Effects of Baccharis dracunculifolia DC on an Innovative Animal Model of Cardiometabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Baccharis dracunculifolia Extract and Phytochemical Composition
2.2. Pharmacological Studies
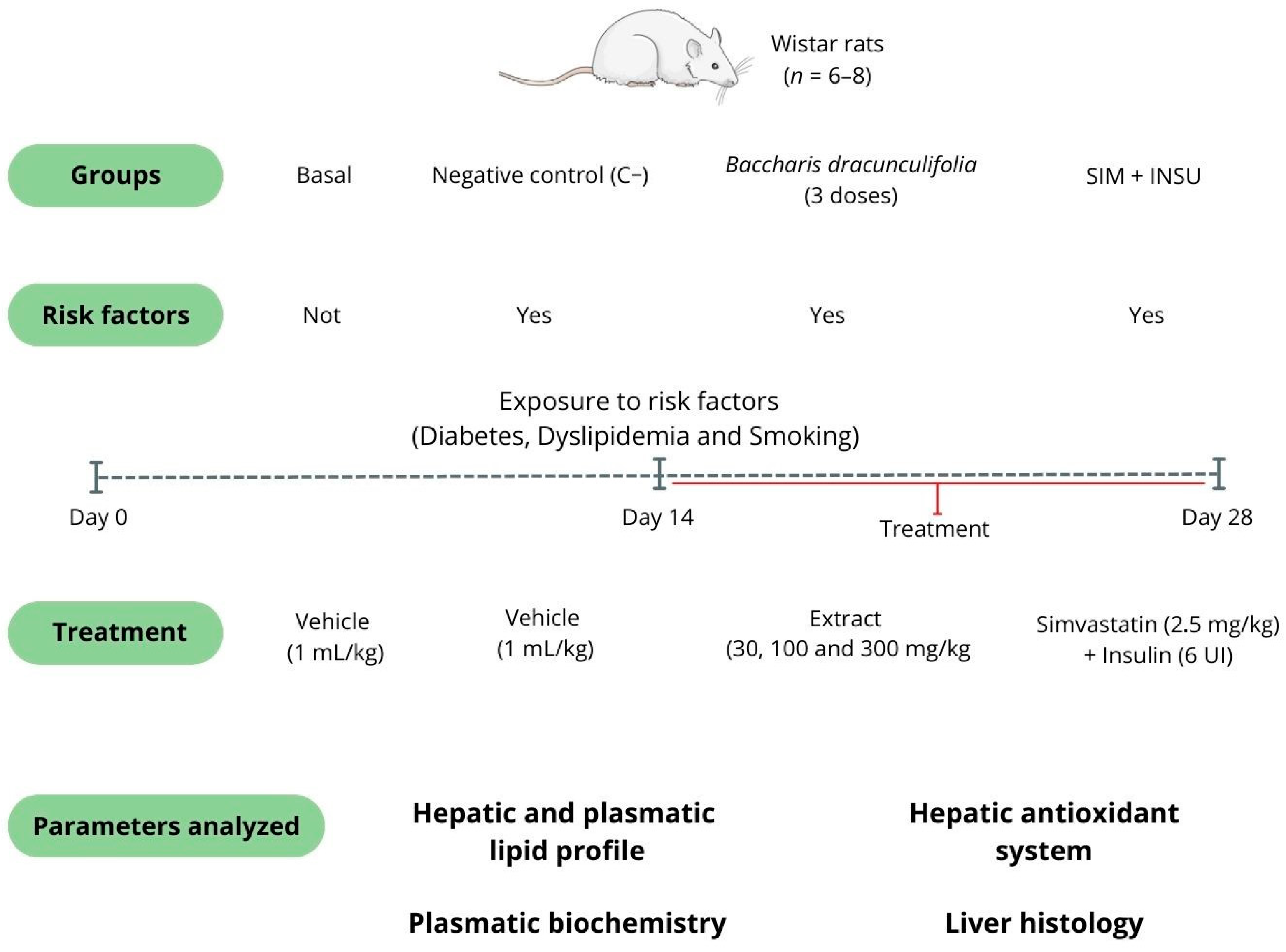
2.2.1. Animals
2.2.2. Experimental Design and Treatments
2.2.3. Euthanasia, Sample Collection, and Biochemical Analysis
2.2.4. Measurement of Hepatic Cholesterol and Triglycerides
2.2.5. Investigation of the Hepatic Antioxidant System
2.2.6. Histopathological Analysis
2.3. Blinding Clarification and Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Baccharis dracunculifolia Did Not Alter the Body Weight and Chow Consumption of Rats
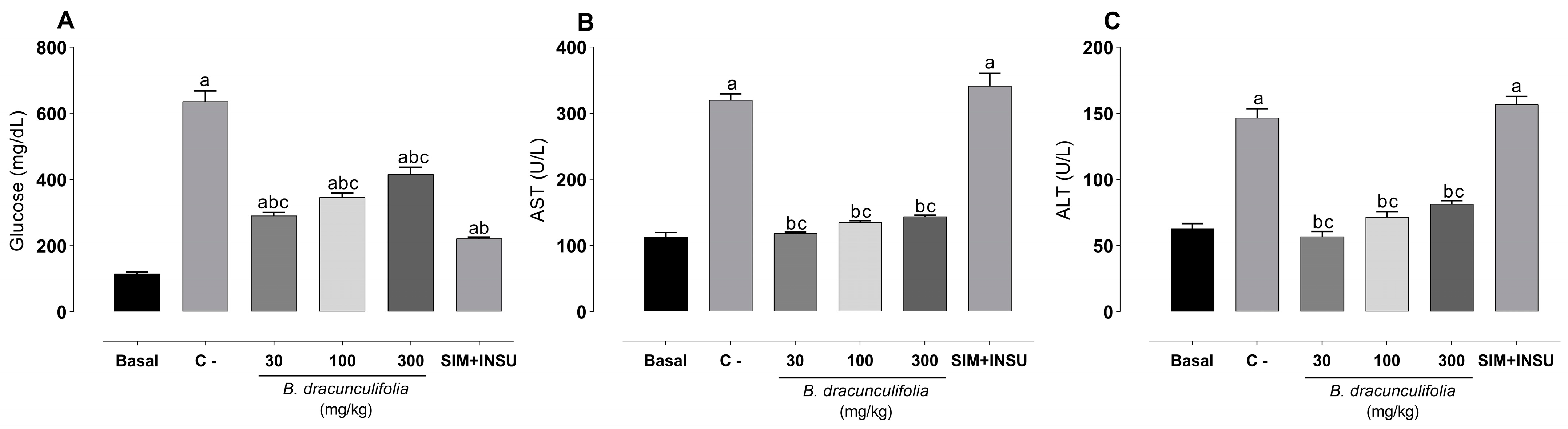
3.3. Baccharis dracunculifolia Exhibit a Median Hypoglycemic Effect and Demonstrated Significant Hepatoprotective Properties
3.4. Baccharis dracunculifolia Extract Exerted Lipid-Lowering Effects
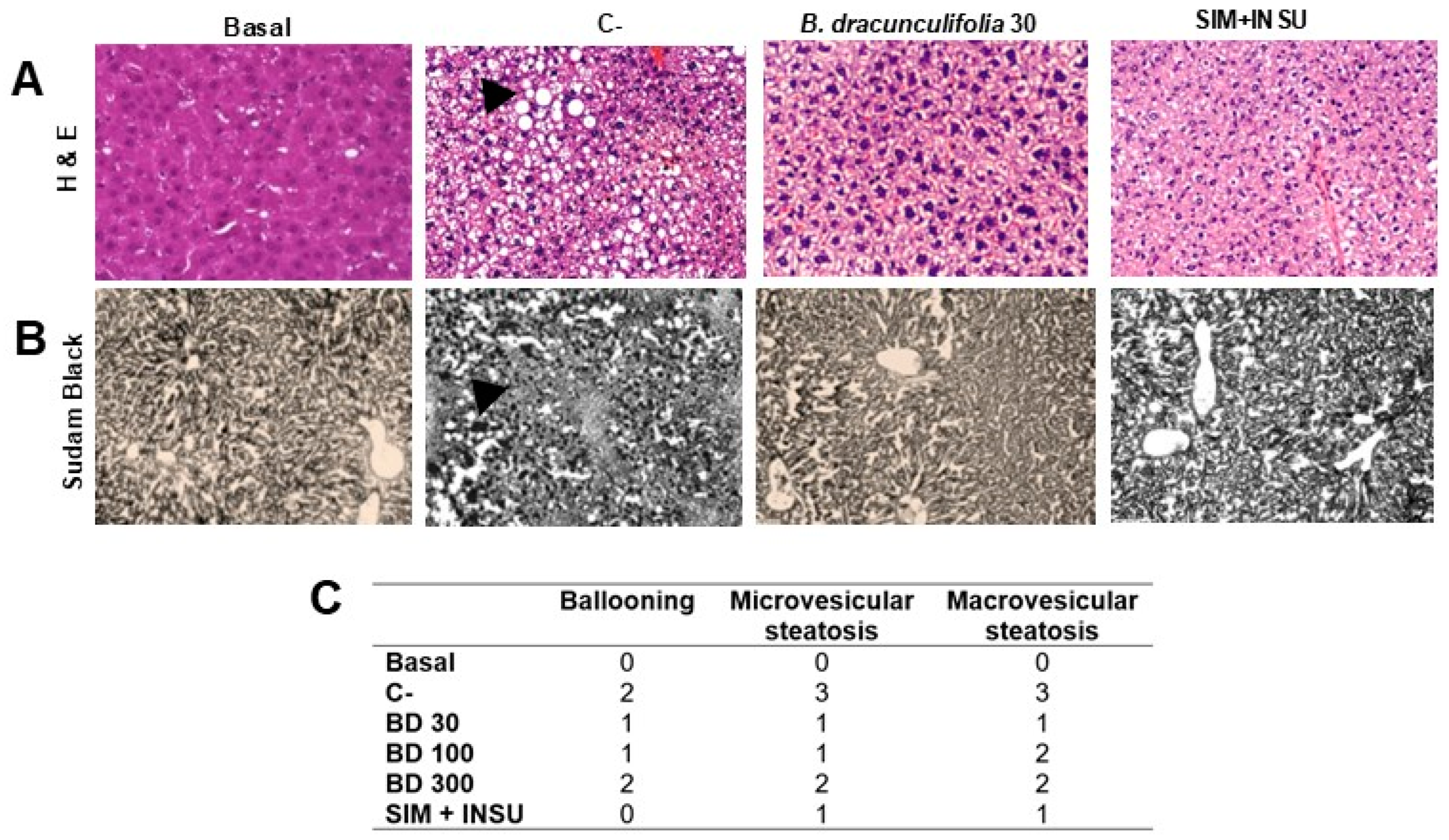
3.5. Baccharis dracunculifolia Reversed Hepatocellular Alterations
3.6. Effects of Baccharis dracunculifolia on Hepatic Redox State
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Verma, A.; Garg, R.; Singh, J.; Verma, H. Cardiometabolic risk factors associated with type 2 diabetes mellitus: A mechanistic insight. Clin. Med. Insights 2023, 16, 11795514231220780. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Gill, J.M.; Alazawi, W. Improving prevention strategies for cardiometabolic disease. Nat. Med. 2020, 26, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and cardiovascular disease: Inter-relation of risk factors and treatment. Future J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Marzoog, B.A. The metabolic syndrome puzzles; possible pathogenesis and management. Curr. Diabetes Rev. 2023, 19, 59–66. [Google Scholar] [CrossRef]
- Kelli, H.M.; Kassas, I.; Lattouf, O.M. Cardio metabolic syndrome: A global epidemic. J. Diabetes Metab. 2015, 6, 2–14. [Google Scholar] [CrossRef]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011–18. