Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors
Abstract
1. Introduction
2. Mucosal Vaccines: Prospects and Obstacles
3. Distinctive Elements of Mucosal Immune Response: An Introduction to Nasal Vaccines
3.1. Immunological Significance of the Nasal Mucosa
3.2. The Immune System Specific to the Nasal Cavity’s Mucosal Region
3.3. Key Aspects of Immune Response Mechanisms in Vaccinations
3.4. APCs in Human Nasal Mucosal Tissues
3.4.1. Macrophages
3.4.2. Dendritic Cells
4. Molecular Bridge of Immunity: Unveiling Common CLRs in Macrophages and Dendritic Cells
- Toll-like receptors (TLRs)
- Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs)
- Absent in melanoma 2 (AIM2)-like receptors (ALRs)
- Cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway
- Nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs)
- Scavenger receptors (SRs)
- Complement receptors (CRs)
- C-type lectin-like receptors (CLRs)
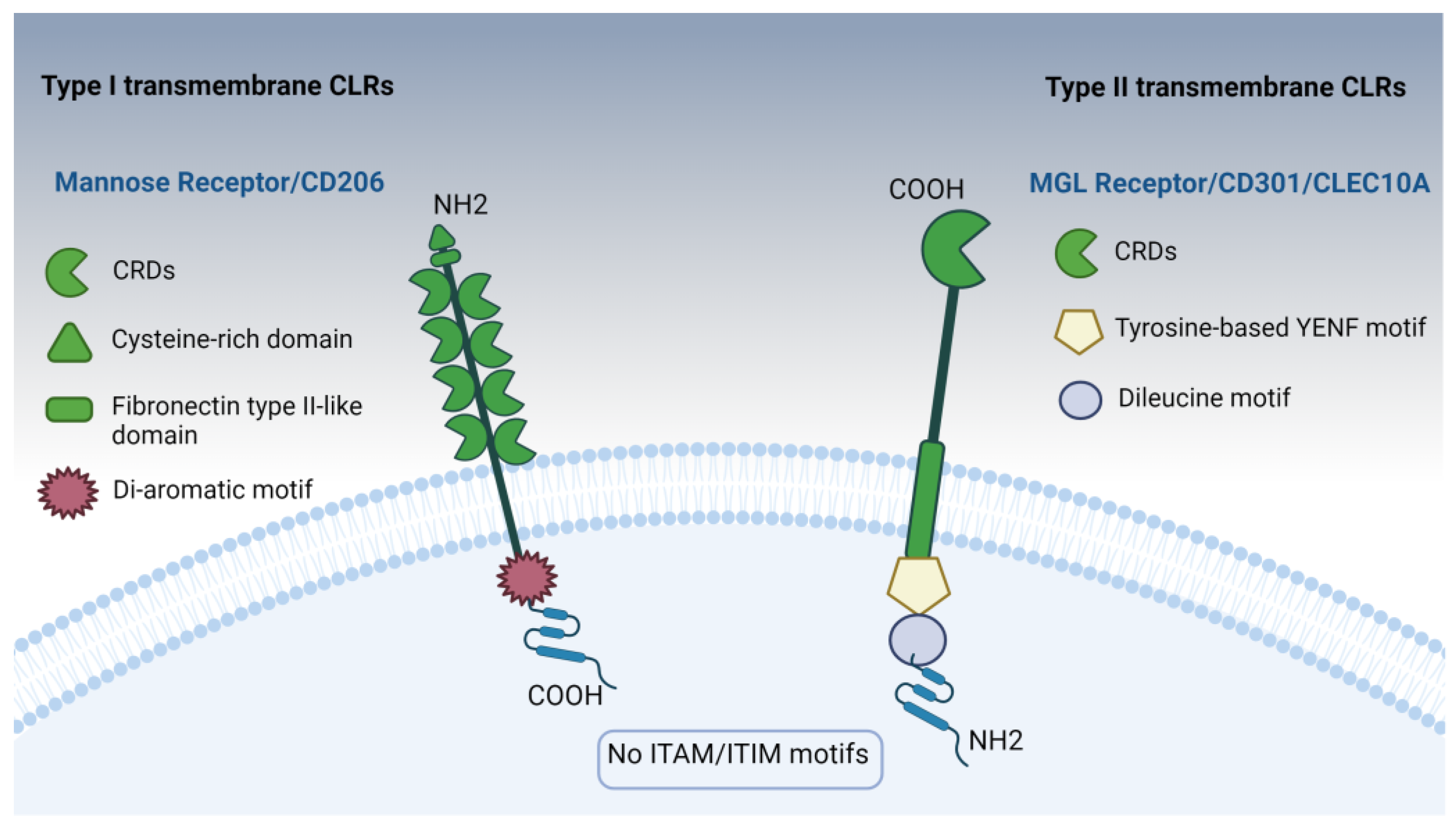
4.1. Mannose Receptors: Insights into Type I Transmembrane CLR Receptors
4.1.1. Ligand Recognition Mechanisms of the MR
4.1.2. Mannose Receptor Signaling and the Role of Immunopotentiators in Vaccines: A Key to Enhanced Immune Responses
4.2. Macrophage Galactose-Type Lectin (MGL) Receptor (CD301/CLEC10A): Insights into Type II Transmembrane CLR Receptors
4.2.1. Understanding MGL Signaling Networks: Shaping the Immune Reaction
4.2.2. Unlocking MGL’s Potential: Lactobionic Acid as a Promising Ligand for Vaccination
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 5 August 2023).
- Nahar, U.J.; Toth, I.; Skwarczynski, M. Mannose in vaccine delivery. J. Control Release 2022, 351, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.T.; Skwarczynski, M.; Toth, I. Towards the development of subunit vaccines against tuberculosis: The key role of adjuvant. Tuberculosis 2023, 139, 102307. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.A.; Paul, S.; Dellosa, G.K.; Jaraquemada, D.; Bello, M.B. Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. npj Vaccines 2022, 7, 71. [Google Scholar] [CrossRef]
- Miquel-Clopés, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef]
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Martínez, N.; Guillen, D.; Moreno-Mendieta, S.A.; Sanchez, S.; Rodríguez-Sanoja, R. The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants. Polymers 2023, 15, 1615. [Google Scholar] [CrossRef]
- Shamsuzzaman, S.; Ahmed, T.; Mannoor, K.; Begum, Y.A.; Bardhan, P.K.; Sack, R.B.; Sack, D.A.; Svennerholm, A.; Holmgren, J.; Qadri, F. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: Comparison with other mucosal and systemic responses. Vaccine 2009, 27, 1386–1392. [Google Scholar] [CrossRef]
- Song, K.R.; Lim, J.K.; Park, S.E.; Saluja, T.; Cho, S.; Wartel, T.A.; Lynch, J. Oral Cholera Vaccine Efficacy and Effectiveness. Vaccines 2021, 9, 1482. [Google Scholar] [CrossRef]
- Alam, M.M.; Riyadh, M.A.; Fatema, K.; Rahman, M.A.; Akhtar, N.; Ahmed, T.; Chowdhury, M.I.; Chowdhury, F.; Calderwood, S.B.; Harris, J.B.; et al. Antigen-Specific Memory B-Cell Responses in Bangladeshi Adults after One- or Two-Dose Oral Killed Cholera Vaccination and Comparison with Responses in Patients with Naturally Acquired Cholera. Clin. Vaccine Immunol. 2011, 18, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Alexander, T.S. Critical Analysis of Compositions and Protective Efficacies of Oral Killed Cholera Vaccines. Clin. Vaccine Immunol. 2014, 21, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Bhuiyan, T.R.; Sack, D.A.; Svennerholm, A. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 2013, 31, 452–460. [Google Scholar] [CrossRef]
- Odevall, L.; Hong, D.; Digilio, L.; Sahastrabuddhe, S.; Mogasale, V.; Baik, Y.; Choi, S.; Kim, J.H.; Lynchb, J. The Euvichol story—Development and licensure of a safe, effective and affordable oral cholera vaccine through global public private partnerships. Vaccine 2018, 36, 6606–6614. [Google Scholar] [CrossRef]
- Baik, Y.O.; Choi, S.K.; Olveda, R.M.; Espos, R.A.; Ligsay, A.D.; Montellano, M.B.; Yeam, J.S.; Yang, J.S.; Park, J.Y.; Kim, D.R. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine 2015, 33, 6360–6365. [Google Scholar] [CrossRef]
- Saluja, T.; Mogasale, V.V.; Excler, J.; Kim, J.H.; Mogasale, V. An overview of VaxchoraTM, a live attenuated oral cholera vaccine. Hum. Vaccines Immunother. 2019, 16, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Mosley, J.F., 2nd; Smith, L.L.; Brantley, P.; Locke, D.; Como, M. Vaxchora: The First FDA-Approved Cholera Vaccination in the United States. Pharm. Ther. 2017, 42, 638–640. [Google Scholar]
- Lee, B.; Kader, M.A.; Colgate, E.R.; Carmolli, M.; Dickson, D.M.; Diehl, S.A.; Alam, M.; Afreen, S.; Mychaleckyj, J.C.; Nayak, U.; et al. Oral rotavirus vaccine shedding as a marker of mucosal immunity. Sci. Rep. 2021, 11, 21760. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Sashina, T.A.; Fomina, S.G.; Novikova, N.A. Comparative characteristics of the VP7 and VP4 antigenic epitopes of the rotaviruses circulating in Russia (Nizhny Novgorod) and the Rotarix and RotaTeq vaccines. Arch. Virol. 2015, 160, 1693–1703. [Google Scholar] [CrossRef]
- Dennehy, P.H. Rotavirus Vaccines: An Overview. Clin. Microbiol. Rev. 2008, 21, 198–208. [Google Scholar] [CrossRef]
- Nair, N.; Feng, N.; Blum, L.K.; Sanyal, M.; Ding, S.; Jiang, B.; Sen, A.; Morton, J.M.; He, X.; Robinson, W.H.; et al. VP4- and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci. Transl. Med. 2017, 9, eaam5434. [Google Scholar] [CrossRef] [PubMed]
- Skansberg, A.; Sauer, M.; Tan, M.; Santosham, M.; Jennings, M.C. Product review of the rotavirus vaccines ROTASIIL, ROTAVAC, and Rotavin-M1. Hum. Vaccines Immunother. 2020, 17, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.; Gardner, R.C.; Debrus, S.; Benninghoff, B.; Pereira, P. Established and new rotavirus vaccines: A comprehensive review for healthcare professionals. Hum. Vaccines Immunother. 2021, 18, 1870395. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Chatterjee, P.; Bavdekar, A.; Murhekar, M.; Babji, S.; Garg, R.; Samanta, S.; Nandy, R.K.; Kawade, A.; Boopathi, K.; et al. Safety and immunogenicity of the Rotavac and Rotasiil rotavirus vaccines administered in an interchangeable dosing schedule among healthy Indian infants: A multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial. Lancet Infect. Dis. 2022, 22, 1191–1199. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Desai, S.; Tewari, T.; Kawade, A.; Goyal, N.; Garg, B.S.; Kumar, D.; Kanungo, S.; Kamat, V.; Kang, G.; et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine 2017, 35, 6228–6237. [Google Scholar] [CrossRef]
- Asturias, E.J.; Bandyopadhyay, A.S.; Self, S.; Rivera, L.; Saez-Llorens, X.; Lopez, E.; Melgar, M.; Gaensbauer, J.T.; Weldon, W.C.; Oberste, M.S.; et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: An open-label randomised controlled trial. Lancet 2016, 388, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yang, Y.; Huang, L.; Wang, L.; Jiang, Z.; Gong, J.; Wang, W.; Wang, H.; Guo, S.; Li, C.; et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: A randomized controlled non-inferiority clinical trial. Hum. Vaccines Immunother. 2017, 13, 1359–1368. [Google Scholar] [CrossRef][Green Version]
- Guzman, C.A.; Borsutzky, S.; Griot-Wenk, M.; Metcalfe, I.C.; Pearman, J.; Collioud, A.; Favre, D.; Dietrich, G. Vaccines against typhoid fever. Vaccine 2006, 24, 3804–3811. [Google Scholar] [CrossRef]
- Bhuiyan, T.R.; Choudhury, F.K.; Khanam, F.; Saha, A.; Sayeed, M.A.; Salma, U.; Lundgren, A.; Sack, D.A.; Svennerholm, A.; Qadri, F. Evaluation of immune responses to an oral typhoid vaccine, Ty21a, in children from 2 to 5 years of age in Bangladesh. Vaccine 2014, 32, 1055–1060. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- He, X.-S.; Holmes, T.H.; Zhang, C.; Mahmood, K.; Kemble, G.W.; Lewis, D.B.; Dekker, C.L.; Greenberg, H.B.; Arvin, A.M. Cellular Immune Responses in Children and Adults Receiving Inactivated or Live Attenuated Influenza Vaccines. J. Virol. 2006, 80, 11756–11766. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Curran, M.P. Live Attenuated Influenza Vaccine (FluMist®; Fluenz™). Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Raut, S.K.; Dhere, R.M. A post-marketing surveillance study of a human live-virus pandemic influenza A (H1N1) vaccine (Nasovac®) in India. Hum. Vaccines Immunother. 2014, 9, 122–124. [Google Scholar] [CrossRef]
