Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Standards and Chemicals
2.2. Instrumentation and Analytical Conditions
2.3. Plasma Sample Preparation
2.4. Method Validation
2.5. Statistical Analysis
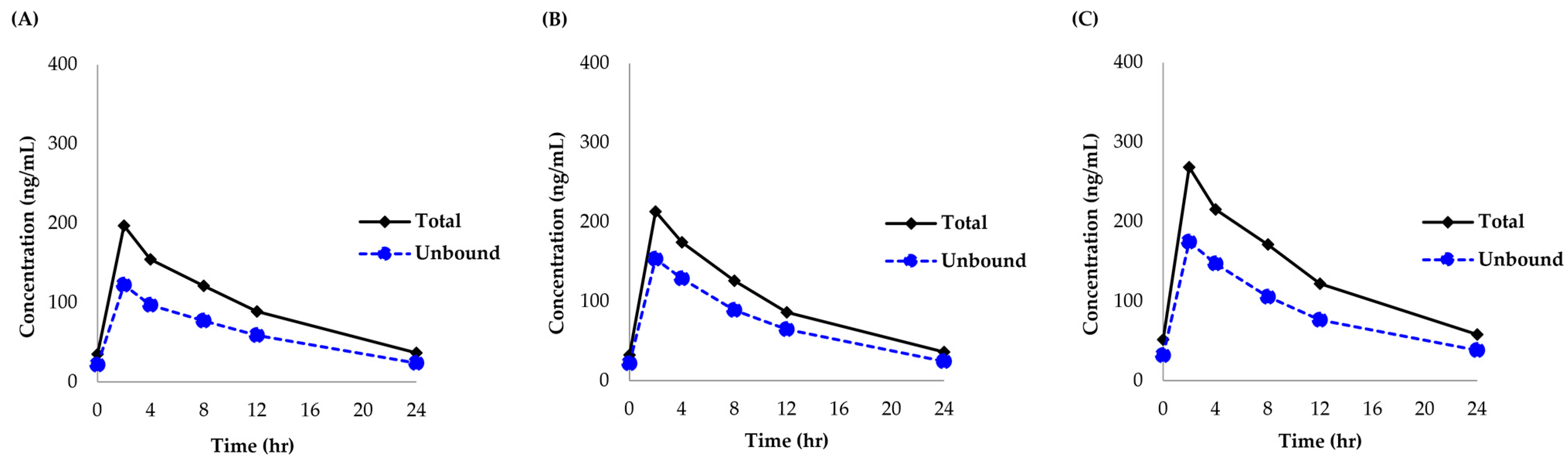
2.6. Application of the Method to an Ongoing Pharmacokinetic Study
3. Results and Discussion
3.1. Optimization of the HPLC–MS/MS Method
3.2. Sample Preparation and IS Selection
3.3. Method Validation
3.3.1. Selectivity and Sensitivity (LLOQ)
3.3.2. Linearity
- Total lenalidomide: y = 0.00557 (±0.00014) x + 0.00121 (±0.000180)
- Unbound lenalidomide: y = 0.00558 (±0.00009) x + 0.00556 (±0.00157)
3.3.3. Precision and Accuracy
3.3.4. Recovery, Matrix Effect, and Carryover
3.3.5. Stability
3.4. Method Applications and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.-W.; Wang, Y.-N.; Ge, X.-L. Lenalidomide use in multiple myeloma (Review). Mol. Clin. Oncol. 2024, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Prescribing Information for REVLIMID® (lenalidomide) Capsules, for Oral Use. 2005. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021880s034lbl.pdf (accessed on 12 July 2024).
