Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Oligonucleotide Synthesis
- [Alkyne]-GGTCGCTTATCTGCACTCGGA (A1),
- [Alkyne]ACGCACCATTTGTTTAATATGTTTTTTAATTCCCCTTGTGGTGTGT (GR20).
2.3. General Method for Click Reaction of Oligonucleotides with Polyazides and MMAE Alkyne, One-Pot Procedure
2.4. General Method for Conjugate Purification
2.5. Cell Lines
2.6. Flow Cytometry
2.7. Cell Viability Assay
3. Results
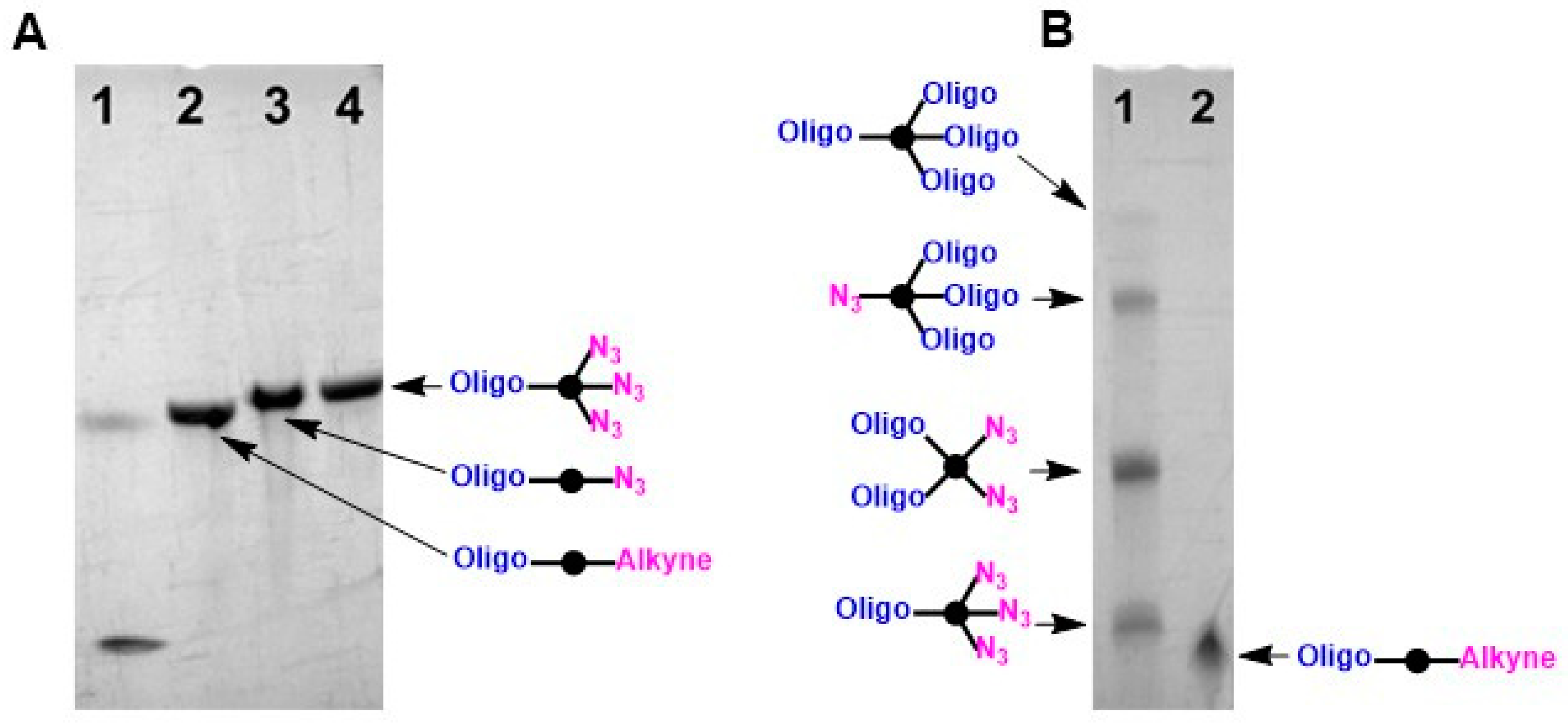
3.1. Synthesis of Conjugates
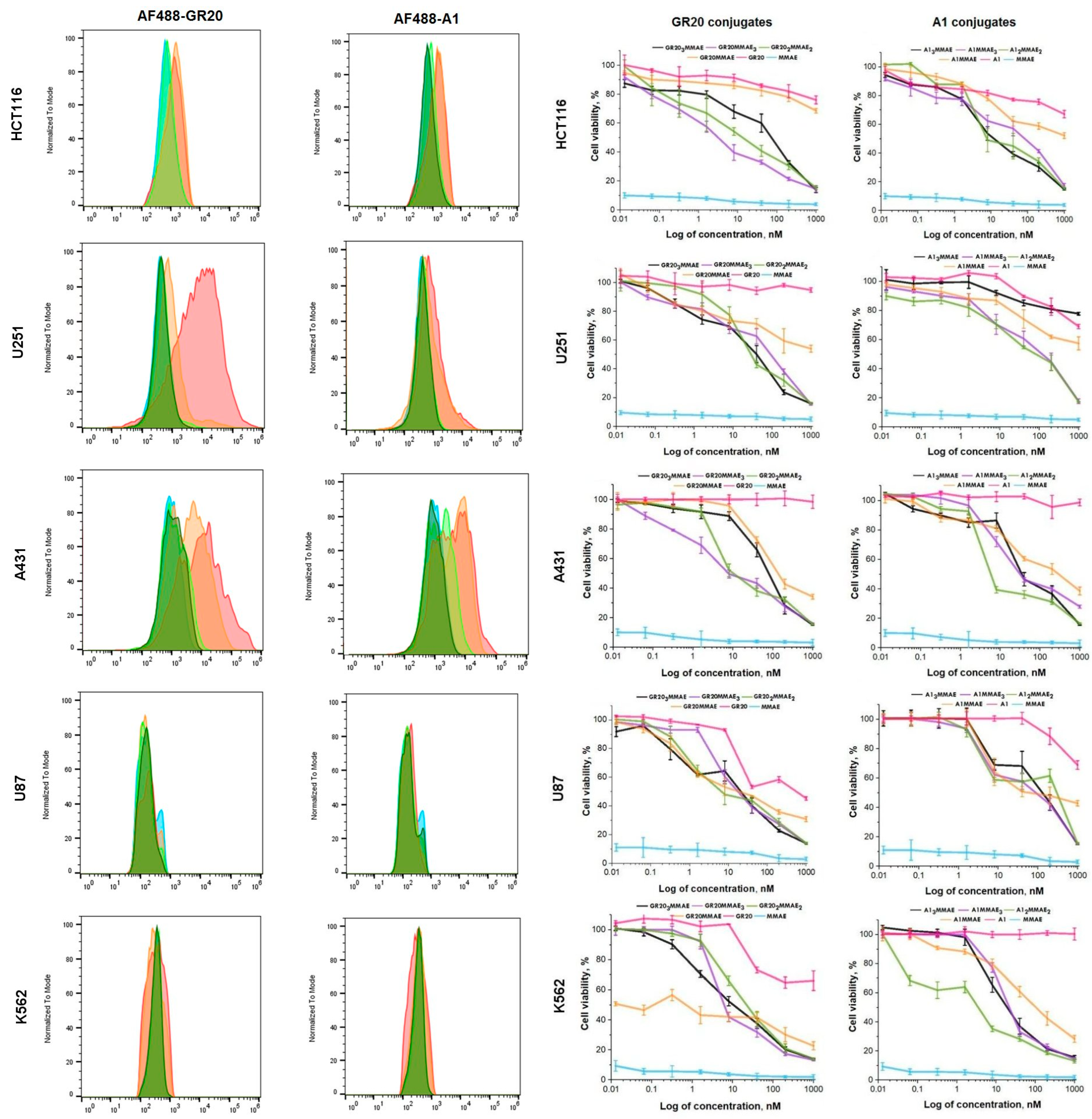
3.2. Flow Cytometry and Cell Viability Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenberger, M.L.; Jenkins, R.B. Genetics of Adult Glioma. Cancer Genet. 2012, 205, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.A.; Kern, M.; Fehrenbach, U.; Tanyldizi, Y.; Misch, M.; Wiener, E. T2 Mapping of the Peritumoral Infiltration Zone of Glioblastoma and Anaplastic Astrocytoma. Neuroradiol. J. 2021, 34, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Dewdney, B.; Jenkins, M.R.; Best, S.A.; Freytag, S.; Prasad, K.; Holst, J.; Endersby, R.; Johns, T.G. From Signalling Pathways to Targeted Therapies: Unravelling Glioblastoma’s Secrets and Harnessing Two Decades of Progress. Signal Transduct. Target. Ther. 2023, 8, 400. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Ezzati, S.; Salib, S.; Balasubramaniam, M.; Aboud, O. Epidermal Growth Factor Receptor Inhibitors in Glioblastoma: Current Status and Future Possibilities. Int. J. Mol. Sci. 2024, 25, 2316. [Google Scholar] [CrossRef]
- Hu, X.; Wang, R.; Jin, J.; Liu, X.; Cui, A.; Sun, L.; Li, Y.; Li, Y.; Wang, Y.; Zhen, Y.; et al. An EGFR-targeting Antibody–Drug Conjugate LR 004-VC-MMAE: Potential in Esophageal Squamous Cell Carcinoma and Other Malignancies. Mol. Oncol. 2019, 13, 246–263. [Google Scholar] [CrossRef]
- Ho, E.C.H.; Qiu, R.; Miller, E.; Bilotta, M.T.; FitzGerald, D.; Antignani, A. Antibody Drug Conjugates, Targeting Cancer-Expressed EGFR, Exhibit Potent and Specific Antitumor Activity. Biomed. Pharmacother. 2023, 157, 114047. [Google Scholar] [CrossRef] [PubMed]