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodriguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New insights and potential therapeutic interventions in metabolic diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Bugger, H.; Byrne, N.J.; Abel, E.D. Animal models of dysregulated cardiac metabolism. Circ. Res. 2022, 130, 1965–1993. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic effects and safe uses of plant-derived polyphenolic compounds in cardiovascular diseases: A review. Drug Des. Dev. Ther. 2021, 2021, 4713–4732. [Google Scholar] [CrossRef] [PubMed]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Bastos, E.M.A.F.; Santana, R.A.; Calaça-Costa, A.G.F.; Thiago, P.S. Interaction between Apis mellifera L. and Baccharis dracunculifolia DC, that favours green propolis production in Minas Gerais. Braz. J. Biol. 2011, 71, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.J.T.; de Sousa, J.P.; Lima, C.N.F.; Lemos, I.C.S.; da Silva, A.R.P.; de Freitas, T.S.; Coutinho, H.D.M.; Silva, L.E.; Deschamps, C. Phytochemical characterization of the Baccharis dracunculifolia DC (Asteraceae) essential oil and antibacterial activity evaluation. Ind. Crops Prod. 2018, 122, 591–595. [Google Scholar] [CrossRef]
- Figueiredo-Rinhel, A.S.G.; de Andrade, M.F.; Landi-Librandi, A.P.; Azzolini, A.E.C.S.; Kabeya, L.M.; Bastos, J.K.; Lucisano-Valim, Y.M. Incorporation of Baccharis dracunculifolia DC (Asteraceae) leaf extract into phosphatidylcholine-cholesterol liposomes improves its anti-inflammatory effect in vivo. Nat. Prod. Res. 2019, 33, 2521–2525. [Google Scholar] [CrossRef]
- Boeing, T.; Costa, P.; Venzon, L.; Meurer, M.; Mariano, L.N.B.; França, T.C.S.; Gouveia, L.; Bassi, A.C.; Steimbach, V.; Souza, P.; et al. Gastric healing effect of p-coumaric acid isolated from Baccharis dracunculifolia DC on animal model. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 49–57. [Google Scholar] [CrossRef]
- Bonin, E.; Carvalho, V.M.; Avila, V.D.; dos Santos, N.C.A.; Benassi-Zanqueta, É.; Lancheros, C.A.C.; Previdelli, I.T.S.; Ueda-Nakamura, T.; Prado, I.N. Baccharis dracunculifolia: Chemical constituents, cytotoxicity and antimicrobial activity. Lebensm. Wiss. Technol. 2020, 120, 108920. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Júnior, A.W.; Busso, C.; Wouk, J.; Iurckevicz, G.; Montanher, P.F.; Yamashita, F.; Malfatti, C.R.M. Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Ind. Crops Prod. 2018, 125, 207–219. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.D.A.; Costa, S.S.; Silva, D.F.D.; Brandao, H.N.; Rocha, J.L.C.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Bachiega, T.F.; de Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. Immunomodulatory/anti-inflammatory effects of Baccharis dracunculifolia leaves. Nat. Prod. Res. 2013, 27, 1646–1650. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Dias, J.H.; Semedo, J.G.; da Silva, J.; Ferraz, A.B.; Picada, J.N. Mutagenic and genotoxic effects of Baccharis dracunculifolia (DC). J. Ethnopharmacol. 2009, 124, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.C.; Machado, M.D.A.; Sakumoto, K.; Inumaro, R.S.; Gonçalves, J.E.; Mandim, F.; Vaz, J.; Valle, J.S.; Faria, M.G.I.; Ruiz, S.P.; et al. Cellular antioxidant, anti-inflammatory, and antiproliferative activities from the flowers, leaves and fruits of Gallesia integrifolia Spreng Harms. Molecules 2023, 28, 5406. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.A.D.S.; Bueno, P.C.P.; Gregório, L.E.; Silva, M.L.A.E.; Albuquerque, S.; Bastos, J.K. In vitro trypanocidal activity evaluation of crude extract and isolated compounds from Baccharis dracunculifolia D.C. (Asteraceae). J. Pharm. Pharmacol. 2004, 59, 1195–1199. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Neto, M.A.M.; Leandro, L.F.; Bastos, J.K.; da Silva Filho, A.A.; Tavares, D.C. In vivo antigenotoxicity of baccharin, an important constituent of Baccharis dracunculifolia DC (Asteraceae). Basic Clin. Pharmacol. Toxicol. 2011, 109, 35–41. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Valle, J.S.; Santos, I.C.; Rahal, I.L.; Silva, G.C.C.; Lopes, A.D.; Ruiz, S.P.; Faria, M.G.I.; Piau Junior, R.; Gonçalves, D.D. Ethnomedicinal, phytochemical and pharmacological investigations of Baccharis dracunculifolia DC. (Asteraceae). Front. Pharmacol. 2022, 13, 1048688. [Google Scholar] [CrossRef]
