Pharmacodynamics of Rivaroxaban and Dabigatran in Adults with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Size
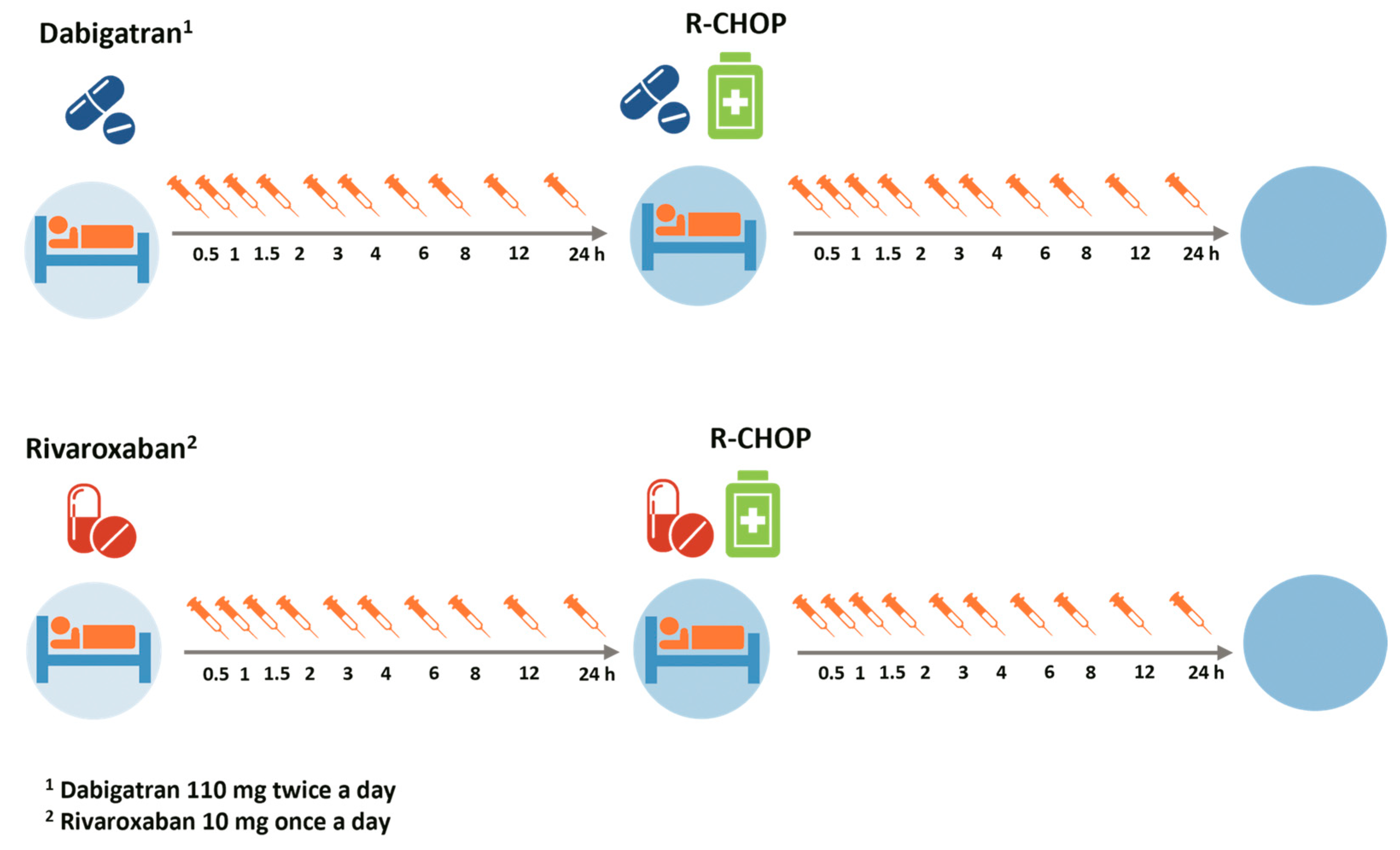
2.3. Study Medication
2.4. Blood Sampling for Pharmacodynamic Assessment
2.5. Measurement of Anti-Xa and Diluted Thrombin Time
2.6. Statistical Analysis
3. Results
Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of Cancer-Associated Venous Thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Monreal, M.; Falgá, C.; Valdés, M.; Suárez, C.; Gabriel, F.; Tolosa, C.; Montes, J. Fatal Pulmonary Embolism and Fatal Bleeding in Cancer Patients with Venous Thromboembolism: Findings from the Riete Registry. J. Thromb. Haemost. 2006, 4, 1950–1956. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., III. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism: A Population-Based Case-Control Study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef]
- Sørensen, H.T.; Mellemkjær, L.; Olsen, J.H.; Baron, J.A. Prognosis of Cancers Associated with Venous Thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and Its Effect on Survival among Patients with Common Cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Horsted, F.; West, J.; Grainge, M.J. Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism Is a Leading Cause of Death in Cancer Patients Receiving Outpatient Chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef]
- Blom, J.W.; Vanderschoot, J.P.; Oostindiër, M.J.; Osanto, S.; van der Meer, F.J.; Rosendaal, F.R. Incidence of Venous Thrombosis in a Large Cohort of 66,329 Cancer Patients: Results of a Record Linkage Study. J. Thromb. Haemost. 2006, 4, 529–535. [Google Scholar] [CrossRef]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous Thromboembolism Risk in Patients with Cancer Receiving Chemotherapy: A Real-World Analysis. Oncologist 2013, 18, 1321–1329. [Google Scholar] [CrossRef]
