Antiproliferative and Morphological Effects of Fenretinide Lipid Nanosystems in Colon Adenocarcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of PCN-Fen
2.3. Characterization of PCN-Fen
2.4. Cell Lines
2.5. MTT Assay
2.6. Quantitative Phase Imaging Microscopy
2.7. Confocal Laser-Scanning Fluorescence Microscopy
2.8. Flow Cytometry
2.8.1. Cell-Cycle Analysis
2.8.2. Mitochondrial Potential Analysis
2.8.3. Evaluation of Reactive Oxygen Species Production
2.9. Statistical Analysis
3. Results
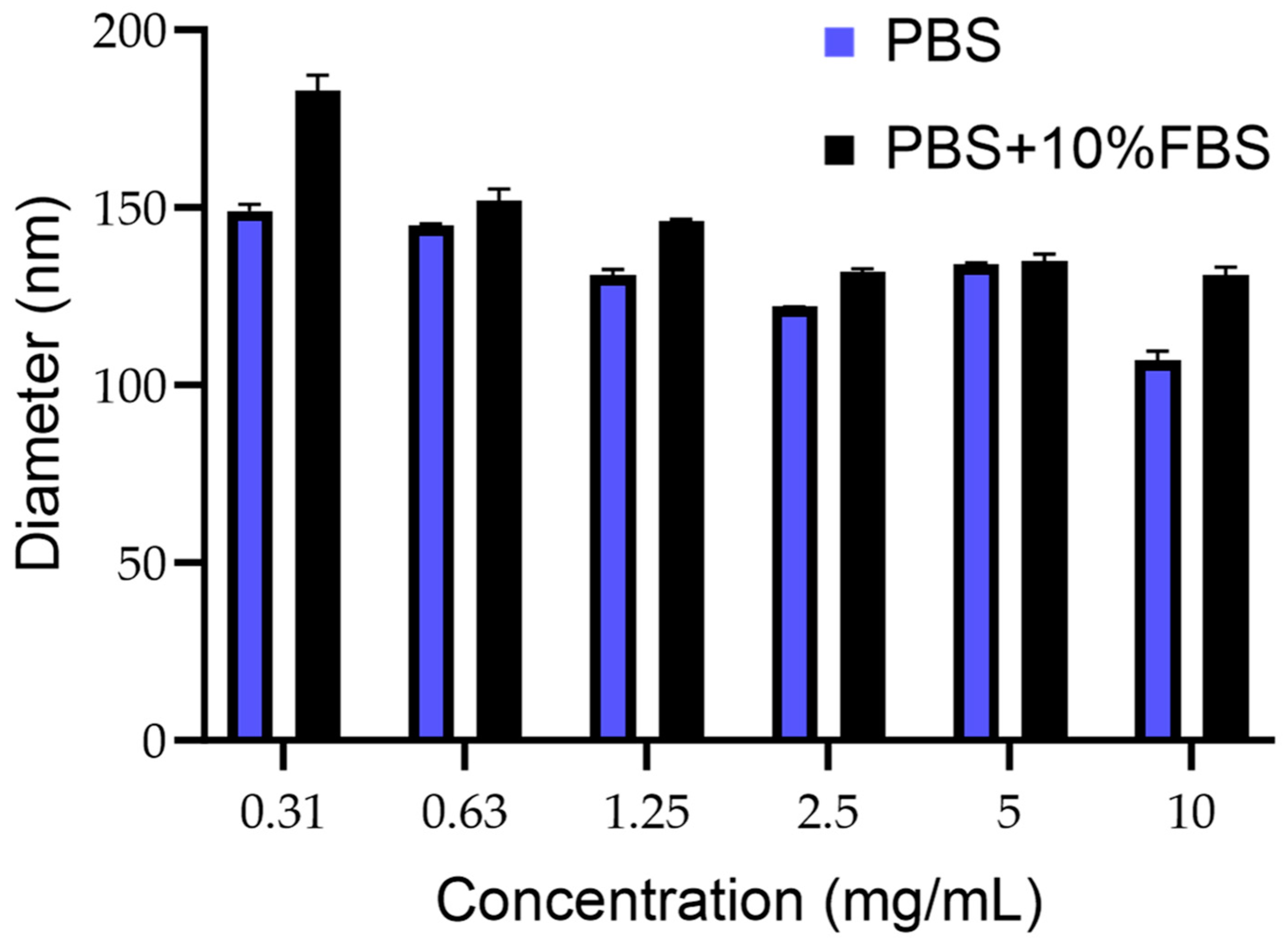
3.1. PCN-Fen Characteristics
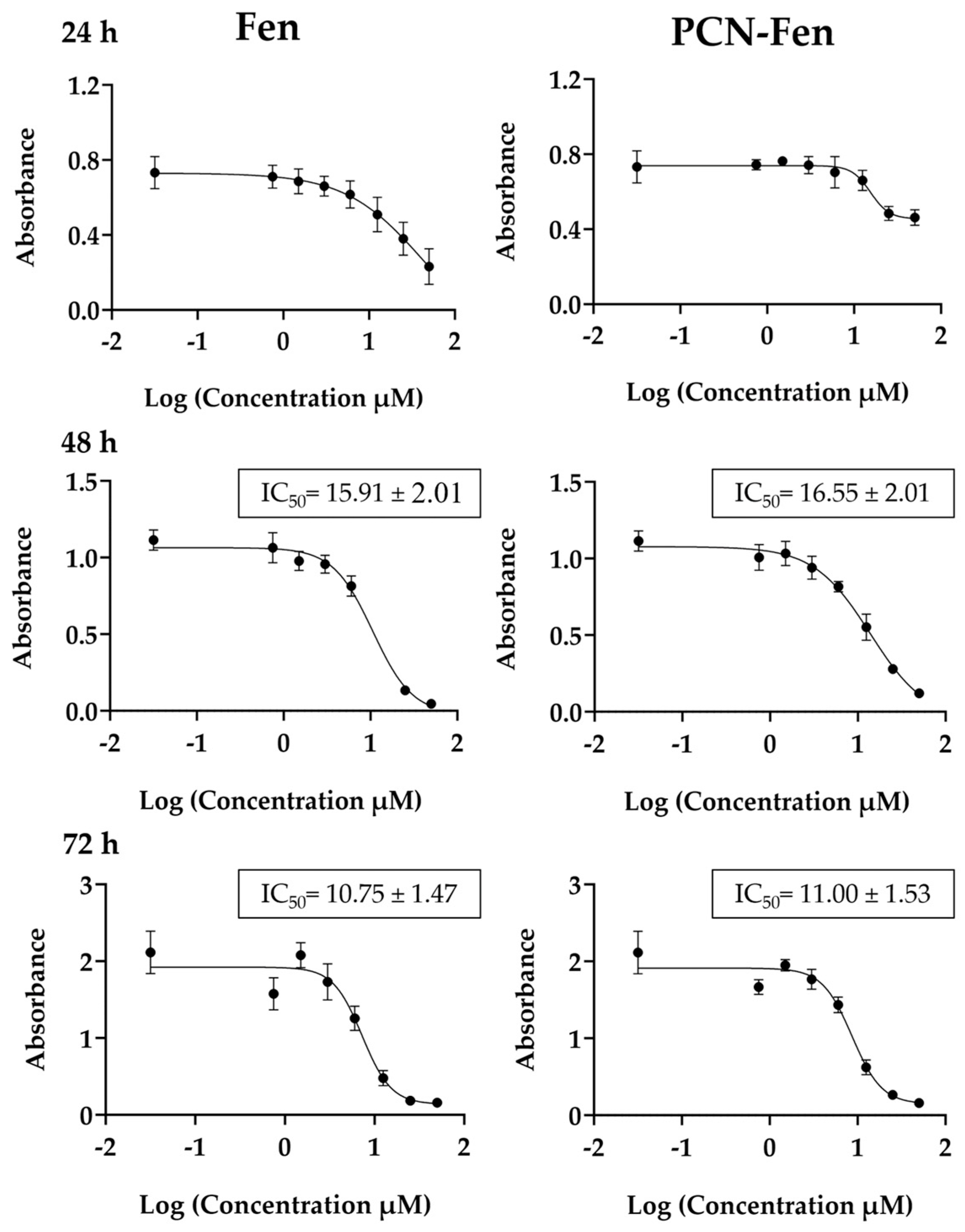
3.2. Effect of Fen and PCN-Fen on Cell Viability
3.3. Quantitative Phase Imaging
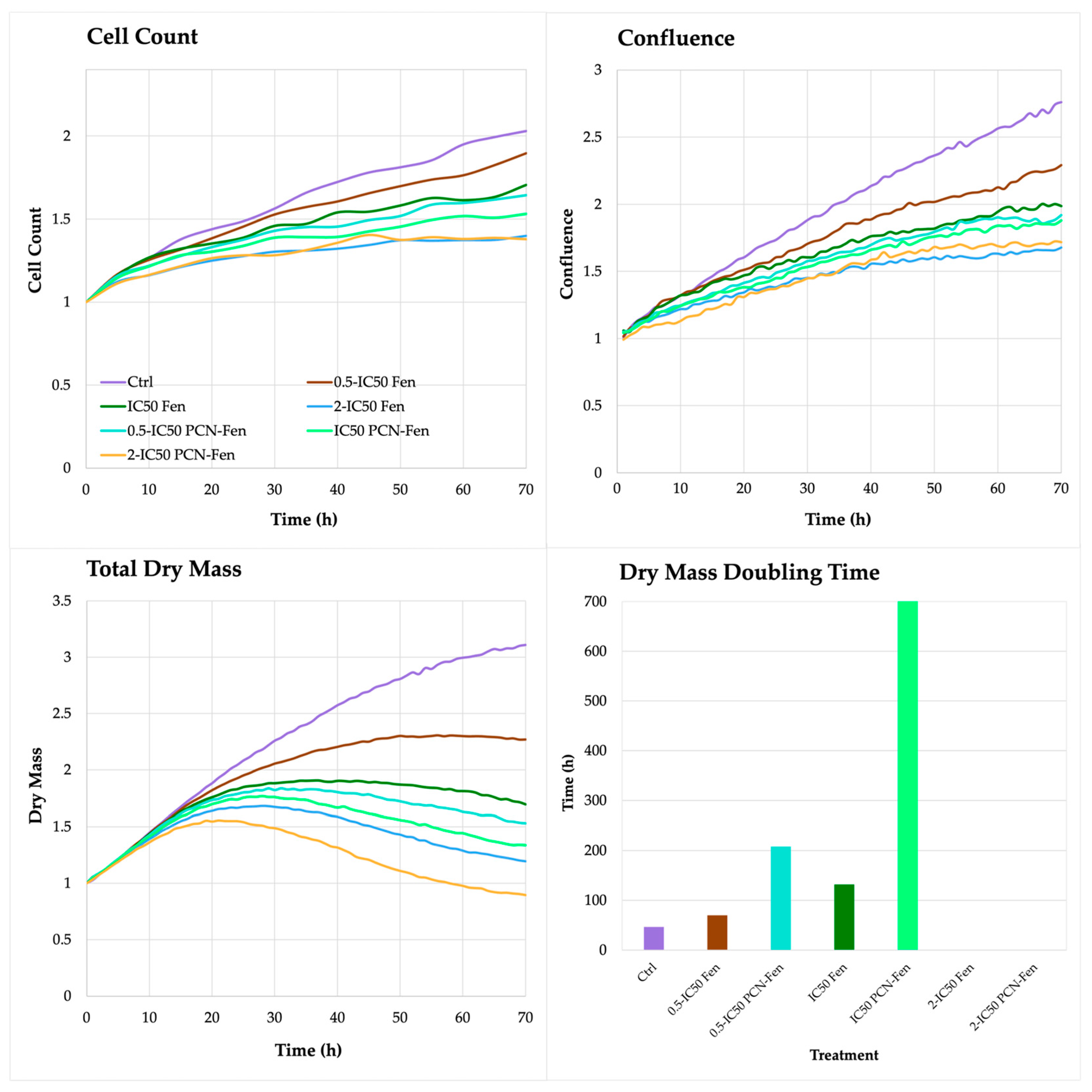
3.3.1. Cell Proliferation
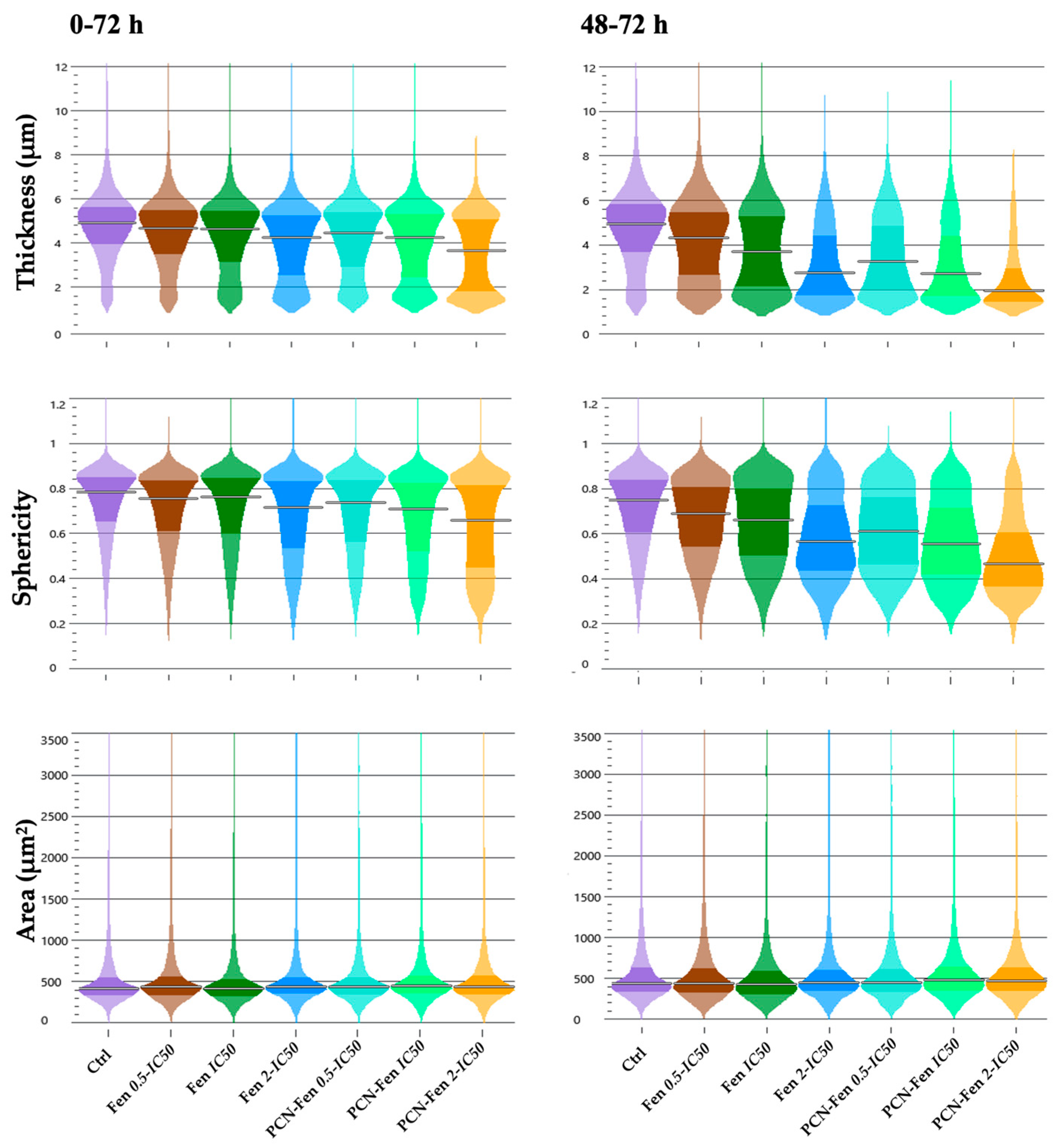
3.3.2. Cell Morphology
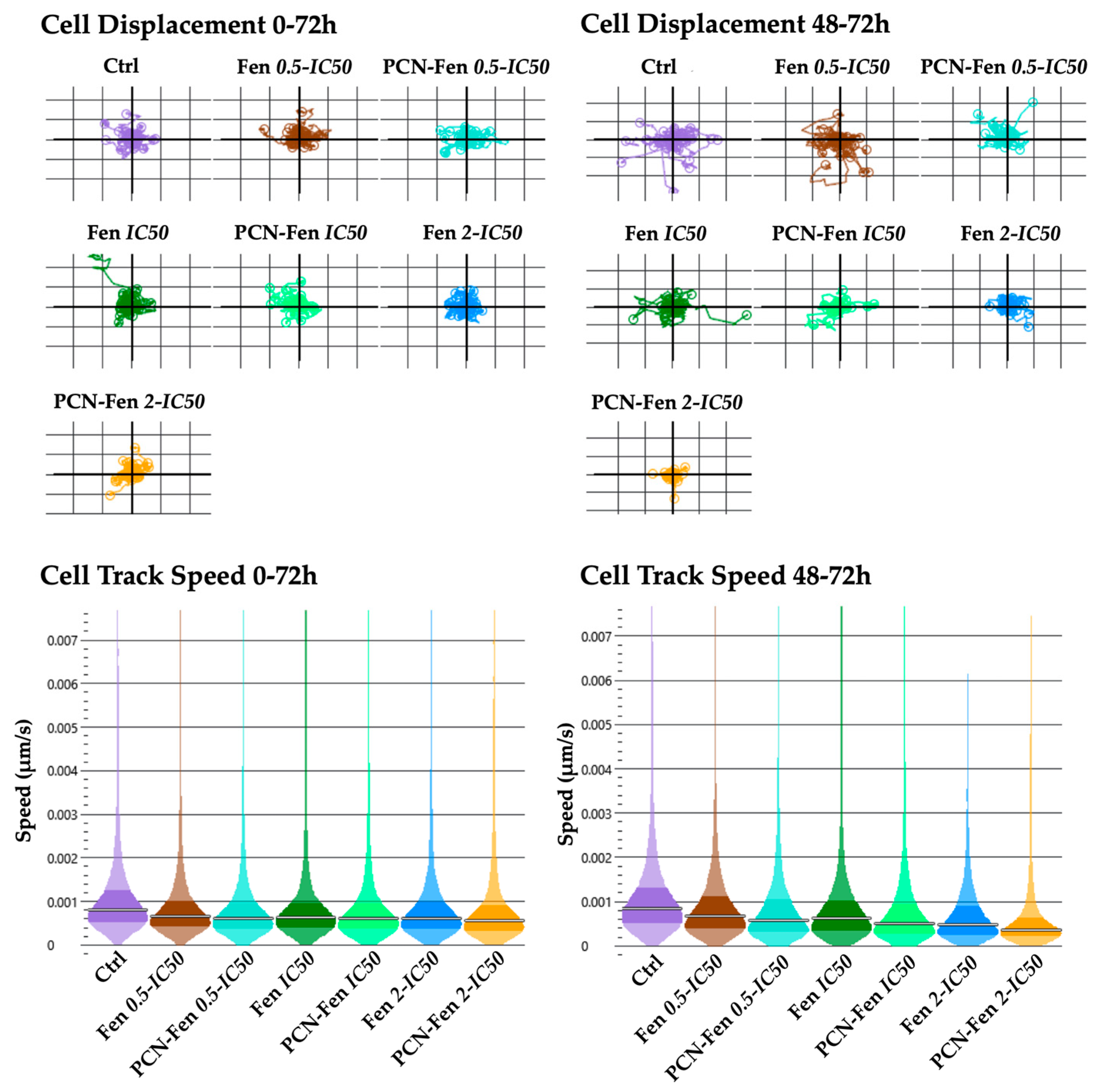
3.3.3. Cell Motility
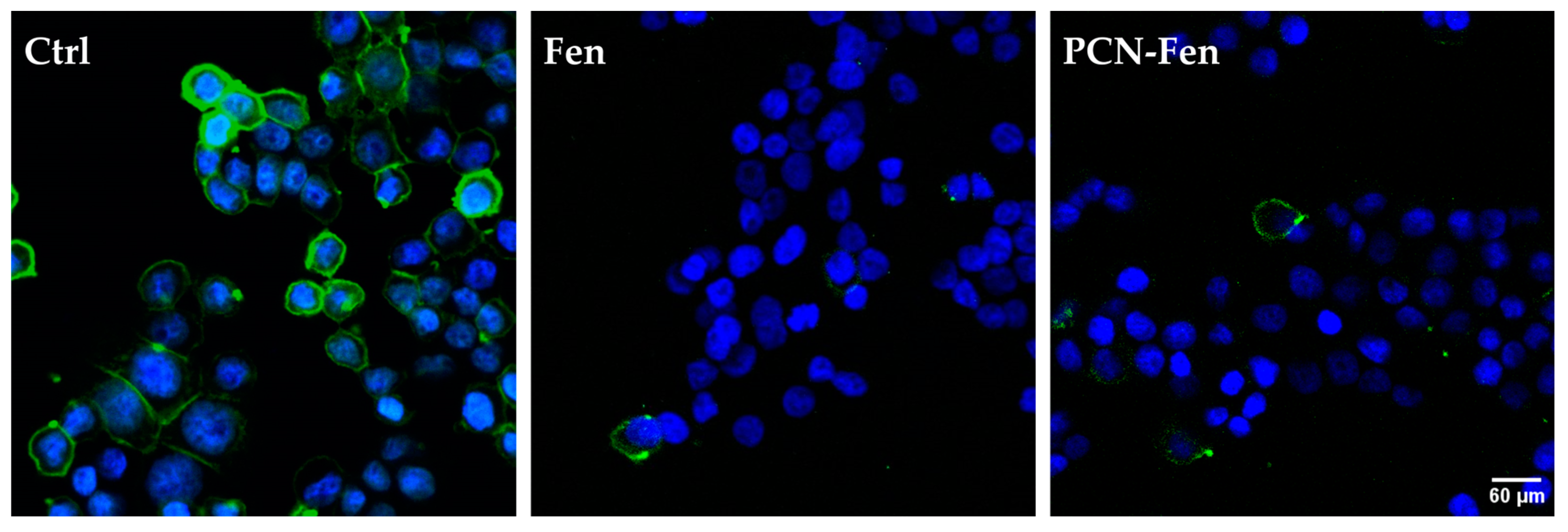
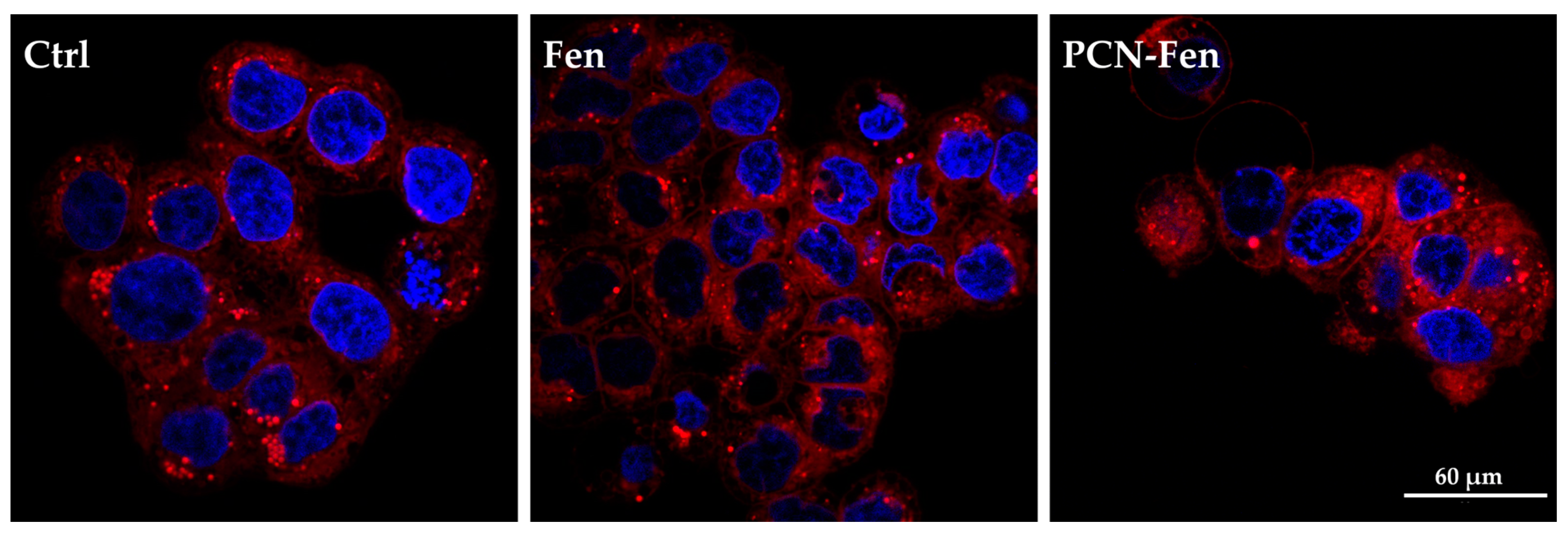
3.4. Confocal Fluorescence Microscopy
3.5. Flow Cytometry Data
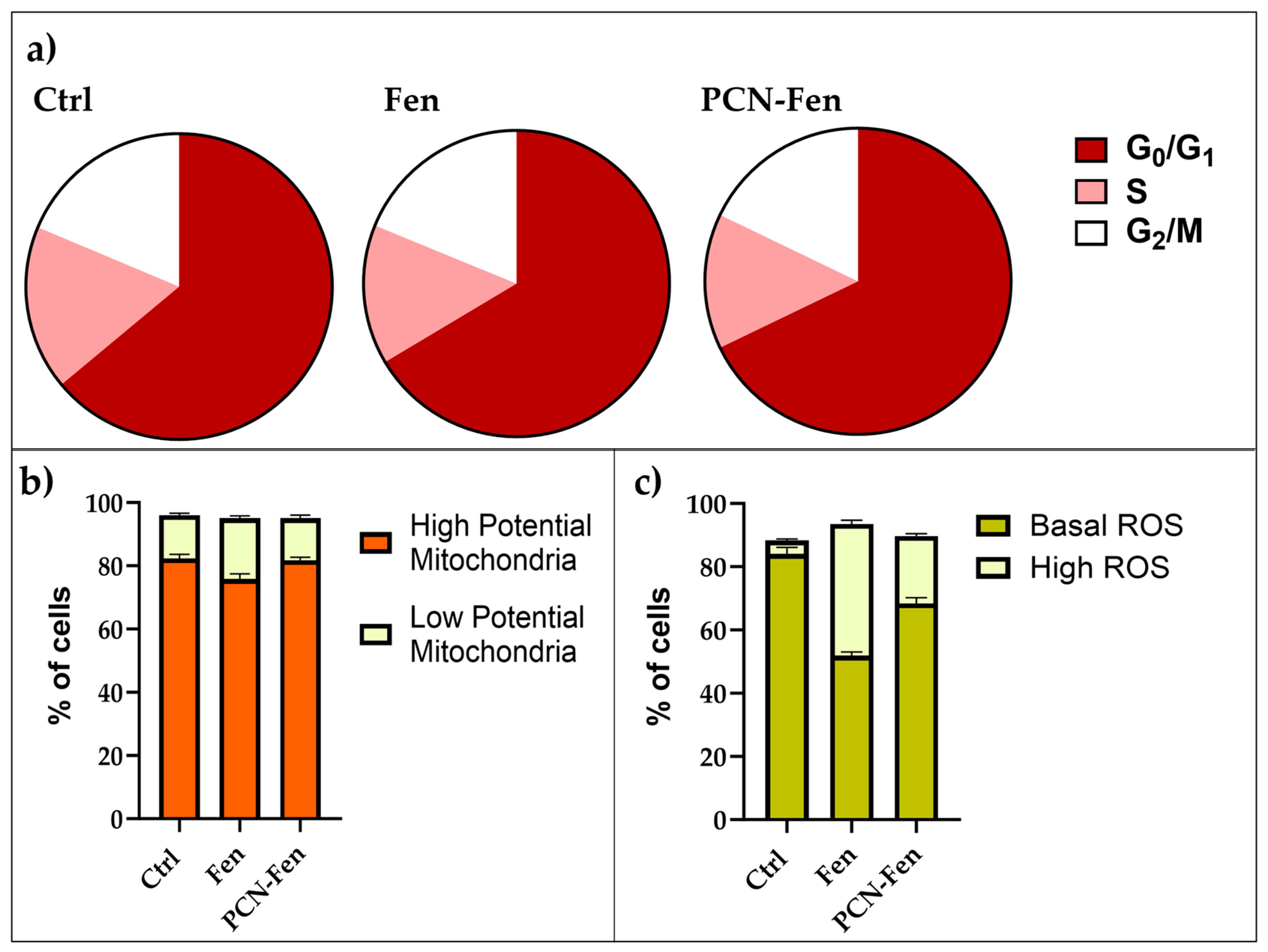
3.5.1. Cell-Cycle Analysis
3.5.2. Mitochondrial Potential
3.5.3. ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, H.B. Effect of Retinoic Acid on Cell Proliferation Kinetics and Retinoic Acid Receptor Expression of Colorectal Mucosa. World J. Gastroenterol. 