- Velasco, M.; Ortiz-Huidobro, R.I.; Larqué, C.; Sánchez-Zamora, Y.I.; Romo-Yáñez, J.; Hiriart, M. Sexual dimorphism in insulin resistance in a metabolic syndrome rat model. Endocr. Connect. 2020, 9, 890–902. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.M.Q.; Silva, G.R.; Cola, I.M.; Silva, A.O.; Schaedler, M.I.; Guarnier, L.P.; Palozi, R.A.C.; Barboza, L.N.; Menetrier, J.V.; Froelich, D.L.; et al. Baccharis trimera (Less.) DC: An innovative cardioprotective herbal medicine against multiple risk factors for cardiovascular disease. J. Med. Food 2020, 23, 676–684. [Google Scholar] [CrossRef]
- Vit, P.; de Jesús, R.O.S.A.; Gudiño, M.; Jacob, T. Inducción de cataratas experimentales en ratas diabetizadas con estreptozotocina. Rev. Fac. Farm. 2002, 43, 15–18. [Google Scholar]
- Albuquerque, E.R.; Silva, G.R.; Braga, F.A.; Silva, E.P.; Negrini, K.S.; Fracasso, J.A.R.; Guarnier, L.P.; Jacomassi, E.; Ribeiro-Paes, J.T.; Gomes, R.S.; et al. Bridging the gap: Exploring the preclinical potential of Pereskia grandifolia in metabolic-associated fatty liver disease. Evid.-Based Complement. Alternat. Med. 2023, 2023, 8840427. [Google Scholar] [CrossRef]
- Barbosa, R.J.; Silva, G.R.; Cola, I.M.; Kuchler, J.C.; Coelho, N.; Barboza, L.N.; Menetriel, J.V.; Souza, R.; Zonta, F.N.; Froehlich, D.L.; et al. Promising therapeutic use of Baccharis trimera (Less.) DC. as a natural hepatoprotective agent against hepatic lesions that are caused by multiple risk factors. J. Ethnopharmacol. 2020, 254, 112729. [Google Scholar] [CrossRef] [PubMed]
- Zago, P.M.J.J.; Silva, G.R.; Amaral, E.C.; Barboza, L.N.; Braga, F.A.; Lorençone, B.R.; Marques, A.A.M.; Moreno, K.G.T.; Leite, P.R.T.; Veiga, A.A.; et al. Multiple risk factors for heart disease: A challenge to the ethnopharmacological use of Croton urucurana Baill. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6580458. [Google Scholar] [CrossRef] [PubMed]
- Auth, P.A.; Silva, G.R.; Amaral, E.C.; Bortoli, V.F.; Manzano, M.I.; Souza, L.M.; Lovato, E.C.W.; Ribeiro-Paes, J.T.; Gasparotto Junior, A.; Lívero, F.A.D.R. Croton urucurana Baill. ameliorates metabolic associated fatty liver disease in rats. Front. Pharmacol. 2022, 13, 886122. [Google Scholar] [CrossRef]
- Lívero, F.A.R.; Martins, G.G.; Telles, J.E.Q.; Beltrame, O.C.; Biscaia, S.M.P.; Franco, C.R.C.; Elferink, R.P.J.O.; Acco, A. Hydroethanolic extract of Baccharis trimera ameliorates alcoholic fatty liver disease in mice. Chem. Biol. Interact. 2016, 260, 22–32. [Google Scholar] [CrossRef]
