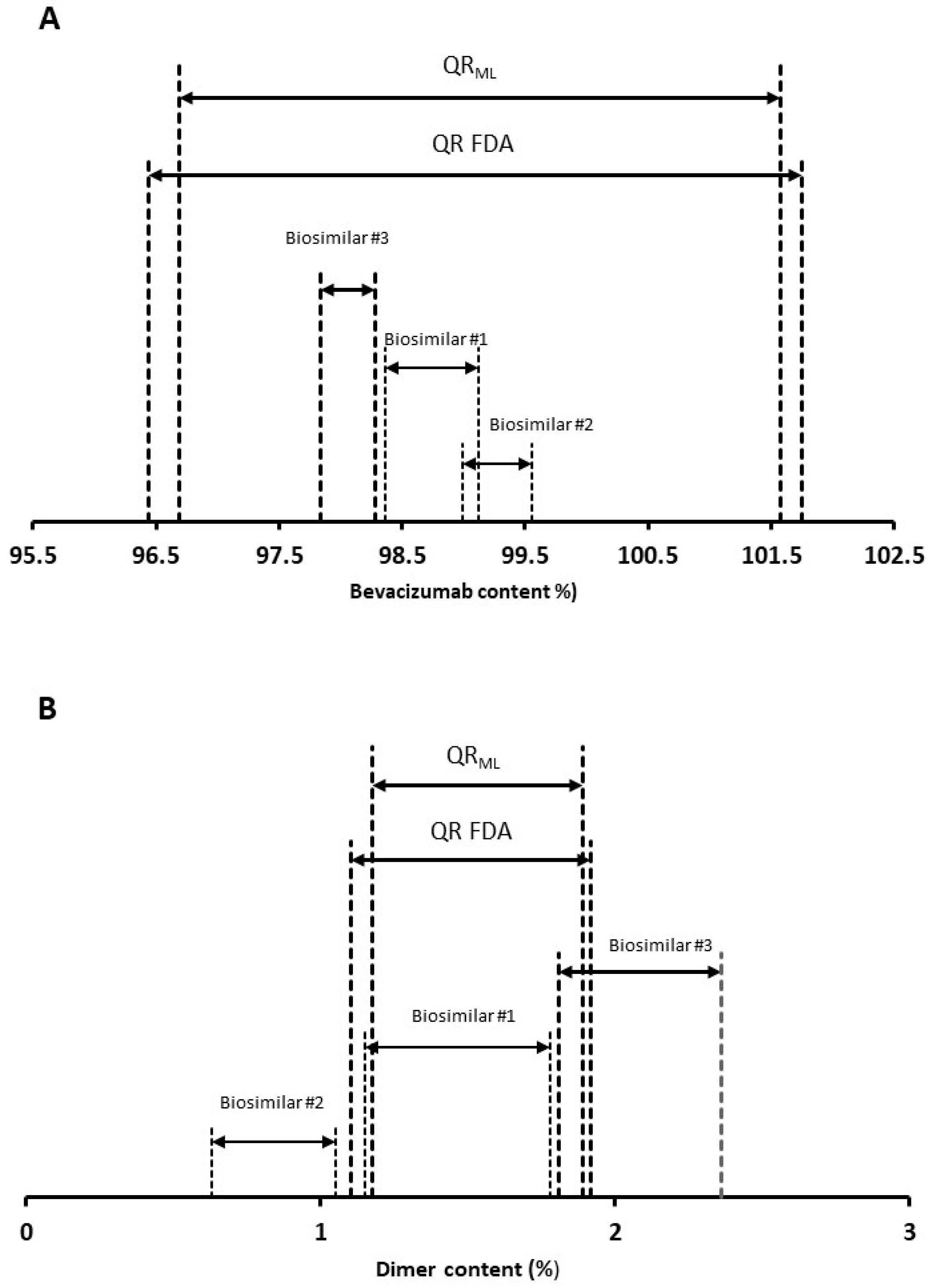
3.2. Analytical Similarity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Vooren, K.; Curto, A.; Garattini, L. Biosimilar versus Generic Drugs: Same but Different? Appl. Health Econ. Health Policy 2015, 13, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.F.; Wang, X.Z.M.; Conlon, H.D.; Anderson, S.; Ryan, A.M.; Bose, A. Biosimilars: Key Regulatory Considerations and Similarity Assessment Tools. Biotechnol. Bioeng. 2017, 114, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guideline on Similar Biological Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf (accessed on 13 March 2024).