2003, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Zhang, Y.; Brewer, M.; Bryant, T.; Lightfoot, S.; Steele, V.E.; Rao, C.V. Chemopreventive Efficacy of Raloxifene, Bexarotene, and Their Combination on the Progression of Chemically Induced Colon Adenomas to Adenocarcinomas in Rats. Cancer Prev. Res. 2013, 6, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Samad, R.; Aouad, P.; Darwiche, N. Natural and Synthetic Retinoids in Preclinical Colorectal Cancer Models. Anticancer Drugs 2019, 30, 655–669. [Google Scholar] [CrossRef] [PubMed]
- di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic Acid Receptors: From Molecular Mechanisms to Cancer Therapy. Mol. Asp. Med. 2015, 41, 1–115. [Google Scholar] [CrossRef]
- Connolly, R.M.; Nguyen, N.K.; Sukumar, S. Molecular Pathways: Current Role and Future Directions of the Retinoic Acid Pathway in Cancer Prevention and Treatment. Clin. Cancer Res. 2013, 19, 1651–1659. [Google Scholar] [CrossRef]
- Garattini, E.; Bolis, M.; Garattini, S.K.; Fratelli, M.; Centritto, F.; Paroni, G.; Gianni’, M.; Zanetti, A.; Pagani, A.; Fisher, J.N.; et al. Retinoids and Breast Cancer: From Basic Studies to the Clinic and Back Again. Cancer Treat. Rev. 2014, 40, 739–749. [Google Scholar] [CrossRef]
- Cooper, J.P.; Reynolds, C.P.; Cho, H.; Kang, M.H. Clinical Development of Fen as an Antineoplastic Drug: Pharmacology Perspectives. Exp. Biol. Med. 2017, 242, 1178–1184. [Google Scholar] [CrossRef]