- Dhere, R.; Yeolekar, L.; Kulkarni, P.; Menon, R.; Vaidya, V.; Ganguly, M.; Tyagi, P.; Barde, P.; Jadhav, S. A pandemic influenza vaccine in India: From strain to sale within 12 months. Vaccine 2011, 29, A16–A21. [Google Scholar] [CrossRef]
- Lindsey, B.B.; Jagne, Y.J.; Armitage, E.P.; Singanayagam, A.; Sallah, H.J.; Drammeh, S.; Senghore, E.; Mohammed, N.I.; Jeffries, D.; Höschler, K.; et al. Effect of a Russian-backbone live-attenuated influenza vaccine with an updated pandemic H1N1 strain on shedding and immunogenicity among children in The Gambia: An open-label, observational, phase 4 study. Lancet Respir. Med. 2019, 7, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin®). npj Vaccines 2023, 8, 125. [Google Scholar] [CrossRef]
- Wang, F.; Huang, B.; Deng, Y.; Zhang, S.; Liu, X.; Wang, L.; Liu, Q.; Zhao, L.; Tang, L.; Wang, W.; et al. Neutralizing antibody levels associated with injectable and aerosolized Ad5-nCoV boosters and BA.2 infection. BMC Med. 2023, 21, 233. [Google Scholar] [CrossRef]
- Pilapitiya, D.; Wheatley, A.K.; Tan, H.-X. Mucosal vaccines for SARS-CoV-2: Triumph of hope over experience. eBioMedicine 2023, 92, 104585. [Google Scholar] [CrossRef]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics 2020, 12, 965. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Peng, M.; Zhu, S.; Wu, Y.; Ji, R.; Shen, C. Screening of Efficient Adjuvants for the RBD-Based Subunit Vaccine of SARS-CoV-2. Vaccines 2023, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Foged, C. Nanoparticles for mucosal vaccine delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 603–646. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 279–301. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Jha, N.K.; Gupta, P.K.; Gupta, G.; Chellappan, D.K.; Zacconi, F.; de Jesus Andreoli Pinto, T.; et al. Targeting mucus barrier in respiratory diseases by chemically modified advanced delivery systems. Chem.-Biol. Interact. 2022, 365, 110048. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.; Martins, C.; Azevedo, C.; Sarmento, B. Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv. Drug Deliv. Rev. 2018, 124, 98–106. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Feng, F.; Wen, Z.; Chen, J.; Yuan, Y.; Wang, C.; Sun, C. Strategies to Develop a Mucosa-Targeting Vaccine against Emerging Infectious Diseases. Viruses 2022, 14, 520. [Google Scholar] [CrossRef]
- Kammona, O.; Bourganis, V.; Karamanidou, T.; Kiparissides, C. Recent developments in nanocarrier-aided mucosal vaccination. Nanomedicine 2017, 12, 1057–1074. [Google Scholar] [CrossRef]
- Rose, M.A.; Zielen, S.; Baumann, U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines 2014, 11, 595–607. [Google Scholar] [CrossRef]
- Srivastava, A.; Gowda, D.V.; Madhunapantula, S.V.; Shinde, C.G.; Iyer, M. Mucosal vaccines: A paradigm shift in the development of mucosal adjuvants and delivery vehicles. Apmis 2015, 123, 275–288. [Google Scholar] [CrossRef]
- Lobaina Mato, Y. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-T.; Yamamoto, F.Y.; Low, C.-F.; Loh, J.-Y.; Chong, C.-M. Gut Immune System and the Implications of Oral-Administered Immunoprophylaxis in Finfish Aquaculture. Front. Immunol. 2021, 12, 773193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Ichimiya, S.; Matsumoto, M.; Seya, T. Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J. Innate Immun. 2018, 10, 515–521. [Google Scholar] [CrossRef]
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. [Google Scholar] [CrossRef]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021, 14, 305–316. [Google Scholar] [CrossRef]
- Chen, Z.; Aspelund, A.; Kemble, G.; Jin, H. Genetic mapping of the cold-adapted phenotype of B/Ann Arbor/1/66, the master donor virus for live attenuated influenza vaccines (FluMist®). Virology 2006, 345, 416–423. [Google Scholar] [CrossRef]
- Costa, A.; Andrade, F. Tissue-based in vitro and ex vivo models for pulmonary permeability studies. In Concepts and Models for Drug Permeability Studies; Woodhead Publishing: Cambridge, UK, 2016; pp. 255–272. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef]
- Shrewsbury, S.B. The Upper Nasal Space: Option for Systemic Drug Delivery, Mucosal Vaccines and “Nose-to-Brain”. Pharmaceutics 2023, 15, 1720. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.G.; Zezoff, D.; Lasrado, S. Anatomy, Head and Neck, Tonsils; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nakahashi-Ouchida, R.; Fujihashi, K.; Kurashima, Y.; Yuki, Y.; Kiyono, H. Nasal vaccines: Solutions for respiratory infectious diseases. Trends Mol. Med. 2023, 29, 124–140. [Google Scholar] [CrossRef]
- Dillon, A.; Lo, D.D. M Cells: Intelligent Engineering of Mucosal Immune Surveillance. Front. Immunol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Himi, T.; Takano, K.; Ogasawara, N.; Go, M.; Kurose, M.; Koizumi, J.; Kamekura, R.; Kondo, A.; Ohkuni, T.; Masaki, T.; et al. Mucosal Immune Barrier and Antigen-Presenting System in Human Nasal Epithelial Cells. In Recent Advances in Tonsils and Mucosal Barriers of the Upper Airways; Karger Publishers: Basel, Switzerland, 2011; Volume 72, pp. 28–30. [Google Scholar] [CrossRef]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; González-Ortega, O. The Mucosal Immune System: An Outlook for Nanovaccines Development. In Nanovaccines: An Innovative Technology to Fight Human and Animal Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 15–35. [Google Scholar] [CrossRef]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef]
- Bemark, M.; Angeletti, D. Know your enemy or find your friend?—Induction of IgA at mucosal surfaces. Immunol. Rev. 2021, 303, 83–102. [Google Scholar] [CrossRef]
- Joseph, J. Harnessing Nasal Immunity with IgA to Prevent Respiratory Infections. Immuno 2022, 2, 571–583. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Bendelac, A. IgA Responses to Microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef]
- Bates, J.T. Naïve CD4+ T Cell Activation in the Nasal-Associated Lymphoid Tissue following Intranasal Immunization with a Flagellin-Based Subunit Vaccine. Int. J. Mol. Sci. 2022, 23, 15572. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kimura, S.; Hase, K. M cell-dependent antigen uptake on follicle-associated epithelium for mucosal immune surveillance. Inflamm. Regen. 2018, 38, 15. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cellshenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L.; Cote, R. Stories from the Dendritic Cell Guardhouse. Front. Immunol. 2019, 10, 2880. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Tacken, P.J.; de Vries, I.J.M.; Torensma, R.; Figdor, C.G. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2016, 27, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2019, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yang, B.; He, Q.; Wang, J.; Weng, Q. New Insights of CCR7 Signaling in Dendritic Cell Migration and Inflammatory Diseases. Front. Pharmacol. 2022, 13, 841687. [Google Scholar] [CrossRef] [PubMed]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef] [PubMed]
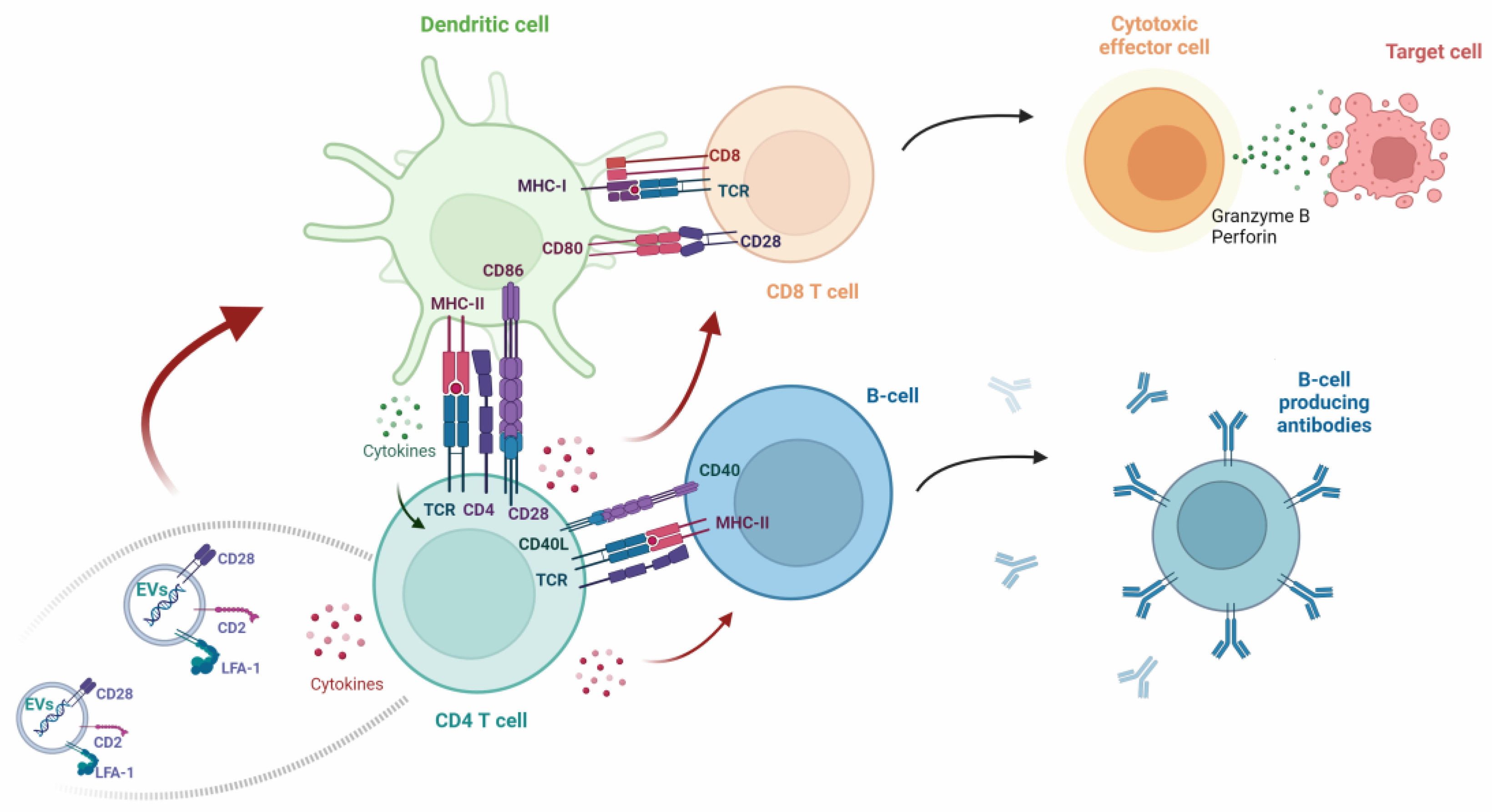
- Tavasolian, F.; Pastrello, C.; Ahmed, Z.; Jurisica, I.; Inman, R.D. Vesicular traffic-mediated cell-to-cell signaling at the immune synapse in Ankylosing Spondylitis. Front. Immunol. 2023, 13, 1102405. [Google Scholar] [CrossRef]
- Hivroz, C.; Saitakis, M. Biophysical Aspects of T Lymphocyte Activation at the Immune Synapse. Front. Immunol. 2016, 7, 46. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Ulivieri, C.; Baldari, C. Regulation of T Cell Activation and Differentiation by Extracellular Vesicles and Their Pathogenic Role in Systemic Lupus Erythematosus and Multiple Sclerosis. Molecules 2017, 22, 225. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2022, 23, 236–250. [Google Scholar] [CrossRef]
