Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Virus Propagation
2.3. Toxicity of Drug Combinations
2.4. Antiviral Testing
2.5. RNA Extraction and qRT-PCR
2.6. Determination of Infectious Titer
2.7. Statistical Analysis
3. Results
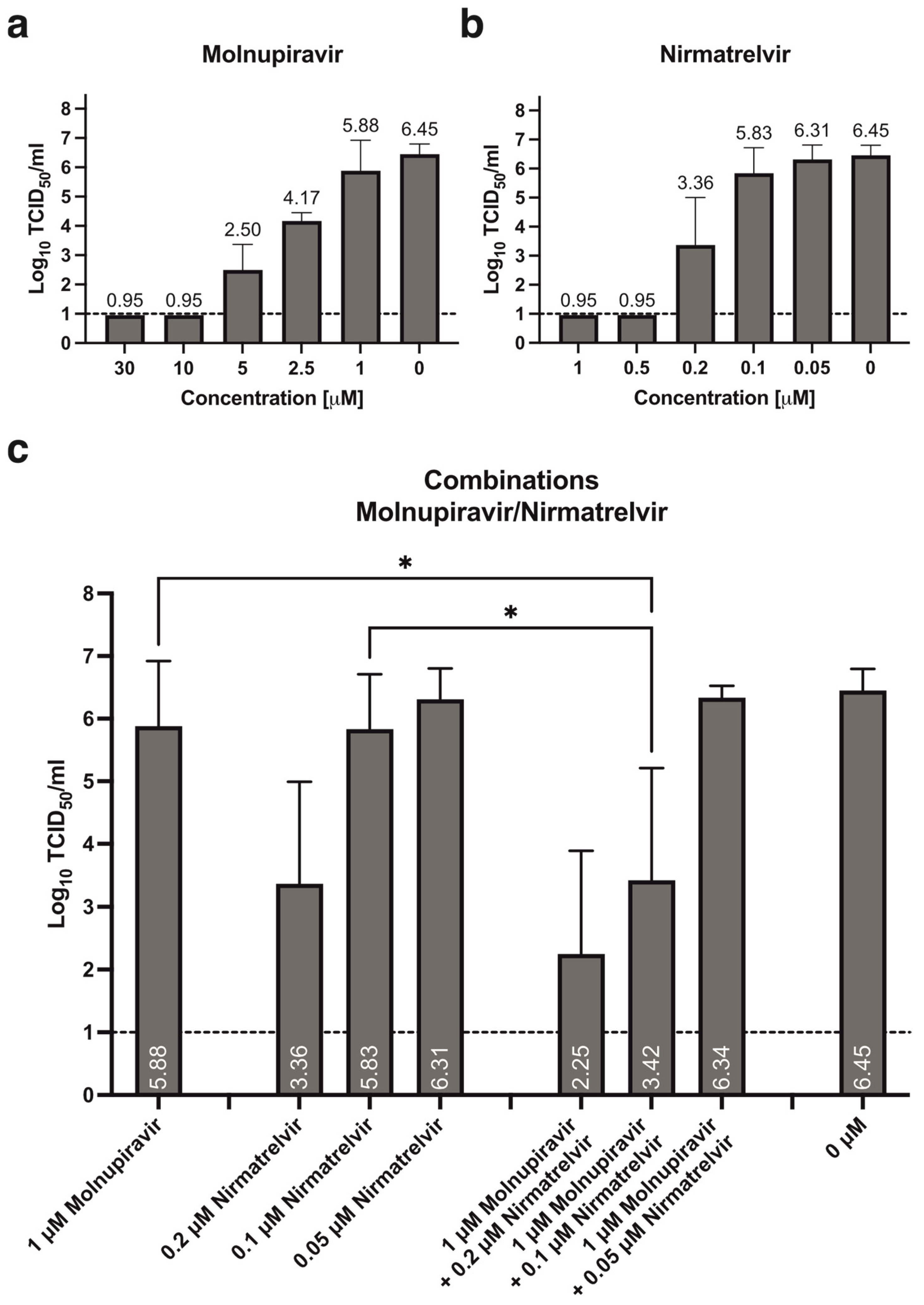
3.1. Antiviral Activity of Molnupiravir and Nirmatrelvir, Alone or in Combination
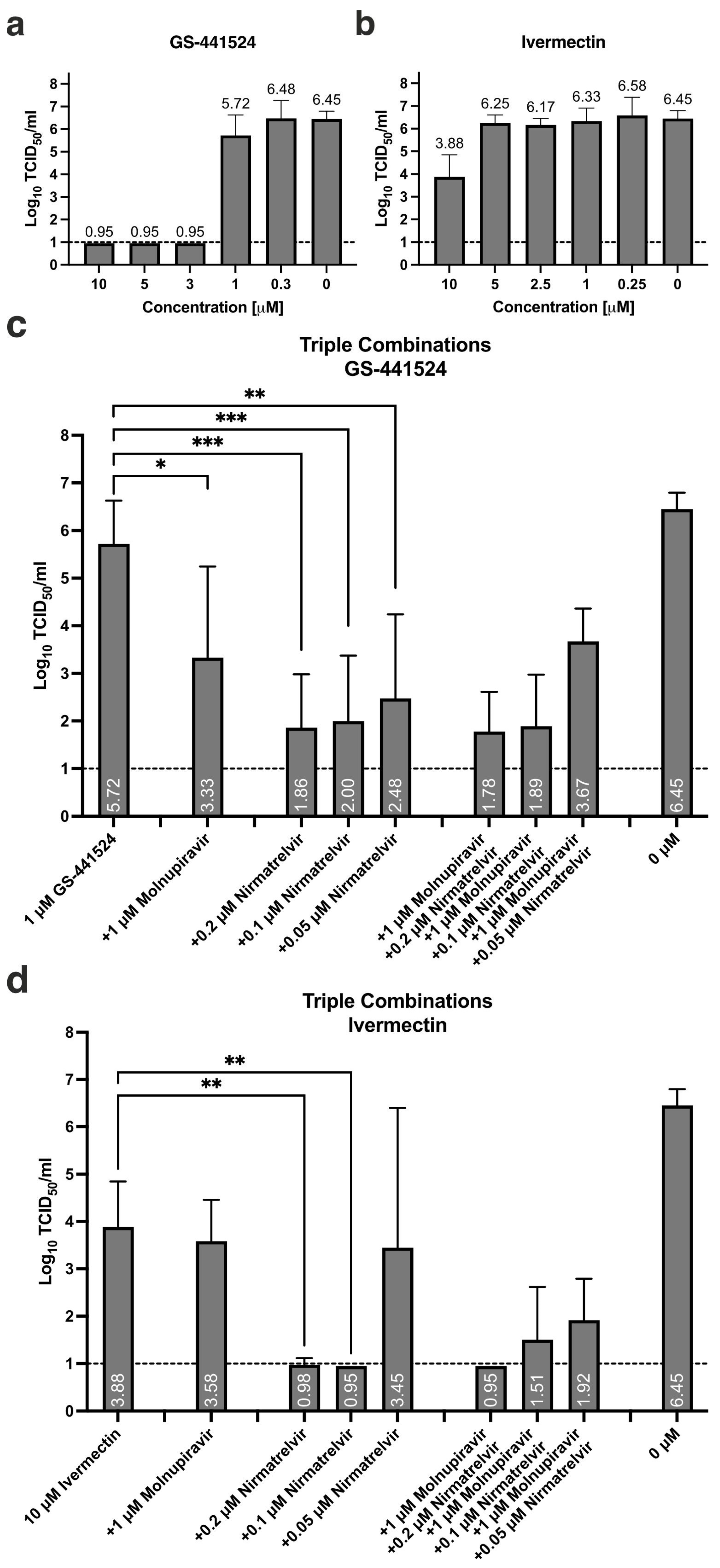
3.2. Antiviral Activity of GS-441524, Alone or in Combination with Molnupiravir and/or Nirmatrelvir
3.3. Antiviral Activity of Ivermectin, Alone or in Combination with Molnupiravir and/or Nirmatrelvir
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Richard Peto, F.R.; Karim, Q.A.; Marissa Alejandria, M.D.; Henao-Restrepo, A.M.; García, C.H.; Kieny, M.P.; Reza Malekzadeh, M.D.; Murthy, S.; Srinath Reddy, M.D.; et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [PubMed]
- Beigel, J.H. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hobbs, F.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2023, 401, 281–293. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.X.; Shuai, H.; Xia, A.J.; Hou, Y.; Zeng, R.; Liu, X.L.; Lin, G.F.; Qiao, J.X.; Li, W.P.; Wang, F.L.; et al. An orally available M(pro) inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron. Nat. Microbiol. 2022, 7, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Tada, S.; Fujita, S.; Hirakawa, T.; Matsumura, M.; Isoyama, S.; Ueno, S.; Hamai, K.; Tsuji, N.; Hirosawa, H.; et al. Effect of baricitinib in patients with coronavirus disease 2019 and respiratory failure: A propensity score-matched retrospective cohort study. Respir. Investig. 2022, 60, 418–424. [Google Scholar] [CrossRef]
- Rubin, R. Baricitinib Is First Approved COVID-19 Immunomodulatory Treatment. JAMA 2022, 327, 2281. [Google Scholar] [CrossRef] [PubMed]
- Kodde, C.; Timmen, F.; Hohenstein, S.; Bollmann, A.; Bonsignore, M.; Kuhlen, R.; Nachtigall, I.; Tasci, S. Impact of Dexamethasone on the Pathogen Profile of Critically Ill COVID-19 Patients. Viruses 2023, 15, 1076. [Google Scholar] [CrossRef] [PubMed]