- Lívero, F.A.R.; da Silva, L.M.; Ferreira, D.M.; Galuppo, L.F.; Borato, D.G.; Prando, T.B.; Lourenço, E.L.; Strapasson, R.L.; Stefanello, M.É.; Werner, M.F.; et al. Hydroethanolic extract of Baccharis trimera promotes gastroprotection and healing of acute and chronic gastric ulcers induced by ethanol and acetic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 985–998. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.C.; Silva, G.R.; Silva, A.O.; Schaedler, M.I.; Guarnier, L.P.; Palozi, R.A.C.; Signor, C.T.; Bosco, J.D.D.; Auth, P.A.; Amaral, E.C.; et al. Hepato-and cardioprotective effects of Baccharis trimera (Less.) DC. against multiple risk factors for chronic noncommunicable diseases. An. Acad. Bras. Cienc. 2021, 93, e20200899. [Google Scholar] [CrossRef]
- Guerra, J.V.S.; Dias, M.M.G.D.; Brilhante, A.J.V.C.B.; Terra, M.F.; García-Arévalo, M.; Figueira, A.C.M. Multifactorial basis and therapeutic strategies in metabolism-related diseases. Nutrient 2021, 13, 2830. [Google Scholar] [CrossRef]
- Franks, P.W.; Cefalu, W.T.; Dennis, J.; Florez, J.C.; Mathieu, C.; Morton, R.W. Precision medicine for cardiometabolic disease: A framework for clinical translation. Lancet Diabetes Endocrinol. 2023, 11, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012, 1259, 87–94. [Google Scholar] [CrossRef]
- Barnaba, C.; Medina-Meza, I.G. Flavonoids ability to disrupt inflammation mediated by lipid and cholesterol oxidation. Adv. Exp. Med. Biol. 2019, 1161, 243–253. [Google Scholar] [CrossRef]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensmitt Rundsch. 2007, 103, 369–377. [Google Scholar]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.J.; et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahmad, W.A.N.W.; Budin, S.B.; Zainalabidin, S. Implication of dietary phenolic acids on inflammation in cardiovascular disease. Rev. Cardiovasc. Med. 2020, 21, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, X.; Wang, J.; Wen, R.; Jia, L.; Zhu, Y.; Li, R.; Wang, R.; Li, J.; Wang, L.; et al. Large dose means significant effect--dose and effect