- Davies, L.C.; Taylor, P.R. Tissue-resident macrophages: Then and now. Immunology 2015, 144, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.; Schiwon, M.; Kurts, C. Macrophages: Sentinels and regulators of the immune system. Cell. Microbiol. 2016, 18, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Allen, J.E. The expanding world of tissue-resident macrophages. Eur. J. Immunol. 2021, 51, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Lavin, Y.; Mortha, A.; Rahman, A.; Merad, M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015, 15, 731–744. [Google Scholar] [CrossRef]
- Ball, S.L.; Mann, D.A.; Wilson, J.A.; Fisher, A.J. The Role of the Fibroblast in Inflammatory Upper Airway Conditions. Am. J. Pathol. 2016, 186, 225–233. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Lu, J.-H. Autophagy and Macrophage Functions: Inflammatory Response and Phagocytosis. Cells 2019, 9, 70. [Google Scholar] [CrossRef]
- Buxadé, M.; Huerga Encabo, H.; Riera-Borrull, M.; Quintana-Gallardo, L.; López-Cotarelo, P.; Tellechea, M.; Martínez-Martínez, S.; Redondo, J.M.; Martín-Caballero, J.; Flores, J.M.; et al. Macrophage-specific MHCII expression is regulated by a remote Ciita enhancer controlled by NFAT5. J. Exp. Med. 2018, 215, 2901–2918. [Google Scholar] [CrossRef]
- Mann, E.R. Intestinal antigen-presenting cells in mucosal immune homeostasis: Crosstalk between dendritic cells, macrophages and B-cells. World J. Gastroenterol. 2014, 20, 9653. [Google Scholar] [CrossRef]
- Zhou, M.; Li, W.; Wen, Z.; Sheng, Y.; Ren, H.; Dong, H.; Cao, M.; Hu, H.-M.; Wang, L.-x. Macrophages enhance tumor-derived autophagosomes (DRibbles)-induced B cells activation by TLR4/MyD88 and CD40/CD40L. Exp. Cell Res. 2015, 331, 320–330. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Andón, F.T.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Hamilton, T.A.; Zhao, C.; Pavicic, P.G.; Datta, S. Myeloid Colony-Stimulating Factors as Regulators of Macrophage Polarization. Front. Immunol. 2014, 5, 554. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.W.; de Boer, T.; Langenberg, D.M.L.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H.M. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.W.; de Boer, T.; Langenberg, D.M.L.; van der Zanden, L.; Ottenhoff, T.H.M. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J. Leukoc. Biol. 2006, 79, 285–293. [Google Scholar] [CrossRef]
- Xu, N.; Tang, X.-H.; Pan, W.; Xie, Z.-M.; Zhang, G.-F.; Ji, M.-H.; Yang, J.-J.; Zhou, M.-T.; Zhou, Z.-Q. Spared Nerve Injury Increases the Expression of Microglia M1 Markers in the Prefrontal Cortex of Rats and Provokes Depression-like Behaviors. Front. Neurosci. 2017, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.-B.; Lü, M.-H.; Fan, Y.-H.; Cao, Y.-L.; Zhang, Z.-R.; Yang, S.-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef]
- Wang, L.-X.; Zhang, S.-x.; Wu, H.-j.; Rong, X.-l.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, J.; Yi, L.; Qin, J.; Tulake, W.; Teng, F.; Tang, W.; Wei, Y.; Dong, J. The Role of T Cells and Macrophages in Asthma Pathogenesis: A New Perspective on Mutual Crosstalk. Mediat. Inflamm. 2020, 2020, 7835284. [Google Scholar] [CrossRef]
- Pastore, M.; Grimaudo, S.; Pipitone, R.M.; Lori, G.; Raggi, C.; Petta, S.; Marra, F. Role of Myeloid-Epithelial-Reproductive Tyrosine Kinase and Macrophage Polarization in the Progression of Atherosclerotic Lesions Associated with Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2019, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef]
- Otsuka, M.; Egawa, G.; Kabashima, K. Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis. Front. Immunol. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
- Lutz, M.B.; Strobl, H.; Schuler, G.; Romani, N. GM-CSF Monocyte-Derived Cells and Langerhans Cells as Part of the Dendritic Cell Family. Front. Immunol. 2017, 8, 1388. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef]
- Price, J.G.; Idoyaga, J.; Salmon, H.; Hogstad, B.; Bigarella, C.L.; Ghaffari, S.; Leboeuf, M.; Merad, M. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 2015, 16, 1060–1068. [Google Scholar] [CrossRef]
- Haniffa, M.; Collin, M.; Ginhoux, F. Ontogeny and Functional Specialization of Dendritic Cells in Human and Mouse. Adv. Immunol. 2013, 120, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.H. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010, 31, 446–451. [Google Scholar] [CrossRef] [PubMed]
- De Monte, A.; Olivieri, C.-V.; Vitale, S.; Bailleux, S.; Castillo, L.; Giordanengo, V.; Maryanski, J.L.; Segura, E.; Doglio, A. CD1c-Related DCs that Express CD207/Langerin, but Are Distinguishable from Langerhans Cells, Are Consistently Present in Human Tonsils. Front. Immunol. 2016, 7, 197. [Google Scholar] [CrossRef]
- Carenza, C.; Calcaterra, F.; Oriolo, F.; Di Vito, C.; Ubezio, M.; Della Porta, M.G.; Mavilio, D.; Della Bella, S. Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front. Immunol. 2019, 10, 1325. [Google Scholar] [CrossRef]
- Dress, R.J.; Dutertre, C.-A.; Giladi, A.; Schlitzer, A.; Low, I.; Shadan, N.B.; Tay, A.; Lum, J.; Kairi, M.F.B.M.; Hwang, Y.Y.; et al. Plasmacytoid dendritic cells develop from Ly6D+ lymphoid progenitors distinct from the myeloid lineage. Nat. Immunol. 2019, 20, 852–864. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and Monocyte-Derived CD11b+ Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef]
- Chow, K.V.; Sutherland, R.M.; Zhan, Y.; Lew, A.M. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol. Cell Biol. 2016, 95, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.G.J.V.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Schreibelt, G.; Rabold, K.; Wculek, S.K.; Schwarze, J.K.; Dzionek, A.; Teijeira, A.; Kandalaft, L.E.; Romero, P.; Coukos, G.; et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J. ImmunoTherapy Cancer 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yang, M.; Wang, Y.-H.; Lande, R.; Gregorio, J.; Perng, O.A.; Qin, X.-F.; Liu, Y.-J.; Gilliet, M. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007, 204, 105–115. [Google Scholar] [CrossRef]
- Li, G.; Cheng, L.; Su, L. Phenotypic and Functional Study of Human Plasmacytoid Dendritic Cells. Curr. Protoc. 2021, 1, e50. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Cueto, F.J.; del Fresno, C.; Sancho, D. DNGR-1, a Dendritic Cell-Specific Sensor of Tissue Damage That Dually Modulates Immunity and Inflammation. Front. Immunol. 2020, 10, 3146. [Google Scholar] [CrossRef]
- Calmeiro, J.; Carrascal, M.A.; Tavares, A.R.; Ferreira, D.A.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics 2020, 12, 158. [Google Scholar] [CrossRef]
- Hubert, M.; Gobbini, E.; Couillault, C.; Manh, T.-P.V.; Doffin, A.-C.; Berthet, J.; Rodriguez, C.; Ollion, V.; Kielbassa, J.; Sajous, C.; et al. IFN-III is selectively produced by cDC1 and predicts good clinical outcome in breast cancer. Sci. Immunol. 2020, 5, eaav3942. [Google Scholar] [CrossRef]
- Lauterbach, H.; Bathke, B.; Gilles, S.; Traidl-Hoffmann, C.; Luber, C.A.; Fejer, G.; Freudenberg, M.A.; Davey, G.M.; Vremec, D.; Kallies, A.; et al. Mouse CD8α+ DCs and human BDCA3+ DCs are major producers of IFN-λ in response to poly IC. J. Exp. Med. 2010, 207, 2703–2717. [Google Scholar] [CrossRef]
- Minoda, Y.; Virshup, I.; Leal Rojas, I.; Haigh, O.; Wong, Y.; Miles, J.J.; Wells, C.A.; Radford, K.J. Human CD141+ Dendritic Cell and CD1c+ Dendritic Cell Undergo Concordant Early Genetic Programming after Activation in Humanized Mice In Vivo. Front. Immunol. 2017, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.L.; Grajales-Reyes, G.E.; Wu, X.; Tussiwand, R.; Briseño, C.G.; Iwata, A.; Kretzer, N.M.; Durai, V.; Murphy, K.M. Transcriptional Control of Dendritic Cell Development. Annu. Rev. Immunol. 2016, 34, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Heger, L.; Hofer, T.P.; Bigley, V.; de Vries, I.J.M.; Dalod, M.; Dudziak, D.; Ziegler-Heitbrock, L. Subsets of CD1c+ DCs: Dendritic Cell Versus Monocyte Lineage. Front. Immunol. 2020, 11, 559166. [Google Scholar] [CrossRef]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, G.; Buschow, S.I.; Gorris, M.A.J.; Halilovic, A.; Hato, S.V.; Sköld, A.E.; Schreibelt, G.; Sittig, S.P.; Torensma, R.; Duiveman-de Boer, T.; et al. Expansion of a BDCA1+CD14+ Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines. Cancer Res. 2016, 76, 4332–4346. [Google Scholar] [CrossRef]
- Meyerson, H.J.; Osei, E.; Schweitzer, K.; Blidaru, G.; Edinger, A.; Schlegelmilch, J.; Awadallah, A.; Goyal, T. CD1c(+) myeloid dendritic cells in myeloid neoplasia. Cytom. Part B Clin. Cytom. 2015, 90, 337–348. [Google Scholar] [CrossRef]