- Arcani, R.; Cauchois, R.; Suchon, P.; Jean, R.; Jarrot, P.A.; Gomes De Pinho, Q.; Dalmas, J.B.; Jean, E.; Andre, B.; Veit, V.; et al. Factors associated with dexamethasone efficacy in COVID-19. A retrospective investigative cohort study. J. Med. Virol. 2022, 94, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Tolzali, M.M.R.; Noori, M.; Shokri, P.; Rahmani, S.; Khanzadeh, S.; Nejadghaderi, S.A.; Fazlollahi, A.; Sullman, M.J.M.; Singh, K.; Kolahi, A.; et al. Efficacy of tocilizumab in the treatment of COVID-19: An umbrella review. Rev. Med. Virol. 2022, 32, e2388. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, O.; Barg, W.; Slifirczyk, A.; Oliynyk, Y.; Gurianov, V.; Rorat, M. Efficacy of Tocilizumab Therapy in Different Subtypes of COVID-19 Cytokine Storm Syndrome. Viruses 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Diabetes and coronavirus (SARS-CoV-2): Molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLoS Pathog. 2021, 17, e1009634. [Google Scholar] [CrossRef]
- Cory, T.J.; Emmons, R.S.; Yarbro, J.R.; Davis, K.L.; Pence, B.D. Metformin Suppresses Monocyte Immunometabolic Activation by SARS-CoV-2 Spike Protein Subunit 1. Front. Immunol. 2021, 12, 733921. [Google Scholar] [CrossRef]
- Chaccour, C.; Casellas, A.; Blanco-Di Matteo, A.; Pineda, I.; Fernandez-Montero, A.; Ruiz-Castillo, P.; Richardson, M.A.; Rodríguez-Mateos, M.; Jordán-Iborra, C.; Brew, J.; et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021, 32, 100720. [Google Scholar] [CrossRef]
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Martínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.J.; Drusano, G.L.; Hanrahan, K.C.; Warfield, K.L.; Brown, A.N. Combination Therapy with UV-4B and Molnupiravir Enhances SARS-CoV-2 Suppression. Viruses 2023, 15, 1175. [Google Scholar] [CrossRef] [PubMed]
- Orth, H.M.; Flasshove, C.; Berger, M.; Hattenhauer, T.; Biederbick, K.D.; Mispelbaum, R.; Klein, U.; Stemler, J.; Fisahn, M.; Doleschall, A.D.; et al. Early combination therapy of COVID-19 in high-risk patients. Infection 2024, 52, 877–889. [Google Scholar] [CrossRef]