- Haas, S.; Ageno, W.; Weitz, J.I.; Goldhaber, S.Z.; Turpie, A.G.G.; Goto, S.; Angchaisuksiri, P.; Nielsen, J.D.; Kayani, G.; Zaghdoun, A.; et al. Anticoagulation Therapy Patterns for Acute Treatment of Venous Thromboembolism in GARFIELD–VTE Patients. J. Thromb. Haemost. 2019, 17, 1694–1706. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients with Cancer with Venous Thromboembolism: Results of a Randomized Trial (Select-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Delluc, A.; Wang, T.; Yap, E.; Ay, C.; Schaefer, J.; Carrier, M.; Noble, S. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1247–1252. [Google Scholar] [CrossRef]
- Cuker, A. Laboratory Measurement of the Non-Vitamin K Antagonist Oral Anticoagulants: Selecting the Optimal Assay Based on Drug, Assay Availability, and Clinical Indication. J. Thromb. Haemost. 2016, 41, 241–247. [Google Scholar] [CrossRef]
- Studt, J.; Alberio, L.; Angelillo-Scherrer, A.; Asmis, L.M.; Fontana, P.; Korte, W.; Mendez, A.; Schmid, P.; Stricker, H.; Tsakiris, D.A.; et al. Accuracy and Consistency of Anti-Xa Activity Measurement for Determination of Rivaroxaban Plasma Levels. J. Thromb. Haemost. 2017, 15, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Ageno, W.; Samama, C.; Lessire, S.; Cate, H.T.; Verhamme, P.; Dogné, J.; Mullier, F. Laboratory Testing in Patients Treated with Direct Oral Anticoagulants: A Practical Guide for Clinicians. J. Thromb. Haemost. 2018, 16, 209–219. [Google Scholar] [CrossRef]
- Marlowe, C.K.; Sinha, U.; Gunn, A.C.; Scarborough, R.M. Design, Synthesis and Structure-Activity Relationship of a Series of Arginine Aldehyde Factor Xa Inhibitors. Part 1: Structures Based on the (D)-Arg-Gly-Arg Tripeptide Sequence. Bioorg. Med. Chem. Lett. 2000, 10, 13–16. [Google Scholar] [CrossRef]
- van Ryn, J.; Haertter, S.; Liesenfeld, K.-H.; Wienen, W.; Feuring, M.; Clemens, A.; van Ryn, J. Dabigatran Etexilate—A Novel, Reversible, Oral Direct Thrombin Inhibitor: Interpretation of Coagulation Assays and Reversal of Anticoagulant Activity. Thromb. Haemost. 2010, 103, 1116–1127. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. Pksolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Stangier, J.; Rathgen, K.; Stähle, H.; Gansser, D.; Roth, W. The Pharmacokinetics, Pharmacodynamics and Tolerability of Dabigatran Etexilate, a New Oral Direct Thrombin Inhibitor, in Healthy Male Subjects. Br. J. Clin. Pharmacol. 