- Torkashvand, F.; Vaziri, B. Main quality attributes of monoclonal antibodies and effect of cell culture components. Iran. Biomed. J. 2017, 21, 131–141. [Google Scholar] [CrossRef]
- Joshi, S.; Rathore, A.S. Assessment of Structural and Functional Comparability of Biosimilar Products: Trastuzumab as a Case Study. BioDrugs 2020, 34, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H. Stability testing in monoclonal antibodies. Crit. Rev. Biotechnol. 2021, 41, 692–714. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Lu, H. Antibody glycosylation: Impact on antibody drug characteristics and quality control. Appl. Microbiol. Biotechnol. 2020, 104, 1905–1914. [Google Scholar] [CrossRef]
- Beyer, B.; Walch, N.; Jungbauer, A.; Lingg, N. How Similar Is Biosimilar? A Comparison of Infliximab Therapeutics in Regard to Charge Variant Profile and Antigen Binding Affinity. Biotechnol. J. 2019, 14, 1800340. [Google Scholar] [CrossRef]
- FDA. Postapproval Manufacturing Changes to Biosimilar and Interchangeable Biosimilar Products Questions and Answers Guidance for Industry. 2024. Available online: https://www.fda.gov/media/180206/download (accessed on 24 August 2024).
- Zhang, E.; Xie, L.; Qin, P.; Lu, L.; Xu, Y.; Gao, W.; Wand, L.; Xie, M.; Jiang, W.; Liu, S. Quality by Design–Based Assessment for Analytical Similarity of Adalimumab Biosimilar HLX03 to Humira®. AAPS J. 2020, 22, 69. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, O.; Zhang, L.; Zhu, X.; Du, C.; Zhang, Z. Development and validation of aggregates analysis method in analytical similarity assessment of HLX04 vs. Avastin®. J. Pharm. Biomed. Anal. 2023, 223, 115121. [Google Scholar] [CrossRef]
- Oliva, A.; Llabrés, M.; Fariña, J.B. Fitting bevacizumab aggregation kinetic data with the Finke–Watzky two-step model: Effect of thermal and mechanical stress. Eur. J. Pharm. Sci. 2015, 77, 170–179. [Google Scholar] [CrossRef]
- Seo, N.; Polozova, A.; Zhang, M.; Yates, Z.; Cao, S.; Li, H.; Kuhns, S.; Maher, G.; McBride, H.J.; Liu, J. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. In Mabs; Taylor & Francis: Milton Park, UK, 2018; Volume 10, pp. 678–691. [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry on Biosimilars: Q & As Regarding Implementation of the BPCI Act of 2009: Questions and Answers Part I. Available online: https://www.fda.gov/media/119258/download (accessed on 28 October 2024).
- FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product (accessed on 13 March 2024).
- World Health Organization Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs). Available online: https://cdn.who.int/media/docs/default-source/biologicals/who-guidelines-on-evaluation-of-biosimilars---4-nov-2021.pdf?sfvrsn=f17799ae_5 (accessed on 19 March 2024).
- Q6B ICH Harmonized Tripartite Guideline Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-6-b-test-procedures-and-acceptance-criteria-biotechnologicalbiological-products-step-5_en.pdf (accessed on 30 May 2024).
- Chow, S.-C.; Song, F.; Bai, H. Analytical similarity assessment in biosimilar studies. ASPS J. 2016, 18, 670–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Medicines Agency, Workshop on the Reflection Paper on Statistical Methodology for the Comparative Assessment of Quality Attributes in Drug Development, London, 2018. Available online: https://www.ema.europa.eu/en/statistical-methodology-comparative-assessment-quality-attributes-drug-development-scientific-guideline (accessed on 12 March 2024).