relationship of Chi-Dan-Tui-Huang decoction on alpha-naphthylisothiocyanate-induced cholestatic hepatitis in rats. BMC Complement. Altern. Med. 2015, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, H.; Chen, D.; Gu, Y. A dose-effect relationship of Ginkgo biloba extract to nerve regeneration in a rat model. Microsurgery 2007, 27, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Qin, C.S.; Xuan, S.T.S.; Ying, P.J.; Le, H.Y.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic effects of plant flavonoids: A review. Evid. Based Complement. Alternat. Med. 2021, 2021, 2057333. [Google Scholar] [CrossRef]
- Long, H.P.; Liu, J.; Xu, P.S.; Xu, K.P.; Li, J.; Tan, G.S. Hypoglycemic flavonoids from Selaginella tamariscina (P. Beauv.) Spring. Phytochemistry 2022, 195, 113073. [Google Scholar] [CrossRef]
- Shaikhomar, O.A.; Bahattab, O.S. Physiological effect of quercetin as a natural flavonoid to be used as hypoglycemic agent in diabetes mellitus type II rats. Saudi J. Biomed. Res. 2021, 6, 10–17. [Google Scholar] [CrossRef]
- Angelico, F.; Alcantara-Payawal, D.; Rani, R.A.; Mustafa, N.; Thongtang, N.; Chaiteerakij, R.; Bunchorntavakul, C.; Sukonthasarn, A. Review and expert opinion on MAFLD, oxidative stress and multifunctional management. Drugs Context 2024, 13, 2023. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; de Pádua Lúcio, K.; Araújo, C.M.; de Araújo, G.R.; de Amorim Miranda, P.H.; Carneiro, A.C.A.; de Castro Ribeiro, É.M.; de Melo Silva, B.; de Lima, W.G.; Costa, D.C. Baccharis trimera protects against ethanol induced hepatotoxicity in vitro and in vivo. J. Ethnopharmacol. 2018, 215, 1–13. [Google Scholar] [CrossRef]
- Pádua, B.C.; Silva, L.D.; Rossoni Júnior, J.V.; Humberto, J.L.; Chaves, M.M.; Silva, M.E.; Pedrosa, M.L.; Costa, D.C. Antioxidant properties of Baccharis trimera in the neutrophils of Fisher rats. J. Ethnopharmacol. 2010, 129, 381–386. [Google Scholar] [CrossRef]
- Sabir, S.M.; Athayde, M.L.; Boligon, A.A.; Rocha, J.B.T. Antioxidant activities and phenolic profile of Baccharis trimera, a commonly used medicinal plant from Brazil. S. Afr. J. Bot. 2017, 113, 318–323. [Google Scholar] [CrossRef]
