Electrospun Antimicrobial Drug Delivery Systems and Hydrogels Used for Wound Dressings
Abstract
1. Introduction
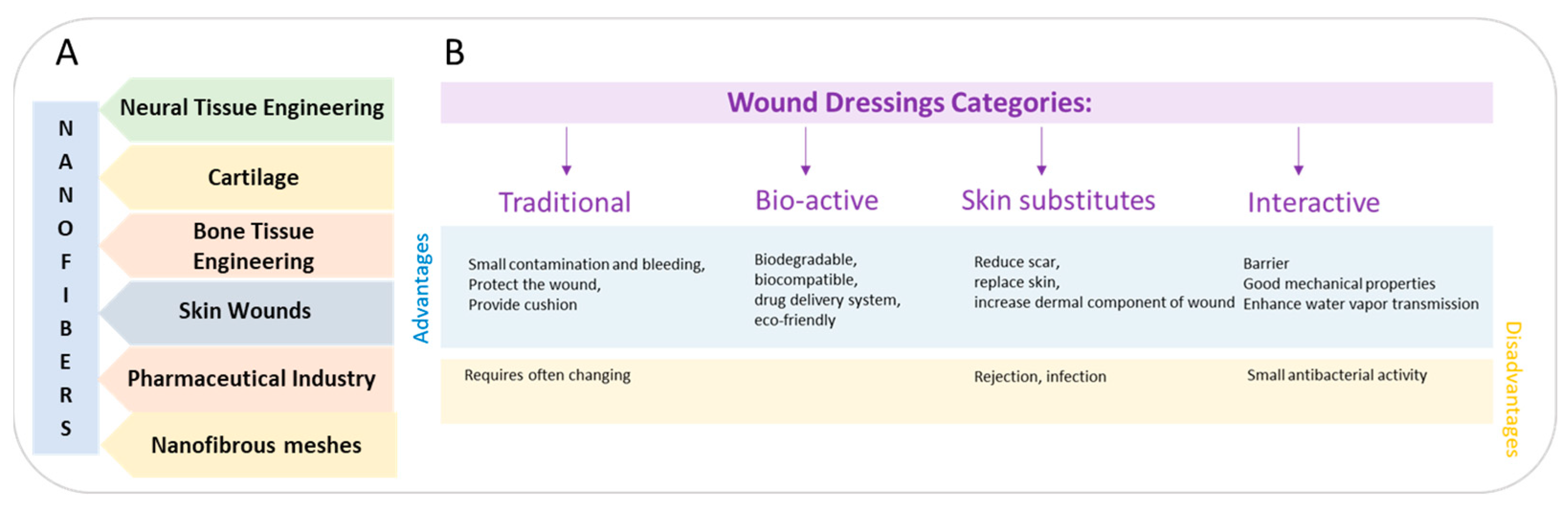
2. Commercial Wound Dressings
2.1. Traditional Wound Dressings
2.2. Interactive and Bioactive Wound Dressings
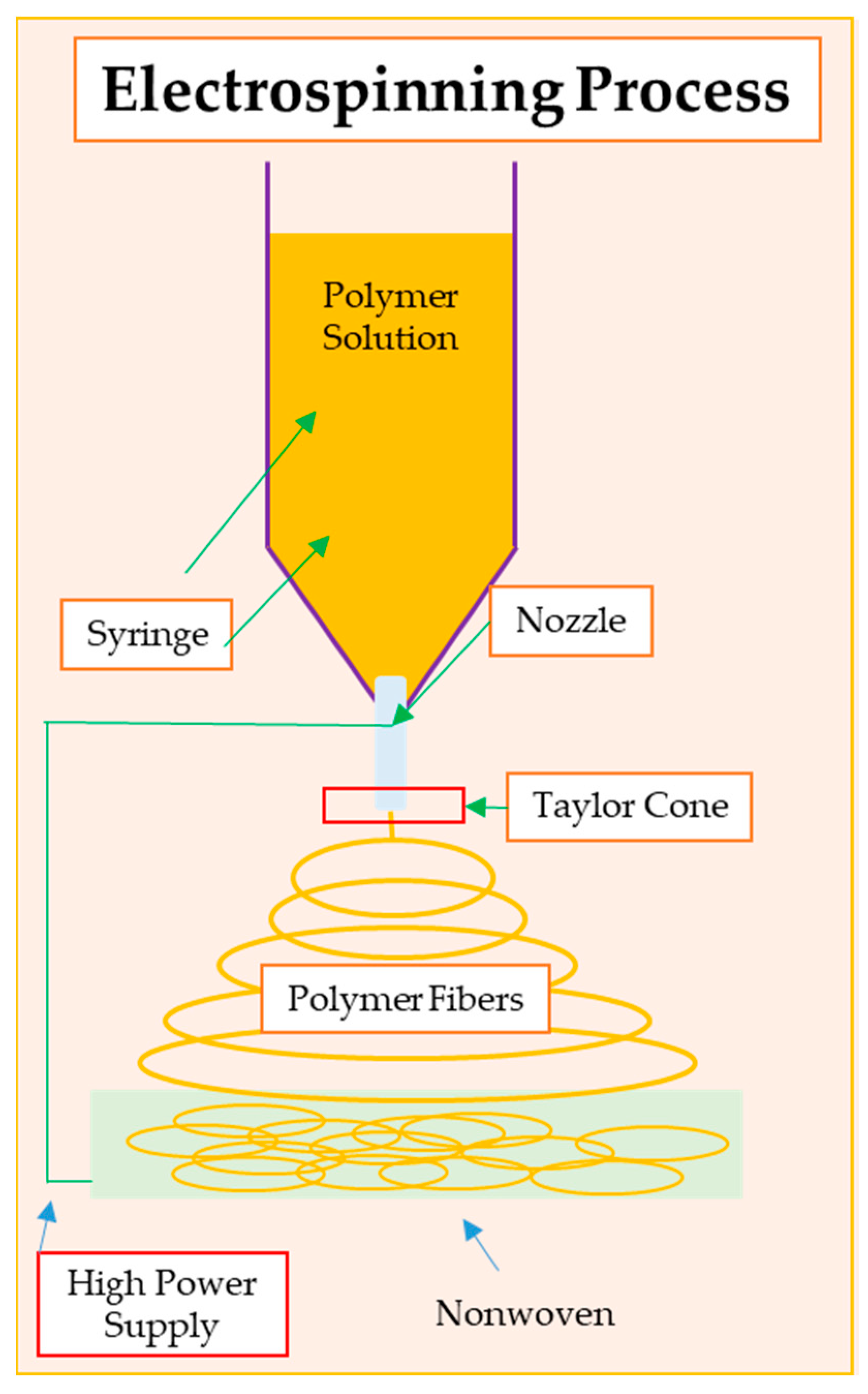
3. Electrospinning as a Method for Wound Dressing Formation
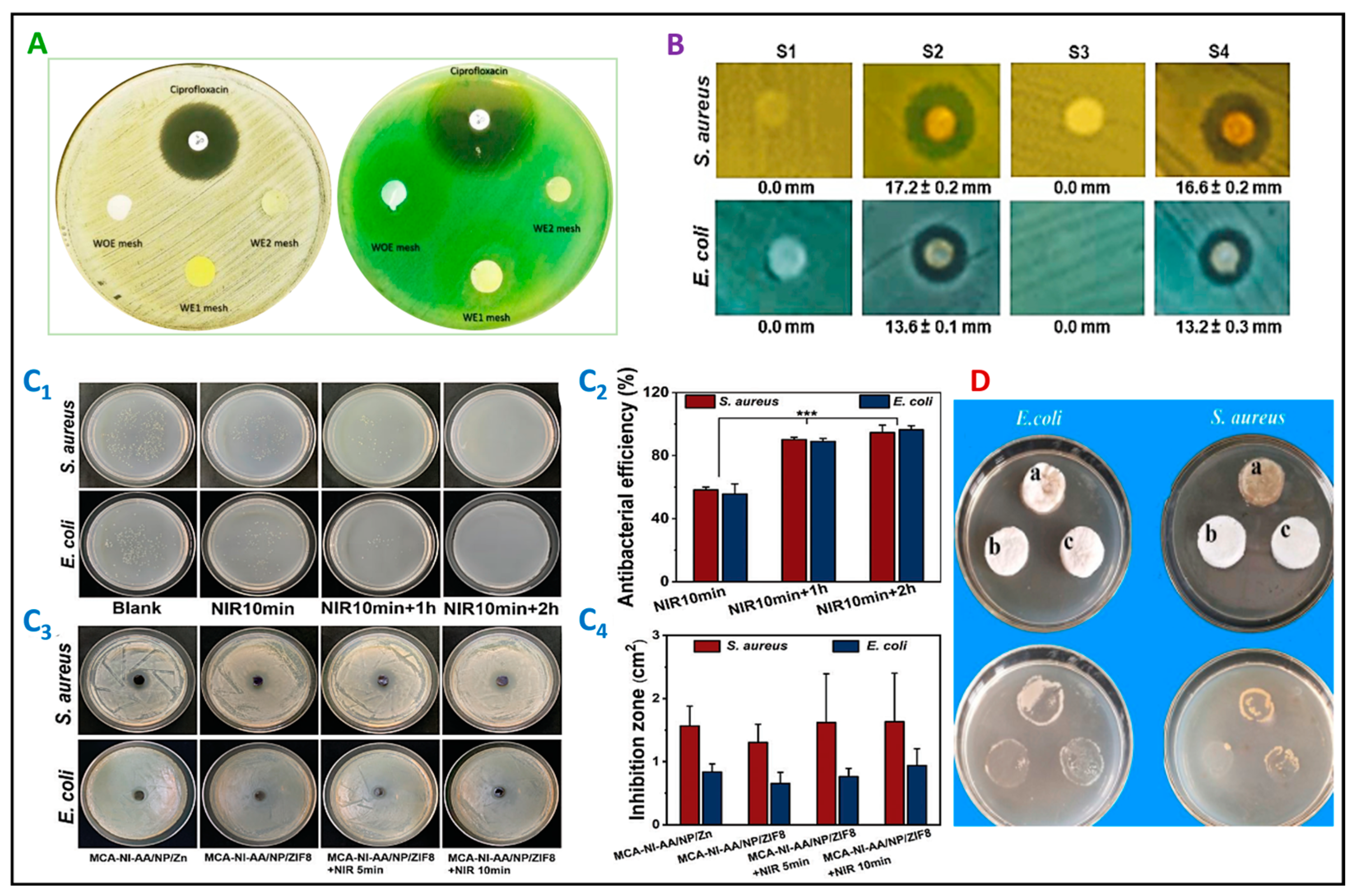
4. Antibacterial Nanofibers for Wound Dressing
4.1. Biopolymeric Nanofibrous Wound Dressings Containing Antibacterial Nanoparticles
| Polymeric Component | Antibacterial/Antimicrobial Component/Polymer | Aim and Application | Ref. |
|---|---|---|---|
| PMMA | Ag NPs, OCT | Control the drug release, 3D multilayered porous scaffold diabetic wounds, and repair skin | [76] |
| PVA/PLA | (PVA-CTX/PLA) and tranexamic acid coagulant (PVA-CTX-TXA/PLA) | Drug release, scaffold can be used to treat burns and chronic and diabetic wound infections | [121] |
| PCL nanofibers | Photoresponsive nanogel containing Ag NPs | Clinical application as wound dressing activated by light | [114] |
| PVA and carbon nanotubes | Ag NPs | Antibacterial wound dressing | [125] |
| PCL, HAP | Ag+ | Scaffold with appropriate characteristics for wound healing | [111] |
| PLA and PCL | Ag-chitosan NPs | Tissue regeneration and wound healing | [126] |
| PLA/chitosan | Ag+ | High antibacterial activity and a high potential for applications in biomedical fields | [85] |
| PLA | Chitosan, copper, or silver-doped bioactive glasses | Good in vitro bioactivity of the fibers and possible bone tissue regeneration | [120] |
| Chitosan, PVA | ZnO NPs | Accelerated wound healing. Nanofibrous mat with antibacterial and antioxidant properties for diabetic wound healing | [116] |