- Son, S.; Oh, M.; Choo, M.; Chow, S.C.; Lee, S.J. Some thoughts on the QR method for analytical similarity evaluation. J. Biopharm. Stat. 2020, 30, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; LLabrés, M. Limitations of the quality range approach in analytical similarity assessment: Effect of mean shift and relative variability. J. Pharm. Biomed. Anal. 2021, 198, 114017. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.-C.; Lee, S.J. Current issues in analytical similarity assessment. Stat. Biopharm. Res. 2020, 13, 203–209. [Google Scholar] [CrossRef]
- Yang, H.; Burdick, R.K.; Cheng, A.; Montes, R.O. In statistical considerations for demonstrating of analytical similarity. In Biosimilars, AAPS Advances in the Pharmaceutical Sciences, Series 34; Guka, H.J., Yang, H., Kakar, S., Eds.; Springer: Cham, Switzerland, 2018; Chapter 17; pp. 431–468. [Google Scholar]
- Tsong, Y.; Dong, X.; Shen, M. Development of statistical methods for analytical similarity assessment. J. Biopharm. Stat. 2017, 27, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.C. Challenging issues in assessing analytical similarity in biosimilar studies. Biosimilars 2015, 5, 33–39. [Google Scholar] [CrossRef]
- Oliva, A.; Llabrés, M. New Quality-Range -setting method based on between-and within-batch variability for biosimilarity assessment. Pharmaceuticals 2021, 14, 527. [Google Scholar] [CrossRef]
- Wang, Y.; Fei, D.; Vanderlaan, M.; Song, A. Biological activity of Bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 2004, 7, 335–345. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Assoun, S.; Brosseau, S.; Steinmetz, C.; Gounant, V.; Zalcman, G. Bevacizumab in advanced lung cancer: State of the art. Future Oncol. 2017, 13, 2515–2535. [Google Scholar] [CrossRef]
- Pfaendler, K.S.; Liu, M.C.; Tewari, K.S. Bevacizumab in cervical cancer: 5 years after. Cancer J. 2018, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of Bevacizumab in the treatment of glioblastoma. J. Neurooncol. 2017, 133, 455–467. [Google Scholar] [CrossRef]
- Prakash, A.; Mishra, N.N.; Utpreksha, V.; Sharma, S.; Anand, A.; Mahajan, R.V.; Prasad, J.P.; Chand, S. Comparative analytical profiling of bevacizumab biosimilars marketed in India: A national control laboratory study. 3 Biotech 2020, 10, 516. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Available online: https://cima.aemps.es/cima/publico/home.html (accessed on 12 May 2024).