- Günthard, H.F.; Calvez, V.; Paredes, R.; Pillay, D.; Shafer, R.W.; Wensing, A.M.; Jacobsen, D.M.; Richman, D.D. Human Immunodeficiency Virus Drug Resistance: 2018 Recommendations of the International Antiviral Society-USA Panel. Clin. Infect. Dis. 2019, 68, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-H.; Liu, C.-J. Combination Therapy for Chronic Hepatitis B: Current Updates and Perspectives. Gut Liver 2017, 11, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Monto, A.S. Resistance of Influenza Virus to Antiviral Medications. Clin. Infect. Dis. 2020, 71, 1092–1094. [Google Scholar] [CrossRef]
- Rong, L.; Dahari, H.; Ribeiro, R.M.; Perelson, A.S. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci. Transl. Med. 2010, 2, 30ra32. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Siegrist, D.; Julien, T.; Padey, B.; Bouveret, M.; Terrier, O.; Pizzorno, A.; Huang, S.; Samby, K.; Wells, T.N.; et al. Molnupiravir combined with different repurposed drugs further inhibits SARS-CoV-2 infection in human nasal epithelium in vitro. Biomed. Pharmacother. 2022, 150, 113058. [Google Scholar] [CrossRef] [PubMed]
- Maas, B.M.; Strizki, J.; Miller, R.R.; Kumar, S.; Brown, M.; Johnson, M.G.; Cheng, M.; De Anda, C.; Rizk, M.L.; Stone, J.A. Molnupiravir: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2024, 17, e13732. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Lavrijsen, M.; Lamers, M.M.; de Vries, A.C.; Rottier, R.J.; Bruno, M.J.; Peppelenbosch, M.P.; Haagmans, B.L.; Pan, Q.; et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022, 32, 322–324. [Google Scholar] [CrossRef]
- Alvarez, J.-C.; Moine, P.; Etting, I.; Annane, D.; Larabi, I.A. Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a COVID-19 treated patient. Clin. Chem. Lab. Med. 2020, 58, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The mechanisms of action of ivermectin against SARS-CoV-2—An extensive review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewski, L.; Jornot, L.; Dudez, T.; Pagano, A.; Rochat, T.; Lacroix, J.S.; Suter, S.; Chanson, M. Long-term cultures of polarized airway epithelial cells from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2006, 34, 39–48. [Google Scholar] [CrossRef]