- Potenza, R.L.; Lodeserto, P.; Orienti, I. Fen in Cancer and Neurological Disease: A Two-Face Janus Molecule. Int. J. Mol. Sci. 2022, 23, 7426. [Google Scholar] [CrossRef] [PubMed]
- Merritt, G.; Aliprandis, E.T.; Prada, F.; Rigas, B.; Kashfi, K. The Retinoid Fen Inhibits Proliferation and Downregulates Cyclooxygenase-2 Gene Expression in Human Colon Adenocarcinoma Cell Lines. Cancer Lett. 2001, 164, 15–23. [Google Scholar] [CrossRef]
- Kouhara, J.; Yoshida, T.; Nakata, S.; Horinaka, M.; Wakada, M.; Ueda, Y.; Yamagishi, H.; Sakai, T. Fen Up-Regulates DR5/TRAIL-R2 Expression via the Induction of the Transcription Factor CHOP and Combined Treatment with Fen and TRAIL Induces Synergistic Apoptosis in Colon Cancer Cell Lines. Int. J. Oncol. 2007, 30, 679–687. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Wang, H.; Zhao, H.; Du, Y. Fen Targeting of Human Colon Cancer Sphere Cells through Cell Cycle Regulation and Stress-responsive Activities. Oncol. Lett. 2018, 16, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A New Bioavailable Fen Formulation with Antiproliferative, Antimetabolic, and Cytotoxic Effects on Solid Tumors. Cell Death Dis. 2019, 10, 529. [Google Scholar] [CrossRef]