- Katsiki, N.; Perez-Martinez, P.; Anagnostis, P.; Mikhailidis, D.P.; Karagiannis, A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr. Vasc. Pharmacol. 2018, 16, 219–227. [Google Scholar] [CrossRef]
- Nassir, F. NAFLD: Mechanisms, treatments, and biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef]
- Adams, S.P.; Alaeiilkhchi, N.; Wright, J.M. Simvastatin for lowering lipids. Cochrane Database Syst. Rev. 2023, 2023, CD014857. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Deedwania, P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr. Hypertens. Rep. 2016, 18, 76. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes mellitus and its metabolic complications: The role of adipose tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Satapati, S.; Fu, X.; He, T.; Mehdibeigi, R.; Spring-Robinson, C.; Duarte, J.; Potthoff, M.J.; Browning, J.D.; Burgess, S.C. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1226–E1235. [Google Scholar] [CrossRef]
- Newsholme, P.; Brennan, L.; Rubi, B.; Maechler, P. New insights into amino acid metabolism, β-cell function and diabetes. Clin. Sci. 2005, 108, 185–194. [Google Scholar] [CrossRef]
- Chandrasekera, P.C.; Pippin, J.J. Of rodents and men: Species-specific glucose regulation and type 2 diabetes research. ALTEX 2014, 31, 157–176. [Google Scholar] [CrossRef]



| Compounds | Theoretical Mass m/z [M-H] | Experimental Mass m/z [M-H] | Retention Time (min) | Error |
|---|---|---|---|---|
| Phenolic acids | ||||
| Quinic acid | 191.05 | 191.05 | 0.84 | −3.66 |
| Chlorogenic acid | 353.08 | 353.08 | 4.25 | 0 |
| 4-Hydroxybenzoic acid | 137.02 | 137.02 | 4.10 | −5.11 |
| Caffeic acid | 179.03 | 179.03 | 4.15 | 4.47 |
| p-Coumaric acid | 163.03 | 163.03 | 4.60 | −4.29 |
| Ferulic acid | 193.04 | 193.05 | 5.51 | −3.62 |
| Protocatechuic acid | 153.01 | 153.01 | 1.28 | −3.92 |
| Flavonoids | ||||
| Isoquercetin | 463.08 | 463.08 | 4.40 | 2.59 |
| Quercetin | 301.03 | 301.03 | 5.16 | 0.33 |
| Isokaempferide | 299.05 | 299.05 | 5.45 | −2.01 |
| Kaempferol | 285.03 | 285.03 | 5.48 | 0 |
| 3-Methoxy-quercetin | 315.05 | 315.05 | 5.51 | 0.32 |
| Apigenin | 269.04 | 269.04 | 5.43 | −0.74 |
| Groups | Initial | At 2 Weeks | At 4 Weeks | ||
|---|---|---|---|---|---|