- Gandullo-Sánchez, L.; Pandiella, A. An Anti-EGFR Antibody-Drug Conjugate Overcomes Resistance to HER2-Targeted Drugs. Cancer Lett. 2023, 554, 216024. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Chatterjee, J.; Patil, K.T.; Arora, S.; Katiyar, M.K.; Kumar, M.; Samarbakhsh, A.; Joshi, G.; Bhutani, P.; Chugh, M.; et al. Targeting the Epidermal Growth Factor Receptor with Molecular Degraders: State-of-the-Art and Future Opportunities. J. Med. Chem. 2023, 66, 3135–3172. [Google Scholar] [CrossRef] [PubMed]
- Pisheh, L.; Matis, S.; Taglieri, M.; Di Gregorio, L.; Benelli, R.; Poggi, A. EGFR-Targeted Antibody–Drug Conjugate to Different Aminobisphosphonates: Direct and Indirect Antitumor Effects on Colorectal Carcinoma Cells. Cancers 2024, 16, 1256. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Chenoweth, A.M.; Johansson, A.; Laddach, R.; Guppy, N.; Trendell, J.; Esapa, B.; Mavousian, A.; Navarro-Llinas, B.; Haider, S.; et al. Anti-EGFR Antibody–Drug Conjugate Carrying an Inhibitor Targeting CDK Restricts Triple-Negative Breast Cancer Growth. Clin. Cancer Res. 2024, 30, 3298–3315. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Papadopoulos, K.P.; Strickler, J.H.; Lassman, A.B.; Waqar, S.N.; Chae, Y.K.; Patel, J.D.; Shacham-Shmueli, E.; Kelly, K.; Khasraw, M.; et al. Phase I Study of Anti-Epidermal Growth Factor Receptor Antibody-Drug Conjugate Serclutamab Talirine: Safety, Pharmacokinetics, and Antitumor Activity in Advanced Glioblastoma. Neuro-Oncol. Adv. 2023, 5, vdac183. [Google Scholar] [CrossRef]
- Li, D.; Sun, X.; Li, Y.; Shang, C.; Dong, Y.; Zhao, R.; Zhang, H.; Wang, Z.; Fan, S.; Ma, C.; et al. AGCM-22, a Novel Cetuximab-Based EGFR-Targeting Antibody-Drug-Conjugate with Highly Selective Anti-Glioblastoma Efficacy. Bioorg. Med. Chem. 2024, 102, 117657. [Google Scholar] [CrossRef]
- Hu, M.; Liu, H.; Zhang, Y.; Lu, D.; Zheng, L.; Wang, Y.; Chen, S.; Liu, T. Preparation and Evaluation of the PD0721-DOX Antibody-drug Conjugate Targeting EGFRvIII to Inhibit Glioblastoma. Exp. Ther. Med. 2024, 27, 254. [Google Scholar] [CrossRef]
- Jain, S.; Griffith, J.I.; Porath, K.A.; Rathi, S.; Le, J.; Pasa, T.I.; Decker, P.A.; Gupta, S.K.; Hu, Z.; Carlson, B.L.; et al. Bystander Effects, Pharmacokinetics, and Linker-Payload Stability of EGFR-Targeting Antibody-Drug Conjugates Losatuxizumab Vedotin and Depatux-M in Glioblastoma Models. Clin. Cancer Res. 2024, 30, 3287–3297. [Google Scholar] [CrossRef]