- Oliva, A.; Llabrés, M. Uncertainty o Size-Exclusion Chromatography Method in Quality Control of Bevacizumab Batches. Separations 2021, 8, 133. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 10 March 2021).
- Hoffman, D.; Kringle, R. Two-sided tolerance intervals for balanced and unbalanced random effects models. J. Biopharm. Stat. 2005, 15, 283–293. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on ABP 215 Biosimilar. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mvasi (accessed on 25 June 2024).
- Burdick, R.; Coffey, T.; Gutka, H.; Gratzl, G.; Conlon, H.D.; Huang, C.; Boyne, M.; Kuehne, H. Statistical approaches to assess biosimilarity from analytical data. AAPS J. 2016, 19, 4–14. [Google Scholar] [CrossRef]
- Liao, J.J.; Darken, P.F. Comparability of critical quality attributes for establishing biosimilarity. Stat. Med. 2013, 32, 462–469. [Google Scholar] [CrossRef]
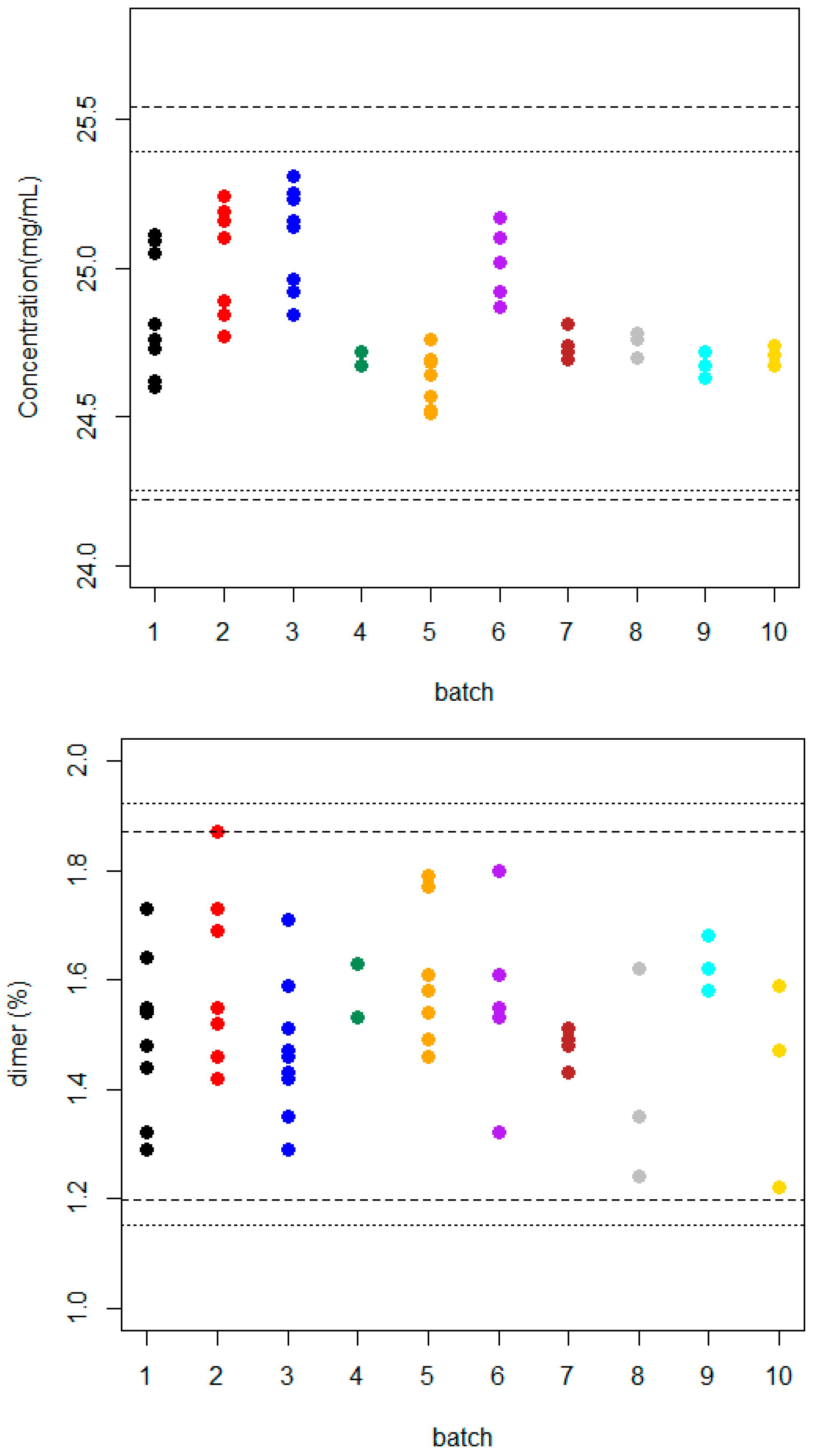
| Content (mg/mL) | Dimer (%) | ||||||
|---|---|---|---|---|---|---|---|
| Batch | Sample Size | Mean | SD | CV (%) | Mean | SD | CV (%) |
| 1 | 8 | 24.85 | 0.209 | 0.84 | 1.499 | 0.150 | 10.01 |
| 2 | 7 | 25.03 | 0.189 | 0.76 | 1.606 | 0.162 | 10.09 |
| 3 | 9 | 25.11 | 0.167 | 0.67 | 1.470 | 0.125 | 8.50 |
| 4 | 2 | 24.69 | 0.035 | 0.14 | 1.580 | 0.071 | 4.49 |
| 5 | 8 | 24.61 | 0.096 | 0.39 | 1.587 | 0.130 | 8.19 |
| 6 | 5 | 25.02 | 0.124 | 0.50 | 1.562 | 0.172 | 11.01 |
| 7 | 4 | 24.74 | 0.051 | 0.21 | 1.478 | 0.034 | 2.30 |
| 8 | 3 | 24.75 | 0.042 | 0.17 | 1.403 | 0.195 | 13.90 |
| 9 | 3 | 24.67 | 0.045 | 0.18 | 1.621 | 0.050 | 3.08 |
| 10 | 3 | 24.71 | 0.035 | 0.14 | 1.427 | 0.189 | 13.24 |
| 6 Batches | ||||
|---|---|---|---|---|
| CQA | Parameter | Estimation | Lower Bound | Upper Bound |
| Content (mg/mL) | 0.190 | 0.093 | 0.364 | |
| 0.162 | 0.130 | 0.210 | ||
| 24.89 | 24.71 | 25.06 | ||
| Dimer (%) | 0.029 | 0.000 | 0.098 | |
| 0.142 | 0.114 | 0.181 | ||
| 1.543 | 1.490 | 1.600 | ||
| 10 Batches | ||||
| CQA | Parameter | Estimation | Lower Bound | Upper Bound |
| Content (mg/mL) | 0.167 | 0.098 | 0.276 | |
| 0.145 | 0.119 | 0.182 | ||
| 24.82 | 24.70 | 24.94 | ||
| Dimer (%) | 0.034 | 0.000 | 0.096 | |
| 0.141 | 0.116 | 0.176 | ||
| 1.523 | 1.478 | 1.573 | ||
| 6 Batches | ||||
|---|---|---|---|---|
| QR Bounds | Lower | Estimate | Upper | |
| Bevacizumab content (%) | lower | 96.56 | 97.06 | 97.56 |
| upper | 101.56 | 102.06 | 102.56 | |