2007, 64, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Stangier, J.; Stähle, H.; Rathgen, K.; Fuhr, R. Pharmacokinetics and Pharmacodynamics of the Direct Oral Thrombin Inhibitor Dabigatran in Healthy Elderly Subjects. Clin. Pharmacokinet. 2008, 47, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, K.H.; Lehr, T.; Dansirikul, C.; Reilly, P.A.; Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Wallentin, L.; Haertter, S.; Staab, A. Population Pharmacokinetic Analysis of the Oral Thrombin Inhibitor Dabigatran Etexilate in Patients with Non-Valvular Atrial Fibrillation from the Re-Ly Trial. J. Thromb. Haemost. 2011, 9, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Toorop, M.M.A.; van Rein, N.; Nierman, M.C.; Vermaas, H.W.; Huisman, M.V.; van der Meer, F.J.M.; Cannegieter, S.C.; Lijfering, W.M. Inter- and Intra-Individual Concentrations of Direct Oral Anticoagulants: The Kidoac Study. J. Thromb. Haemost. 2022, 20, 92–103. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: Asco Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Wang, T.; Zwicker, J.I.; Ay, C.; Pabinger, I.; Falanga, A.; Antic, D.; Noble, S.; Khorana, A.A.; Carrier, M.; Meyer, G. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2019, 17, 1772–1778. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
| Baseline Characteristics | Total (n = 26) | Group of DOAC Administration (n = 26) | |
|---|---|---|---|
| Rivaroxaban 10 mg (n = 12) | Dabigatran 110 mg (n = 14) | ||
| Sex, n (%) | |||
| Male | 14 (53.9) | 6 (50.00) | 8 (57.14) |
| Age—mean ± SD | 59.4 ± 14.4 | 61.8 ± 14.9 | 57.3 ± 14.1 |
| Weight (kg)—mean ± SD | 52.9 ± 8.8 | 49.1 ± 7.1 | 56.0 ± 9.0 |
| Height (cm)—mean ± SD | 157.5 ± 6.6 | 155.5 ± 7.2 | 159.1 ± 5.8 |
| BMI (kg/m2)—mean ± SD | 21.30 ± 3.1 | 20.3 ± 2.6 | 22.1 ± 3.4 |
| BSA—mean ± SD | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 |
| Ann Arbor stage, n (%) | |||
| I | 2 (7.7) | 0 (0) | 2 (14.3) |
| II | 8 (30.8) | 3 (25.0) | 5 (35.7) |
| III | 4 (15.4) | 2 (16.7) | 2 (14.3) |
| IV | 12 (46.1) | 7 (58.3) | 5 (35.7) |
| Khorana score, n (%) | |||
| ≤2 | 23 (88.5) | 10 (83.3) | 13 (92.9) |
| >2 | 3 (11.5) | 2 (16.7) | 1 (7.1) |
