Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue
Abstract
1. Introduction
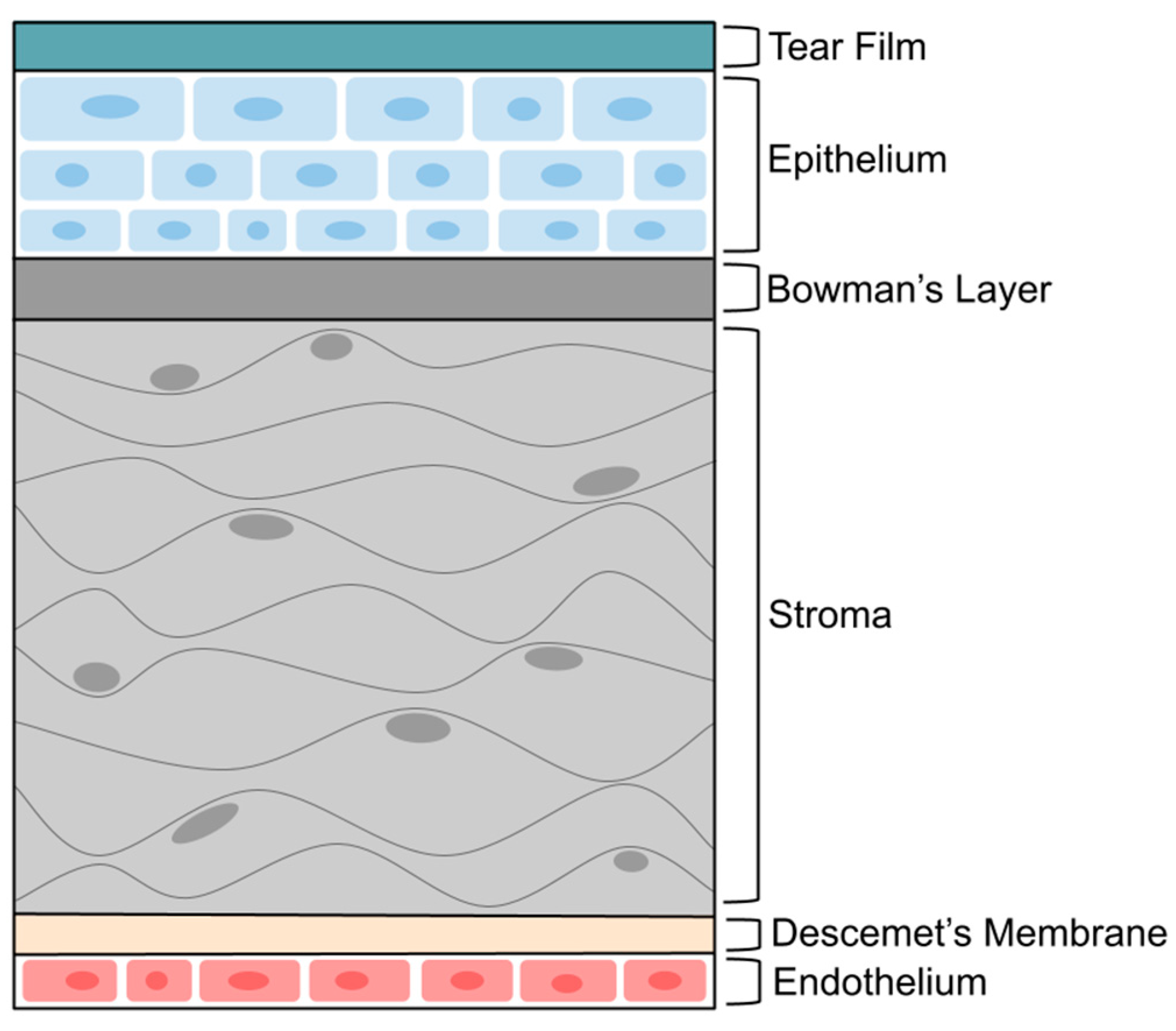
2. Cornea Structure and Barrier
3. Corneal Wound-Healing Processes
- (1)
- Stromal injury
- (a)
- Stromal injury involves epithelial injury.
- (b)
- Epithelial injury + damage to basement membrane → stromal haze
- (i)
- Stromal haze → scarring, opacity → reduced transparency
- (c)
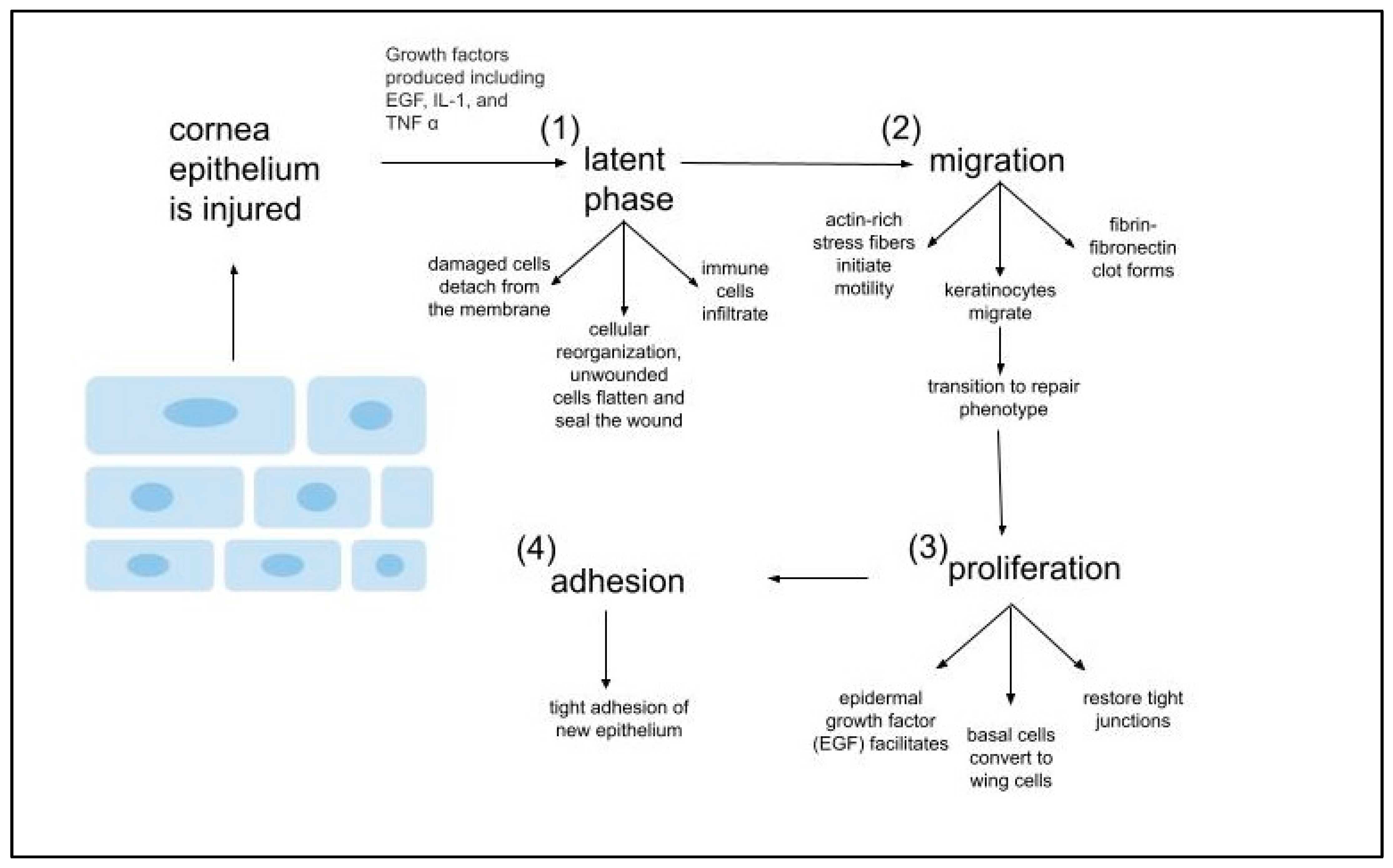
- Epithelial Healing Cascade
- (i)
- Upon injury, epithelial cells proliferate.
