A Novel Poly(ε-Caprolactone)-Based Photo-Crosslinkable Liquid Copolymer as a Versatile Drug Delivery Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
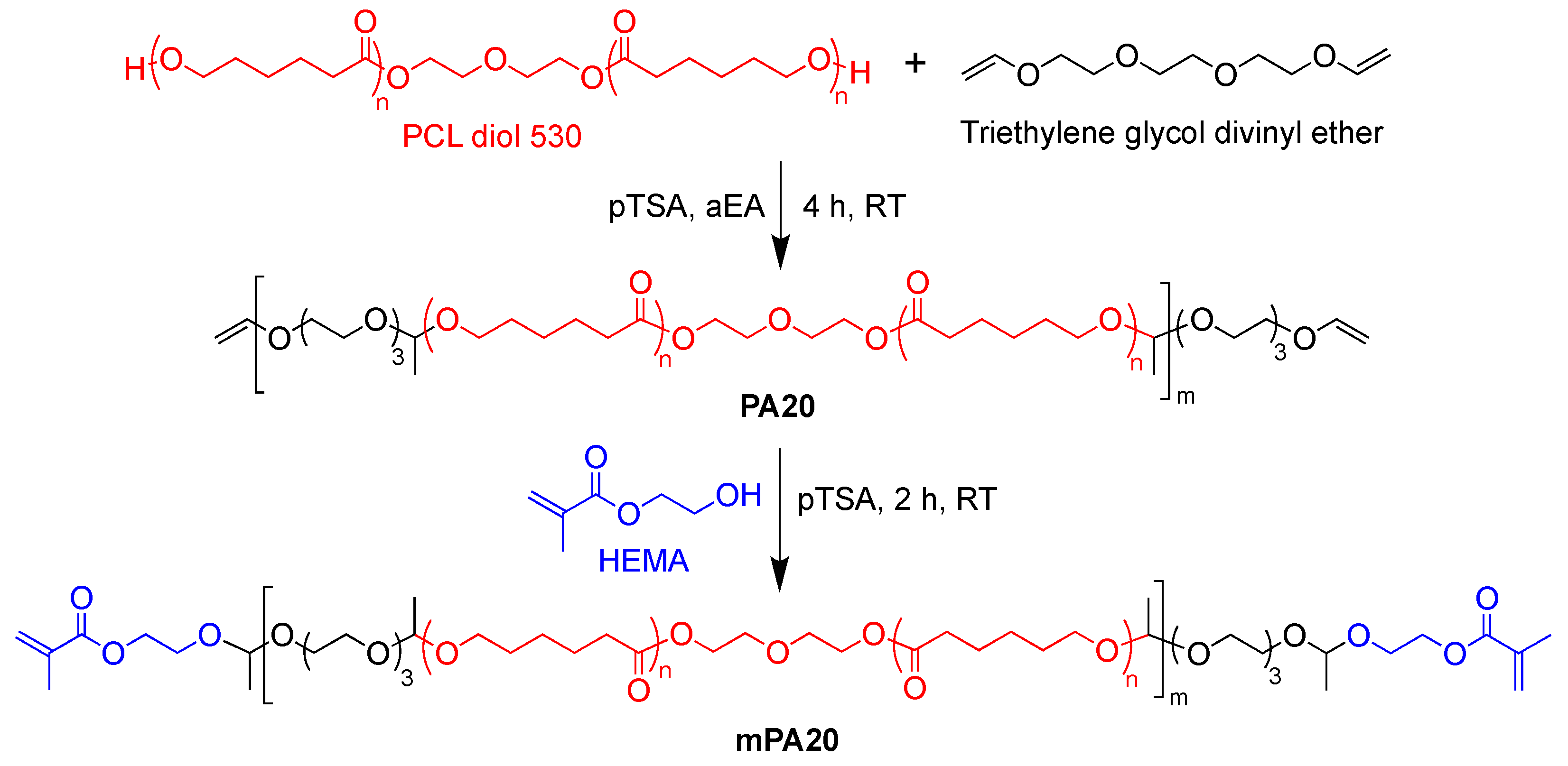
2.2. One-Pot Synthesis of mPA20
2.3. Polymer Characterization
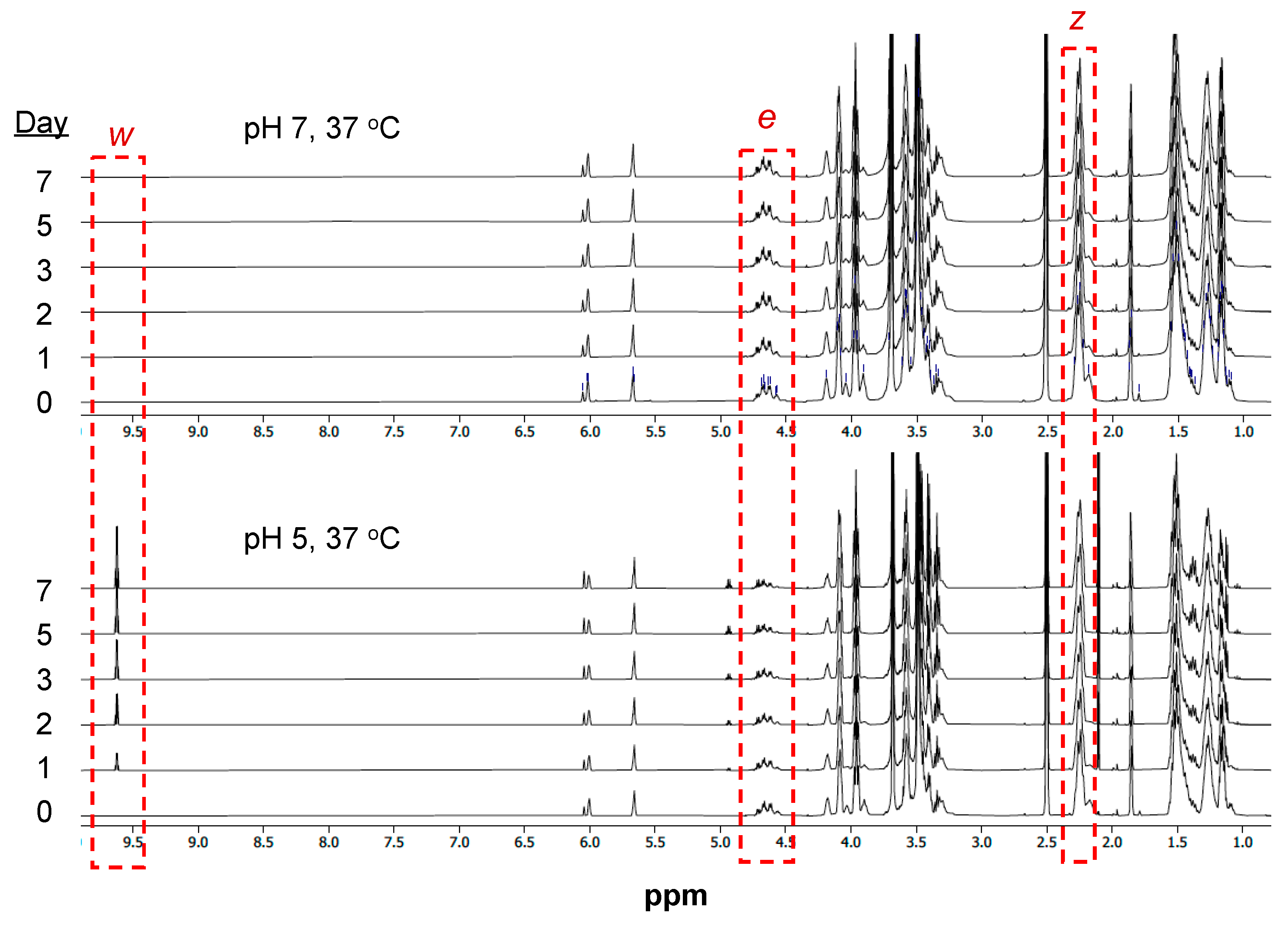
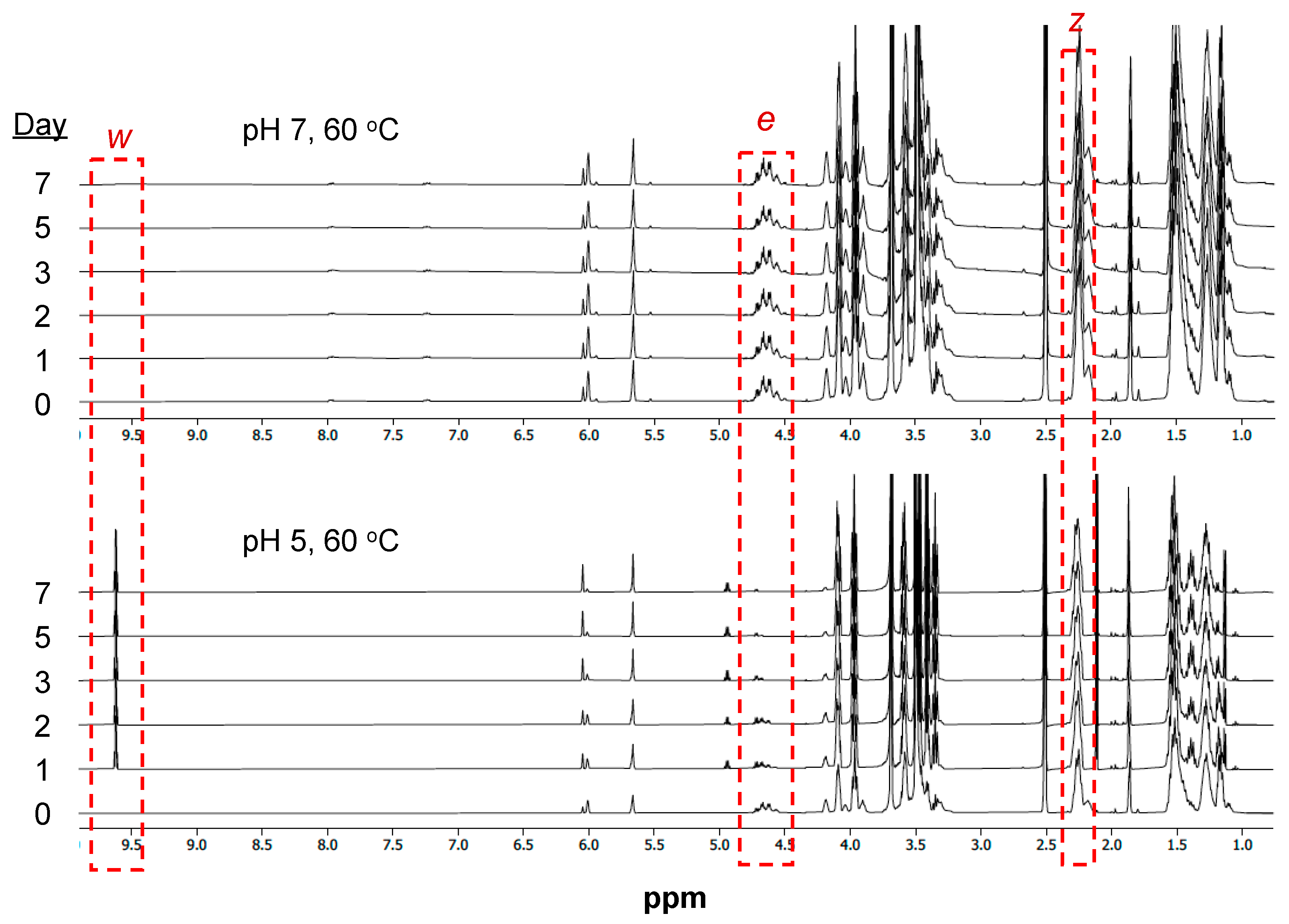
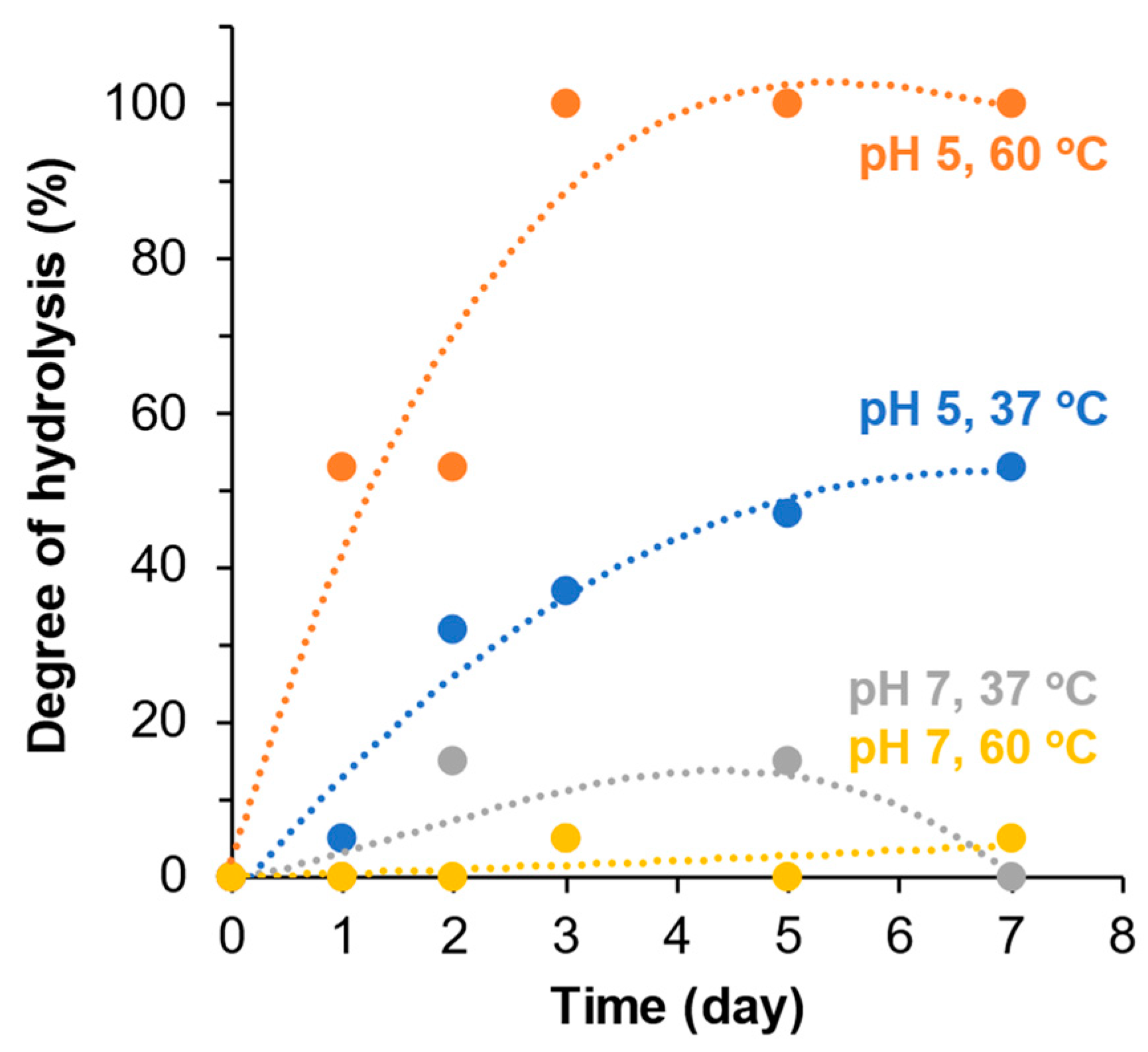
2.4. Polymer Degradation Kinetics
2.5. Photo-Crosslinking and Quantification of Gel Fraction
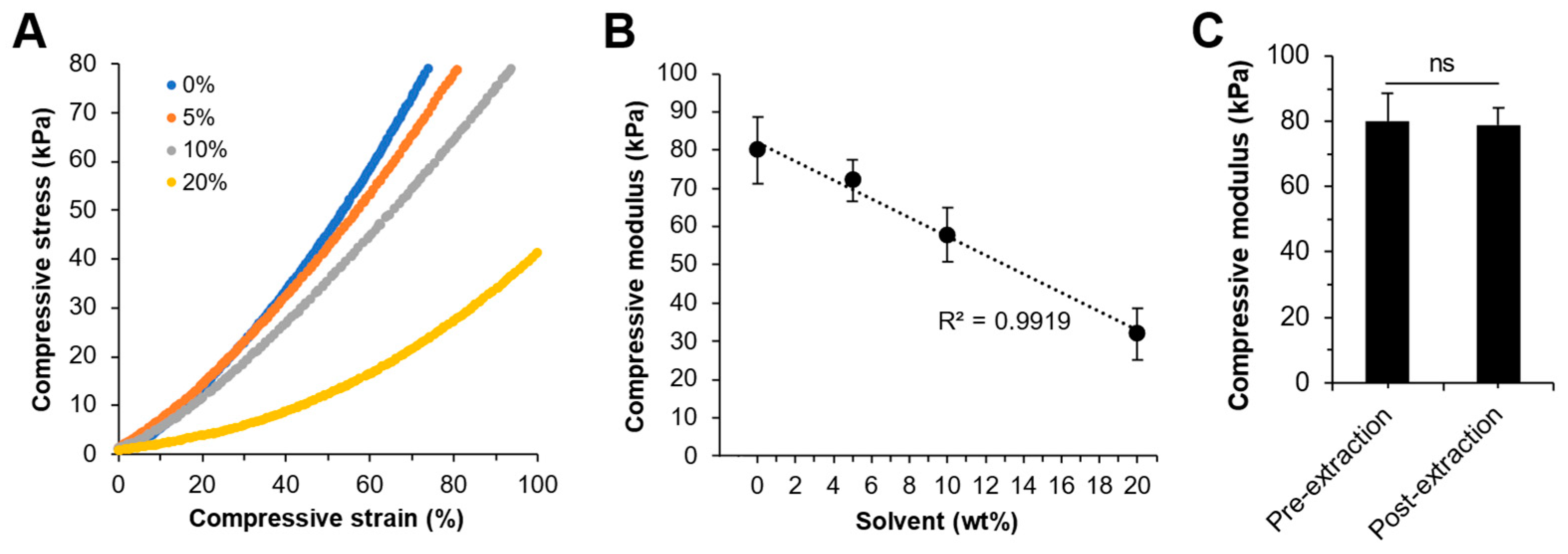
2.6. Mechanical Compression Test
2.7. Accelerated Polymer Erosion Test
2.8. NR Release Kinetics
2.9. Preparation of Nanodroplets and Subsequent Photo-Crosslinking to Produce Nanogels
2.10. Intracellular Uptake and Cytotoxicity Assay
3. Results
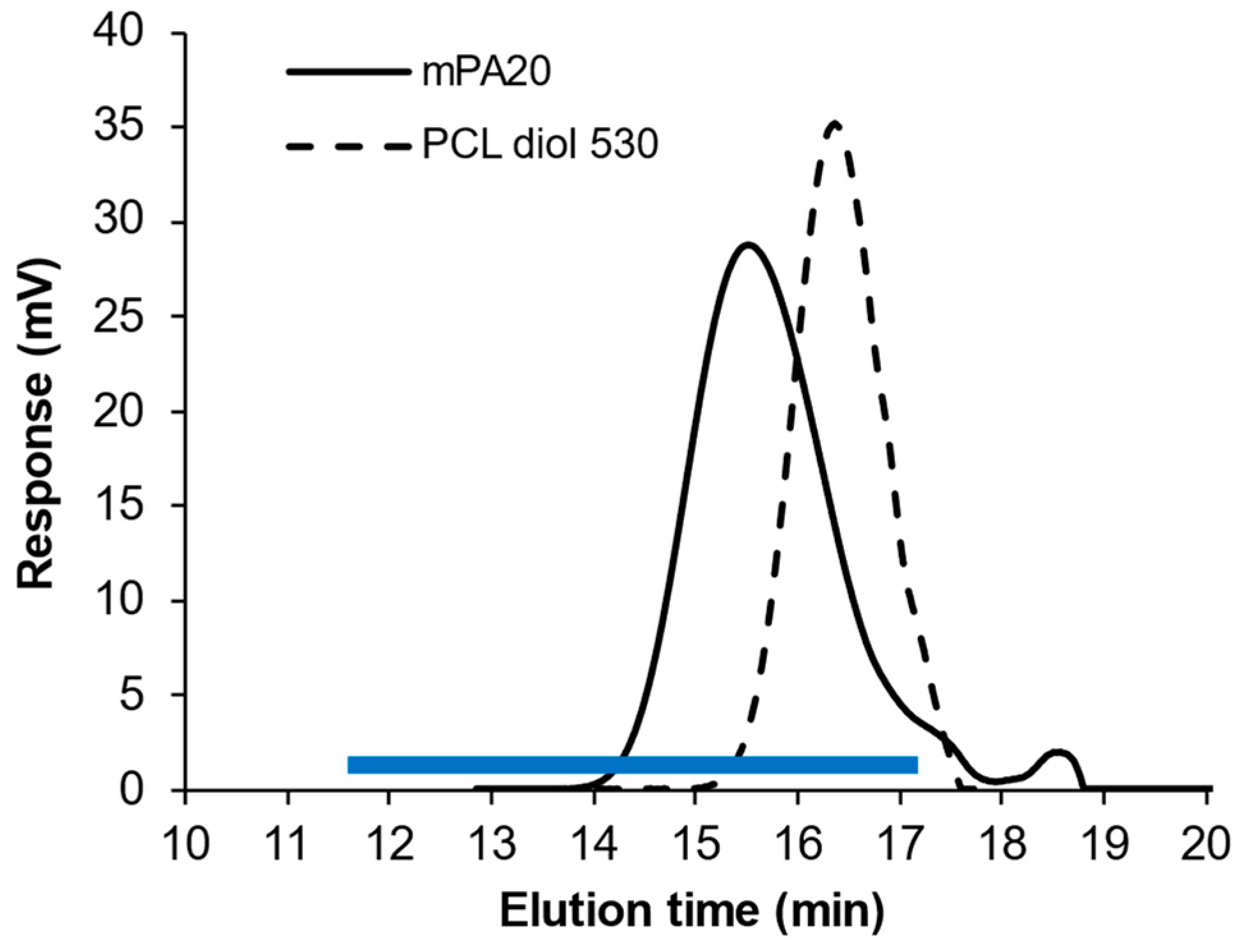
3.1. Polymer Synthesis and Characterization
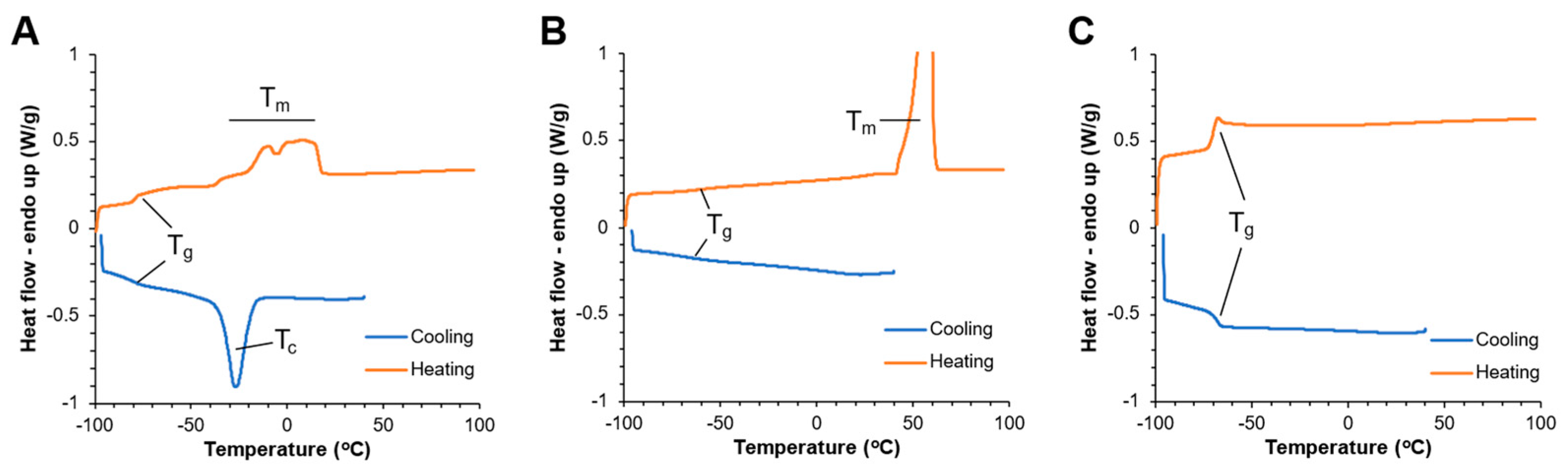
3.2. mPA20 Is an Amorphous, Low-Melt, Viscous Newtonian Liquid
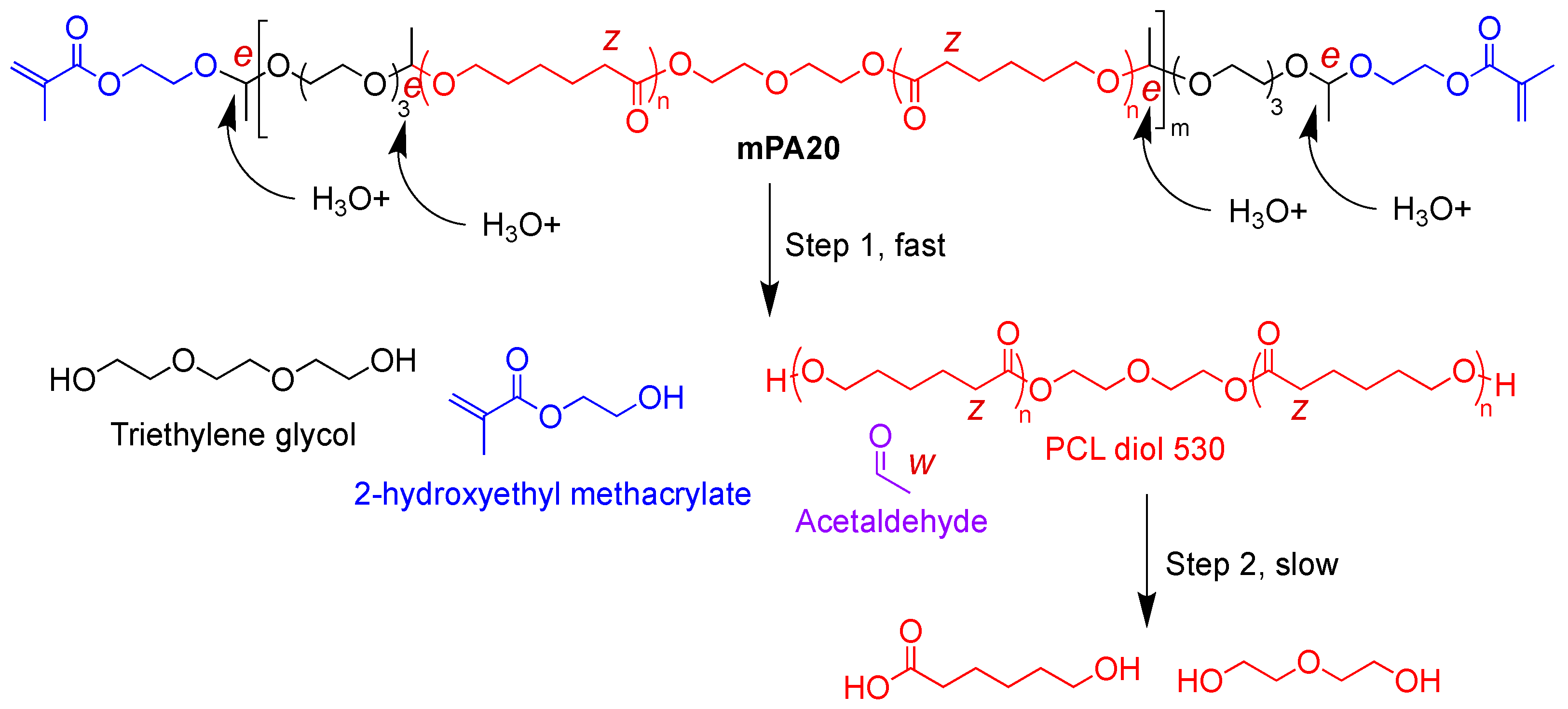
3.3. Hydrolytic Degradation of mPA20
3.4. Photo-Crosslinking of mPA20
3.5. Mechanical Properties of the Photo-Crosslinked mPA20
3.6. Accelerated Erosion of Photo-Crosslinked mPA20
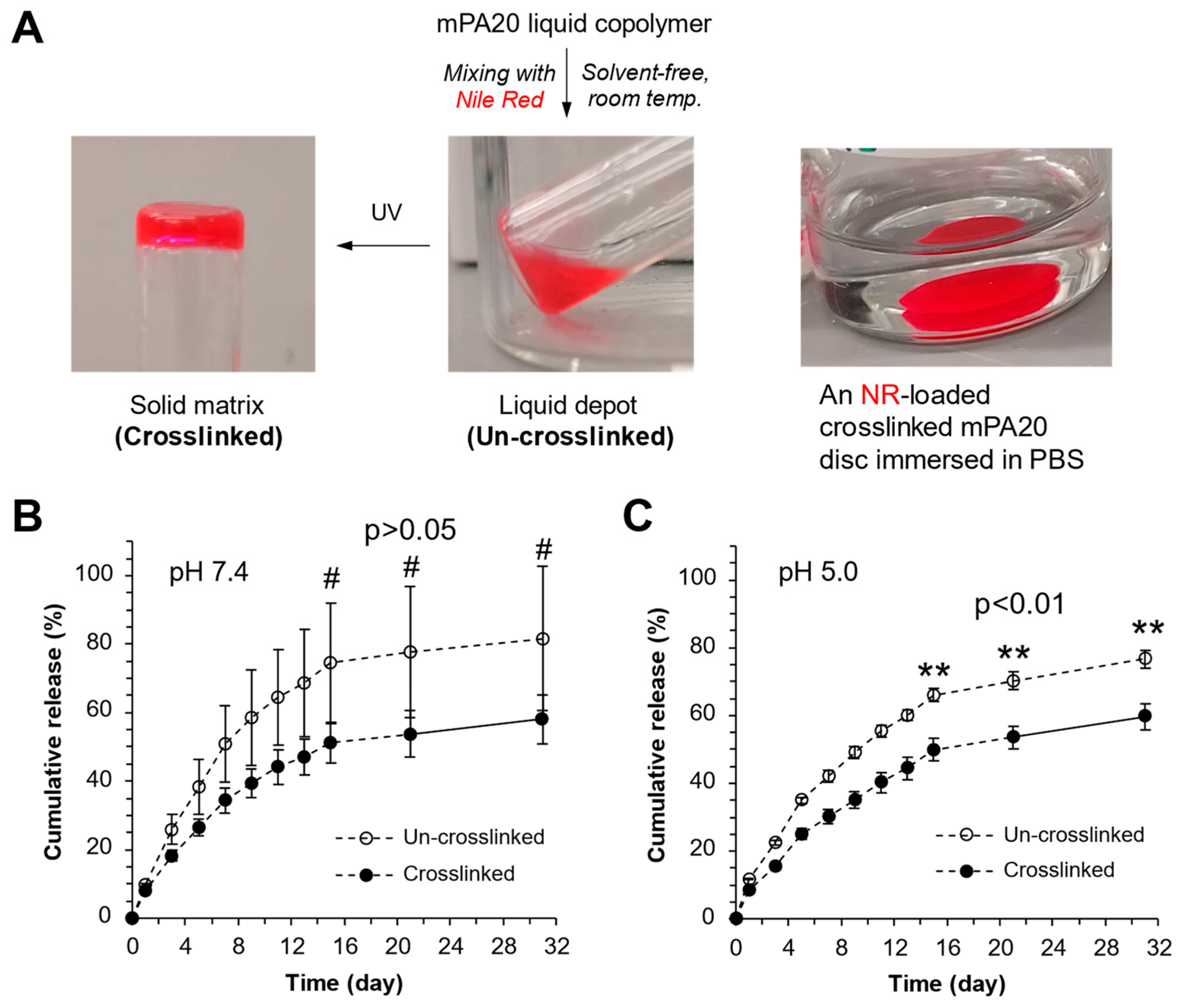
3.7. Loading and Release Kinetics of a Poorly Water-Soluble Compound