- Mair, M.J.; Bartsch, R.; Le Rhun, E.; Berghoff, A.S.; Brastianos, P.K.; Cortes, J.; Gan, H.K.; Lin, N.U.; Lassman, A.B.; Wen, P.Y.; et al. Understanding the Activity of Antibody–Drug Conjugates in Primary and Secondary Brain Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 372–389. [Google Scholar] [CrossRef]
- Xi, J.; Liu, K.; Peng, Z.; Dai, X.; Wang, Y.; Cai, C.; Yang, D.; Yan, C.; Li, X. Toxic Warhead-Armed Antibody for Targeted Treatment of Glioblastoma. Crit. Rev. Oncol. Hematol. 2024, 193, 104205. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lin, X.; Gao, X.; Khan, R.U.; Liao, J.-Y.; Du, S.; Ge, J.; Zeng, S.; Yao, S.Q. The Dawn of a New Era: Targeting the “Undruggables” with Antibody-Based Therapeutics. Chem. Rev. 2023, 123, 7782–7853. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, V.; Engelholm, L.H. Antibody–Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers 2024, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next Generation of Antibody–Drug Conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, X.; Yu, C.; Wang, L. Antibody-Drug Conjugate Overview: A State-of-the-Art Manufacturing Process and Control Strategy. Pharm. Res. 2024, 41, 419–440. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Gou, L.; Li, W.; Wang, Y. Antibody–Drug Conjugates: Recent Advances in Payloads. Acta Pharm. Sin. B 2023, 13, 4025–4059. [Google Scholar] [CrossRef]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload Diversification: A Key Step in the Development of Antibody–Drug Conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef]
- Lu, N.; Wu, J.; Tian, M.; Zhang, S.; Li, Z.; Shi, L. Comprehensive Review on the Elaboration of Payloads Derived from Natural Products for Antibody-Drug Conjugates. Eur. J. Med. Chem. 2024, 268, 116233. [Google Scholar] [CrossRef]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y.; et al. Site-Selective Modification Strategies in Antibody–Drug Conjugates. Chem. Soc. Rev. 2021, 50, 1305–1353. [Google Scholar] [CrossRef]
- Journeaux, T.; Bernardes, G.J.L. Homogeneous Multi-Payload Antibody–Drug Conjugates. Nat. Chem. 2024, 16, 854–870. [Google Scholar] [CrossRef]
- Parakh, S.; Nicolazzo, J.; Scott, A.M.; Gan, H.K. Antibody Drug Conjugates in Glioblastoma—Is There a Future for Them? Front. Oncol. 2021, 11, 718590. [Google Scholar] [CrossRef]