- Kumar, S.K.; Callander, N.S.; Hillengass, J.; Liedtke, M.; Baljevic, M.; Campagnaro, E.; Cornell, R.F.; Costello, C.; Domm, J.; Faiman, B.; et al. Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1281–1301. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Davies, F.; Pawlyn, C.; Davies, F.E.; Cairns, D.A.; Kaiser, M.; Cook, G.; Drayson, M.T.; Gregory, W.; Jackson, G.H.; et al. Selinexor, Lenalidomide and Dexamethasone (SRd) for Patients with Relapsed/Refractory and Newly Diagnosed Multiple Myeloma. Blood 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Pourkhosravani, S.; Khodakarim, S.; Nozari, M.; Mahaki, B. A population-based study on incidence trends of myeloma in the United States over 2000–2020. Sci. Rep. 2023, 13, 20705. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.H.; Mace, S.; Francis, K.; Sutton, L.; Tse, S.; McWilliams, M.; Morgan, J.; Wilson, A.; Kwok, J. Population pharmacokinetics of lenalidomide in patients with B-cell malignancies. Br. J. Clin. Pharmacol. 2019, 85, 924–934. [Google Scholar] [CrossRef]
- Liang, X.; Wu, Y.; Chen, X.; Liu, J.; Yang, C.; Liu, Y.; Zhou, G.; Ma, W.; Qiao, R. Population pharmacokinetics of lenalidomide in Chinese patients with influence of genetic polymorphisms of ABCB1. Sci. Rep. 2024, 14, 2577. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, S.; Palmisano, M. Population Pharmacokinetics and Exposure-Safety of Lenalidomide in Patients with Multiple Myeloma, Myelodysplastic Syndromes and Mantle Cell Lymphoma. Blood 2013, 122, 3234. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, S.; Palmisano, M. Clinical Pharmacokinetics and Pharmacodynamics of Lenalidomide. Clin. Pharmacokinet. 2017, 56, 139–152. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Chanan-Khan, A.; Leung, N.; Ludwig, H.; Jagannath, S.; Niesvizky, R.; Palumbo, A.; Sezer, O.; Schots, R.; et al. Renal impairment in patients with multiple myeloma: A consensus statement on behalf of the International Myeloma Working Group. J. Clin. Oncol. 2010, 28, 4976–4984. [Google Scholar] [CrossRef]
- Nolin, T.D. A Synopsis of Clinical Pharmacokinetic Alterations in Advanced CKD. Semin. Dial. 2015, 28, 325–329. [Google Scholar] [CrossRef]
- Dasgupta, A. Clinical utility of free drug monitoring. Clin. Chem. Lab. Med. 2002, 40, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Ali, R.A.; Ebeid, R.S.; El-Walily, A.M. Synthesis of hapten and preparation of specific polyclonal antibody with high affinity for lenalidomide, the potent drug for treatment of multiple myeloma. Chem. Cent. J. 2012, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Tariman, J.D. Lenalidomide: A new agent for patients with relapsed or refractory multiple myeloma. Clin. J. Oncol. Nurs. 2007, 11, 569–574. [Google Scholar] [CrossRef][Green Version]
- Hussein, M.A. Lenalidomide: Patient management strategies. Semin. Hematol. 2005, 42 (Suppl. 4), S22–S25. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, W.; Liu, S.; Li, J.; Zhang, Y.; Yang, L. Detection of lenalidomide metabolites in urine to discover drug-resistant compounds. Clin. Chim. Acta 2024, 553, 117707. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Liu, Z.; Li, X.; Chen, Y.; Ma, J. Simultaneous quantification of thalidomide, lenalidomide and pomadomide in plasma by LC-MS/MS. J. Pharmacol. Toxicol. Methods 2023, 120, 107250. [Google Scholar] [CrossRef]
- Vardhan, G.; Reddy, C.; Kumar, V.; Rao, J.V. Development and validation of a novel chiral chromatographic method for separation of lenalidomide enantiomers in human plasma. Chirality 2023, 35, 83–91. [Google Scholar] [CrossRef]
- Ranganathan, P.; Shetty, N.; Anbazhagan, S. Development and validation of Lenalidomide in human plasma by LC-MS/MS. Saudi J. Biol. Sci. 2019, 26, 1843–1847. [Google Scholar] [CrossRef]
- Gopinath, R.; Rao, B.M.; Raju, V.B.; Kumari, K.D.; Reddy, S.B.; Kumar, P.K. Development and validation of a liquid chromatography-tandem mass spectrometric method for the determination of lenalidomide in human plasma and its application on bioequivalence studies. J. Anal. Sci. Technol. 2019, 10, 33. [Google Scholar] [CrossRef]