- (ii)
- Actin-rich stress fibers initiate migration to the wound
- (iii)
- Generation of adhesion structures that anchor the regenerated epithelium to the underlying connective tissue.
- (d)
- Epithelial healing cascade is regulated by GFs and cytokines, which primary function is to mediate interactions corneal epithelium and stroma via the basement membrane. These interactions lead to keratocyte apoptosis, keratocyte activation, and keratocyte trans-differentiation into myofibroblasts.
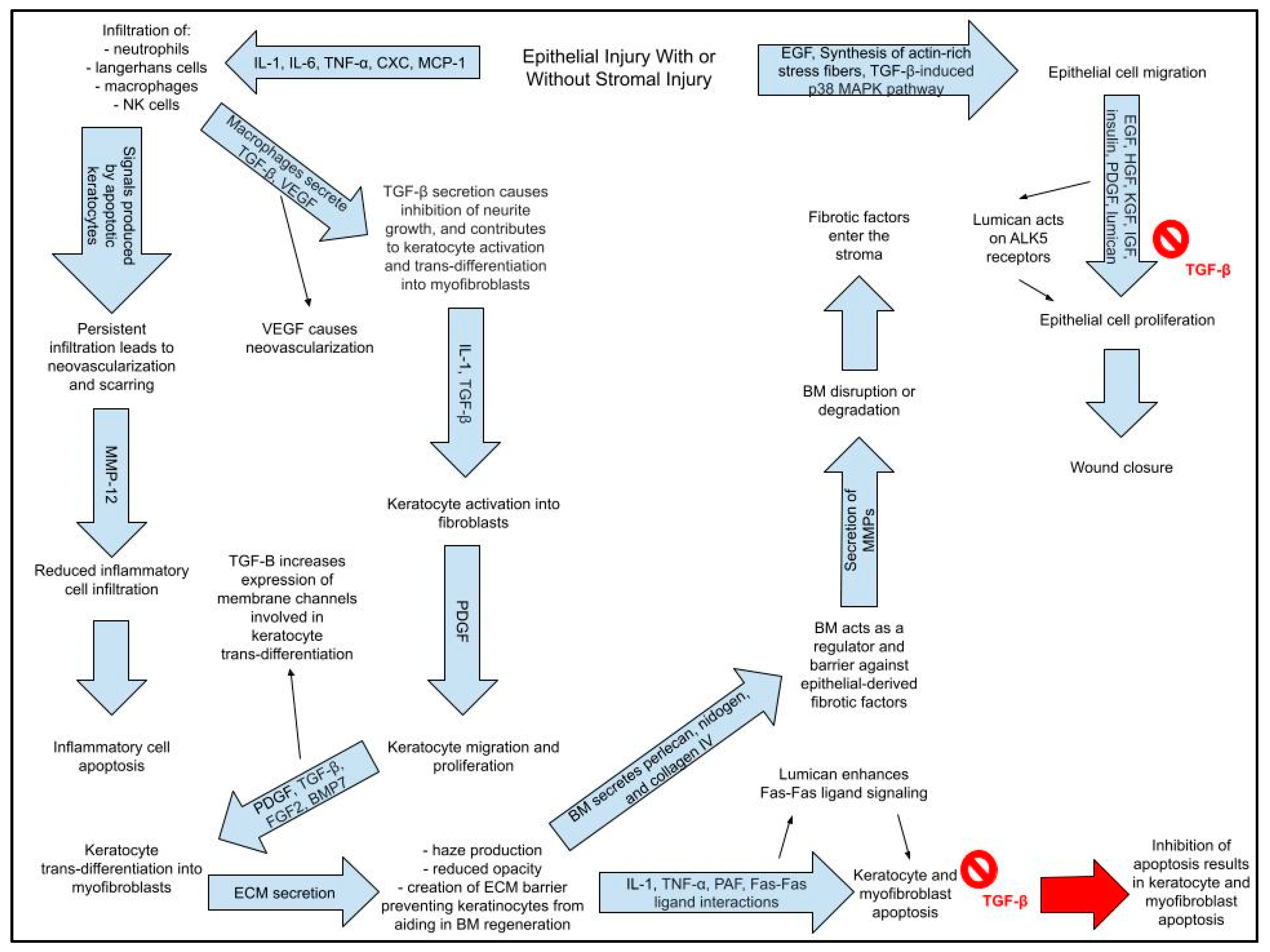
- (2)
- IL-1, TNF-α, PAF, and Fas–Fas ligand interactions cause immediate apoptosis of keratocytes beneath corneal epithelium within the zone of injury.
- (a)
- Apoptosis causes infiltration of inflammatory cells via release of IL-1, IL-6, TNF-α, CXC chemokines, and MCP-1 (macrophage chemotactic protein-1) released from epithelial and stromal cells
- (3)
- Some keratocytes undergo necrosis.
- (4)
- Other keratocytes near the area of necrosis/apoptosis proliferate, migrate, and undergo activation into fibroblasts.
- (5)
- Fibroblasts have the potential to transform into myofibroblasts.
- (6)
- Fibroblasts and myofibroblasts secrete a provisional matrix scaffold.
- (7)
- Epithelial and stromal cells secrete chemokines, which triggers an influx of inflammatory cells;
- (a)
- Inflammatory cells clear apoptotic and necrotic (#2 and #3) debris.
- (8)
- Epithelial cells, inflammatory cells, fibroblasts, and myofibroblasts secrete collagenolytic metalloproteinases that cause stromal repair and remodeling
- (9)
- Resorption of abnormal ECM and apoptosis or reversal of myofibroblast phenotype restores normal form and function of the stroma.