- Mao, Y.; Wei, D.; Fu, F.; Wang, H.; Sun, Z.; Huang, Z.; Wang, Y.; Zhang, G.; Zhang, X.; Jiang, B.; et al. Development of a MMAE-Based Antibody-Drug Conjugate Targeting B7–H3 for Glioblastoma. Eur. J. Med. Chem. 2023, 257, 115489. [Google Scholar] [CrossRef]
- Dufrusine, B.; Capone, E.; Ponziani, S.; Lattanzio, R.; Lanuti, P.; Giansanti, F.; De Laurenzi, V.; Iacobelli, S.; Ippoliti, R.; Mangiola, A.; et al. Extracellular LGALS3BP: A Potential Disease Marker and Actionable Target for Antibody–Drug Conjugate Therapy in Glioblastoma. Mol. Oncol. 2023, 17, 1460–1473. [Google Scholar] [CrossRef]
- Uchida, S.; Serada, S.; Suzuki, Y.; Funajima, E.; Kitakami, K.; Dobashi, K.; Tamatani, S.; Sato, Y.; Beppu, T.; Ogasawara, K.; et al. Glypican-1-Targeted Antibody–Drug Conjugate Inhibits the Growth of Glypican-1-Positive Glioblastoma. Neoplasia 2024, 50, 100982. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Parakh, S.; Osellame, L.D.; Cher, L.; Uccellini, A.; Hafeez, U.; Menon, S.; Scott, A.M. Antibody Drug Conjugates for Glioblastoma: Current Progress towards Clinical Use. Expert Opin. Biol. Ther. 2023, 23, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Narsinh, K.H.; Perez, E.; Haddad, A.F.; Young, J.S.; Savastano, L.; Villanueva-Meyer, J.E.; Winkler, E.; De Groot, J. Strategies to Improve Drug Delivery Across the Blood–Brain Barrier for Glioblastoma. Curr. Neurol. Neurosci. Rep. 2024, 24, 123–139. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as Therapeutics. Nat. Rev. Drug. Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, G.; Carnes, E. Therapeutic Applications of Aptamers. Int. J. Mol. Sci. 2024, 25, 6742. [Google Scholar] [CrossRef]
- Safarkhani, M.; Ahmadi, S.; Ipakchi, H.; Saeb, M.R.; Makvandi, P.; Ebrahimi Warkiani, M.; Rabiee, N.; Huh, Y. Advancements in Aptamer-Driven DNA Nanostructures for Precision Drug Delivery. Adv. Sci. 2024, 11, 2401617. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, J.; Ma, Y.; Zhu, X.; Zhang, C. Aptamers Entirely Built from Therapeutic Nucleoside Analogues for Targeted Cancer Therapy. J. Am. Chem. Soc. 2022, 144, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yin, J.; Chen, Y.; Guo, C.; Hu, H.; Su, J. Recent Advances in Aptamer-Based Targeted Drug Delivery Systems for Cancer Therapy. Front. Bioeng. Biotechnol. 2022, 10, 972933. [Google Scholar] [CrossRef]