- Chen, N.; Lau, H.; Kong, L.; Kumar, G.; Zeldis, J.B.; Knight, R.; Laskin, O.L. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J. Clin. Pharmacol. 2007, 47, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wen, L.; Lau, H.; Surapaneni, S.; Kumar, G. Pharmacokinetics, metabolism, and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother. Pharmacol. 2012, 69, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Kasserra, C.; Reyes, J.; Liu, L.; Lau, H. Single-dose pharmacokinetics of lenalidomide in healthy volunteers: Dose proportionality, food effect, and racial sensitivity. Cancer Chemother. Pharmacol. 2012, 70, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Shida, S.; Takahashi, N.; Miura, M.; Niioka, T.; Matsumoto, M.; Hagihara, M.; Kobayashi, T.; Abumiya, M.; Kameoka, Y.; Fujishima, N.; et al. A limited sampling model to estimate exposure to lenalidomide in multiple myeloma patients. Ther. Drug Monit. 2014, 36, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Nayak, A.K.; Beg, S.; Swain, S.; Awasthi, R.; Singh, B.; Rahman, M.; Choudhury, H.; Panda, S.S.; Paik, P. Development and validation of LC-MS/MS method for the quantitation of lenalidomide in human plasma using Box-Behnken experimental design. Analyst 2013, 138, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Guglieri-López, B.; García-Domenech, R.; Bermejo, M.; González-Álvarez, M.; González-Álvarez, I. A Wide Linearity Range Method for the Determination of Lenalidomide in Plasma by High-Performance Liquid Chromatography: Application to Pharmacokinetic Studies. J. Lab. Autom. 2016, 21, 806–810. [Google Scholar] [CrossRef]
- Shu, C.; Zeng, T.; Gao, S.; Xia, T.; Huang, L.; Zhang, F.; Chen, W. LC-MS/MS method for simultaneous determination of thalidomide, lenalidomide, cyclophosphamide, bortezomib, dexamethasone and adriamycin in serum of multiple myeloma patients. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2016, 1028, 111–119. [Google Scholar] [CrossRef]
- Veeraraghavan, S.; Viswanadha, S.; Thappali, S.; Govindarajulu, B.; Vakkalanka, S.; Rangasamy, M. Simultaneous quantification of lenalidomide, ibrutinib, and its active metabolite PCI-45227 in rat plasma by LC-MS/MS: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 107, 151–158. [Google Scholar] [CrossRef]
- Iqbal, M.; Wani, T.A.; Khalil, N.Y.; Darwish, I.A. Development and validation of ultra-performance liquid chromatographic method with tandem mass spectrometry for determination of lenalidomide in rabbit and human plasma. Chem. Cent. J. 2013, 7, 7. [Google Scholar] [CrossRef]
- Liu, Q.; Farley, K.L.; Johnson, A.J.; Muthusamy, N.; Hofmeister, C.C.; Blum, K.A.; Schaaf, L.J.; Grever, M.R.; Byrd, J.C.; Dalton, J.T.; et al. Development and validation of a highly sensitive liquid chromatography/mass spectrometry method for simultaneous quantification of lenalidomide and flavopiridol in human plasma. Ther. Drug Monit. 2008, 30, 620–627. [Google Scholar] [CrossRef]
- Tohnya, T.M.; Hwang, K.; Lepper, E.R.; Fine, H.A.; Dahut, W.L.; Venitz, J.; Sparreboom, A.; Figg, W.D. Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 811, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, S.; Thappali, S.; Viswanadha, S.; Nalla, S.; Chennupati, S.; Golla, M.; Vakkalanka, S.; Rangasamy, M. Simultaneous quantification of idelalisib, fludarabine, and lenalidomide in rat plasma by using high-performance liquid chromatography coupled with heated electrospray ionization tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 949–950, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Vuignier, K.; Schappler, J.; Veuthey, J.L.; Carrupt, P.A.; Martel, S. Drug-protein binding: A critical review of analytical tools. Anal. Bioanal. Chem. 2010, 398, 53–66. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Bioanalytical Method Validation, Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 23 August 2024).