- Spearman, C. The method of ‘right and wrong cases’ (‘constant stimuli’) without gauss’s formulae. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Günthard, H.F.; Saag, M.S.; Benson, C.A.; Del Rio, C.; Eron, J.J.; Gallant, J.E.; Hoy, J.F.; Mugavero, M.J.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA 2016, 316, 191–210. [Google Scholar] [CrossRef]
- Naggie, S.; Muir, A.J. Oral Combination Therapies for Hepatitis C Virus Infection: Successes, Challenges, and Unmet Needs. Annu. Rev. Med. 2017, 68, 345–358. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 drug discovery and treatment options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, R.; Cerchia, C.; Beccari, A.R.; Di Leva, F.S.; Summa, V. Targeting SARS-CoV-2 Proteases and Polymerase for COVID-19 Treatment: State of the Art and Future Opportunities. J. Med. Chem. 2022, 65, 2716–2746. [Google Scholar] [CrossRef] [PubMed]
- Martonik, D.; Parfieniuk-Kowerda, A.; Starosz, A.; Grubczak, K.; Moniuszko, M.; Flisiak, R. Effect of antiviral and immunomodulatory treatment on a cytokine profile in patients with COVID-19. Front. Immunol. 2023, 14, 1222170. [Google Scholar] [CrossRef] [PubMed]
- Moshawih, S.; Jarrar, Q.; Bahrin, A.A.; Lim, A.F.; Ming, L.; Goh, H.P. Evaluating NSAIDs in SARS-CoV-2: Immunomodulatory mechanisms and future therapeutic strategies. Heliyon 2024, 10, e25734. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Dhawan, M.; Saied, A.A.; Kaur, G.; Kumar, R.; Bin Emran, T. Immunomodulatory approaches in managing lung inflammation in COVID-19: A double-edge sword. Immun. Inflamm. Dis. 2023, 11, e1020. [Google Scholar] [CrossRef]
- Rosenke, K.; Lewis, M.C.; Feldmann, F.; Bohrnsen, E.; Schwarz, B.; Okumura, A.; Bohler, W.F.; Callison, J.; Shaia, C.; Bosio, C.M.; et al. Combined molnupiravir-nirmatrelvir treatment improves the inhibitory effect on SARS-CoV-2 in macaques. JCI Insight 2023, 8, e166485. [Google Scholar] [CrossRef]
- Wang, X.; Sacramento, C.Q.; Jockusch, S.; Chaves, O.A.; Tao, C.; Fintelman-Rodrigues, N.; Chien, M.; Temerozo, J.R.; Li, X.; Kumar, S.; et al. Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture. Commun. Biol. 2022, 5, 154. [Google Scholar] [CrossRef]