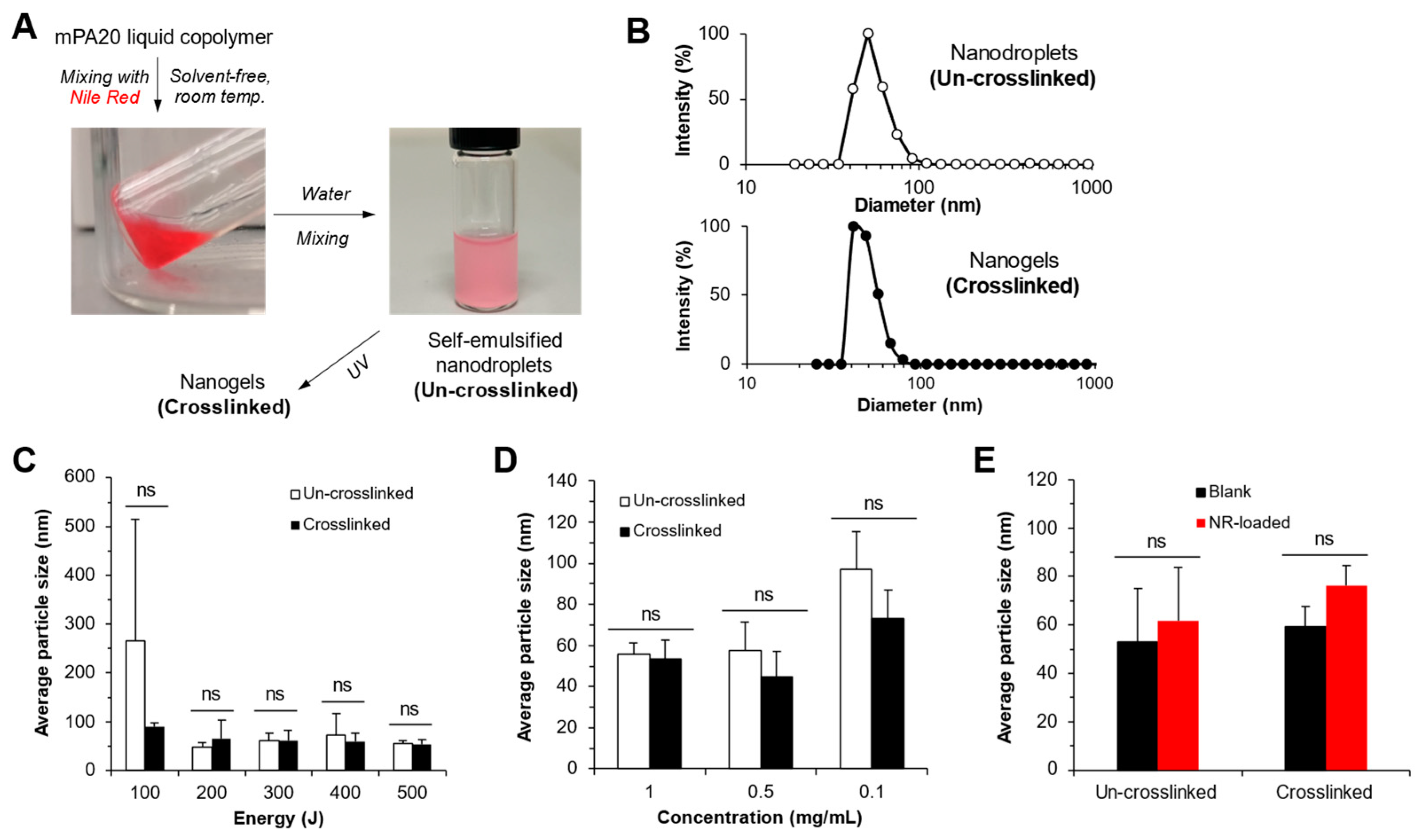
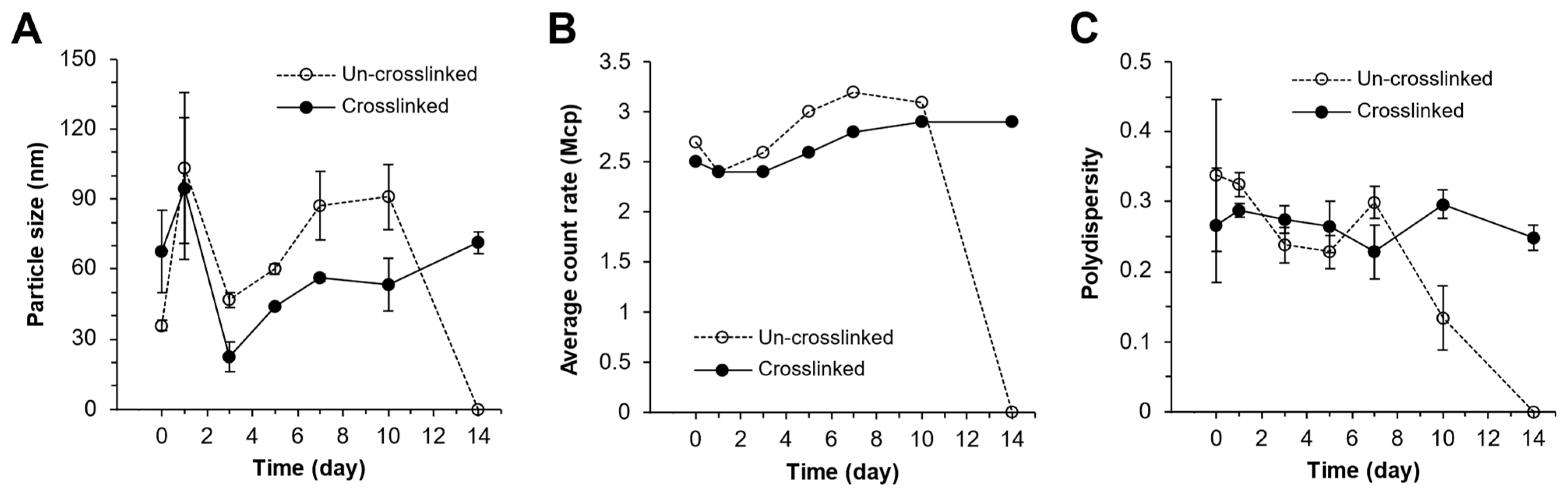
3.8. mPA20 Self-Emulsified in Water to Form Nanodroplets That Were Subsequently Photo-Crosslinked into Nanogels
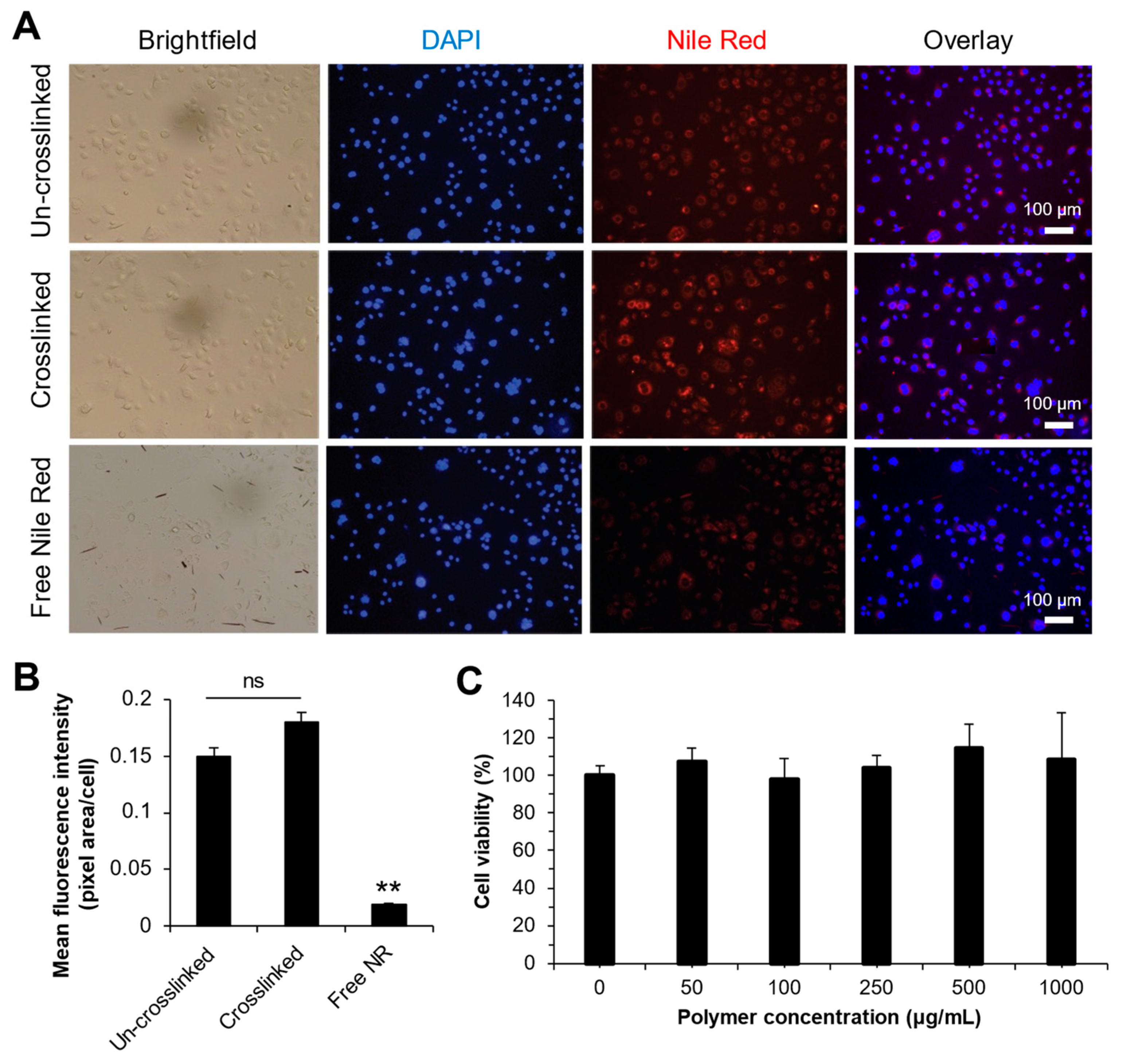
3.9. Intracellular Uptake and Cytotoxicity
4. Discussion
4.1. Polymer Design, One-Pot Synthesis, and Injectability
4.2. Acid-Labile Hydrolytic Degradation
4.3. Photo-Crosslinking, Tunable Mechanics, and Material Erosion
4.4. Solvent-Free Drug Loading and Mechanism of Drug Release
4.5. Dynamics of the mPA20 Nanodroplets and Nanogels in Aqueous Solution
4.6. Intracellular Uptake of Liquid and Solid Nanoparticles
4.7. Implications for Drug Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amsden, B.G. Liquid, Injectable, Hydrophobic and Biodegradable Polymers as Drug Delivery Vehicles. Macromol. Biosci. 2010, 10, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Tran, M.; Wang, C. Semi-solid materials for controlled release drug formulation: Current status and future prospects. Front. Chem. Sci. Eng. 2014, 8, 225–232. [Google Scholar] [CrossRef]
- Einmahl, S.; Capancioni, S.; Schwach-Abdellaoui, K.; Moeller, M.; Behar-Cohen, F.; Gurny, R. Therapeutic applications of viscous and injectable poly(ortho esters). Adv. Drug Deliv. Rev. 2001, 53, 45–73. [Google Scholar] [CrossRef]