- Dong, R.; Gong, Y.; Meng, W.; Yuan, M.; Zhu, H.; Ying, M.; He, Q.; Cao, J.; Yang, B. The Involvement of M2 Macrophage Polarization Inhibition in Fen-Mediated Chemopreventive Effects on Colon Cancer. Cancer Lett. 2017, 388, 43–53. [Google Scholar] [CrossRef]
- Orienti, I.; Farruggia, G.; Nguyen, F.; Guan, P.; Calonghi, N.; Kolla, V.; Chorny, M.; Brodeur, G.M. Nanomicellar Lenalidomide-Fen Combination Suppresses Tumor Growth in an MYCN Amplified Neuroblastoma Tumor. Int. J. Nanomed. 2020, 15, 6873–6886. [Google Scholar] [CrossRef]
- Apolinário, A.C.; Naser, Y.A.; Volpe-Zanutto, F.; Vora, L.K.; Sabri, A.H.; Li, M.; Hutton, A.R.J.; McCarthy, H.O.; Lopes, L.B.; Donnelly, R.F. Novel lipid nanovesicle-loaded dissolving microarray patches for fenretinide in breast cancer chemoprevention. J. Control. Release 2024, 374, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Salvati, V.; Sette, G.; Zucchetti, M.; Bongiorno-Borbone, L.; Peschiaroli, A.; Zolla, L.; Francescangeli, F.; Ferrari, M.; Matteo, C.; et al. A Novel Oral Micellar Fen Formulation with Enhanced Bioavailability and Antitumour Activity against Multiple Tumours from Cancer Stem Cells. J. Exp. Clin. Cancer Res. 2019, 38, 373. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Development of Mixed Micelles for Enhancing Fenretinide Apparent Solubility and Anticancer Activity Against Neuroblastoma Cells. Curr. Drug Deliv. 2024. [Google Scholar] [CrossRef]