- Division of Drug Evaluation, Ministry of Food and Drug Safety. Guideline for Bioanalytical Method. Validation and Study Sample Analysis. 2023. Available online: https://www.mfds.go.kr/brd/m_1060/down.do?brd_id=data0011&seq=15366&data_tp=A&file_seq=1 (accessed on 12 July 2024).
- Ministry of Food and Drug Safety. A Prospective Investigator-Initiated Study to Explore the Optimal Dose of Lenalidomide in Combination Therapy for Patients with Multiple Myeloma Featuring Renal Dysfunction. KCT0009561. 2024. Available online: https://cris.nih.go.kr/cris/search/detailSearch.do?seq=27100&search_page=L (accessed on 12 July 2024).
- Szabó, Z.I.; Foroughbakhshfasaei, M.; Gál, R.; Horváth, P.; Komjáti, B.; Noszál, B.; Tóth, G. Chiral separation of lenalidomide by liquid chromatography on polysaccharide-type stationary phases and by capillary electrophoresis using cyclodextrin selectors. J. Sep. Sci. 2018, 41, 1414–1423. [Google Scholar] [CrossRef]
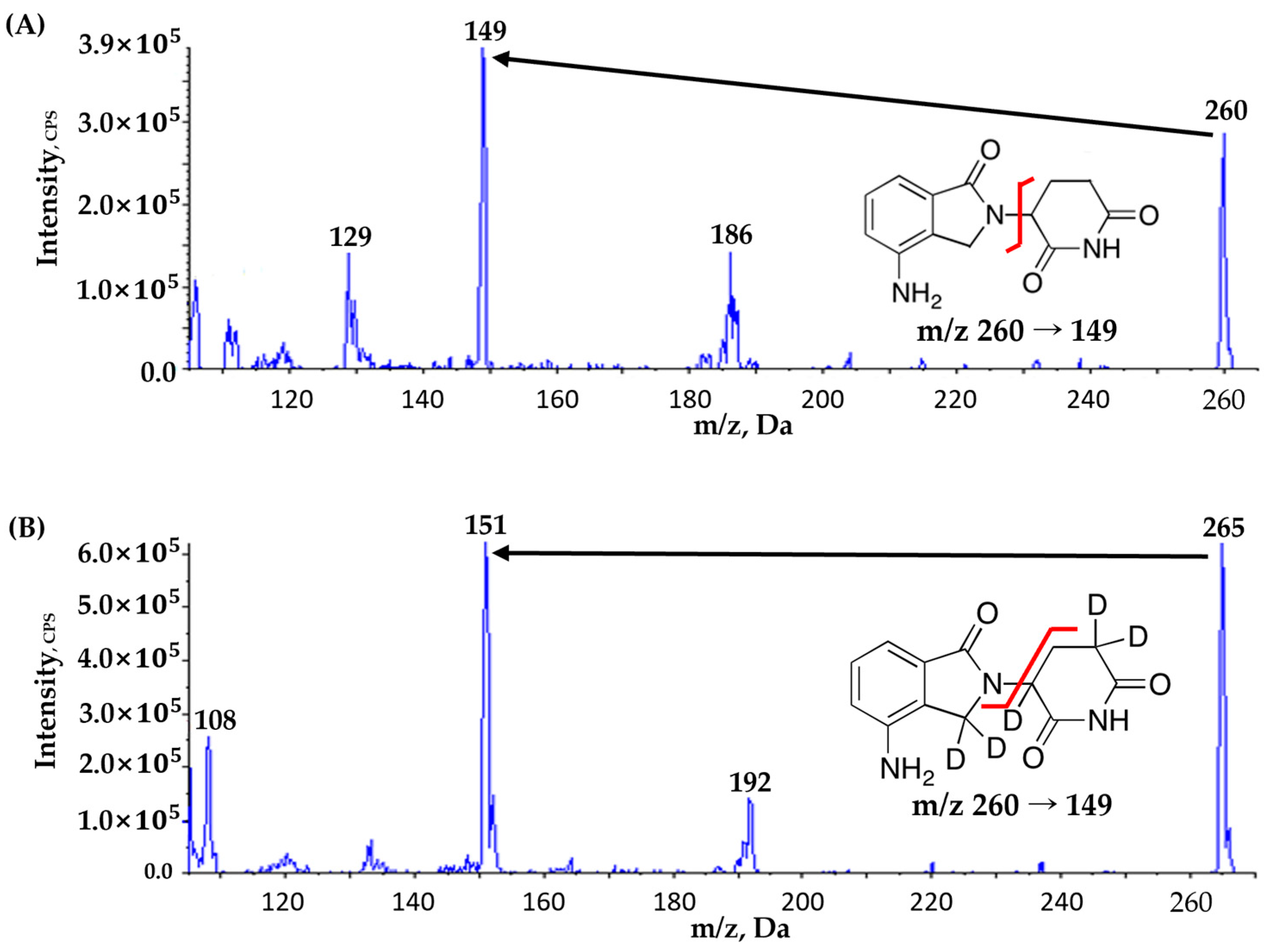