- Peña-Silva, R.; Duffull, S.B.; Steer, A.C.; Jaramillo-Rincon, S.X.; Gwee, A.; Zhu, X. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19. Br. J. Clin. Pharmacol. 2021, 87, 1589–1590. [Google Scholar] [CrossRef]
- Canga, A.G.; Prieto, A.M.S.; Liébana, M.J.D.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J.G. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef]
| Concentration (µM) | Log10 Fold Change RNA | Infectious Titer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molnupiravir | PF-07321332 | GS-441524 | Ivermectin | 48 h | ±SD | 72 h | ±SD | IC | ±SD | Log10 TCID50/mL | ±SD |
| 30 | −3.69 | 0.33 | −3.94 | 0.07 | −4.62 | 0.04 | LOD | n/a | |||
| 10 | −4.13 | 1.23 | −3.20 | 0.43 | −3.43 | 0.34 | LOD | n/a | |||
| 5 | −2.02 | 0.38 | −0.85 | 0.16 | −0.83 | 0.26 | 2.50 | 0.71 | |||
| 2.5 | −0.74 | 0.06 | −0.47 | 0.02 | −0.28 | 0.14 | 4.17 | 0.24 | |||
| 1 | −0.21 | 0.10 | −0.21 | 0.19 | −0.23 | 0.20 | 5.88 | 0.96 | |||
| 0.5 | −0.17 | 0.01 | −0.07 | 0.07 | −0.08 | 0.04 | - | - | |||
| 0.1 | −0.32 | 0.06 | −0.03 | 0.01 | 0.06 | 0.05 | - | - | |||
| 5 | −4.79 | 0.13 | −4.17 | 0.64 | −4.96 | 0.03 | - | - | |||
| 1 | −4.14 | 0.83 | −3.99 | 0.16 | −4.80 | 0.38 | LOD | n/a | |||
| 0.5 | −3.80 | 0.23 | −4.10 | 0.18 | −4.82 | 0.31 | LOD | n/a | |||
| 0.2 | −3.20 | 0.10 | −2.97 | 0.67 | −3.39 | 0.74 | 3.36 | 1.41 | |||
| 0.1 | −2.28 | 0.86 | −0.93 | 0.60 | −0.73 | 0.56 | 5.83 | 0.81 | |||
| 0.05 | −0.44 | 0.21 | −0.19 | 0.12 | 0.00 | 0.16 | 6.31 | 0.46 | |||
| 0.01 | 0.22 | 0.06 | −0.13 | 0.26 | −0.05 | 0.15 | 6.00 | 0.00 | |||
| 10 | −3.89 | 0.12 | −3.85 | 0.74 | −4.79 | 0.21 | LOD | n/a | |||
| 5 | −3.07 | 0.22 | −4.07 | 0.27 | −5.31 | 0.17 | LOD | n/a | |||
| 3 | −3.33 | 0.34 | −3.38 | 0.33 | −4.40 | 0.38 | LOD | n/a | |||
| 1 | −1.12 | 0.11 | −0.88 | 0.10 | −1.67 | 0.15 | 5.72 | 0.84 | |||
| 0.3 | −0.01 | 0.08 | −0.16 | 0.12 | −0.26 | 0.19 | 6.48 | 0.73 | |||
| 10 | −0.83 | 0.08 | −0.55 | 0.17 | −0.69 | 0.19 | 3.89 | 0.97 | |||
| 5 | −0.70 | 0.09 | 0.27 | 0.05 | 0.30 | 0.07 | 6.25 | 0.25 | |||
| 2.5 | −0.78 | 1.07 | 0.06 | 0.24 | 0.45 | 0.14 | 6.17 | 0.24 | |||
| 1 | −0.31 | 0.24 | 0.17 | 0.14 | 0.29 | 0.19 | 6.33 | 0.47 | |||
| 0.5 | −0.08 | 0.08 | 0.02 | 0.12 | 0.26 | 0.19 | 6.33 | 0.47 | |||