| Body Weight (g) | Body Weight (g) | Chow Consumption (g) | Body Weight (g) | Chow Consumption (g) | |
| Basal | 274.30 ± 1.87 | 283.70 ± 3.55 | 108.90 ± 2.93 | 287.90 ± 3.58 | 101.60 ± 4.98 |
| C− | 244.30 ± 5.57 | 245.40 ± 7.94 | 135.90 ± 3.23 | 254.50 ± 6.53 | 126.4 ± 6.43 |
| BD 30 | 240.30 ± 2.71 | 233.80 ± 5.83 | 142.80 ± 5.06 | 230.30 ± 10.53 | 136.4 ± 4.88 |
| BD 100 | 227.30 ± 4.25 | 228.90 ± 2.77 | 128.30 ± 8.57 | 230.40 ± 6.51 | 132.9 ± 8.53 |
| BD 300 | 205.90 ± 7.36 | 223.10 ± 7.35 | 112.20 ± 3.72 | 231.10 ± 8.77 | 103.7 ± 5.10 |
| SIM + INSU | 241.90 ± 5.13 | 246.50 ± 4.71 | 133.80 ± 7.16 | 287.00 ± 4.98 | 153.60 ± 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.R.d.; Kluck, A.J.; Albuquerque, E.R.; Guarnier, L.P.; Braga, F.d.A.; Silva, E.P.; Negrini, K.S.; Mendonça, J.A.; Gazim, Z.C.; Gasparotto Junior, A.; et al. Effects of Baccharis dracunculifolia DC on an Innovative Animal Model of Cardiometabolic Syndrome. Pharmaceutics 2024, 16, 1446. https://doi.org/10.3390/pharmaceutics16111446
Silva GRd, Kluck AJ, Albuquerque ER, Guarnier LP, Braga FdA, Silva EP, Negrini KS, Mendonça JA, Gazim ZC, Gasparotto Junior A, et al. Effects of Baccharis dracunculifolia DC on an Innovative Animal Model of Cardiometabolic Syndrome. Pharmaceutics. 2024; 16(11):1446. https://doi.org/10.3390/pharmaceutics16111446
Chicago/Turabian StyleSilva, Gustavo Ratti da, Arianne Jung Kluck, Edilson Rodrigues Albuquerque, Lucas Pires Guarnier, Fernanda de Abreu Braga, Ester Pelegrini Silva, Karina Sposito Negrini, Juliana Aparecida Mendonça, Zilda Cristiani Gazim, Arquimedes Gasparotto Junior, and et al. 2024. "Effects of Baccharis dracunculifolia DC on an Innovative Animal Model of Cardiometabolic Syndrome" Pharmaceutics 16, no. 11: 1446. https://doi.org/10.3390/pharmaceutics16111446
APA StyleSilva, G. R. d., Kluck, A. J., Albuquerque, E. R., Guarnier, L. P., Braga, F. d. A., Silva, E. P., Negrini, K. S., Mendonça, J. A., Gazim, Z. C., Gasparotto Junior, A., Ribeiro-Paes, J. T., & Lívero, F. A. d. R. (2024). Effects of Baccharis dracunculifolia DC on an Innovative Animal Model of Cardiometabolic Syndrome. Pharmaceutics, 16(11), 1446. https://doi.org/10.3390/pharmaceutics16111446