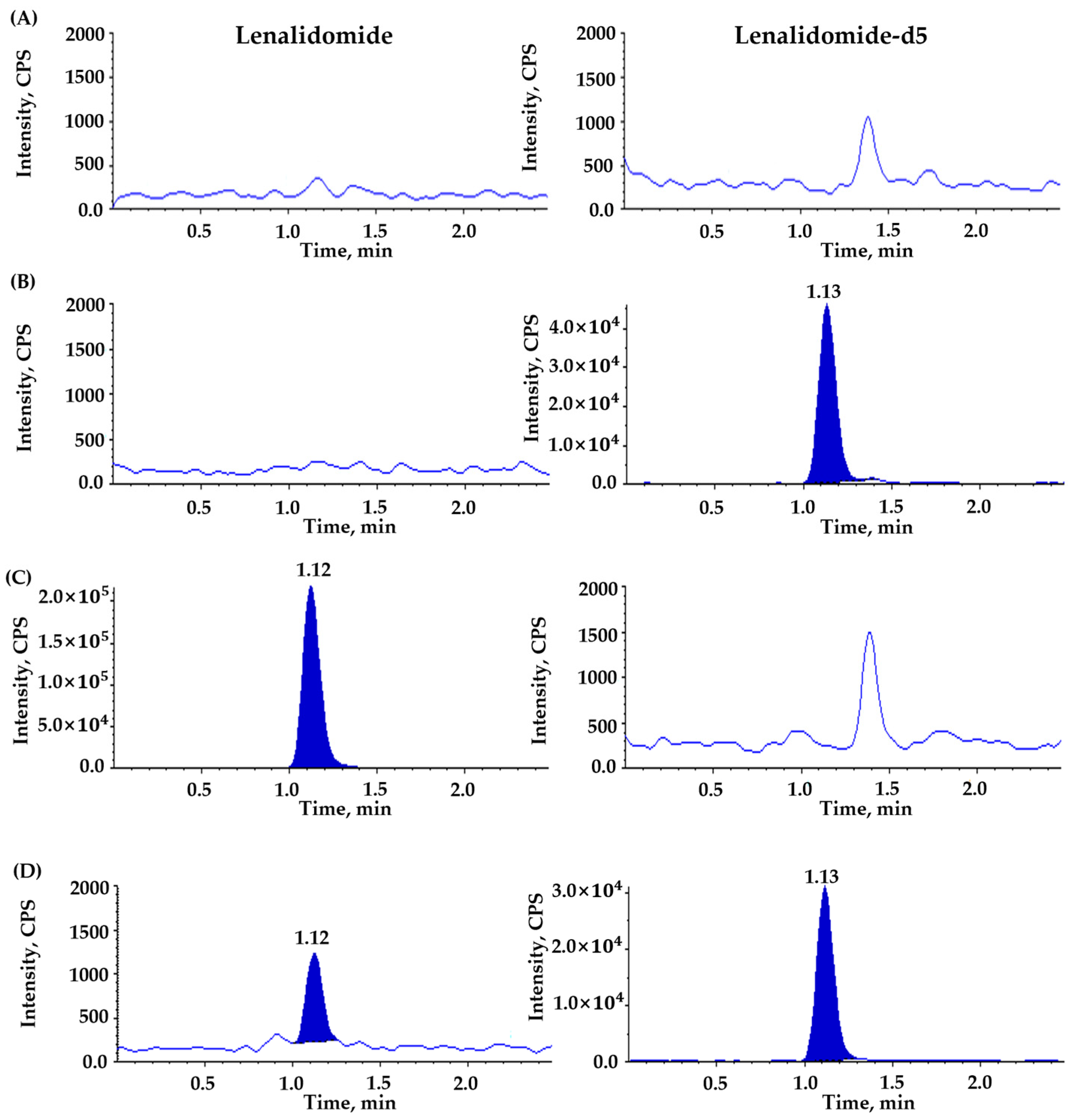
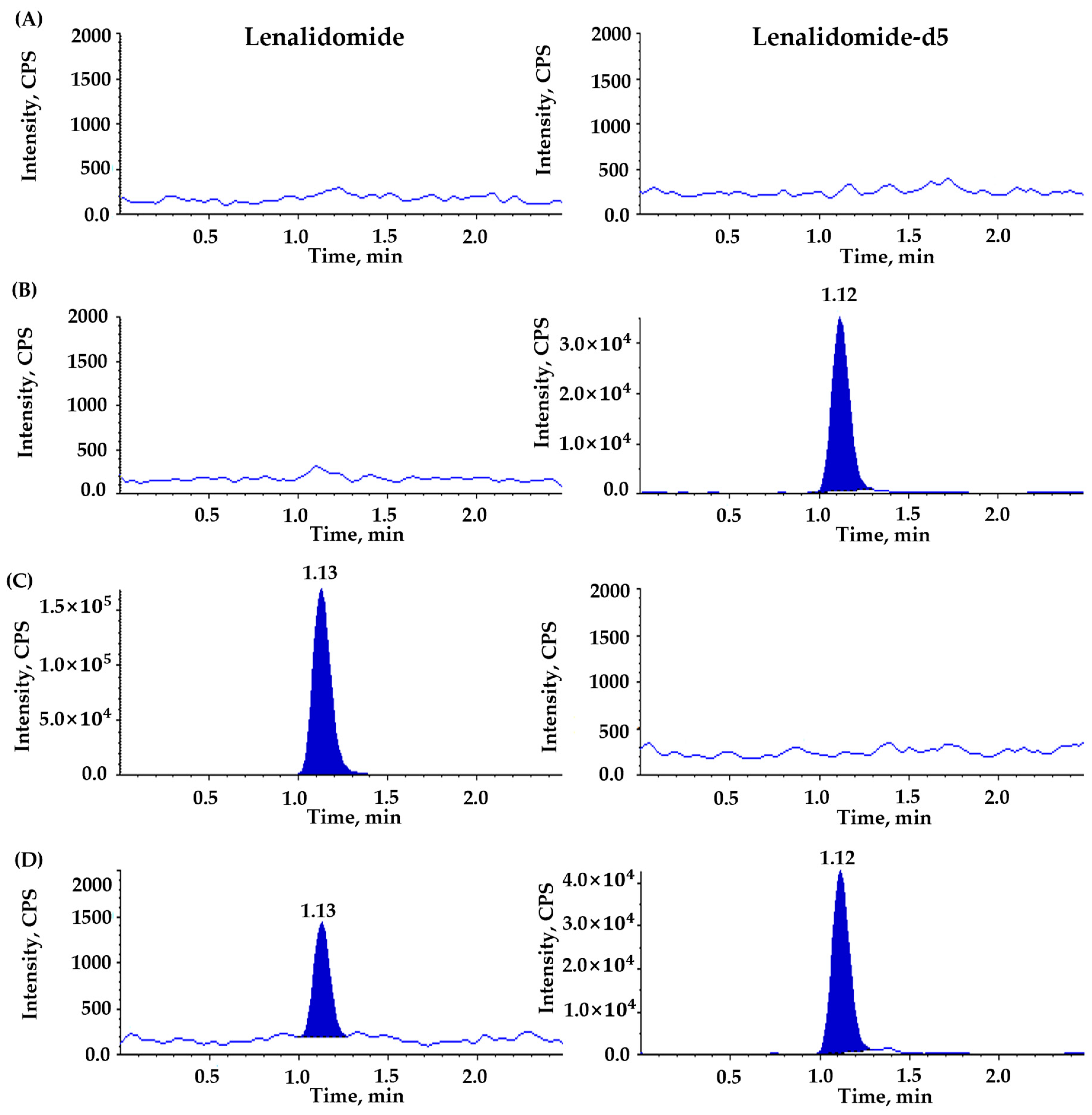
| Compounds | Ion Transition (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) | RT (min) | Source Temperature (°C) | Curtain Gas (psi) | GS1 (L/hr) | GS2 (L/hr) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenalidomide | 260 → 149 | 71.0 | 10.0 | 21.0 | 24.0 | 1.12 | 350 | 20 | 40 | 50 |
| Lenalidomide -d5 | 265 → 151 | 96.0 | 10.0 | 21.0 | 26.0 | 1.13 |
| Nominal Concentration (ng/mL) | Total Lenalidomide | Unbound Lenalidomide | ||||
|---|---|---|---|---|---|---|
| Predicted Concentration (Mean ± SD) (ng/mL) | Precision (CV, %) a | Accuracy (%) b | Predicted Concentration (Mean ± SD) (ng/mL) | Precision (CV, %) a | Accuracy (%) b | |
| Within-run accuracy and precision (n = 5 sample replicates) | ||||||
| 5 | 5.29 ± 0.17 | 3.29 | 105.86 | 4.61 ± 0.19 | 4.06 | 92.14 |
| 15 | 15.20 ± 0.31 | 2.04 | 101.36 | 14.39 ± 0.72 | 5.01 | 95.91 |
| 300 | 293.75 ± 1.89 | 0.65 | 97.92 | 298.26 ± 5.51 | 1.85 | 99.42 |