- Jabbari, A.; Sameiyan, E.; Yaghoobi, E.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Aptamer-Based Targeted Delivery Systems for Cancer Treatment Using DNA Origami and DNA Nanostructures. Int. J. Pharm. 2023, 646, 123448. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, F.; Ahmari, A.; Shabani, S.; Sadeghi, B.; Fahimirad, S.; Fattahi, F. Aptamers as an Approach to Targeted Cancer Therapy. Cancer Cell Int. 2024, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, Y.; He, S. Aptamer-Mediated Therapeutic Strategies Provide a Potential Approach for Cancer. Int. Immunopharmacol. 2024, 136, 112356. [Google Scholar] [CrossRef]
- Li, N.; Nguyen, H.H.; Byrom, M.; Ellington, A.D. Inhibition of Cell Proliferation by an Anti-EGFR Aptamer. PLoS ONE 2011, 6, e20299. [Google Scholar] [CrossRef]
- Esposito, C.L.; Passaro, D.; Longobardo, I.; Condorelli, G.; Marotta, P.; Affuso, A.; De Franciscis, V.; Cerchia, L. A Neutralizing RNA Aptamer against EGFR Causes Selective Apoptotic Cell Death. PLoS ONE 2011, 6, e24071. [Google Scholar] [CrossRef]
- Ray, P.; Cheek, M.A.; Sharaf, M.L.; Li, N.; Ellington, A.D.; Sullenger, B.A.; Shaw, B.R.; White, R.R. Aptamer-Mediated Delivery of Chemotherapy to Pancreatic Cancer Cells. Nucleic Acid Ther. 2012, 22, 295–305. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Zhao, Z.; Liu, Q.; Tan, W.; Fang, X. Cellular Internalization and Cytotoxicity of Aptamers Selected from Lung Cancer Cell. Am. J. Biomed. Sci. 2013, 5, 47–58. [Google Scholar] [CrossRef]
- Wang, D.-L.; Song, Y.-L.; Zhu, Z.; Li, X.-L.; Zou, Y.; Yang, H.-T.; Wang, J.-J.; Yao, P.-S.; Pan, R.-J.; Yang, C.J.; et al. Selection of DNA Aptamers against Epidermal Growth Factor Receptor with High Affinity and Specificity. Biochem. Biophys. Res. Commun. 2014, 453, 681–685. [Google Scholar] [CrossRef]
- Wu, X.; Liang, H.; Tan, Y.; Yuan, C.; Li, S.; Li, X.; Li, G.; Shi, Y.; Zhang, X. Cell-SELEX Aptamer for Highly Specific Radionuclide Molecular Imaging of Glioblastoma in vivo. PLoS ONE 2014, 9, e90752. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Vermeulen, J.F.; Fereidouni, F.; Bader, A.N.; Voortman, J.; Roovers, R.C.; Gerritsen, H.C.; van Bergen en Henegouwen, P.M.P. Endocytosis of EGFR Requires its Kinase Activity and N-Terminal Transmembrane Dimerization Motif. J. Cell Sci. 2013, 126, 4900–4912. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef]