- Yin, X.; Yu, H.; Jin, X.; Li, J.; Guo, H.; Shi, Q.; Yin, Z.; Xu, Y.; Wang, X.; Liu, R.; et al. Human Blood CD1c+ Dendritic Cells Encompass CD5high and CD5low Subsets That Differ Significantly in Phenotype, Gene Expression, and Functions. J. Immunol. 2017, 198, 1553–1564. [Google Scholar] [CrossRef]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.-P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef]
- Askmyr, D.; Abolhalaj, M.; Gomez Jimenez, D.; Greiff, L.; Lindstedt, M.; Lundberg, K. Pattern recognition receptor expression and maturation profile of dendritic cell subtypes in human tonsils and lymph nodes. Hum. Immunol. 2021, 82, 976–981. [Google Scholar] [CrossRef]
- Solano-Gálvez, S.; Tovar-Torres, S.; Tron-Gómez, M.; Weiser-Smeke, A.; Álvarez-Hernández, D.; Franyuti-Kelly, G.; Tapia-Moreno, M.; Ibarra, A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018, 6, 88. [Google Scholar] [CrossRef]
- Bakdash, G.; Schreurs, I.; Schreibelt, G.; Tel, J. Crosstalk between dendritic cell subsets and implications for dendritic cell-based anticancer immunotherapy. Expert Rev. Clin. Immunol. 2014, 10, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Tongeren, J.; Egmond, D.; Groot, E.; Fokkens, W.; Drunen, C. Dendritic Cell Subsets in Oral Mucosa of Allergic and Healthy Subjects. PLoS ONE 2016, 11, e0154409. [Google Scholar] [CrossRef] [PubMed]
- Froidure, A.; Shen, C.; Pilette, C. Dendritic cells revisited in human allergic rhinitis and asthma. Allergy 2016, 71, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Sichien, D.; Lambrecht, B.N.; Guilliams, M.; Scott, C.L. Development of conventional dendritic cells: From common bone marrow progenitors to multiple subsets in peripheral tissues. Mucosal Immunol. 2017, 10, 831–844. [Google Scholar] [CrossRef]
- Schnell, U.; Cirulli, V.; Giepmans, B.N.G. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1989–2001. [Google Scholar] [CrossRef]
- Roe, M.M.; Swain, S.; Sebrell, T.A.; Sewell, M.A.; Collins, M.M.; Perrino, B.A.; Smith, P.D.; Smythies, L.E.; Bimczok, D. Differential regulation of CD103 (αE integrin) expression in human dendritic cells by retinoic acid and Toll-like receptor ligands. J. Leukoc. Biol. 2017, 101, 1169–1180. [Google Scholar] [CrossRef]
- Lee, H.; Ruane, D.; Law, K.; Ho, Y.; Garg, A.; Rahman, A.; Esterházy, D.; Cheong, C.; Goljo, E.; Sikora, A.G.; et al. Phenotype and function of nasal dendritic cells. Mucosal Immunol. 2015, 8, 1083–1098. [Google Scholar] [CrossRef]
- Janeway, C.A. Approaching the Asymptote? Evolution and Revolution in Immunology. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 54, pp. 1–13. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C.; Bottazzi, B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 2003, 21, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Inohara, N.; Koseki, T.; del Peso, L.; Hu, Y.; Yee, C.; Chen, S.; Carrio, R.; Merino, J.; Liu, D.; Ni, J.; et al. Nod1, an Apaf-1-like Activator of Caspase-9 and Nuclear Factor-κB. J. Biol. Chem. 1999, 274, 14560–14567. [Google Scholar] [CrossRef]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M.; Niedźwiedzka-Rystwej, P. Toll-like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2010, 239, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, X.; Hess, N.J.; Guan, Y.; Tapping, R.I. TLR10 Is a Negative Regulator of Both MyD88-Dependent and -Independent TLR Signaling. J. Immunol. 2016, 196, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ranoa, D.R.E.; Jiang, S.; Mutha, S.K.; Li, X.; Baudry, J.; Tapping, R.I. Human TLRs 10 and 1 Share Common Mechanisms of Innate Immune Sensing but Not Signaling. J. Immunol. 2010, 184, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y.J. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol. Cells 2007, 23, 1–10. [Google Scholar] [CrossRef]
- Tanji, H.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Structural Reorganization of the Toll-Like Receptor 8 Dimer Induced by Agonistic Ligands. Science 2013, 339, 1426–1429. [Google Scholar] [CrossRef]
- Krummen, M.; Balkow, S.; Shen, L.; Heinz, S.; Loquai, C.; Probst, H.-C.; Grabbe, S. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J. Leukoc. Biol. 2010, 88, 189–199. [Google Scholar] [CrossRef]
- Weighardt, H.; Jusek, G.; Mages, J.; Lang, R.; Hoebe, K.; Beutler, B.; Holzmann, B. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur. J. Immunol. 2004, 34, 558–564. [Google Scholar] [CrossRef]
- Stack, J.; Doyle, S.L.; Connolly, D.J.; Reinert, L.S.; O’Keeffe, K.M.; McLoughlin, R.M.; Paludan, S.R.; Bowie, A.G. TRAM Is Required for TLR2 Endosomal Signaling to Type I IFN Induction. J. Immunol. 2014, 193, 6090–6102. [Google Scholar] [CrossRef]
- Horng, T.; Barton, G.M.; Flavell, R.A.; Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002, 420, 329–333. [Google Scholar] [CrossRef]
- An, H.; Xu, H.; Yu, Y.; Zhang, M.; Qi, R.; Yan, X.; Liu, S.; Wang, W.; Guo, Z.; Qin, Z.; et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-κB, ERK and p38 MAPK signal pathways. Immunol. Lett. 2002, 81, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Fujita, T. Structural Mechanism of RNA Recognition by the RIG-I-like Receptors. Immunity 2008, 29, 178–181. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Benedict, C.A.; Eichholz, K.; Bru, T.; Tran, T.T.P.; Fernandes, P.; Welles, H.; Mennechet, F.J.D.; Manel, N.; Alves, P.; Perreau, M.; et al. Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog. 2016, 12, e1005871. [Google Scholar] [CrossRef]
- Jin, T.; Perry, A.; Smith, P.; Jiang, J.; Xiao, T.S. Structure of the Absent in Melanoma 2 (AIM2) Pyrin Domain Provides Insights into the Mechanisms of AIM2 Autoinhibition and Inflammasome Assembly. J. Biol. Chem. 2013, 288, 13225–13235. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Hopfner, K.-P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.B.; Kacew, A.J.; Sweis, R.F. The Development of STING Agonists and Emerging Results as a Cancer Immunotherapy. Curr. Oncol. Rep. 2023, 25, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Su, J. Progresses on three pattern recognition receptor families (TLRs, RLRs and NLRs) in teleost. Dev. Comp. Immunol. 2021, 122, 104131. [Google Scholar] [CrossRef] [PubMed]
- Tenthorey, J.L.; Kofoed, E.M.; Daugherty, M.D.; Malik, H.S.; Vance, R.E. Molecular Basis for Specific Recognition of Bacterial Ligands by NAIP/NLRC4 Inflammasomes. Mol. Cell 2014, 54, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U. Activation and regulation mechanisms of NOD-like receptors based on structural biology. Front. Immunol. 2022, 13, 953530. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Gorp, H.V.; Kuchmiy, A.; Hauwermeiren, F.V.; Lamkanfi, M. NOD-like receptors interfacing the immune and reproductive systems. FEBS J. 2014, 281, 4568–4582. [Google Scholar] [CrossRef]
- Hatscher, L.; Lehmann, C.H.K.; Purbojo, A.; Onderka, C.; Liang, C.; Hartmann, A.; Cesnjevar, R.; Bruns, H.; Gross, O.; Nimmerjahn, F.; et al. Select hyperactivating NLRP3 ligands enhance the TH1- and TH17-inducing potential of human type 2 conventional dendritic cells. Sci. Signal. 2021, 14, eabe1757. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.; Simões, I.; Consuegra-Fernández, M.; Aranda, F.; Lozano, F.; Berraondo, P. Exploiting scavenger receptors in cancer immunotherapy: Lessons from CD5 and SR-B1. Eur. J. Immunol. 2017, 47, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, J.G.; Wang, X.Y.; Subjeck, J.R. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur. J. Immunol. 2007, 37, 2268–2279. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Aldred, S.; Dale, C.; Nakano, E.; Kitas, G.D.; Grant, M.G.; Nugent, D.; Taiwo, F.A.; Li, L.; Powers, H.J. Homocysteine from endothelial cells promotes LDL nitration and scavenger receptor uptake. Free. Radic. Biol. Med. 2006, 40, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Delneste, Y. Scavenger receptors and heat-shock protein-mediated antigen cross-presentation. Biochem. Soc. Trans. 2004, 32, 633–635. [Google Scholar] [CrossRef][Green Version]