| 800 | 744.01 + 12.09 | 1.62 | 93.00 | 776.20 ± 5.63 | 0.73 | 97.02 |
| Between-run accuracy and precision (n = 3 runs with 5 replicates/run) | ||||||
| 5 | 5.06 ± 0.39 | 7.65 | 101.10 | 4.92 ± 0.52 | 10.55 | 98.48 |
| 15 | 14.97 ± 0.48 | 3.22 | 99.80 | 14.09 ± 0.82 | 5.80 | 93.95 |
| 300 | 297.42 ± 5.04 | 1.70 | 99.14 | 292.15 ± 6.59 | 2.26 | 97.38 |
| 800 | 755.59 ± 15.45 | 2.05 | 94.45 | 763.95 ± 15.15 | 1.98 | 95.49 |
| Nominal Concentration (ng/mL) | Extraction Recovery (Mean ± SD, %) | Matrix Effect (Mean ± SD, %) | ||
|---|---|---|---|---|
| Total | Unbound Fraction | Total | Unbound Fraction | |
| Lenalidomide | ||||
| 15 | 49.35 ± 1.65 | 45.26 ± 1.18 | 106.82 ± 3.08 | 100.04 ± 1.50 |
| 300 | 55.23 ± 0.86 | 42.83 ± 0.84 | 103.15 ± 0.67 | 98.16 ± 3.39 |
| 800 | 58.43 ± 0.95 | 46.75 ± 0.66 | 99.69 ± 0.89 | 95.81 ± 0.92 |
| Lenalidomide-d5 (internal standard, IS) | ||||
| 1000 | 35.48 ± 1.16 | 27.73 ± 1.06 | 99.15 ± 2.72 | 97.33 ± 1.94 |
| Stability Condition | Nominal Concentration (ng/mL) | |||||