- Vorobyeva, M.; Vorobjev, P.; Venyaminova, A. Multivalent Aptamers: Versatile Tools for Diagnostic and Therapeutic Applications. Molecules 2016, 21, 1613. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Napolitano, E.; Musumeci, D.; Montesarchio, D. Dimeric and Multimeric DNA Aptamers for Highly Effective Protein Recognition. Molecules 2020, 25, 5227. [Google Scholar] [CrossRef]
- Omer, M.; Andersen, V.L.; Nielsen, J.S.; Wengel, J.; Kjems, J. Improved Cancer Targeting by Multimerizing Aptamers on Nanoscaffolds. Mol. Ther. Nucleic Acids 2020, 22, 994–1003. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Gu, J.; Amini, R.; Mansfield, A.G.; Xia, J.; White, D.; Stacey, H.D.; Ang, J.C.; Panesar, G.; et al. Three on Three: Universal and High-Affinity Molecular Recognition of the Symmetric Homotrimeric Spike Protein of SARS-CoV-2 with a Symmetric Homotrimeric Aptamer. J. Am. Chem. Soc. 2022, 144, 23465–23473. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Lee, N.Z.; Cao, X. Multivalent Aptamer Approach: Designs, Strategies, and Applications. Micromachines 2022, 13, 436. [Google Scholar] [CrossRef]
- Zou, Z.; Younas, T.; Dumsday, G.; Haritos, V.S.; He, L. Rapid Production of Multimeric RNA Aptamers Stabilized by a Designed Pseudo-circular Structure in E. coli. Biotechnol. J. 2023, 18, 2200390. [Google Scholar] [CrossRef]
- Hamidi, S.V.; Jahromi, A.K.; Hosseini, I.I.; Moakhar, R.S.; Collazos, C.; Pan, Q.; Liang, C.; Mahshid, S. Surface-Based Multimeric Aptamer Generation and Bio-Functionalization for Electrochemical Biosensing Applications. Angew. Chem. Int. Ed. 2024, 63, e202402808. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; EI-Sagheer, A.H.; Li, S.; Vallis, K.A.; Tan, W.; Brown, T. Engineering Enzyme-Cleavable Oligonucleotides by Automated Solid-Phase Incorporation of Cathepsin B Sensitive Dipeptide Linkers. Angew. Chem. Int. Ed. 2022, 61, e202114016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, X.; Mu, G.; Zhao, X.; Wang, Q.; Wang, J.; Tang, X. Cathepsin