| 0.25 | 0.16 | 0.20 | 0.04 | 0.08 | −0.13 | 0.17 | 6.58 | 0.73 | |||
| 0 | 0 | 0 | 0 | 0.16 | 0.20 | 0.01 | 0.06 | −0.01 | 0.06 | 6.45 | 0.32 |
| Concentration (µM) | Log10 Fold Change RNA | Infectious Titer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Molnupiravir | PF-07321332 | GS-441524 | Ivermectin | 48 h | ±SD | 72 h | ±SD | IC | ±SD | Log10 TCID50/mL | ±SD |
| 1 | −0.21 | 0.10 | −0.21 | 0.19 | −0.23 | 0.20 | 5.88 | 0.96 | |||
| 0.2 | −3.20 | 0.10 | −2.97 | 0.67 | −3.39 | 0.74 | 3.36 | 1.41 | |||
| 0.1 | −2.28 | 0.86 | −0.93 | 0.60 | −0.73 | 0.56 | 5.83 | 0.81 | |||
| 0.05 | −0.44 | 0.21 | −0.19 | 0.12 | 0.00 | 0.16 | 6.31 | 0.46 | |||
| 1 | −1.12 | 0.11 | −0.88 | 0.10 | −1.67 | 0.15 | 5.72 | 0.84 | |||
| 0.3 | −0.01 | 0.08 | −0.16 | 0.12 | −0.26 | 0.19 | 6.48 | 0.73 | |||
| 10 | −0.83 | 0.08 | −0.55 | 0.17 | −0.69 | 0.19 | 3.89 | 0.97 | |||
| 0.25 | 0.16 | 0.20 | 0.04 | 0.08 | −0.13 | 0.17 | 6.58 | 0.73 | |||
| 1 | 0.2 | −2.97 | 0.12 | −3.40 | 0.94 | −3.90 | 0.79 | 2.25 | 1.64 | ||
| 1 | 0.1 | −2.62 | 0.51 | −2.41 | 1.47 | −2.48 | 1.49 | 3.42 | 1.79 | ||
| 1 | 0.05 | −0.98 | 0.13 | −0.50 | 0.11 | −0.18 | 0.29 | 6.33 | 0.19 | ||
| 1 | 1 | −2.25 | 1.49 | −2.09 | 1.26 | −2.81 | 1.87 | 3.33 | 1.91 | ||
| 1 | 0.3 | −1.74 | 0.93 | −0.55 | 0.11 | −0.95 | 0.38 | 5.83 | 0.54 | ||
| 1 | 10 | −1.76 | 0.13 | −2.23 | 0.40 | −2.80 | 0.35 | 3.58 | 0.88 | ||
| 1 | 0.25 | −0.35 | 0.31 | −0.32 | 0.30 | −0.55 | 0.14 | 6.00 | 0.33 | ||
| 0.2 | 1 | −3.11 | 0.19 | −3.84 | 0.51 | −4.58 | 0.18 | 1.86 | 1.12 | ||
| 0.2 | 0.3 | −3.07 | 0.26 | −3.41 | 0.61 | −4.08 | 0.65 | 2.31 | 1.57 | ||
| 0.2 | 10 | −3.03 | 0.24 | −3.73 | 0.51 | −4.67 | 0.41 | 0.98 | 0.14 | ||
| 0.2 | 0.25 | −2.83 | 0.37 | −3.13 | 0.69 | −3.63 | 0.81 | 2.92 | 1.55 | ||
| 0.1 | 1 | −3.09 | 0.26 | −3.43 | 0.64 | −4.42 | 0.29 | 2.00 | 1.37 | ||
| 0.1 | 0.3 | −2.46 | 0.31 | −1.66 | 0.66 | −1.85 | 0.43 | 4.83 | 0.27 | ||
| 0.1 | 10 | −3.06 | 0.18 | −3.60 | 0.65 | −4.13 | 0.75 | LOD | n/a | ||
| 0.1 | 0.25 | −2.73 | 0.40 | −2.43 | 1.12 | −2.29 | 0.77 | 4.42 | 1.34 | ||
| 0.05 | 1 | −2.79 | 0.85 | −2.90 | 0.91 | −3.53 | 1.47 | 2.48 | 1.76 | ||
| 0.05 | 0.3 | −1.95 | 1.25 | −1.10 | 0.63 | −1.59 | 0.74 | 5.83 | 0.27 | ||