4. Exosomes for Preserving Cornea
5. Immunogenicity and Toxicity of Exosomes
6. Exosomes Combined with Other Therapeutic Approaches
6.1. Exosomes with Hydrogels
6.2. Exosomes with Gene Therapy
6.3. Stem Cell-Derived Exosomes for Corneal-Related Investigations
| Exosome Source | Type of Cornea Wound | Method of Research | Results | Ref |
|---|---|---|---|---|
| Human corneal epithelial cells (hCECs), corneal fibroblasts (hCFs), corneal endothelial cells (hCEnCs) | Corneal injury | In vitro—Exosomes derived from the three different corneal cell types were characterized using TEM, DLS, and Western blot analyses. Uptake of the three different exosome types into hCECs was examined using immunofluorescence staining; effects on hCEC proliferation was examined using immunofluorescence staining in combination with Ki-67 assay. To determine how the exosomes affected signal transduction pathways, phosphorylation levels of protein mediators were examined using the human phosphor-kinase proteome profiler array. Gene expression of hCECs, hCFs, and hCEnCs post-treatment were analyzed using robust multi-array analysis (RMA). Gene Interaction Network Analysis was used to predict the biological functions affected by exosomes in HCECs and hCFs. | Exosomes from all three cell types increased the rate of wound closure of scratch-wounded HCECs. Profiling of activated kinases show that corneal cell-derived exosomes activate signal transduction mediators for the HSP27, STAT, β-catenin, GSK-3β and p38 pathways. The mean gene expression profile of hCECs cultured with exosomes would likely promote cell proliferation and migration while also reducing differentiation. | [27] |
| Induced pluripotent stem cell-derived mesenchymal stem cells (iPSC-MSCs) Modification: exosomes derived from iPSC-MSCs transfected with miR-432-5p were combined with a thermosensitive, chitosan-based hydrogel (CHI hydrogel) | Unspecified corneal disease | In vitro—iPSC-MSC-Exos were isolated via centrifugation and characterized via TEM, DLS, and Western blot assay. CHI hydrogel and exosome-loaded CHI hydrogel were subjected to rheological studies and release properties were analyzed using a BCA assay. Biocompatibility studies were conducted via co-culture with HCECs and MSCs to examine effects on proliferation and mRNA contents via CCK-8 assay and RT-qPCR-based miRNA microarray analysis, respectively. In vivo—An anterior lamellar damage model was established in 42 rats using an operating knife. Ofloxacin eye drops were used for three days post-op. Corneal epithelial defect was monitored using fluorescent staining paper, and clarity was monitored via a slit-lamp microscope. The rat model was used for histological analysis via H&E staining, protein expression via immunofluorescence staining, and detection of miRNA expression of related genes via microarray analysis. | iPSC-MSC-Exos were found to encapsulate miR-432-5p, which modulates collagen biosynthesis in corneal stromal stem cells. miR-432-5p prevented ECM deposition via repression of TRAM-2. The formulated exosome-loaded CHI hydrogel allowed for sustained release of exosomes and encouraged regeneration of the corneal epithelium and stromal layer. Stroma regeneration was achieved via downregulation of mRNA expression encoding the three most enriched collagens. Additionally, in vivo experiments confirmed the ability of iPSC-MSC-Exo-loaded CHI hydrogel reduced corneal scarring. | [45] |
| Human mesenchymal stromal cells (hMSCs) Cargo: c-Rel-specific siRNA | Regular and diabetic corneal injury | In vitro—Effects of loaded-exosomes were examined via immunofluorescence staining and flow cytometry. In vivo/ex vivo—c-Rel-deficient and wild-type mice were used; scraping was used to cause a 2.5-mm diameter injury; wounds were staining with fluorescent dye and examined under a slit lamp microscope at specific time points and production of inflammatory cytokines was examined using ELISA. Both regular and diabetic corneal injury mouse models were used to examine the effects of topical treatment with c-Rel-specific siRNA, either encapsulated with nano-polymers or exosomes, effects of treatment were examined using rates of wound closure. | Expression of c-Rel and its inflammatory targets are increased with corneal injury in mice; c-Rel-deficient mice exhibit accelerated corneal wound-healing. Topical treatment of the corneal surface with exosomes or nano-polymers loaded with c-Rel-specific siRNA can accelerate corneal wound-healing. However, this study shows that siRel-loaded exosomes show better efficacy than nano-polymers. | [47] |
| Mesenchymal stem cells from corneal stromal stem cells (CSSC) Cargo: nonmammalian miR159a | Corneal inflammation and fibrosis after corneal wound | In vitro—EVs from CSSCs were characterized via estimating protein concentration via UV absorbance, TRPS, immunoblotting, TEM, and flow cytometry. EVs were labeled via fluorescent tag and loaded with miR159a via incubation with PBS. To determine if EVs transfected miRNA to HCECs, qPCR and RNA sequencing were used. In vivo/Ex vivo—A corneal wound model was established in mice using an Algerbrush. Two weeks after corneal debridement, eyes were collected and subjected to imaging, determination of gene expression via RT-PCR, immunoblotting, and neutrophil myeloperoxidase assay. | CSSC EVs labeled with fluorescent successfully fused with HCECs and CSSCs in vitro to transfer miRNA causing Alix knockdown. EVs with Alix knockdown exhibited lower amounts of miRNA, were ineffective at reducing fibrotic scarring, and lost regenerative function. In vivo, EVs from CSSCs reduced fibrotic scarring, and stimulated regeneration of stromal tissue; it is thought these effects result from CSSC EVs ability to reduce expression of fibrotic genes, block neutrophil infiltration, and restore normal corneal tissue morphology. | [48] |
| Mesenchymal stromal/stem cells | Corneal epithelial wound | In vitro—Effects of the MSC-conditioned media (MSC-CM; containing the secretome and exosomes of MSCs without the MSCs themselves) and EV-depleted MSC-CM on HCECs and human corneal limbal epithelial cells was examined; HCEC viability was examined via trypan blue incubation with microscopic examination; Cell proliferation and cytotoxicity were determined by measuring cellular DNA content via fluorescence and LDH assay, respectively; HCLE cells underwent a cell scratch assay using a spinning disc confocal microscope; In vivo—Mouse model of corneal epithelial wound-healing was conducted using an Algerbrush; images illustrating effects on wound-healing on corneas were obtained after staining with fluorescent dye; intensity fold change of corneal fluorescein staining was examined. | In vitro studies using HCECs showed that MSC-CM increased cell proliferation in HCEC and HCLE cells, while EV-depleted MSC-CM showed lower cell proliferation in both cell lines. In vitro and in vivo experiments revealed MSC-CM promoted wound-healing in a dose-dependent manner, while exosome deprivation delayed wound-healing. Incubation period of MSC-CM also influences its therapeutic effects, showing 72H incubation is more effective than 48H. Stability evaluation showed that after one cycle of free-thawing, MSC-S is stable at 4 °C for up to 4 weeks. This study elucidated that MSC-CM is the active ingredient in the MSC secretome and improved corneal barrier and decreased corneal haze/edema. | [54] |