| PCL, collagen, zein | Aloe vera, and ZnO NPs | Biocompatible and non-toxic materials for wound dressing | [127] |
| PCL, gelatin | ZnO NPs, amoxicillin | treatment of full-thickness wounds | [128] |
| PMMA | Ag NPs, Octenidin (OCT) | Control the drug release, 3D multilayered porous scaffold diabetic wounds, and repair skin | [129] |
| Polymeric Component | Antibacterial/Antimicrobial Component | Method of Preparing | Aim and Application | Ref. |
|---|---|---|---|---|
| Chitosan, bacterial cellulose | Chitooligosaccharide | Composite membranes | Good antioxidant activity and wound-dressing applications | [130] |
| Collagen (type I) | Tobramycin (Tob) | Film casting | Potential application in corneal repair | [131] |
| Chitosan | Quaternary ammonium chitosan NPs (TMC NPs)/chitosan | Lyophilization (sponge composite) | Promising dressing material for chronic wounds | [37] |
| Curcumin-β-cycyclodextrin inclusion complex (CMx) and cellulose | Chitosan | Freeze drying (sponge composite) | Wound-dressing materials for the treatment of wounds (especially chronic wounds) | [132] |
| Chitosan | Hydroxybutyl chitosan | Freeze drying (sponge composite) | Excellent antibacterial activity of composite sponge to be applied for wound dressings | [133] |
| Chitosan | Chitosan | 3D printing | Improving the quality of the restored tissue concerning both commercial patches and spontaneous healing | [134] |
| PCL and silk sericin | Chitosan/sodium alginate hydrogel | Electrospin ning and 3D bioprinting | Promoting the healing process and skin tissue engineering | [135] |