- Galassi, L.; Rossi, M.; Lodeserto, P.; Lenzi, M.; Borsetti, F.; Voltattorni, M.; Farruggia, G.; Blasi, P.; Orienti, I. Naxitamab Activity in Neuroblastoma Cells Is Enhanced by NanoFen and Nanospermidine. Pharmaceutics 2023, 15, 648. [Google Scholar] [CrossRef]
- Lodeserto, P.; Rossi, M.; Blasi, P.; Farruggia, G.; Orienti, I. Nanospermidine in Combination with NanoFen Induces Cell Death in Neuroblastoma Cell Lines. Pharmaceutics 2022, 14, 1215. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Maiden, A.M.; Rodenburg, J.M.; Humphry, M.J. A New Method of High Resolution, Quantitative Phase Scanning Microscopy. In Proceedings of the Scanning Microscopy 2010, Monterey, CA, USA, 2 June 2010; Postek, M.T., Newbury, D.E., Platek, S.F., Joy, D.C., Eds.; SPIE: Bellingham, WA, USA, 2010; p. 77291I. [Google Scholar]
- Stylianopoulos, T. EPR-Effect: Utilizing Size-Dependent Nanoparticle Delivery to Solid Tumors. Ther. Deliv. 2013, 4, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S. Thinking about Enhanced Permeability and Retention Effect (EPR). J. Pers. Med. 2022, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Karaz, S.; Senses, E. Liposomes Under Shear: Structure, Dynamics, and Drug Delivery Applications. Adv. Nanobiomed. Res. 2023, 3, 2200101. [Google Scholar] [CrossRef]