- Nicoletti, A.; Caligiuri, G.; Törnberg, I.; Kodama, T.; Stemme, S.; Hansson, G.K. The macrophage scavenger receptor type A directs modified proteins to antigen presentation. Eur. J. Immunol. 1999, 29, 512–521. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Arbore, G.; Kemper, C. A novel “complement–metabolism–inflammasome axis” as a key regulator of immune cell effector function. Eur. J. Immunol. 2016, 46, 1563–1573. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Evolution and Function of Thioester-Containing Proteins and the Complement System in the Innate Immune Response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef]
- West, E.E.; Kunz, N.; Kemper, C. Complement and human T cell metabolism: Location, location, location. Immunol. Rev. 2020, 295, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Murali, M. Analysis of the Complement System in the Clinical Immunology Laboratory. Clin. Lab. Med. 2019, 39, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; Friec, G.L.; Kemper, C. Complement—Tapping into new sites and effector systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Speakman, E.A.; Dambuza, I.M.; Salazar, F.; Brown, G.D. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2020, 41, 61–76. [Google Scholar] [CrossRef]
- Jung, S.; Kim, S.; Kim, Y.; Chung, H.; Kim, S.; Yeo, S. Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review. Molecules 2018, 23, 734. [Google Scholar] [CrossRef]
- Mnich, M.E.; van Dalen, R.; van Sorge, N.M. C-Type Lectin Receptors in Host Defense Against Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 309. [Google Scholar] [CrossRef]
- Yan, H.; Kamiya, T.; Suabjakyong, P.; Tsuji, N.M. Targeting C-Type Lectin Receptors for Cancer Immunity. Front. Immunol. 2015, 6, 408. [Google Scholar] [CrossRef]
- Ruland, J.; Hartjes, L. CARD–BCL-10–MALT1 signalling in protective and pathological immunity. Nat. Rev. Immunol. 2018, 19, 118–134. [Google Scholar] [CrossRef]
- Hoving, J.C.; Wilson, G.J.; Brown, G.D. Signalling C-Type lectin receptors, microbial recognition and immunity. Cell. Microbiol. 2014, 16, 185–194. [Google Scholar] [CrossRef]
- Sosa Cuevas, E.; Valladeau-Guilemond, J.; Mouret, S.; Roubinet, B.; de Fraipont, F.; Landemarre, L.; Charles, J.; Bendriss-Vermare, N.; Chaperot, L.; Aspord, C. Unique CLR expression patterns on circulating and tumor-infiltrating DC subsets correlated with clinical outcome in melanoma patients. Front. Immunol. 2022, 13, 1040600. [Google Scholar] [CrossRef]
- Tone, K.; Stappers, M.H.T.; Willment, J.A.; Brown, G.D. C-type lectin receptors of the Dectin-1 clusterhysiological roles and involvement in disease. Eur. J. Immunol. 2019, 49, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Scur, M.; Parsons, B.D.; Dey, S.; Makrigiannis, A.P. The diverse roles of C-type lectin-like receptors in immunity. Front. Immunol. 2023, 14, 1126043. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; Bianchi, G.; Doni, A.; Del Prete, A.; Sironi, M.; Laskarin, G.; Monti, P.; Piemonti, L.; Biondi, A.; Mantovani, A.; et al. Cross-Linking of the Mannose Receptor on Monocyte-Derived Dendritic Cells Activates an Anti-Inflammatory Immunosuppressive Program. J. Immunol. 2003, 171, 4552–4560. [Google Scholar] [CrossRef]
- Anselmi, G.; Vaivode, K.; Dutertre, C.-A.; Bourdely, P.; Missolo-Koussou, Y.; Newell, E.; Hickman, O.; Wood, K.; Saxena, A.; Helft, J.; et al. Engineered niches support the development of human dendritic cells in humanized mice. Nat. Commun. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Angelina, A.; Martín-Cruz, L.; de la Rocha-Muñoz, A.; Lavín-Plaza, B.; Palomares, O. C-Type Lectin Receptor Mediated Modulation of T2 Immune Responses to Allergens. Curr. Allergy Asthma Rep. 2023, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, H.J.P.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef]
- Sheikh, H.; Yarwood, H.; Ashworth, A.; Isacke, C.M. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 2000, 113, 1021–1032. [Google Scholar] [CrossRef]
- Jiang, W.; Swiggard, W.J.; Heufler, C.; Peng, M.; Mirza, A.; Steinman, R.M.; Nussenzweig, M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 1995, 375, 151–155. [Google Scholar] [CrossRef]
- Cummings, R.D. The mannose receptor ligands and the macrophage glycome. Curr. Opin. Struct. Biol. 2022, 75, 102394. [Google Scholar] [CrossRef]
- Higashino, K.I.; Ishizaki, J.; Kishino, J.; Ohara, O.; Arita, H. Structural Comparison of phospholipase-A2-binding Regions in phospholipase-A2 Receptors from Various Mammals. Eur. J. Biochem. 2005, 225, 375–382. [Google Scholar] [CrossRef]
- Taylor, M.E.; Conary, J.T.; Lennartz, M.R.; Stahl, P.D.; Drickamer, K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J. Biol. Chem. 1990, 265, 12156–12162. [Google Scholar] [CrossRef] [PubMed]
- Leteux, C.; Chai, W.; Loveless, R.W.; Yuen, C.-T.; Uhlin-Hansen, L.; Combarnous, Y.; Jankovic, M.; Maric, S.C.; Misulovin, Z.; Nussenzweig, M.C.; et al. The Cysteine-Rich Domain of the Macrophage Mannose Receptor Is a Multispecific Lectin That Recognizes Chondroitin Sulfates a and B and Sulfated Oligosaccharides of Blood Group Lewisa and Lewisx Types in Addition to the Sulfated N-Glycans of Lutropin. J. Exp. Med. 2000, 191, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Shukla, N. Attributes of alternatively activated (M2) macrophages. Life Sci. 2019, 224, 222–231. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J. Biol. Chem. 1993, 268, 399–404. [Google Scholar] [CrossRef]
- Feinberg, H.; Jégouzo, S.A.F.; Lasanajak, Y.; Smith, D.F.; Drickamer, K.; Weis, W.I.; Taylor, M.E. Structural analysis of carbohydrate binding by the macrophage mannose receptor CD206. J. Biol. Chem. 2021, 296, 100368. [Google Scholar] [CrossRef]
- Taylor, P.; Gordon, S.; Martinezpomares, L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef]
- Tietze, C.; Schlesinger, P.; Stahl, P. Mannose-specific endocytosis receptor of alveolar macrophages: Demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J. Cell Biol. 1982, 92, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, R.A.; Sastry, K.; Bailly, P.; Warner, A. Molecular characterization of the human macrophage mannose receptor: Demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 1990, 172, 1785–1794. [Google Scholar] [CrossRef]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pomares, L.; Mahoney, J.A.; Káposzta, R.; Linehan, S.A.; Stahl, P.D.; Gordon, S. A Functional Soluble Form of the Murine Mannose Receptor Is Produced by Macrophages in Vitro and Is Present in Mouse Serum. J. Biol. Chem. 1998, 273, 23376–23380. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; van der Zande, H.J.P.; Hussaarts, L.; Schulte-Schrepping, J.; Pelgrom, L.R.; García-Tardón, N.; Schlautmann, L.; Stoetzel, I.; Händler, K.; Lambooij, J.M.; et al. Soluble mannose receptor induces proinflammatory macrophage activation and metaflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e210330411. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, X.; Yu, B.; Li, N.; Huang, Y.; He, Y. Structural Insights into the pH-Dependent Conformational Change and Collagen Recognition of the Human Mannose Receptor. Structure 2018, 26, 60–71.e3. [Google Scholar] [CrossRef] [PubMed]
- Kreer, C.; Kuepper, J.M.; Zehner, M.; Quast, T.; Kolanus, W.; Schumak, B.; Burgdorf, S. N-glycosylation converts non-glycoproteins into mannose receptor ligands and reveals antigen-specific T cell responses in vivo. Oncotarget 2016, 8, 6857–6872. [Google Scholar] [CrossRef]
- Burgdorf, S.; Kautz, A.; Böhnert, V.; Knolle, P.A.; Kurts, C. Distinct Pathways of Antigen Uptake and Intracellular Routing in CD4 and CD8 T Cell Activation. Science 2007, 316, 612–616. [Google Scholar] [CrossRef]
- Yin, X.; Bai, H.; Mu, L.; Chen, N.; Qi, W.; Huang, Y.; Xu, H.; Jian, J.; Wang, A.; Ye, J. Expression and functional characterization of the mannose receptor (MR) from Nile tilapia (Oreochromis niloticus) in response to bacterial infection. Dev. Comp. Immunol. 2022, 126, 104257. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Ouyang, A.; Wang, H.; Su, J.; Liu, X. Mannose Receptor Mediates the Activation of Chitooligosaccharides on Blunt Snout Bream (Megalobrama amblycephala) Macrophages. Front. Immunol. 2021, 12, 686846. [Google Scholar] [CrossRef]