| Dimer (%) | lower | 1.118 | 1.193 | 1.268 |
| upper | 1.866 | 1.951 | 2016 | |
| 10 Batches | ||||
| Bevacizumab content (%) | lower | 96.64 | 97.08 | 97.53 |
| upper | 101.07 | 101.51 | 101.95 | |
| Dimer (%) | lower | 1.103 | 1.174 | 1.245 |
| upper | 1.812 | 1.883 | 1.955 | |
| 6 Batches | ||||
|---|---|---|---|---|
| QR Bounds | Lower | Estimate | Upper | |
| Bevacizumab content (%) | lower | 95.91 | 96.69 | 97.47 |
| upper | 101.22 | 102.28 | 103.35 | |
| Dimer (%) | lower | 0.972 | 1.172 | 1.372 |
| upper | 1.742 | 1.972 | 2.203 | |
| 10 Batches | ||||
| Bevacizumab content (%) | lower | 96.30 | 96.91 | 97.52 |
| upper | 100.65 | 101.56 | 102.48 | |
| Dimer (%) | lower | 0.978 | 1.112 | 1.246 |
| upper | 1.777 | 1.917 | 2.057 | |
| Bevacizumab (%) | Dimer (%) | |||||
|---|---|---|---|---|---|---|
| Product | Mean | Sigma | Min–Max | Mean | Sigma | Min–Max |
| Biosimilar #1 | 98.72 | 0.259 | 98.36–99.12 | 1.405 | 0.242 | 1.145–1.780 |
| Biosimilar #2 | 99.22 | 0.220 | 98.99–99.56 | 0.808 | 0.176 | 0.630–1.048 |
| Biosimilar #3 | 98.17 | 0.270 | 97.84–98.52 | 2.083 | 0.195 | 1.808–2.356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, A.; Echezarreta, M.; Santana-Mayor, Á.; Conde-Díaz, A.; Goncalves, J.; Chow, S.-C.; Llabrés, M. Assessing Protein Content and Dimer Formation in the Bevacizumab Reference Product and Biosimilar Versions Marketed in Spain. Pharmaceutics 2024, 16, 1520. https://doi.org/10.3390/pharmaceutics16121520
Oliva A, Echezarreta M, Santana-Mayor Á, Conde-Díaz A, Goncalves J, Chow S-C, Llabrés M. Assessing Protein Content and Dimer Formation in the Bevacizumab Reference Product and Biosimilar Versions Marketed in Spain. Pharmaceutics. 2024; 16(12):1520. https://doi.org/10.3390/pharmaceutics16121520
Chicago/Turabian StyleOliva, Alexis, Magdalena Echezarreta, Álvaro Santana-Mayor, Adrían Conde-Díaz, Joao Goncalves, Shein-Chung Chow, and Matías Llabrés. 2024. "Assessing Protein Content and Dimer Formation in the Bevacizumab Reference Product and Biosimilar Versions Marketed in Spain" Pharmaceutics 16, no. 12: 1520. https://doi.org/10.3390/pharmaceutics16121520
APA StyleOliva, A., Echezarreta, M., Santana-Mayor, Á., Conde-Díaz, A., Goncalves, J., Chow, S.-C., & Llabrés, M. (2024). Assessing Protein Content and Dimer Formation in the Bevacizumab Reference Product and Biosimilar Versions Marketed in Spain. Pharmaceutics, 16(12), 1520. https://doi.org/10.3390/pharmaceutics16121520