| Human MSCs derived from cadaver corneas | Non-specific corneal epithelial wound | In vitro—Human corneal epithelial cells (HCECs) were scratch-wounded and then treated with 1.0 × 105, 1.0 × 106, or 1.0 × 107 exosome or phosphate buffered saline, (PBS) which served as a vehicle control In vivo—Exosomes were applied topically to mice corneas with 2-mm epithelial debridement wounds | Corneal MSCs (cMSCs) exosomes were successfully taken up by human cMSCs in vivo and in vitro cMSC exosome treated HCECs had improved corneal wound-healing compared to the control group | [55] |
| Saliva from healthy human subjects—commercially purchased salivary exosomes | Diabetic cornea and keratoconus | Ex vivo—Scratch and cell migration assays were performed over 48 h on primary human corneal fibroblast cells which were isolated from 4 donor human corneas | Higher concentrations of salivary exosomes with high anti-inflammatory biomarkers (CD-9, CD-63, CD-81) promoted wound-healing through late stages of disease when fibronectin was downregulated | [56] |
| Bone marrow mesenchymal stem cells (BM-MSCs) | Diabetic corneal epithelium wound/diabetic keratopathy | In vivo—Diabetic mouse models were established using streptozocin (STZ) and their central cornea was scratched. Exosomes were traced with PKH-26 | Corneal epithelium healing rates in the experimental groups (subconjunctival injection of exosomes derived from BM-MSCs or subconjunctival injection of BM-MSCs) were significantly higher at 24, 48 and 72 h. There was no significant difference in healing rates between the two experimental groups | [57] |
| Human limbal mesenchymal stem cells (hLMSCs) Modification: hLMSCs were treated with various concentrations of melatonin (Mel) to induce exosome production | Corneal scarring | In vitro—The effects of exosomes procured from melatonin-treated hLMSCs (Mel-prExos) on cell viability was evaluated using the MTT assay; expression of miR-155 (associated with α-SMA expression), miR-29 (associated with TGFβ1/β3 expression), and PPARγ miRNAs and genes were compared between melatonin-treated hLMSCs and Mel-prExo-treated hLMSCs. | At 1 μM of melatonin and in the presence of Mel-prExos, TGFβ1 was expressed 0.001-fold, while TGFβ3 was expressed 0.6-fold. miR-29 expression was increased 38-fold in the control-Exo group compared to that of the control group. Melatonin and mel-prExos increased the expression of anti-fibrotic genes and miRNAs and promoted the pro-regenerative effects of naïve hLMSCs, as evidenced in increased TGFβ3 and PPARγ expression and the inhibition of TGFβ1. | [58] |
| Wharton’s jelly mesenchymal stem cells (WJ-MSC) Modification: WJ-MSC-Exos are combined in a solution with amniotic membrane extract (AME) | Corneal wounds | In vitro—Characterization of AME and WJ-MSC-Exos was conducted using DLS and SEM; Cytocompatibility was determined using MTT assay and alamarBlue cell proliferation assay; Actin cytoskeleton and nuclei staining was used to determine cellular morphology of adherent corneal keratocytes following AME/WJ-MSC-Exo treatment; wound-healing potential of the formulation was investigated with scratch and cell migration assays. | The presence of both WJ-MSC-Exos and AME synergistically enhance the proliferation of corneal keratocytes increased wound closure rates. | [59] |
| M1 macrophages Modification: M1 macrophages were conditioned with epidermal growth factor (EGF) | Ocular surface inflammation | In vitro—Proteome analysis, pathway analysis, and QT-PCR was conducted on M1 macrophages to determine if the derived exosomes carried inflammation-relieving signals. Ex vivo—Effects of the M1 and EGF-M1 exosome eye drops on inflammatory signals and vasculogenic factors were examined using immunofluorescence staining of pan-macrophage markers. In vivo—Absorption of exosome eye drops was investigated using immunofluorescence assays in a mouse model. | Exosomes derived from EGF-treated M1 macrophages had enriched proteomic profiles contributing to their ability to regulate the immune system and inflammation. When applied as eye drops in mouse corneas, the exosomes reduced inflammation and increased M2-related ARG1 expression. The effects of EGF-M1-Exo eye drops differed from that of M1-Exo eye drops in their ability to suppress IL-1B, IL-6, VCAM1, ICAM1, VEGFA, PDGFA, MMP2 and MMP9 gene expression, while causing activation of ARG1 gene expression. | [60] |
| Bone Marrow Mesenchymal stem cells (BMSCs) Cargo: miRNA-29b-3p agomir/antagomir | Inflammation secondary to corneal injury | In vitro—Exosome tracking on immortal HCECs (iHCECs) was performed via fluorescence assay; effects on cell migration, proliferation, and viability were examined using fluorescence assays, flow cytometry and TEM, and CCK-8 assay, respectively. In vivo—Exosome tracking on injured murine corneas was performed via fluorescence assay; the effects of Exos on corneal inflammation, haze production, gene expression, and autophagy were determined using fluorescein staining and bright field photographs, H&E staining, corneal haze grading, TEM, Western blot analysis, and RT-qPCR; effects of the exosomes on cytokine expression were examined using immunohistochemical analysis. | Compared to PBS, Exos-29b-agomir, Exos-29b-antagomir, and Exos-control all could produce therapeutic effects on corneal inflammation and fibrosis. Exos-29b-agomir, however, encapsulated large amounts of miR-29b-3p and had profound therapeutic effects on autophagy activation and corneal inflammation via inhibition of PI3k/AKT/mTOR pathways and inhibition of mTOR/NF-κB/IL-1β pathways, respectively. Exos-29b-agomir decreased expression of collagen type III, α-smooth muscle actin, fibronectin, and vimentin. Additionally, the overexpression of miR-29b-3p in Exos-29b-agomir prevented autophagy impairment and inflammatory injury in iHCECs. | [61] |
| Myeloid-derived suppressor cells (MDSCs) Modification: MDSCs were conditioned with rapamycin | Corneal allograft rejection | In vitro and Ex vivo—Determination of the active component contributing to Rapa-Exos anti-rejection effects, as well as the mechanism of action, were determined using RT-qPCR, slit-lamp images of corneal allografts, survival curves via the Kaplan-Meier approach, H&E staining, and ELISA. In vivo—The effects of Rapa-Exos on corneal allograft rejection were examined in a well-characterized murine corneal transplantation model using slit-lamp images of corneal grafts, histopathological examination via H&E staining, RT-qPCR, and immunofluorescence staining. | The Rapa-Exos derived from rapamycin-conditioned MDSCs exerted superior effects when compared to Exos derived from untreated MDSCs. In vitro assays revealed that the anti-rejection effects are due to functions of miR-181d-5p, which causes KLF6 knockdown. KLF6 knockdown resolves inflammation and prolongs survival of corneal allografts through immunosuppressive effects. | [62] |