- Sil, A.; Wagener, J.; Malireddi, R.K.S.; Lenardon, M.D.; Köberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; et al. Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation. PLoS Pathog. 2014, 10, e1004050. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Marijnissen, R.J.; Kullberg, B.J.; Koenen, H.J.P.M.; Cheng, S.-C.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.M.; Joosten, L.A.B.; et al. The Macrophage Mannose Receptor Induces IL-17 in Response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef]
- Kooij, G.; Braster, R.; Koning, J.J.; Laan, L.C.; van Vliet, S.J.; Los, T.; Eveleens, A.M.; van der Pol, S.M.A.; Förster-Waldl, E.; Boztug, K.; et al. Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: Implications for multiple sclerosis. Acta Neuropathol. Commun. 2015, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, M.V.S.; Arnett, E.; Azad, A.K.; Guirado, E.; Ni, B.; Gerberick, A.D.; He, L.-Z.; Keler, T.; Thomas, L.J.; Lafuse, W.P.; et al. M. tuberculosis-Initiated Human Mannose Receptor Signaling Regulates Macrophage Recognition and Vesicle Trafficking by FcRγ-Chain, Grb2, and SHP-1. Cell Rep. 2017, 21, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Schuette, V.; Embgenbroich, M.; Ulas, T.; Welz, M.; Schulte-Schrepping, J.; Draffehn, A.M.; Quast, T.; Koch, K.; Nehring, M.; König, J.; et al. Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4. Proc. Natl. Acad. Sci. USA 2016, 113, 10649–10654. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, F.; Ji, Z.; Fei, W.; Tan, Z.; Hu, Y.; Zheng, C. Mannose-Modified PLGA Nanoparticles for Sustained and Targeted Delivery in Hepatitis B Virus Immunoprophylaxis. AAPS PharmSciTech 2019, 21, 13. [Google Scholar] [CrossRef]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Pei, M.; Xu, R.; Zhang, C.; Wang, X.; Li, C.; Hu, Y. Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids Surf. B Biointerfaces 2021, 197, 111378. [Google Scholar] [CrossRef]
- Su, F.-Y.; Chen, J.; Son, H.-N.; Kelly, A.M.; Convertine, A.J.; West, T.E.; Skerrett, S.J.; Ratner, D.M.; Stayton, P.S. Polymer-augmented liposomes enhancing antibiotic delivery against intracellular infections. Biomater. Sci. 2018, 6, 1976–1985. [Google Scholar] [CrossRef]
- Wilson, D.S.; Hirosue, S.; Raczy, M.M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Kwissa, M.; Franetich, J.-F.; Broggi, M.A.S.; Diaceri, G.; et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat. Mater. 2019, 18, 175–185. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Lu, L.; Wang, H.; Sun, H.; Leng, X.; et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials 2019, 206, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Han, D.; Sun, X.; Zhang, M.; Feng, X.; Sun, C.; Gu, J.; Tong, C.; Lei, L.; Han, W. Mannose-modified chitosan microspheres enhance OprF-OprI-mediated protection of mice against Pseudomonas aeruginosa infection via induction of mucosal immunity. Appl. Microbiol. Biotechnol. 2014, 99, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhen, Y.; Ma, X.; Wei, B.; Li, S.; Wang, N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf. B Biointerfaces 2015, 126, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Heger, L.; Balk, S.; Lühr, J.J.; Heidkamp, G.F.; Lehmann, C.H.K.; Hatscher, L.; Purbojo, A.; Hartmann, A.; Garcia-Martin, F.; Nishimura, S.-I.; et al. CLEC10A Is a Specific Marker for Human CD1c+ Dendritic Cells and Enhances Their Toll-Like Receptor 7/8-Induced Cytokine Secretion. Front. Immunol. 2018, 9, 744. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y.; Lin, X.; Wang, H.; Yong, L.; Zhang, H.; Cai, F. CLEC10A can serve as a potential therapeutic target and its level correlates with immune infiltration in breast cancer. Oncol. Lett. 2022, 24, 285. [Google Scholar] [CrossRef]
- Oo-puthinan, S.; Maenuma, K.; Sakakura, M.; Denda-Nagai, K.; Tsuiji, M.; Shimada, I.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Bovin, N.V.; Irimura, T. The amino acids involved in the distinct carbohydrate specificities between macrophage galactose-type C-type lectins 1 and 2 (CD301a and b) of mice. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Denda-Nagai, K.; Aida, S.; Saba, K.; Suzuki, K.; Moriyama, S.; Oo-puthinan, S.; Tsuiji, M.; Morikawa, A.; Kumamoto, Y.; Sugiura, D.; et al. Distribution and Function of Macrophage Galactose-type C-type Lectin 2 (MGL2/CD301b). J. Biol. Chem. 2010, 285, 19193–19204. [Google Scholar] [CrossRef]
- Gabba, A.; Bogucka, A.; Luz, J.G.; Diniz, A.; Coelho, H.; Corzana, F.; Cañada, F.J.; Marcelo, F.; Murphy, P.V.; Birrane, G. Crystal Structure of the Carbohydrate Recognition Domain of the Human Macrophage Galactose C-Type Lectin Bound to GalNAc and the Tumor-Associated Tn Antigen. Biochemistry 2021, 60, 1327–1336. [Google Scholar] [CrossRef]
- Fu, Y.L.; Harrison, R.E. Microbial Phagocytic Receptors and Their Potential Involvement in Cytokine Induction in Macrophages. Front. Immunol. 2021, 12, 662063. [Google Scholar] [CrossRef]
- Napoletano, C.; Rughetti, A.; Agervig Tarp, M.P.; Coleman, J.; Bennett, E.P.; Picco, G.; Sale, P.; Denda-Nagai, K.; Irimura, T.; Mandel, U.; et al. Tumor-Associated Tn-MUC1 Glycoform Is Internalized through the Macrophage Galactose-Type C-Type Lectin and Delivered to the HLA Class I and II Compartments in Dendritic Cells. Cancer Res. 2007, 67, 8358–8367. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-G.; Beatson, R.; Maurstad, G.; Picco, G.; Arulappu, A.; Coleman, J.; Wandell, H.H.; Clausen, H.; Mandel, U.; Taylor-Papadimitriou, J.; et al. The Breast Cancer-Associated Glycoforms of MUC1, MUC1-Tn and sialyl-Tn, Are Expressed in COSMC Wild-Type Cells and Bind the C-Type Lectin MGL. PLoS ONE 2015, 10, e0125994. [Google Scholar] [CrossRef]
- Saeland, E.; van Vliet, S.J.; Bäckström, M.; van den Berg, V.C.M.; Geijtenbeek, T.B.H.; Meijer, G.A.; van Kooyk, Y. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol. Immunother. 2006, 56, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Zaal, A.; Li, R.J.E.; Lübbers, J.; Bruijns, S.C.M.; Kalay, H.; van Kooyk, Y.; van Vliet, S.J. Activation of the C-Type Lectin MGL by Terminal GalNAc Ligands Reduces the Glycolytic Activity of Human Dendritic Cells. Front. Immunol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.; van Vliet, S.J.; Carasi, P.; Frigerio, S.; García, P.A.; Croci, D.O.; Festari, M.F.; Costa, M.; Landeira, M.; Rodríguez-Zraquia, S.A.; et al. The Tn antigen promotes lung tumor growth by fostering immunosuppression and angiogenesis via interaction with Macrophage Galactose-type lectin 2 (MGL2). Cancer Lett. 2021, 518, 72–81. [Google Scholar] [CrossRef]
- van der Meijs, N.L.; Travecedo, M.A.; Marcelo, F.; van Vliet, S.J. The pleiotropic CLEC10A: Implications for harnessing this receptor in the tumor microenvironment. Expert Opin. Ther. Targets 2024, 28, 601–612. [Google Scholar] [CrossRef]
- Napoletano, C.; Steentoff, C.; Battisti, F.; Ye, Z.; Rahimi, H.; Zizzari, I.G.; Dionisi, M.; Cerbelli, B.; Tomao, F.; French, D.; et al. Investigating Patterns of Immune Interaction in Ovarian Cancerrobing the O-glycoproteome by the Macrophage Galactose-Like C-Type Lectin (MGL). Cancers 2020, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Jégouzo, S.A.F.; Quintero-Martínez, A.; Ouyang, X.; dos Santos, Á.; Taylor, M.E.; Drickamer, K. Organization of the extracellular portion of the macrophage galactose receptor: A trimeric cluster of simple binding sites for N-acetylgalactosamine. Glycobiology 2013, 23, 853–864. [Google Scholar] [CrossRef]
- Higashi, N.; Fujioka, K.; Denda-Nagai, K.; Hashimoto, S.-i.; Nagai, S.; Sato, T.; Fujita, Y.; Morikawa, A.; Tsuiji, M.; Miyata-Takeuchi, M.; et al. The Macrophage C-type Lectin Specific for Galactose/N-Acetylgalactosamine Is an Endocytic Receptor Expressed on Monocyte-derived Immature Dendritic Cells. J. Biol. Chem. 2002, 277, 20686–20693. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Aarnoudse, C.A.; Broks-van den Berg, V.C.M.; Boks, M.; Geijtenbeek, T.B.H.; van Kooyk, Y. MGL-mediated internalization and antigen presentation by dendritic cells: A role for tyrosine-5. Eur. J. Immunol. 2007, 37, 2075–2081. [Google Scholar] [CrossRef]