| Medical history, n (%) | |||
| Hypertension | 5 (19) | 3 (25) | 2 (14.3) |
| Diabetes mellitus | 1 (4) | 0 (0) | 1 (7.1) |
| Dyslipidemia | 1 (4) | 0 (0) | 1 (7.1) |
| Concomitant medications, n (%) | |||
| Calcium channel blockers | 3 (11.5) | 1 (8.3) | 2 (14.3) |
| ACE inhibitors | 1(3.8) | 1 (8.3) | 0 (0.0) |
| Diuretic | 1(3.8) | 1 (8.3) | 0 (0.0) |
| Statin | 1(3.8) | 0 (0) | 1 (7.1) |
| Metformin | 1 (3.8) | 0 (0.0) | 1 (7.1) |
| Sulfonylureas | 1 (3.8) | 0 (0.0) | 1 (7.1) |
| Laboratory—mean ± SD | |||
| Hemoglobin (g/dL) | 10.9 ± 1.5 | 10.5 ± 1.7 | 11.2 ± 1.4 |
| WBC (109/L) | 7192.3 ± 4954.2 | 7515.0 ± 4496.0 | 6915.7 ± 5469.6 |
| Platelet (109/L) | 338.5 ± 151.9 | 372.7 ± 201.0 | 309.1 ± 90.4 |
| Creatinine clearance (ml/min) | 68.9 ± 21.8 | 61.1 ± 22.3 | 75.7 ± 19.7 |
| LDH (U/L) * | 240.4 ± 69.5 | 241.8 ± 64.4 | 239.1 ± 76.5 |
| Uric (mg/dL) | 5.6 ± 1.2 | 5.5 ± 1.4 | 5.7 ± 1.1 |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.2 ± 0.2 |
| Aspartate aminotransferase (U/L) | 26.1 ± 10.3 | 29.5 ± 13.0 | 23.2 ± 6.7 |
| Alanine aminotransferase (U/L) | 26.0 ± 18.1 | 32.5 ± 24.5 | 20.5 ± 6.9 |
| Alkaline phosphatase (U/L) | 115.1 ± 116.2 | 90.8 ± 35.9 | 136.0 ± 154.5 |
| Total bilirubin (mg/dL) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.2 |
| Direct bilirubin (mg/dL) | 0.7 ± 2.7 | 0.2 ± 0.1 | 1.1 ± 3.7 |
| Parameter | DOAC Alone | DOAC with R-CHOP | p Value | ||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | ||
| Rivaroxaban | |||||
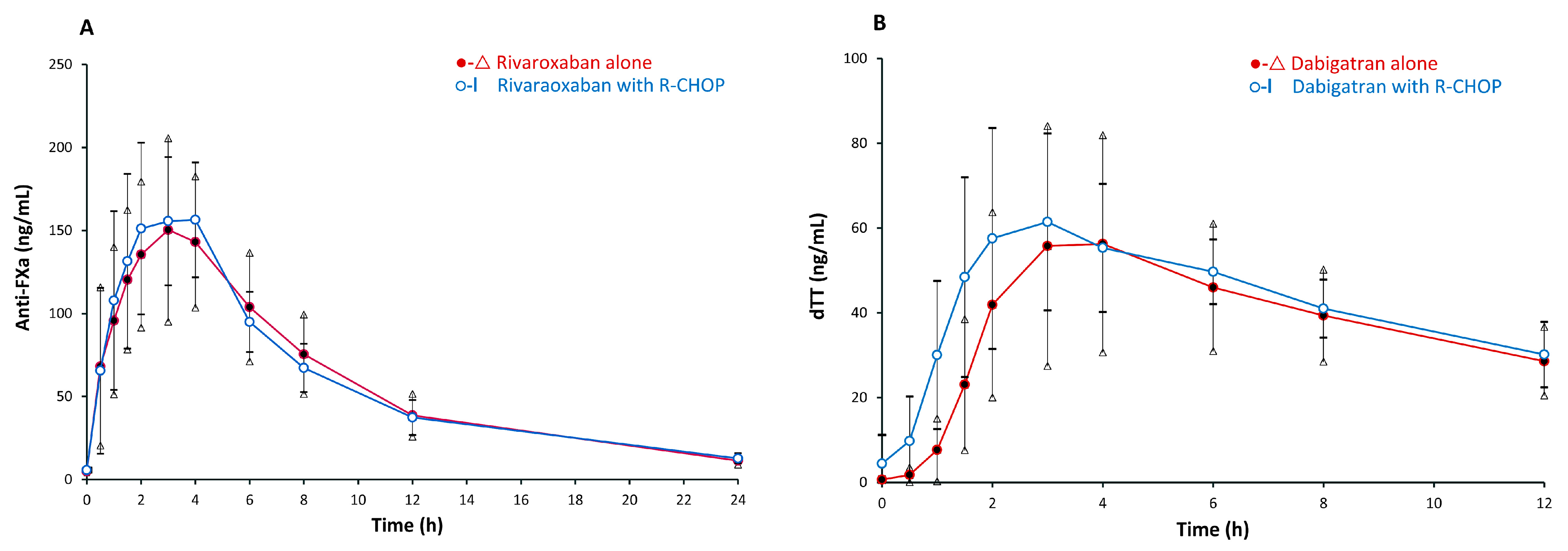
| Arithmetic AUEC0–24 (ng·h/mL) | 1422.0 ± 457.1 | 32 | 1425.9 ± 375.1 | 26 | 0.96 |
| Arithmetic AUEC0–∞ (ng·h/mL) | 1565.0 ± 485.2 | 31 | 1576.6± 415.4 | 26 | 0.88 |
| Geometric AUEC0–24 (ng·h/mL) | 1354.0 | 33 | 1384.2 | 25 | 0.85 |
| Geometric AUEC₀–∞(ng·h/mL) | 1464.7 | 36 | 1520.9 | 27 | 0.67 |
| Maximum inhibition of FXa (ng/mL) | 187.0 ± 75.7 | 40 | 196.5 ± 61.5 | 31 | 0.55 |
| Emax (ng/mL) | 138.87 ± 75.7 | 42 | 165.79 ± 91.87 | 33 | 0.55 |
| Time to maximum inhibition of FXa (h) * | 2.5 (0.5–6) | 60 | 1.75 (1.0–4.0) | 50 | 0.78 |
| Dabigatran etixalate | |||||