| Adipose-derived mesenchymal stem cells (ASCs) | Corneal endothelial injury | In vitro—The effects of ASC-derived exosomes (ASC-Exos) on the regenerative capacity of human corneal endothelial cells was examined using cell viability and cell-cycle analyses; to examine the difference in expression of miRNAs between ASC-derived and human corneal endothelial cell-derived exosomes, RT-qPCR was performed; uptake, cell viability, cytotoxicity, and cell cycles of corneal endothelial cells treated with ASC-Exos was determined using fluorescence microscopy, CCK-8 assay, LDH assay, and propidium iodide staining with flow cytometry, respectively; To examine the effects of ASC-Exos on induced endothelial-to-mesenchymal transition (EMT) and mitophagy, Western blot analyses, immunofluorescence staining, and determination of mitochondrial membrane potential were performed, In vivo—ASC-Exos were introduced into rat corneal endothelial cells using electroporation after corneal injury was induced via cryoinjury. To determine the effects of ASC-Exos on rat corneal endothelium, fluorescence microscopy, TUNEL staining, and Alizarin Red S staining, Ki67 staining, immunofluorescence staining, was performed. Differential miRNA expression was determined via RT-qPCR. | ASC-Exos induced corneal endothelial cell proliferation and suppressed or protected against TGFβ- or H2O2-induced oxidative stress/senescence and induced EMT and mitophagy. In vivo studies demonstrated the wound-healing effects of ASC-Exos in rat corneal endothelial cells and protected those cells from cryoinjury and related damage. RNA sequencing analysis showed that the miRNAs expressed by ASC-Exos and human corneal endothelial cell-derived Exos influence lysine degradation, adherens junction, TGFβ, p53, Hippo, FoxO signaling, actin cytoskeleton regulation, and RNA degradation. | [63] |
| Human mesenchymal stem cells (MSCs) | Corneal scarring | In Vitro—Cellular uptake of MSC-Exos, as well as their immunomodulatory effects, were examined in both corneal stromal fibroblasts and myofibroblasts; cellular uptake was examined using fluorescence intensity and immunoassays were used to determine immunomodulatory effects; wound-healing effects were examined via a scratch wound assay on HCET cell culture, determined via rate of wound closure and fluorescein staining. Ex vivo—Doses of MSC-Exo were applied to epithelium-on and -off corneas to determine if a relationship exists between dosing and retention after topical administration, this was accomplished via use of a confocal laser scanning ophthalmoscope and fluorescent microscopy; immunohistochemistry, immunoassay and RT-qPCR were used to assess relative molecular changes and immunomodulatory effects in rat corneas after 5 days of topical administration, respectively. In vivo—Rat corneas underwent irregular phototherapeutic keratectomy to examine MSC-Exos effects on corneal stromal haze, which was determined via slit-lamp imaging, confocal microscopy, anterior segment-optical coherence tomography, and neovascularization score. | When compared to the PBS control group, the MSC-Exos treatment group had faster epithelial wound closure, lower corneal haze score, and reduced haze intensity during the follow-up period. Attenuation of corneal vascularization, based on CD31 and LYVE-1 staining, as well as reduced fibrosis as measured by fibronectin and collagen 3A1 staining was also observed in the MSC-Exo group. MSC-Exo treated corneas exhibited a regenerative immune phenotype characterized by higher infiltration of non-inflammatory immune cells (vs non-inflammatory immune cells), reduced levels of pro-inflammatory cytokines, and increased levels of anti-inflammatory cytokines. This formulation may alleviate corneal injury. | [64] |
| Bone marrow-derived mesenchymal stem cells (BMSCs) | Corneal alkali burn | In vitro—Isolated BMSC-Exos were identified via TEM, Western blot analysis, and NTA; exosome uptake was examined via dye staining and use of laser confocal microscope. HCEC proliferation and migration was examined after scratching wound assay and coculture with BMSC-Exos. The protein expression of p-MEK/MEK and p44/42 MAPK in HCECs was detected via Western blot analysis. In vivo/Ex vivo—An alkali burn model in mice was used via sodium hydroxide exposure. After treatment with BMSC-Exos via injection, pathological changes and protein expression in excised corneas were examined via H&E staining, immunofluorescence, and immunohistochemistry. | In vitro, BMSC-Exos caused dose-dependent enhancement of HCEC proliferation and migration; p44/42 MAPK pathway was activated by BMSC-Exos treatment, and its blocking of U0126 was partially responsible for their ability to enhance HCEC proliferation and migration. In vivo, BMSC-Exos injection reduced pathological changes inducing inflammation, and decreased upregulation of α-SMA and CD31, the proteins responsible for fibrosis and vascularization in corneal tissues, respectively. | [65] |
| Umbilical cord mesenchymal stem cells (HUMSCs) Cargo: miR-21 | Corneal epithelial wound | In vitro—HUMSC small extracellular vesicles (HUMSC-sEVs) were identified via TEM, NTA, and Western blot analyses. HCEC proliferation post-treatment with HUMSC-sEVs were examined using CCK-8 and EdU assays. HCEC migration was evaluated using a scratch wound assay. The genes expressed by HCECs post-treatment with HUMSC-sEVs was examined via full-length transcriptome sequencing, RT-PCR, and Western blot analyses. In vivo/Ex vivo—A corneal mechanical wound model was established in rats via a unilateral corneal injury, rats received a subconjunctival injection of HUMSCs or HUMSC-EVs. Wound residual area was monitored using fluorescein staining and imaging via a slit lamp microscope. H&E staining alongside light microscopy was used to examine corneal structure and degree of re-epithelialization. | In vitro, HUMSCs were less effective when the release of exosomes was blocked, suggesting sEVs/exosomes play a vital role in wound-healing and HUMSC function. HUMSC-sEVs promote HCEC proliferation and migration. In vivo, HUMSC-sEVs promoted corneal epithelial wound-healing, as well as HCEC proliferation and migration. H&E staining revealed that corneas treated with HUMSC/HUMSC-sEVs regained a more regular arrangement and compact structure than those treated with PBS. After miR-21 transfection, beneficial effects of treatment were partially negated. HUMSC-sEVs may potentially enhance the recovery of corneal epithelial wounds via miR-21 transference, causing repression of PTEN expression, which in turn activate the PI3K/Akt signaling pathway in HCECs. | [66] |
| Human umbilical cord mesenchymal stem cells (hucMSC) Modification: used in combination with autophagy activators | Corneal injury | In vitro—hucMSC-Exos were isolated and identified via NTA, TEM, and Western blot analyses. The effects of treatment with hucMSC-Exos combined with autophagy regulators were examined in HCECs via cell viability assays, scratch assays, cell cycle assays and apoptosis assays. In vivo—The corneal injury mouse model was conducted using an AlgerBrush II corneal remover. The effects of hucMSC-Exos combined with autophagy regulators on mice were examined using corneal fluorescein staining, haze grades, H&E staining, immunohistochemical staining, TUNEL assay, TEM, Western blotting, and qPCR. | In vitro results indicate the hucMSC-Exos combined with the autophagy activator increased cell proliferation and migration capacity, in addition to positive effects on the cell cycle via upregulating the proportion of cells in the S phase and increased expression of proteins involved in proliferation. Combination treatment with autophagy activator reduced apoptosis in HCECs. In vivo, hucMSC-Exos with autophagy activator mitigated epithelial wounds and opacity in the stroma. Additionally, the combination treatment reduced levels of apoptotic and inflammatory markers via activation of the AMPK-mTOR-ULK1 signaling pathway. | [67] |