- Heller, J.; Barr, J.; Ng, S.Y.; Abdellauoi, K.S.; Gurny, R. Poly(ortho esters): Synthesis, characterization, properties and uses. Adv. Drug Deliv. Rev. 2002, 54, 1015–1039. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Barr, J. Poly(ortho esters)—From Concept to Reality. Biomacromolecules 2004, 5, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Barr, J.; Ng, S.Y.; Shen, H.-R.; Schwach-Abdellaoui, K.; Gurny, R.; Vivien-Castioni, N.; Loup, P.J.; Baehni, P.; Mombelli, A. Development and applications of injectable poly(ortho esters) for pain control and periodontal treatment. Biomaterials 2002, 23, 4397–4404. [Google Scholar] [CrossRef]
- Einmahl, S.; Behar-Cohen, F.; Tabatabay, C.; Savoldelli, M.; D’Hermies, F.; Chauvaud, D.; Heller, J.; Gurny, R. A viscous bioerodible poly(ortho ester) as a new biomaterial for intraocular application. J. Biomed. Mater. Res. 2000, 50, 566–573. [Google Scholar] [CrossRef]
- Deeks, E.D. Granisetron Extended-Release Injection: A Review in Chemotherapy-Induced Nausea and Vomiting. Drugs 2016, 76, 1779–1786. [Google Scholar] [CrossRef]
- Blair, H.A. Bupivacaine/Meloxicam Prolonged Release: A Review in Postoperative Pain. Drugs 2021, 81, 1203–1211. [Google Scholar] [CrossRef]