| Arithmetic AUEC0–12 (ng·h/mL) | 449.2 ± 275.3 | 61 | 503.6 ± 187.2 | 37 | 0.46 |
| Arithmetic AUEC₀–∞(ng·h/mL) | 791.7 ± 603.6 | 76 | 1703.3 ± 2250.1 | 132 | 0.16 |
| Geometric AUEC0–12 (ng·h/mL) | 350.6 | 80 | 476.2 | 42 | 0.18 |
| Geometric AUEC₀–∞(ng·h/mL) | 552.27 | 109 | 1042.71 | 216 | 0.16 |
| Maximum dTT (ng/mL) | 75.8 ± 34.1 | 45 | 76.2 ± 30.9 | 41 | 0.95 |
| Emax (ng/mL) | 67 ± 42.8 | 42 | 74 ± 35 | 32 | 0.56 |
| Time to maximum dTT (h) * | 4.0 (3.0–8.0) | 64 | 3.0 (1.5–8) | 47 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punnachet, T.; Cressey, T.R.; Apiwatnakorn, P.; Koonarat, A.; Norasetthada, L.; Tantiworawit, A.; Rattarittamrong, E.; Rattanathammethee, T.; Hantrakool, S.; Piriyakhuntorn, P.; et al. Pharmacodynamics of Rivaroxaban and Dabigatran in Adults with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy. Pharmaceutics 2024, 16, 1319. https://doi.org/10.3390/pharmaceutics16101319
Punnachet T, Cressey TR, Apiwatnakorn P, Koonarat A, Norasetthada L, Tantiworawit A, Rattarittamrong E, Rattanathammethee T, Hantrakool S, Piriyakhuntorn P, et al. Pharmacodynamics of Rivaroxaban and Dabigatran in Adults with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy. Pharmaceutics. 2024; 16(10):1319. https://doi.org/10.3390/pharmaceutics16101319
Chicago/Turabian StylePunnachet, Teerachat, Tim R. Cressey, Porntipa Apiwatnakorn, Atisa Koonarat, Lalita Norasetthada, Adisak Tantiworawit, Ekarat Rattarittamrong, Thanawat Rattanathammethee, Sasinee Hantrakool, Pokpong Piriyakhuntorn, and et al. 2024. "Pharmacodynamics of Rivaroxaban and Dabigatran in Adults with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy" Pharmaceutics 16, no. 10: 1319. https://doi.org/10.3390/pharmaceutics16101319
APA StylePunnachet, T., Cressey, T. R., Apiwatnakorn, P., Koonarat, A., Norasetthada, L., Tantiworawit, A., Rattarittamrong, E., Rattanathammethee, T., Hantrakool, S., Piriyakhuntorn, P., Hantrakun, N., Niprapan, P., & Chai-Adisaksopha, C. (2024). Pharmacodynamics of Rivaroxaban and Dabigatran in Adults with Diffuse Large B-Cell Lymphoma Receiving R-CHOP Immunochemotherapy. Pharmaceutics, 16(10), 1319. https://doi.org/10.3390/pharmaceutics16101319