|---|---|---|---|---|---|---|
| 15 | 300 | 800 | ||||
| Total Lenalidomide | Unbound Lenalidomide | Total Lenalidomide | Unbound Lenalidomide | Total Lenalidomide | Unbound Lenalidomide | |
| Freeze–thaw (three cycles) | 97.97 ± 2.56 | 91.98 ± 6.81 | 99.49 ± 0.81 | 97.66 ± 1.29 | 97.08 ± 1.07 | 94.64 ± 0.55 |
| Room temperature (7 h) | 94.00 ± 5.11 | 92.04 ± 6.13 | 99.96 ± 1.51 | 98.45 ± 2.16 | 97.55 ± 1.36 | 96.28 ± 0.52 |
| 4 °C (7 h) | 94.41 ± 0.71 | 96.93 ± 9.15 | 100.36 ± 2.34 | 97.34 ± 2.71 | 96.21 ± 2.61 | 96.40 ± 1.46 |
| −70 °C (7 h) | 96.41 ± 3.41 | 97.33 ± 4.96 | 100.55 ± 1.09 | 99.06 ± 0.95 | 97.14 ± 0.89 | 95.54 ± 2.87 |
| Autosampler, 10 °C (30 h) | 98.34 ± 2.86 | 94.49 ± 7.00 | 101.49 ± 0.68 | 98.53 ± 0.350 | 96.60 ± 1.80 | 93.38 ± 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yang, S.; Shim, W.-S.; Song, E.; Han, S.; Park, S.-S.; Choi, S.; Joo, S.H.; Park, S.J.; Shin, B.; et al. Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma. Pharmaceutics 2024, 16, 1340. https://doi.org/10.3390/pharmaceutics16101340
Lee S, Yang S, Shim W-S, Song E, Han S, Park S-S, Choi S, Joo SH, Park SJ, Shin B, et al. Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma. Pharmaceutics. 2024; 16(10):1340. https://doi.org/10.3390/pharmaceutics16101340
Chicago/Turabian StyleLee, Suhyun, Seungwon Yang, Wang-Seob Shim, Eunseo Song, Seunghoon Han, Sung-Soo Park, Suein Choi, Sung Hwan Joo, Seok Jun Park, Beomjin Shin, and et al. 2024. "Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma" Pharmaceutics 16, no. 10: 1340. https://doi.org/10.3390/pharmaceutics16101340
APA StyleLee, S., Yang, S., Shim, W.-S., Song, E., Han, S., Park, S.-S., Choi, S., Joo, S. H., Park, S. J., Shin, B., Kim, D., Kim, H., Jung, Y., Lee, K.-T., & Chung, E. K. (2024). Development and Validation of an Improved HPLC-MS/MS Method for Quantifying Total and Unbound Lenalidomide in Human Plasma. Pharmaceutics, 16(10), 1340. https://doi.org/10.3390/pharmaceutics16101340