| Mesenchymal stem cells (MSCs) | Corneal allograft rejection | In vitro—MSC-derived exosomes were collected, and then characterized using electron microscopy and Western blot analysis. In vivo—Using the Wistar-Lewis rat corneal allograft rejection model, the rats were divided into four groups with six rats in each group; each group received a different concentration of MSC-exo via two subconjunctival injections: one was given postoperatively, and the other was given two days post-op. All exosomes administered via injection were labeled via PKH26 for tracking. Rats were observed post-op day three via a slit-lap microscope and given an opacity score. Ex vivo—On day 10, rat eyes were excised and subjected to gene expression array, histopathological examination, immunohistochemistry. staining and photography for exosome tracking. Grafts taken from subjects were examined via qPCR. Spleens and lymph nodes were also collected, and monocytes were examined via flow cytometry. | The isolated exosomes expressed the desired exosome biomarkers and were detected in both the cornea and anterior chamber two hours post-injection. The rats that received the 10 μg injection exhibited increased graft survival time, and ex vivo analyses showed inhibition of CD4+ and CD25+ T cell infiltration. In all groups receiving MSC-Exo injection, ex vivo analysis of excised grafts showed reduced levels of the pro-inflammatory cytokines IFN-γ and CXCL11. | [68] |
| Mesenchymal stem cells (MSCs) and blood serum (SER) | Corneal endothelial dystrophy | In vitro—MSC-EVs and SER-EVs (used as a control; thought to possess pro-angiogenic activity) were isolated and characterized using NTA, super-resolution microscopy, MACSPlex flow cytometry, and fluorescent staining. Endoplasmic stress was induced in HCECs via serum deprivation and tunicamycin application; the presence of ER stress biomarkers was determined using RT-PCR, and ER stress-related protein phosphorylation was examined via Western blot analysis. To determine if HCECs expressed apoptotic markers post-treatment, a phosphatidylserine assay was conducted using Annexin V and 7-AAD. To determine if MSC-EV’s miRNA were successfully transferred to HCECs, a Funrich analysis and RT-PCR were performed. | In the in vitro model of corneal dystrophy, the effects MSC-EVs were compared with those of SER-EVs. SER-EVs exhibited minimal or absent effect on modulating endoplasmic reticulum stress and/or apoptosis. MSC-EVs caused downregulation of genes causing endoplasmic reticulum stress in an endoplasmic reticulum stress model in HCECs. MSC-EVs upregulated the Akt pathway, while also decreasing activation of apoptotic markers. The therapeutic effects of MSC-EVs is likely linked to the transfer of endoplasmic reticulum-stress targeting miRNAs to corneal endothelial cells. | [69] |
| Induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs) | Corneal epithelial defect | In vitro—Characterization of exosomes derived from IPSCs and MSCs was conducted using NTA, TEM, and Western blot analyses. To assess cellular uptake of IPSC-/MSC-Exos by HCECs, immunofluorescence staining was employed. The effects of IPSC-/MSC-Exos on HCEC was evaluated using apoptotic assays, live-dead cell determination, scratch wound assay, and CCK-8 cell viability assay. The effects of the IPSC-/MSC-Exos on the HCEC cell cycle were examined using flow cytometry, immunofluorescent staining, RT-qPCR, and Western blot analyses. Ex vivo—Rat eyeballs were harvested at 48H and underwent histological analysis with H&E staining. In vivo—AlgerBrush was used to establish a corneal epithelial defect model in rats. To assess cellular uptake of IPSC-/MSC-Exos after topical administration, immunofluorescent staining and TEM were conducted. The effect of IPSC-/MSC-Exos on corneal epithelial defect healing was monitored every six hours over a 48H time period using fluorescein staining with slit lamp, observing healing rates, and monitoring of central corneal thickness as measured by AS-OCT at 24 and 48H. | In vitro, IPSC-Exos had a more beneficial effect on HCEC proliferation, migration, cell cycle promotion, and inhibition of apoptosis. Both IPSC- and MSC-Exos promoted cell regeneration by increasing upregulation of cyclin A and CDK2, which caused HCECs to enter S phase from G0/G1 phase. In vivo, both exosome types accelerated corneal wound-healing through modulation of cellular metabolism; when compared to a control group, IPSC-/MSC-Exos nearly completely restored corneal integrity. However, iPSC-Exos exhibited a much stronger therapeutic effect on accelerating wound-healing and re-epithelialization of corneal injury. This study suggests that increased therapeutic efficacy of IPSC-Exos may be attributed to their increased expression of proteins shared with their source cell line. | [70] |
| Adipose-derived stem cells (ADSCs) | Corneal fibrosis | Ex vivo—ADSCs and ADSC-Exos were isolated from rabbit adipose tissues and characterized using flow cytometry, NTA, and Western blot analyses. Rabbit corneal keratocytes were isolated and grown for 7 days in an FBS-containing medium (FBS induces differentiation of keratocytes into myofibroblasts). Rabbit corneal keratocytes were incubated with both ADSCs and ADSC-Exos, and then the effects of ADSC and ADSC-Exos were examined using CCK-8 cell viability assay and Western blot analysis. The effects of ADSC-Exos on the differentiation of keratocytes into myofibroblast investigated using QRT-PCR, Targetscan analysis, dual luciferase reporter assay, and Western blot analysis. | Keratocytes grown in FBS-containing medium differentiated into myofibroblasts by increasing HIPK2 kinase expression and activity. ADSCs and ADSC-Exos inhibited FBS-induced differentiation of keratocytes into myofibroblasts causing HIPK2 knockdown due to their miR-19a content. Target scan analysis confirmed that the HIPK2 3′UTR is the direct binding target of miR-19a. | [71] |
| Aqueous humor (AH) | Unspecified corneal wound | In vitro—Human immortalized keratinocyte cells (HaCaT) in a scratch wound assay model were used to investigate the effects of AH-EVs versus the effects of EVs derived from mesenchymal stromal stem cells (MSC-EVs) on viability, proliferation, and wound-healing. Ex vivo—AH was obtained from 10 patients with cataract who were undergoing surgical pharmacoemulsification and insertion of intraocular lenses. The EVs obtained from AH were characterized using NTA, electron microscopy, super resolution microscopy, and bead-based cytofluorimetry. | AH-EVs had a mean size of ~100 nm and expressed tetraspanins, such as CD9, CD63, and CD81, that are used to classify EVs. Co-expression of these tetraspanins was confirmed by super resolution microscopy. Confirmation of AH-EV expression of mesenchymal, stem, epithelial, and endothelial cell markers was determined via cytofluorimetric analysis. In the scratch wound model using HaCaT cells, the effects of AH-EVs were associated with faster wound-healing, as well as increased cell viability and proliferation. | [72] |