- Wang, Y.; Grainger, D. Regulatory Considerations Specific to Liposome Drug Development as Complex Drug Products. Front. Drug Deliv. 2022, 2, 901281. [Google Scholar] [CrossRef]
- Caracciolo, G. Liposome–Protein Corona in a Physiological Environment: Challenges and Opportunities for Targeted Delivery of Nanomedicines. Nanomedicine 2015, 11, 543–557. [Google Scholar] [CrossRef]
- Alizadeh, E.; Castle, J.; Quirk, A.; Taylor, C.D.L.; Xu, W.; Prasad, A. Cellular Morphological Features Are Predictive Markers of Cancer Cell State. Comput. Biol. Med. 2020, 126, 104044. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-M.; Lee, E.-J.; Kim, D.-J.; Hwang, W.-B. Kynurenine Promotes the Goblet Cell Differentiation of HT-29 Colon Carcinoma Cells by Modulating Wnt, Notch and AhR Signals. Oncol. Rep. 2018, 39, 1930–1938. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Kwak, J.-H.; Je, N.K.; Lee, Y.; Jung, Y.-S. Epithelial-Mesenchymal Transition Is Associated with Acquired Resistance to 5-Fluorocuracil in HT-29 Colon Cancer Cells. Toxicol. Res. 2015, 31, 151–156. [Google Scholar] [CrossRef]
- Kraveka, J.M.; Li, L.; Szulc, Z.M.; Bielawski, J.; Ogretmen, B.; Hannun, Y.A.; Obeid, L.M.; Bielawska, A. Involvement of Dihydroceramide Desaturase in Cell Cycle Progression in Human Neuroblastoma Cells. J. Biol. Chem. 2007, 282, 16718–16728. [Google Scholar] [CrossRef]