- Valladeau, J.; Duvert-Frances, V.r.; Pin, J.-J.; Kleijmeer, M.J.; Ait-Yahia, S.; Ravel, O.; Vincent, C.; Vega, F.; Helms, A.; Gorman, D.; et al. Immature Human Dendritic Cells Express Asialoglycoprotein Receptor Isoforms for Efficient Receptor-Mediated Endocytosis. J. Immunol. 2001, 167, 5767–5774. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.T.; Owen, D.J. Endocytic sorting of transmembrane protein cargo. Curr. Opin. Cell Biol. 2011, 23, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, S.; Sawai, E.T.; Radke, K. Dileucine and YXXL Motifs in the Cytoplasmic Tail of the Bovine Leukemia Virus Transmembrane Envelope Protein Affect Protein Expression on the Cell Surface. J. Virol. 2004, 78, 8301–8311. [Google Scholar] [CrossRef][Green Version]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Saeland, E.; van Kooyk, Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer 2011, 128, 1371–1383. [Google Scholar] [CrossRef]
- Gu, C.; Wang, L.; Zurawski, S.; Oh, S. Signaling Cascade through DC-ASGPR Induces Transcriptionally Active CREB for IL-10 Induction and Immune Regulation. J. Immunol. 2019, 203, 389–399. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Bay, S.; Vuist, I.M.; Kalay, H.; García-Vallejo, J.J.; Leclerc, C.; van Kooyk, Y. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-α secretion. J. Leukoc. Biol. 2013, 94, 315–323. [Google Scholar] [CrossRef]
- Weber-Nordt, R.M.; Henschler, R.; Schott, E.; Wehinger, J.; Behringer, D.; Mertelsmann, R.; Finke, J. Interleukin-10 increases Bcl-2 expression and survival in primary human CD34+ hematopoietic progenitor cells. Blood 1996, 88, 2549–2558. [Google Scholar] [CrossRef]
- Unutmaz, D.; Zizzari, I.G.; Martufi, P.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Bellati, F.; Nuti, M.; Rughetti, A.; Napoletano, C. The Macrophage Galactose-Type C-Type Lectin (MGL) Modulates Regulatory T Cell Functions. PLoS ONE 2015, 10, e0132617. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef]
- Ilarregui, J.M.; Kooij, G.; Rodríguez, E.; van der Pol, S.M.A.; Koning, N.; Kalay, H.; van der Horst, J.C.; van Vliet, S.J.; García-Vallejo, J.J.; de Vries, H.E.; et al. Macrophage galactose-type lectin (MGL) is induced on M2 microglia and participates in the resolution phase of autoimmune neuroinflammation. J. Neuroinflammation 2019, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel insights into the immunomodulatory role of the dendritic cell and macrophage-expressed C-type lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef] [PubMed]
- van Sorge, N.M.; Bleumink, N.M.C.; van Vliet, S.J.; Saeland, E.; van der Pol, W.L.; van Kooyk, Y.; van Putten, J.P.M. N-glycosylated proteins and distinct lipooligosaccharide glycoforms ofCampylobacter jejunitarget the human C-type lectin receptor MGL. Cell. Microbiol. 2009, 11, 1768–1781. [Google Scholar] [CrossRef]
- Kremsreiter, S.M.; Kroell, A.-S.H.; Weinberger, K.; Boehm, H. Glycan–Lectin Interactions in Cancer and Viral Infections and How to Disrupt Them. Int. J. Mol. Sci. 2021, 22, 10577. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Sancho, D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology 2015, 220, 175–184. [Google Scholar] [CrossRef]
- Pedro, A.R.V.; Lima, T.; Fróis-Martins, R.; Leal, B.; Ramos, I.C.; Martins, E.G.; Cabrita, A.R.J.; Fonseca, A.J.M.; Maia, M.R.G.; Vilanova, M.; et al. Dectin-1-Mediated Production of Pro-Inflammatory Cytokines Induced by Yeast β-Glucans in Bovine Monocytes. Front. Immunol. 2021, 12, 689879. [Google Scholar] [CrossRef]
- Liu, P.; Gao, C.; Chen, H.; Vong, C.T.; Wu, X.; Tang, X.; Wang, S.; Wang, Y. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies. Acta Pharm. Sin. B 2021, 11, 2798–2818. [Google Scholar] [CrossRef]
- Liu, L.; Yi, H.; He, H.; Pan, H.; Cai, L.; Ma, Y. Tumor associated macrophage-targeted microRNA delivery with dual-responsive polypeptide nanovectors for anti-cancer therapy. Biomaterials 2017, 134, 166–179. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated trimethyl chitosan–cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Gui, S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-ɑ siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur. J. Pharm. Sci. 2018, 125, 232–243. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Kang, Y.; Denning, T.; Merlin, D. TNFα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control Release 2018, 287, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Huang, Z.; Dong, L.; Xu, L.; Zhu, Y.; Zeng, K.; Zhang, C.; Chen, J.; Zhang, J. Targeting delivery of anti-TNF oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut 2009, 59, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wuensch, S.A.; Azadniv, M.; Ebrahimkhani, M.R.; Crispe, I.N. Galactosylated LDL Nanoparticles: A Novel Targeting Delivery System to Deliver Antigen to Macrophages and Enhance Antigen Specific T Cell Responses. Mol. Pharm. 2009, 6, 1506–1517. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J. Control Release 2012, 158, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.-L.; Lin, H.-J.; Wang, H.-W.; Tsai, W.-Y.; Lin, S.-F.; Chien, M.-Y.; Liang, P.-H.; Huang, Y.-Y.; Liu, D.-Z. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015, 11, 356–367. [Google Scholar] [CrossRef]
- Wang, H.-W.; Jiang, P.-L.; Lin, S.-F.; Lin, H.-J.; Ou, K.-L.; Deng, W.-P.; Lee, L.-W.; Huang, Y.-Y.; Liang, P.-H.; Liu, D.-Z. Application of galactose-modified liposomes as a potent antigen presenting cell targeted carrier for intranasal immunization. Acta Biomater. 2013, 9, 5681–5688. [Google Scholar] [CrossRef]
| Vaccine Name | Disease Targeted | Administration Route | Vaccine Components | Major Features: Mode of Action | References |
|---|---|---|---|---|---|
| Dukoral | Cholera | Oral | Killed whole-cell monovalent vaccine composed of formalin or heat inactivated V. cholerae O1 strains with the recombinant cholera toxin B subunit (CTB) | Induction of O1 LPS and CTB-specific IgG and IgA antibodies in serum; mucosal vaccine-specific IgG and IgA antibodies, particularly in the nasal cavity; increased number of memory B lymphocytes in circulation that express markers for homing to the small intestine, colon, and airways; poor cell-mediated immune responses | [10,11,12] |
| ShanChol | Cholera | Oral | Killed whole-cell bivalent vaccine: formalin and heat inactivated V. cholerae O1 and O139 strains | Production of gut O1 and O139 LPS-specific IgA and IgM antibodies; systemic IgG antibodies were also produced in the bloodstream, although to a smaller extent than in the intestinal environment | [13,14] |
| Euvichol | Cholera | Oral | Bivalent inactivated vaccine: formalin and heat inactivated V. cholerae O1 (two Inaba and two Ogawa serotypes) and O139 strains | Robust immune response characterized by high levels of vibriocidal antibodies, similar to those induced by the Shanchol vaccine; reduced production costs | [15,16] |
| Vaxchora | Cholera | Oral | Live attenuated monovalent vaccine: V. cholerae O1 strain, serotype Inaba, where the toxigenic A1 subunit of the cholera toxin was removed, leaving only the non-toxic but immunogenic B subunit; contains cryoprotectants (sucrose and hydrolyzed casein), antioxidant (ascorbic acid), and a stabilizer (sodium chloride) | The live attenuated cholera bacteria undergo replication within the gastrointestinal tract leading to the production of anti-CTB and anti-LPS specific IgG antibodies in the serum; enhancement of anti-O1 LPS IgA and IgG memory B cells; presence of anti-LPS IgA antibodies in the mucosal surface, as indicated by the existence of secretory IgA in stool samples | [17,18], |
| RotaRix | Rotavirus | Oral | Live attenuated monovalent vaccine: human VP7 and VP4 antigens from the G1P1 [8] strain | VP4 and VP7 specific humoral immune responses, mainly by the generation of mucosal IgA antibodies; strong protection against G1 and non-G1 serotypes (except in the G2 serotype), frequently associated with P [8]; poor VP7 specific cytotoxic T-cell immune response | [19,20] |
| RotaTeq | Rotavirus | Oral | Live attenuated pentavalent vaccine containing a mixture of five bovine–human reassortant rotaviruses: four express human VP7 (from strains G1, G2, G3, and G4) with VP4 from the bovine strain, and one expresses human VP4 (from the P1 [8] strain) with VP7 from the bovine strain; contains buffers to protect the viruses from gastric acid and a stabilizer solution (sucrose, sodium phosphate, sodium citrate, and polysorbate 80) | Generation of VP4 and VP7 specific antibodies, leading to robust heterotypic and homotypic neutralizing activity; high IgA seroconversion and systemic IgG antibodies; production of antibodies against nonstructural proteins, such as NSP2 and NSP4; strong protection against G1 and non-G1 serotypes (including the G2 serotype); enhanced levels of rotavirus-specific B cells expressing receptors for intestinal migration | [21,22] |