| Immortalized Human Corneal stromal stem cells (imCSSCs) | Corneal inflammation and fibrosis | In vitro—imCSSCs were grown in a conditioned medium, and EVs were isolated from the imCSSC line via a Total Exosome isolation kit and ultracentrifugation. EVs were cultured with trehalose dissolved in PBS prior to being lyophilized using liquid nitrogen. After snap freezing, EVs were stored for four weeks then evaluated using DLS and TEM. After storage, retained EV markers were determined using Western blot, fluorescent staining, flow cytometry, RT-PCR, and RAW cell differentiation/anti-inflammatory assay. Anti-fibrotic properties of EVs post-storage were examined using immunofluorescence staining and RT-PCR. | EVs stored at −80 °C were also lyophilized with trehalose most closely mimicked their pre-storage morphology; EVs maintained normal protein expression. EV samples that were lyophilized without trehalose exhibited lower particle concentrations, slower recovery rate, and decreased protein concentrations; although, this was remediated via addition of trehalose. Regardless of storage condition, all EV samples reduced inflammation and inhibited expression of fibrotic markers. | [73] |
7. Clinical Trials
8. Future Perspective, Opportunities, and Challenges
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef] [PubMed]
- Tidke, S.C.; Tidake, P. A Review of Corneal Blindness: Causes and Management. Cureus 2022, 14, e30097. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles As Nanomedicine: Hopes And Hurdles In Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Burnouf, P.A.; Chew, C.H.; Burnouf, T. Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol. 2019, 37, 707–729. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Understanding exosomes: Part 1-Characterization, quantification and isolation techniques. Periodontol. 2000 2024, 94, 231–256. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, F.; Jiang, Y.; Wang, Y.; Li, Z.; Shi, X.; Zhu, Y.; Wang, H.; Zhang, Z. Roles of Exosomes in Ocular Diseases. Int. J. Nanomedicine 2020, 15, 10519–10538. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Mandal, A.; Pal, D.; Mitra, A.K. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert. Opin. Drug Deliv. 2017, 14, 1145–1162. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41. [Google Scholar] [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.G.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. CLEAR—Anatomy and physiology of the anterior eye. Cont. Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Bowman’s layer in the cornea- structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Pouw, A.E.; Greiner, M.A.; Coussa, R.G.; Jiao, C.; Han, I.C.; Skeie, J.M.; Fingert, J.H.; Mullins, R.F.; Sohn, E.H. Cell-Matrix Interactions in the Eye: From Cornea to Choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kao, W.W. Corneal Epithelial Wound Healing. Prog. Mol. Biol. Transl. Sci. 2015, 134, 61–71. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; El Haj, A.J.; Yang, Y. Control of scar tissue formation in the cornea: Strategies in clinical and corneal tissue engineering. J. Funct. Biomater. 2012, 3, 642–687. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrosio, R., Jr.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Jeon, K.I.; Hindman, H.B.; Bubel, T.; McDaniel, T.; DeMagistris, M.; Callan, C.; Huxlin, K.R. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci. Rep. 2018, 8, 12945. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef]
- Li, S.F.; Han, Y.; Wang, F.; Su, Y. Progress in exosomes and their potential use in ocular diseases. Int. J. Ophthalmol. 2020, 13, 1493–1498. [Google Scholar] [CrossRef]
- Li, N.; Zhao, L.; Wei, Y.; Ea, V.L.; Nian, H.; Wei, R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res. Ther. 2019, 10, 278. [Google Scholar] [CrossRef]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Sanghani, A.; Andriesei, P.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. Advances in exosome therapies in ophthalmology-From bench to clinical trial. Acta Ophthalmol. 2022, 100, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sheng, M.; Zhang, J.; Yan, G.; Li, B. Plasma exosomal proteomic studies of corneal epithelial injury in diabetic and non-diabetic group. Exp. Eye Res. 2021, 212, 108794. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 2015, 5, 159–164. [Google Scholar] [CrossRef]
- Meng, S.; Hu, H.; Qiao, Y.; Wang, F.; Zhang, B.N.; Sun, D.; Zhou, L.; Zhao, L.; Xie, L.; Zhang, H.; et al. A Versatile Hydrogel with Antibacterial and Sequential Drug-Releasing Capability for the Programmable Healing of Infectious Keratitis. ACS Nano 2023, 17, 24055–24069. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kuo, Y.J.; Hung, K.H.; Peng, C.L.; Chen, K.Y.; Yeh, L.K. Liposomal dexamethasone-moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed. Mater. 2020, 15, 055022. [Google Scholar] [CrossRef]
- Chen, F.; Mundy, D.C.; Le, P.; Seo, Y.A.; Logan, C.M.; Fernandes-Cunha, G.M.; Basco, C.A.; Myung, D. In Situ-Forming Collagen-Hyaluronate Semi-Interpenetrating Network Hydrogel Enhances Corneal Defect Repair. Transl. Vis. Sci. Technol. 2022, 11, 22. [Google Scholar] [CrossRef]
- Feng, L.; Liu, R.; Zhang, X.; Li, J.; Zhu, L.; Li, Z.; Li, W.; Zhang, A. Thermo-Gelling Dendronized Chitosans as Biomimetic Scaffolds for Corneal Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 49369–49379. [Google Scholar] [CrossRef]
- Logan, C.M.; Fernandes-Cunha, G.M.; Chen, F.; Le, P.; Mundy, D.; Na, K.S.; Myung, D. In Situ-forming Collagen Hydrogels Crosslinked by Multifunctional Polyethylene Glycol as a Matrix Therapy for Corneal Defects: 2-Month Follow-up In Vivo. Cornea 2023, 42, 97–104. [Google Scholar] [CrossRef]
- Na, K.S.; Fernandes-Cunha, G.M.; Varela, I.B.; Lee, H.J.; Seo, Y.A.; Myung, D. Effect of mesenchymal stromal cells encapsulated within polyethylene glycol-collagen hydrogels formed in situ on alkali-burned corneas in an ex vivo organ culture model. Cytotherapy 2021, 23, 500–509. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, e2006335. [Google Scholar] [CrossRef]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Zhao, W.; He, X.; Liu, R.; Ruan, Q. Accelerating corneal wound healing using exosome-mediated targeting of NF-kappaB c-Rel. Inflamm. Regen. 2023, 43, 6. [Google Scholar] [CrossRef]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, G.; Lee, J.; Lee, Y.; Kim, J.H. Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming. Tissue Eng. Regen. Med. 2022, 19, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Na, K.S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Di, G.H.; Qi, X.; Xu, J.; Yu, C.Q.; Cao, Q.L.; Xing, Z.J.; Li, Z.C. Therapeutic effect of secretome from TNF-alpha stimulated mesenchymal stem cells in an experimental model of corneal limbal stem cell deficiency. Int. J. Ophthalmol. 2021, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Anwar, K.; Ashraf, M.; Lee, H.; Jung, R.; Koganti, R.; Ghassemi, M.; Djalilian, A.R. Wound-Healing Effects of Mesenchymal Stromal Cell Secretome in the Cornea and the Role of Exosomes. Pharmaceutics 2023, 15, 1486. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef]