- Bikman, B.T.; Guan, Y.; Shui, G.; Siddique, M.M.; Holland, W.L.; Kim, J.Y.; Fabriàs, G.; Wenk, M.R.; Summers, S.A. Fen Prevents Lipid-Induced Insulin Resistance by Blocking Ceramide Biosynthesis. J. Biol. Chem. 2012, 287, 17426–17437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; et al. Ceramides and Other Bioactive Sphingolipid Backbones in Health and Disease: Lipidomic Analysis, Metabolism and Roles in Membrane Structure, Dynamics, Signaling and Autophagy. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Hammerling, U. The Mitochondrial PKCδ/Retinol Signal Complex Exerts Real-Time Control on Energy Homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158614. [Google Scholar] [CrossRef] [PubMed]
- Roese, E.; Bunjes, H. Drug Release Studies from Lipid Nanosystems in Physiological Media by a New DSC Method. J. Control. Release 2017, 256, 92–100. [Google Scholar] [CrossRef]
- Terzi, M.Y.; Izmirli, M.; Gogebakan, B. The Cell Fate: Senescence or Quiescence. Mol. Biol. Rep. 2016, 43, 1213–1220. [Google Scholar] [CrossRef]

| Formulation | Drug Loading (% w/w) | Encapsulation Efficiency (% w/w) | Zeta Potential (mV) | Drug Leakage at 24 h (%) |
|---|---|---|---|---|