B-Activatable Cyclic Antisense Oligonucleotides for Cell-Specific Target Gene Knockdown In Vitro and In Vivo. Mol. Ther. Nucleic Acids 2023, 33, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, S.; Vallis, K.A.; El-Sagheer, A.H.; Brown, T. Modular and Automated Synthesis of Oligonucleotide-Small Molecule Conjugates for Cathepsin B Mediated Traceless Release of Payloads. RSC Chem. Biol. 2024, 5, 738–744. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Uspenskaya, A.A.; Zyk, N.U.; Nimenko, E.A.; Ber, A.P.; Petrov, S.A.; Polshakov, V.I.; Shafikov, R.R.; Skvortsov, D.A.; Plotnikova, E.A.; et al. Synthesis, Characterization, and Preclinical Evaluation of a Small-Molecule Prostate-Specific Membrane Antigen-Targeted Monomethyl Auristatin E Conjugate. J. Med. Chem. 2021, 64, 17123–17145. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Z.; Li, H.; Yang, H.; Zhao, Y.; Fu, H. Bioorthogonal Ligation and Cleavage by Reactions of Chloroquinoxalines with ortho-Dithiophenols. Angew. Chem. Int. Ed. 2020, 59, 3671–3677. [Google Scholar] [CrossRef]
- Ponomarenko, A.I.; Brylev, V.A.; Sapozhnikova, K.A.; Ustinov, A.V.; Prokhorenko, I.A.; Zatsepin, T.S.; Korshun, V.A. Tetrahedral DNA Conjugates from Pentaerythritol-Based Polyazides. Tetrahedron 2016, 72, 2386–2391. [Google Scholar] [CrossRef]
- Farzan, V.M.; Ulashchik, E.A.; Martynenko-Makaev, Y.V.; Kvach, M.V.; Aparin, I.O.; Brylev, V.A.; Prikazchikova, T.A.; Maklakova, S.Y.; Majouga, A.G.; Ustinov, A.V.; et al. Automated Solid-Phase Click Synthesis of Oligonucleotide Conjugates: From Small Molecules to Diverse N-Acetylgalactosamine Clusters. Bioconjugate Chem. 2017, 28, 2599–2607. [Google Scholar] [CrossRef]
- Zavyalova, E.; Turashev, A.; Novoseltseva, A.; Legatova, V.; Antipova, O.; Savchenko, E.; Balk, S.; Golovin, A.; Pavlova, G.; Kopylov, A. Pyrene-Modified DNA Aptamers with High Affinity to Wild-Type EGFR and EGFRvIII. Nucleic Acid Ther. 2020, 30, 175–187. [Google Scholar] [CrossRef]
- Balamkundu, S.; Liu, C.-F. Lysosomal-Cleavable Peptide Linkers in Antibody–Drug Conjugates. Biomedicines 2023, 11, 3080. [Google Scholar] [CrossRef]
- Sheyi, R.; De La Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.proteinatlas.org/ENSG00000146648-EGFR/cell+line (accessed on 2 November 2024).