| 0.05 | 10 | −2.22 | 0.43 | −2.29 | 1.53 | −2.70 | 1.46 | 3.45 | 2.95 | ||
| 0.05 | 0.25 | −0.65 | 0.09 | −0.57 | 0.23 | −0.44 | 0.20 | 6.50 | 0.27 | ||
| 1 | 0.2 | 1 | −3.27 | 0.29 | −3.70 | 0.48 | −4.38 | 0.27 | 1.78 | 0.83 | |
| 1 | 0.1 | 1 | −3.29 | 0.24 | −3.53 | 0.38 | −4.30 | 0.12 | 1.89 | 1.08 | |
| 1 | 0.05 | 1 | −2.81 | 0.54 | −2.59 | 1.08 | −3.05 | 0.90 | 3.67 | 0.69 | |
| 1 | 0.2 | 0.3 | −2.95 | 0.15 | −3.37 | 0.52 | −3.89 | 0.12 | 1.70 | 1.24 | |
| 1 | 0.1 | 0.3 | −2.72 | 0.47 | −2.55 | 1.11 | −2.50 | 1.22 | 4.00 | 1.93 | |
| 1 | 0.05 | 0.3 | −1.27 | 0.36 | −0.73 | 0.65 | −1.20 | 0.68 | 5.50 | 0.86 | |
| 1 | 0.2 | 10 | −3.16 | 0.17 | −3.71 | 0.49 | −4.43 | 0.19 | LOD | n/a | |
| 1 | 0.1 | 10 | −3.14 | 0.25 | −3.26 | 0.40 | −3.64 | 0.51 | 1.51 | 1.11 | |
| 1 | 0.05 | 10 | −2.52 | 0.22 | −3.07 | 0.45 | −3.70 | 0.76 | 1.92 | 0.88 | |
| 1 | 0.2 | 0.25 | −2.89 | 0.33 | −3.20 | 0.52 | −3.55 | 0.43 | 3.08 | 0.69 | |
| 1 | 0.1 | 0.25 | −2.65 | 0.37 | −2.60 | 1.03 | −2.34 | 0.74 | 4.08 | 1.07 | |
| 1 | 0.05 | 0.25 | −1.15 | 0.22 | −0.80 | 0.40 | −0.87 | 0.19 | 5.42 | 0.57 | |
| 0 | 0 | 0 | 0 | 0.16 | 0.20 | 0.01 | 0.06 | −0.01 | 0.06 | 6.45 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siegrist, D.; Jonsdottir, H.R.; Bouveret, M.; Boda, B.; Constant, S.; Engler, O.B. Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia. Pharmaceutics 2024, 16, 1262. https://doi.org/10.3390/pharmaceutics16101262
Siegrist D, Jonsdottir HR, Bouveret M, Boda B, Constant S, Engler OB. Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia. Pharmaceutics. 2024; 16(10):1262. https://doi.org/10.3390/pharmaceutics16101262
Chicago/Turabian StyleSiegrist, Denise, Hulda R. Jonsdottir, Mendy Bouveret, Bernadett Boda, Samuel Constant, and Olivier B. Engler. 2024. "Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia" Pharmaceutics 16, no. 10: 1262. https://doi.org/10.3390/pharmaceutics16101262
APA StyleSiegrist, D., Jonsdottir, H. R., Bouveret, M., Boda, B., Constant, S., & Engler, O. B. (2024). Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia. Pharmaceutics, 16(10), 1262. https://doi.org/10.3390/pharmaceutics16101262