| PCN-Fen | 10.8 ± 0.9 | 95.2 ± 3.2 | −17.1 ± 0.6 | 5.9 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anconelli, L.; Farioli, F.; Lodeserto, P.; Andreadi, A.; Borsetti, F.; Voltattorni, M.; Galassi, L.; Rossi, M.; Farruggia, G.; Blasi, P.; et al. Antiproliferative and Morphological Effects of Fenretinide Lipid Nanosystems in Colon Adenocarcinoma Cells. Pharmaceutics 2024, 16, 1421. https://doi.org/10.3390/pharmaceutics16111421
Anconelli L, Farioli F, Lodeserto P, Andreadi A, Borsetti F, Voltattorni M, Galassi L, Rossi M, Farruggia G, Blasi P, et al. Antiproliferative and Morphological Effects of Fenretinide Lipid Nanosystems in Colon Adenocarcinoma Cells. Pharmaceutics. 2024; 16(11):1421. https://doi.org/10.3390/pharmaceutics16111421
Chicago/Turabian StyleAnconelli, Lorenzo, Francesca Farioli, Pietro Lodeserto, Aikaterini Andreadi, Francesca Borsetti, Manuela Voltattorni, Lucrezia Galassi, Martina Rossi, Giovanna Farruggia, Paolo Blasi, and et al. 2024. "Antiproliferative and Morphological Effects of Fenretinide Lipid Nanosystems in Colon Adenocarcinoma Cells" Pharmaceutics 16, no. 11: 1421. https://doi.org/10.3390/pharmaceutics16111421
APA StyleAnconelli, L., Farioli, F., Lodeserto, P., Andreadi, A., Borsetti, F., Voltattorni, M., Galassi, L., Rossi, M., Farruggia, G., Blasi, P., & Orienti, I. (2024). Antiproliferative and Morphological Effects of Fenretinide Lipid Nanosystems in Colon Adenocarcinoma Cells. Pharmaceutics, 16(11), 1421. https://doi.org/10.3390/pharmaceutics16111421