- El-Sagheer, A.H.; Brown, T. Click Chemistry with DNA. Chem. Soc. Rev. 2010, 39, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Ustinov, A.V.; Stepanova, I.A.; Dubnyakova, V.V.; Zatsepin, T.S.; Nozhevnikova, E.V.; Korshun, V.A. Modification of Nucleic Acids Using [3+2]-Dipolar Cycloaddition of Azides and Alkynes. Russ. J. Bioorg. Chem. 2010, 36, 401–445. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Peng, X. DNA-Associated Click Chemistry. Sci. China Chem. 2014, 57, 215–231. [Google Scholar] [CrossRef]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Sun, X.; Chen, Z.; Guo, Y.; Shen, Z.; Song, Y.; Xin, W.; Ding, H.; Ma, X.; Xu, W.; et al. ADCdb: The Database of Antibody–Drug Conjugates. Nucleic Acids Res. 2024, 52, D1097–D1109. [Google Scholar] [CrossRef]
- Xi, M.; Zhu, J.; Zhang, F.; Shen, H.; Chen, J.; Xiao, Z.; Huangfu, Y.; Wu, C.; Sun, H.; Xia, G. Antibody-Drug Conjugates for Targeted Cancer Therapy: Recent Advances in Potential Payloads. Eur. J. Med. Chem. 2024, 276, 116709. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-Drug Conjugates with Dual Payloads for Combating Breast Tumor Heterogeneity and Drug Resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Sahtoe, D.D.; Coscia, A.; Mustafaoglu, N.; Miller, L.M.; Olal, D.; Vulovic, I.; Yu, T.-Y.; Goreshnik, I.; Lin, Y.-R.; Clark, L.; et al. Transferrin Receptor Targeting by de Novo Sheet Extension. Proc. Natl. Acad. Sci. USA 2021, 118, e2021569118. [Google Scholar] [CrossRef]
- Barker, S.J.; Thayer, M.B.; Kim, C.; Tatarakis, D.; Simon, M.J.; Dial, R.; Nilewski, L.; Wells, R.C.; Zhou, Y.; Afetian, M.; et al. Targeting the Transferrin Receptor to Transport Antisense Oligonucleotides across the Mammalian Blood-Brain Barrier. Sci. Transl. Med. 2024, 16, eadi2245. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brylev, V.A.; Ryabukhina, E.V.; Nazarova, E.V.; Samoylenkova, N.S.; Gulyak, E.L.; Sapozhnikova, K.A.; Dzarieva, F.M.; Ustinov, A.V.; Pronin, I.N.; Usachev, D.Y.; et al. Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker. Pharmaceutics 2024, 16, 1434. https://doi.org/10.3390/pharmaceutics16111434
Brylev VA, Ryabukhina EV, Nazarova EV, Samoylenkova NS, Gulyak EL, Sapozhnikova KA, Dzarieva FM, Ustinov AV, Pronin IN, Usachev DY, et al. Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker. Pharmaceutics. 2024; 16(11):1434. https://doi.org/10.3390/pharmaceutics16111434
Chicago/Turabian StyleBrylev, Vladimir A., Ekaterina V. Ryabukhina, Ekaterina V. Nazarova, Nadezhda S. Samoylenkova, Evgeny L. Gulyak, Ksenia A. Sapozhnikova, Fatima M. Dzarieva, Alexey V. Ustinov, Igor N. Pronin, Dmitry Y. Usachev, and et al. 2024. "Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker" Pharmaceutics 16, no. 11: 1434. https://doi.org/10.3390/pharmaceutics16111434
APA StyleBrylev, V. A., Ryabukhina, E. V., Nazarova, E. V., Samoylenkova, N. S., Gulyak, E. L., Sapozhnikova, K. A., Dzarieva, F. M., Ustinov, A. V., Pronin, I. N., Usachev, D. Y., Kopylov, A. M., Golovin, A. V., Pavlova, G. V., Ryazantsev, D. Y., & Korshun, V. A. (2024). Towards Aptamer-Targeted Drug Delivery to Brain Tumors: The Synthesis of Ramified Conjugates of an EGFR-Specific Aptamer with MMAE on a Cathepsin B-Cleavable Linker. Pharmaceutics, 16(11), 1434. https://doi.org/10.3390/pharmaceutics16111434