| Rotavac | Rotavirus | Oral | Live attenuated monovalent vaccine: human VP7 and VP4 antigens from the G9P [11] strain | Mucosal IgA and systemic IgG neutralizing antibodies specific for the multi-strain rotavirus | [23,24] |
| RotaSiil | Rotavirus | Oral | Live attenuated pentavalent vaccine containing a mixture of five bovine–human reassortant rotaviruses: VP7 gene from G1, G2, G3, G4, and G9 strains; thermostable vaccine | [25,26] | |
| Biopolio (bOPV) | Poliovirus | Oral | Live attenuated bivalent vaccine containing a mixture of poliovirus 1 and 3 serotypes (Sabin strains); contains sucrose as a stabilizer | Generation of poliovirus IgG-specific antibodies in the serum and poliovirus IgA-specific antibodies in the mucosal linings of the intestines and respiratory tract | [27,28] |
| Vivotif | Typhoid fever | Oral | Live attenuated vaccine containing a Salmonella typhi Ty21a strain, in which enzymes essential for lipopolysaccharide biosynthesis were removed; despite this modification, the strain retains the capacity to produce sufficient amounts of LPSs, ensuring that antibodies are still produced against LPS antigens, thereby maintaining its protective properties | Strong humoral response illustrated by the generation of anti-S. typhi O-antigen (from LPSs) IgA and IgG antibodies in the gut and serum, respectively; robust cellular immune response distinguished by CD4+ and CD8+ T cells displaying gut-homing characteristics, resulting in the secretion of IFN-ɤ and cytotoxic T-cell activity | [29,30] |
| FluMist/Fluenz | Influenza type A and B viruses | Nasal spray | Live attenuated tetravalent vaccine with four hemagglutinin and neuraminidase antigens from circulating influenza virus strains: A strain—H1N1 and H3N2 and two B strains; contains an immunostimulant (arginine) and cryoprotectant (sucrose) | Cold-adapted vaccine designated to enable viral replication within the nasal passages while inhibiting replication in the lungs or other regions further down the body’s respiratory system; increased levels of secreted nasal IgA and systemic IgG antibodies; production of strong cell-mediated influenza specific IFN-ɤ+ T-cell responses and enhanced NK cell cytotoxicity activity | [31,32,33] |
| Nasovac-S | Influenza type A and B viruses | Nasal spray | Live attenuated trivalent vaccine: a strain—H1N1 and H3N2 and one B strain; contains several amino acids (L-Alanine, L-Histidine, and L-Arginine) as stabilizers | Robust immune response characterized by the synthesis of mucosal IgA antibodies targeting the two influenza surface proteins (hemagglutinin and neuraminidase), alongside systemic IgG antibodies in the bloodstream; potential CD4+ T-cell responses via IL-2 and IFN-ɤ production | [34,35,36] |
| Pandemic influenza vaccine H5Ν1-AstraZeneca | Influenza type A virus | Nasal spray | Live attenuated monovalent vaccine: a strain—H5N1; contains gelatin (porcine, type A), arginine hydrochloride, and monosodium glutamate monohydrate as stabilizers | Development of antibodies that effectively neutralized multiple clades of H5N1 viruses; possible activation of cellular immunity comparable to the response triggered by natural infection | [37] |
| iNCOVACC (BBV154) | COVID-19 | Nasal drop vaccine | Adenovirus-vectored vaccine for SARS-CoV-2 that encodes a spike protein stabilized in its prefusion state, incorporating two proline modifications in the S2 subunit | Elevated neutralization titers against the original SARS-CoV-2 strain and cross-neutralizing responses to the Omicron BA.5 sub-lineage; high levels of specific IgA-secreting plasmablasts; strong T-cell-mediated immunity, despite a high baseline likely indicating previous adenovirus infection; presence of lung-resident lineage cells, particularly CD103+ CD69+ CD8+ T cells | [38] |
| Convidecia Air (Ad5-nCoV-IH) | COVID-19 | Aerosolized vaccine for inhalation through the mouth | Recombinant adenovirus type 5-vectored vaccine that encodes the spike protein of SARS-CoV-2 | Following a heterologous prime-boost regimen, there is a significant increase in neutralizing antibodies effective against various strains of SARS-CoV-2; promotes elevated sIgA levels and stimulates resident memory B and T cells in respiratory mucosa, enhancing local infection defense | [39,40] |
| Macrophage Subtype | Stimulator Factors | Typical Receptors Expressed | Enzymes Expressed | Cytokines and Chemokines Secreted | Function |
|---|---|---|---|---|---|
| M1 | LPS, IFN-ɤ, TNF-α, GM-CSF | MHCII, CD68, CD80, CD86, CD64 | iNOS, IDO, ROS, MMPs | TNF-α, IL-1β, IL-6, IL-12, IL-23, CXCL5, CXCL9, CXCL10, CCL5 | Pro-inflammatory activity Defense against infections and intracellular pathogens |
| M2a | IL-4, IL-13 | CD163, CD206, CD209/DC-SIGN, Dectin-1, DCIR | Arginase-1, YM1 | IL-10, TGF-β, CCL17, CCL22, CCL24 | Anti-inflammatory activity Enhanced phagocytic capacity Tissue regeneration and repair |
| M2b | Immune complexes combined with TLR and/or IL-1 receptor agonists | CD163, CD206, CD209/DC-SIGN | Arginase-1 | IL-10, TGF-β, IL-1, IL-6, TNF-α, CCL1 | Anti-inflammatory activity Pro-inflammatory activity Modulation of immune responses |
| M2c | IL-10, TGF-β, glucocorticoid hormones | CD163, CD206, CD209/DC-SIGN, MerTK | Arginase-1 | IL-10, TGF-β, CCL16, CCL18 | Recruitment of immune cells Phagocytosis of apoptotic cells Supports angiogenesis |
| M2d | TLR or adenosine receptor agonists | CD163, CD206, CD209/DC-SIGN | Arginase-1 | IL-10, TGF-β, VEGF, CXCL10, CXCL16, CCL5 | Promotion of tumor progression Supports angiogenesis |
| Langerhans cells (LCs) | GM-CSF, TGF-β1, TLR agonists, TNF-α, IL-4 | MHCII, Langerin/CD207, EpCam/CD326, CD1a, CD11c, CCR7, TLR2, TLR3, TLR5, TLR8, TLR9 | IDO, Cathepsins, iNOS | IL-10, TGF-β, IL-23, IL-12, IL-6, TNF-α | Enhanced antigen presentation Immune surveillance Skin barrier maintenance |
| Dendritic Cell Subtype | Key Regulatory Molecules | Typical Receptors Expressed | Cytokines, Chemokines Secreted | Function |
|---|---|---|---|---|
| CD141+ DCs (conventional DCs) | IRF8, ID2, BATF3A | CD141, DNGR-1/CLEC9A, XCR1, Necl2/CADM1, CD80, CD11c, MHCII, TLR2, TLR3. TLR6, TLR8, TLR9 | TNF-α, IL-1β, IL-6, IL-12, IFN-β, IFN-α, IL-15 | Enhanced antigen presentation, particularly in the context of cross-presentation induction of CD8+ T-cell responses |
| CD1c+ DCs (conventional DCs) | IRF4, Notch2 | CD206, CD11c, CD11b, CD1c, MGL/CD301, CD172a/SIRPα, MHCII, DCIR, Dectin-1, Dectin-2, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR8 | IL-10, TNF-α, IL-1β, IL-6, IL-12, IL-18, IL-15, IL-23 | Enhanced antigen presentation to both CD4+ and CD8+ T cells Immunomodulation |
| Plasmacytoid DCs | IRFs | CD303, CD123, CD304, CD45RA, TLR7, TLR9 | IL-10, IL-1β, IL-18, IL-6, TNF-α, IFN-λ, IFN-β, IFN-α | Producers of type I and III interferons Limited ability to present antigens Viral sensing |
| Monocyte-derived DCs | IRF4, IRF8, Notch2 | CD64, CD11c, MHCII, CCR2, CD11b, CCR7, TLR2, TLR3, TLR4, TLR5, TLR7, TLR8, TLR9 | IL-12, IL-6, IL-10, TNF-α, NO | Enhanced antigen presentation Immune regulation Initiation of adaptive immunity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaço, M.; Cruz, M.T.; Almeida, L.P.d.; Borges, O. Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors. Pharmaceutics 2024, 16, 1308. https://doi.org/10.3390/pharmaceutics16101308
Colaço M, Cruz MT, Almeida LPd, Borges O. Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors. Pharmaceutics. 2024; 16(10):1308. https://doi.org/10.3390/pharmaceutics16101308
Chicago/Turabian StyleColaço, Mariana, Maria T. Cruz, Luís Pereira de Almeida, and Olga Borges. 2024. "Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors" Pharmaceutics 16, no. 10: 1308. https://doi.org/10.3390/pharmaceutics16101308
APA StyleColaço, M., Cruz, M. T., Almeida, L. P. d., & Borges, O. (2024). Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors. Pharmaceutics, 16(10), 1308. https://doi.org/10.3390/pharmaceutics16101308