- Timbart, L.; Tse, M.Y.; Pang, S.C.; Babasola, O.; Amsden, B.G. Low Viscosity Poly(trimethylene carbonate) for Localized Drug Delivery: Rheological Properties and in vivo Degradation. Macromol. Biosci. 2009, 9, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B. In Vivo Degradation Mechanisms of Aliphatic Polycarbonates and Functionalized Aliphatic Polycarbonates. Macromol. Biosci. 2021, 21, 2100085. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–36. [Google Scholar]
- Christen, M.-O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Rocha, J.; Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Wetspun Polymeric Fibrous Systems as Potential Scaffolds for Tendon and Ligament Repair, Healing and Regeneration. Pharmaceutics 2022, 14, 2526. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Polymeric Modification and Its Implication in Drug Delivery: Poly-ε-caprolactone (PCL) as a Model Polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Bentolila, N.Y.; Domb, A.J. Effect of Molecular Weight on Gelling and Viscoelastic Properties of Poly(caprolactone)–b-Poly(ethylene glycol)–b-Poly(caprolactone) (PCL–PEG–PCL) Hydrogels. Polymers 2020, 12, 2372. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef]
- Kamenova, K.; Prancheva, A.; Radeva, L.; Yoncheva, K.; Zaharieva, M.M.; Najdenski, H.M.; Petrov, P.D. Nanosized Complexes of the Proteolytic Enzyme Serratiopeptidase with Cationic Block Copolymer Micelles Enhance the Proliferation and Migration of Human Cells. Pharmaceutics 2024, 16, 988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Z.; Wang, H.; Wu, S.; Zhao, K.; Sun, H.; Kong, D.; Wang, C.; Leng, X.; Zhu, D. Preparation and evaluation of PCL–PEG–PCL polymeric nanoparticles for doxorubicin delivery against breast cancer. RSC Adv. 2016, 6, 54727–54737. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Darge, H.F.; Hanurry, E.Y.; Andrgie, A.T.; Mekonnen, T.W.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Fabrication of Core Crosslinked Polymeric Micelles as Nanocarriers for Doxorubicin Delivery: Self-Assembly, In Situ Diselenide Metathesis and Redox-Responsive Drug Release. Pharmaceutics 2020, 12, 580. [Google Scholar] [CrossRef]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG copolymer based injectable thermosensitive hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef]
- Kamenova, K.; Grancharov, G.; Kortenova, V.; Petrov, P.D. Redox-Responsive Crosslinked Mixed Micelles for Controllable Release of Caffeic Acid Phenethyl Ester. Pharmaceutics 2022, 14, 679. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Manivasagan, P.; Nguyen, T.L.; Phan, T.-H.; Luu, C.H.; Ho, D.-K.; Li, Y.; Kim, J.; Lee, D.S.; et al. Injectable Hydrogel Based on Protein-Polyester Microporous Network as an Implantable Niche for Active Cell Recruitment. Pharmaceutics 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, W.; Tabet, A.; Hanson, S.; Zhang, L.; Zhu, D.; Wang, C. Polymer-Based Dual-Responsive Self-Emulsifying Nanodroplets as Potential Carriers for Poorly Soluble Drugs. ACS Appl. Bio Mater. 2021, 4, 4441–4449. [Google Scholar] [CrossRef]
- Field, J.; Haycock, J.W.; Boissonade, F.M.; Claeyssens, F. A Tuneable, Photocurable, Poly(Caprolactone)-Based Resin for Tissue Engineering—Synthesis, Characterisation and Use in Stereolithography. Molecules 2021, 26, 1199. [Google Scholar] [CrossRef]
- Tomlinson, R.; Heller, J.; Brocchini, S.; Duncan, R. Polyacetal−Doxorubicin Conjugates Designed for pH-Dependent Degradation. Bioconjug. Chem. 2003, 14, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Giménez, V.; James, C.; Armiñán, A.; Schweins, R.; Paul, A.; Vicent, M.J. Demonstrating the importance of polymer-conjugate conformation in solution on its therapeutic output: Diethylstilbestrol (DES)-polyacetals as prostate cancer treatment. J. Control. Release 2012, 159, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Schacht, E.; Toncheva, V.; Vandertaelen, K.; Heller, J. Polyacetal and poly(ortho ester)–poly(ethylene glycol) graft copolymer thermogels: Preparation, hydrolysis and FITC-BSA release studies. J. Control. Release 2006, 116, 219–225. [Google Scholar] [CrossRef]
- Liao, Z.; Zheng, C.; Qiu, L. pH-Sensitive Degradable Poly(ortho ester)-Polyesters As Injectable Viscous Fluid for Long-Acting Rheumatoid Arthritis Treatment. ACS Appl. Polym. Mater. 2024, 6, 5763–5777. [Google Scholar] [CrossRef]
- Jacot, J.G.; Martin, J.C.; Hunt, D.L. Mechanobiology of cardiomyocyte development. J. Biomech. 2010, 43, 93–98. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef]
- Ting, J.M.; Porter, W.W.; Mecca, J.M.; Bates, F.S.; Reineke, T.M. Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery. Bioconjug. Chem. 2018, 29, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Mohr, P.C.; Khot, G.; Rabe, J.P.; Mohr, A. Nile Red Dye in Aqueous Surfactant and Micellar Solution. Langmuir 2015, 31, 2639–2648. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Gadalla, H.H.; Yuan, Z.; Chen, Z.; Alsuwayyid, F.; Das, S.; Mitra, H.; Ardekani, A.M.; Wagner, R.; Yeo, Y. Effects of nanoparticle deformability on multiscale biotransport. Adv. Drug