| Chitosan-PEG | Cu NPs | Hydrogel | Sustained drug release with excellent keratinocyte cell response and anti-infection wound dressing | [136] |
4.2. Biofunctionalized Antibacterial Nanofibers for Wound Dressings
4.2.1. Silk Fibroin (SF)
4.2.2. Chitosan
5. Hydrogels with Nanofibers and Functional Thermoresponsive Hydrogels as Wound Dressings
5.1. Hydrogels with Nanofibers
5.2. Thermoresponsive Hydrogels with Antibacterial Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| AE | Allium sativum aqueous extract |
| Ag20 | Ag nanoparticle (core) |
| AgHG | PNIPAM microgel particle (core) |
| AMPs | antimicrobial peptides |
| CE | Cleome droserifolia |
| CIP | ciprofloxacin |
| CMx | cyclodextrin inclusion complex |
| CNFs | cellulose nanofibers |
| CNTs | carbon nanotubes |
| Cys-KR12 | antimicrobial peptide motif |
| DFUs | diabetic foot ulcers |
| ECM | extracellular collagen matrix |
| EGF | epidermal growth factor |
| GelMA | gelatin methacryloyl |
| GEN | gentamicin |
| GSNO | S-nitrosoglutathione |
| HA | hyaluronic acid |
| HAP | hydroxyapatite |
| Hes | hesperidin |
| HPCS | hydroxypropyl chitosan |
| HT | hardystonite |
| L929 | mouse fibroblast cell line |
| LL37 | human cathelicidin peptide |
| LPSs | lipopolysaccharides |
| MA-K-CA | methacrylated κ—carrageenan |
| MCA-NI-AA | methacrylated κ—carrageenan, poly(N-isopropylacrylamide) and acrylic acid |
| NIR | near-infrared |
| NPs | nanoparticles |
| NRs | nanorods |
| OCT | octenidine |
| PCL | poly(ε-caprolactone) |
| PDA | polydopamine |
| PDLLA | poly(d,l-lactic acid) |
| PEG | poly(ethylene glycol) |
| PL | Pluronic F-127 |
| PLA | poly(lactic acid) |
| PMMA | poly(methyl methacrylate) |
| PNIPAM | poly(N-isopropylacrylamide) |
| PPy | polypyrrole |
| PPZn | Zn nanoparticle (shell) |