- Escandon, P.; Liu, A.; Nicholas, S.E.; Khan, A.; Riaz, K.M.; Karamichos, D. Unravelling Novel Roles of Salivary Exosomes in the Regulation of Human Corneal Stromal Cell Migration and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4330. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Zhu, D.; Ma, S. Effect of PKH-26-labeled exosomes derived from bone marrow mesenchymal stem cells on corneal epithelium regeneration in diabetic mice. Ann. Transl. Med. 2023, 11, 167. [Google Scholar] [CrossRef]
- Altug, B.; Soykan, M.N.; Eyubova, S.; Eker Sariboyaci, A.; Dogan, C.; Ozalp, O.; Atalay, E. Crosstalk among miR-29, alpha-SMA, and TGFbeta1/beta3 in melatonin-induced exosome (Mel-prExo) treated human limbal mesenchymal stem cells (hLMSCs): An insight into scarless healing of the cornea. Biofactors 2024. [Google Scholar] [CrossRef]
- Erkoc-Biradli, F.Z.; Erenay, B.; Ozgun, A.; Oztatli, H.; Isik, F.; Ates, U.; Rasier, R.; Garipcan, B. Mesenchymal stem cells derived-exosomes enhanced amniotic membrane extract promotes corneal keratocyte proliferation. Biotechnol. Prog. 2024, 40, e3465. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Koh, A.; Kim, K.W. EGF-conditioned M1 macrophages Convey reduced inflammation into corneal endothelial cells through exosomes. Heliyon 2024, 10, e26800. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Lu, P.; Wang, Y.; Xing, S.; Yan, Y.; Han, R.; Hao, P.; Li, X. Mesenchymal Stem Cell-Derived Exosomes as Drug Carriers for Delivering miRNA-29b to Ameliorate Inflammation in Corneal Injury Via Activating Autophagy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 16. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Sun, Y.; Zeng, F.; Chen, X.; Ma, L.; Liu, X.; Qi, X.; Shi, W.; Gao, H. Exosomal miR-181d-5p Derived from Rapamycin-Conditioned MDSC Alleviated Allograft Rejection by Targeting KLF6. Adv Sci (Weinh) 2023, 10, e2304922. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Hwang, J.S.; Bo Noh, K.; Park, S.H.; Seo, J.H.; Shin, Y.J. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Riau, A.K.; Yam, G.H.; Yusoff, N.; Han, E.J.Y.; Goh, T.W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, Y.; Zhang, Y.; Zheng, D.; Yan, L.; Guo, M.; Mao, Y.; Yang, L. Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 MAPK pathway. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 723–734. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver miR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Ma, S.; Yin, J.; Hao, L.; Liu, X.; Shi, Q.; Diao, Y.; Yu, G.; Liu, L.; Chen, J.; Zhong, J. Exosomes From Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation. Front. Bioeng. Biotechnol. 2022, 10, 879192. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lv, Y.; Zhang, W.; Zhang, X.; Li, F.; Lu, X.; Zhao, S. Mesenchymal stem cell derived exosomes-based immunological signature in a rat model of corneal allograft rejection therapy. Front. Biosci. 2022, 27, 86. [Google Scholar] [CrossRef]
- Buono, L.; Scalabrin, S.; De Iuliis, M.; Tanzi, A.; Grange, C.; Tapparo, M.; Nuzzi, R.; Bussolati, B. Mesenchymal Stem Cell-Derived Extracellular Vesicles Protect Human Corneal Endothelial Cells from Endoplasmic Reticulum Stress-Mediated Apoptosis. Int. J. Mol. Sci. 2021, 22, 4930. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal miR-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging 2020, 12, 4093–4110. [Google Scholar] [CrossRef] [PubMed]
- Verta, R.; Saccu, G.; Tanzi, A.; Grange, C.; Buono, L.; Fagoonee, S.; Deregibus, M.C.; Camussi, G.; Scalabrin, S.; Nuzzi, R.; et al. Phenotypic and functional characterization of aqueous humor derived extracellular vesicles. Exp. Eye Res. 2023, 228, 109393. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Knight, R.; Robertson, S.Y.T.; Dos Santos, A.; Zhang, C.; Ma, C.; Xu, J.; Zheng, J.; Deng, S.X. Stability and Function of Extracellular Vesicles Derived from Immortalized Human Corneal Stromal Stem Cells: A Proof of Concept Study. AAPS J. 2022, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Jeng, B.H.; Hamrah, P.; Kirshner, Z.Z.; Mendez, B.C.; Wessel, H.C.; Brown, L.R.; Steed, D.L. Exploratory Phase II Multicenter, Open-Label, Clinical Trial of ST266, a Novel Secretome for Treatment of Persistent Corneal Epithelial Defects. Transl. Vis. Sci. Technol. 2022, 11, 8. [Google Scholar] [CrossRef]
- Lee, C.S.; Fan, J.; Hwang, H.S.; Kim, S.; Chen, C.; Kang, M.; Aghaloo, T.; James, A.W.; Lee, M. Bone-Targeting Exosome Mimetics Engineered by Bioorthogonal Surface Functionalization for Bone Tissue Engineering. Nano Lett. 2023, 23, 1202–1210. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Lu, Z.; Zhang, L.; Hu, Y.; Li, Q.; Du, W.; Feng, X.; Jia, H.; Liu, B.F. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale 2017, 9, 15598–15605. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Xie, F.; Xu, B.; Xie, P.; Yang, T.; Shi, Q.; Zhang, C.Y.; Zhang, Y.; Chen, J.; et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater. Sci. 2020, 8, 2966–2976. [Google Scholar] [CrossRef]
- El-Andaloussi, S.; Lee, Y.; Lakhal-Littleton, S.; Li, J.; Seow, Y.; Gardiner, C.; Alvarez-Erviti, L.; Sargent, I.L.; Wood, M.J. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 2012, 7, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Singh, A.; Verma, S.; Stephenson, S.; Bhowmick, T.; Sangwan, V.S. Mini Review: Current Trends and Understanding of Exosome Therapeutic Potential in Corneal Diseases. Front. Pharmacol. 2021, 12, 684712. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Peng, Z.; Yuan, L.; Jin, M.; Hu, H.; Peng, X.; Wang, Y.; Zhang, C.; Luo, Z.; Liao, H. Research progress of exosomes in pathogenesis, diagnosis, and treatment of ocular diseases. Front. Bioeng. Biotechnol. 2023, 11, 1100310. [Google Scholar] [CrossRef]
- Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. [Google Scholar] [CrossRef]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Nelson, B.C.; Maragh, S.; Ghiran, I.C.; Jones, J.C.; DeRose, P.C.; Elsheikh, E.; Vreeland, W.N.; Wang, L. Measurement and standardization challenges for extracellular vesicle therapeutic delivery vectors. Nanomedicine 2020, 15, 2149–2170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbins, B.T.; Montreuil, K.A.; Kundu, N.; Kumar, P.; Agrahari, V. Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue. Pharmaceutics 2024, 16, 1424. https://doi.org/10.3390/pharmaceutics16111424
Robbins BT, Montreuil KA, Kundu N, Kumar P, Agrahari V. Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue. Pharmaceutics. 2024; 16(11):1424. https://doi.org/10.3390/pharmaceutics16111424
Chicago/Turabian StyleRobbins, Brooke T., Kate A. Montreuil, Neloy Kundu, Prashant Kumar, and Vibhuti Agrahari. 2024. "Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue" Pharmaceutics 16, no. 11: 1424. https://doi.org/10.3390/pharmaceutics16111424
APA StyleRobbins, B. T., Montreuil, K. A., Kundu, N., Kumar, P., & Agrahari, V. (2024). Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue. Pharmaceutics, 16(11), 1424. https://doi.org/10.3390/pharmaceutics16111424