Deliv. Rev. 2024, 213, 115445. [Google Scholar] [CrossRef] [PubMed]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of polymer micelles affects interactions with tumor cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flowers, M.; Mertens, N.; Billups, A.; Ogle, B.M.; Wang, C. A Novel Poly(ε-Caprolactone)-Based Photo-Crosslinkable Liquid Copolymer as a Versatile Drug Delivery Platform. Pharmaceutics 2024, 16, 1380. https://doi.org/10.3390/pharmaceutics16111380
Flowers M, Mertens N, Billups A, Ogle BM, Wang C. A Novel Poly(ε-Caprolactone)-Based Photo-Crosslinkable Liquid Copolymer as a Versatile Drug Delivery Platform. Pharmaceutics. 2024; 16(11):1380. https://doi.org/10.3390/pharmaceutics16111380
Chicago/Turabian StyleFlowers, Marcus, Nicole Mertens, Amanda Billups, Brenda M. Ogle, and Chun Wang. 2024. "A Novel Poly(ε-Caprolactone)-Based Photo-Crosslinkable Liquid Copolymer as a Versatile Drug Delivery Platform" Pharmaceutics 16, no. 11: 1380. https://doi.org/10.3390/pharmaceutics16111380
APA StyleFlowers, M., Mertens, N., Billups, A., Ogle, B. M., & Wang, C. (2024). A Novel Poly(ε-Caprolactone)-Based Photo-Crosslinkable Liquid Copolymer as a Versatile Drug Delivery Platform. Pharmaceutics, 16(11), 1380. https://doi.org/10.3390/pharmaceutics16111380