| PVA | poly(vinyl alcohol) |
| RhB | rhodamine B |
| SF | silk fibroin |
| TMC | quaternary ammonium chitosan |
| Tob | tobramycin |
| ZIF-8 | zeolitic imidazolate framework |
References
- Park, S. Biochemical, Structural and Physical Changes in Aging Human Skin, and Their Relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent Progress of Antibacterial Hydrogels in Wound Dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Niazi, M.; Ramakrishna, S. The Evolution of Wound Dressings: From Traditional to Smart Dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Gradys, A.; Sajkiewicz, P. Progress in the Applications of Smart Piezoelectric Materials for Medical Devices. Polymers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Sajkiewicz, P.; Gradys, A.; Tymkiewicz, R.; Urbanek, O.; Kołbuk, D. Influence of Process-Material Conditions on the Structure and Biological Properties of Electrospun Polyvinylidene Fluoride Fibers. Bull. Pol. Acad. Sci. Tech. Sci. 2020, 68, 627–633. [Google Scholar] [CrossRef]
- Jundziłł, A.; Pokrywczyńska, M.; Adamowicz, J.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Frontczak-Baniewicz, M.; Mikułowski, G.; Kloskowski, T.; et al. Vascularization Potential of Electrospun Poly(L-Lactide-Co-Caprolactone) Scaffold: The Impact for Tissue Engineering. Med. Sci. Monit. 2017, 23, 1540–1551. [Google Scholar] [CrossRef][Green Version]
- Kloskowski, T.; Jundziłł, A.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Pokrywczyńska, M.; Frontczak-Baniewicz, M.; Kowalewski, T.A.; Chłosta, P.; et al. Ureter Regeneration–The Proper Scaffold Has to Be Defined. PLoS ONE 2014, 9, e106023. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel Drug Delivery Systems Based on Triaxial Electrospinning Based Nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Nakielski, P.; Kowalczyk, T.; Zembrzycki, K.; Kowalewski, T.A. Experimental and Numerical Evaluation of Drug Release from Nanofiber Mats to Brain Tissue. J. Biomed. Mater. Res. 2015, 103, 282–291. [Google Scholar] [CrossRef]
- Moazzami Goudarzi, Z.; Soleimani, M.; Ghasemi-Mobarakeh, L.; Sajkiewicz, P.; Sharifianjazi, F.; Esmaeilkhanian, A.; Khaksar, S. Control of Drug Release from Cotton Fabric by Nanofibrous Mat. Int. J. Biol. Macromol. 2022, 217, 270–281. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sanket, A.S.; Pradhan, S.; Sahoo, R.; Das, S.; Pany, S.; Douglas, T.E.L.; Dandela, R.; Liu, Q.; Rajadas, J.; et al. Designing of Gradient Scaffolds and Their Applications in Tissue Regeneration. Biomaterials 2023, 296, 122078. [Google Scholar] [CrossRef]
- Ura, D.P.; Rosell-Llompart, J.; Zaszczyńska, A.; Vasilyev, G.; Gradys, A.; Szewczyk, P.K.; Knapczyk-Korczak, J.; Avrahami, R.; Šišková, A.O.; Arinstein, A.; et al. The Role of Electrical Polarity in Electrospinning and on the Mechanical and Structural Properties of As-Spun Fibers. Materials 2020, 13, 4169. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, Electrospun and Composite Materials for Bone/Cartilage and Neural Tissue Engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Chang, L.; Du, H.; Xu, F.; Xu, C.; Liu, H. Hydrogel-Enabled Mechanically Active Wound Dressings. Trends Biotechnol. 2023, 42, 31–42. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Bretcanu, O.; Misra, S.K.; Yunos, D.M.; Boccaccini, A.R.; Roy, I.; Kowalczyk, T.; Blonski, S.; Kowalewski, T.A. Electrospun Nanofibrous Biodegradable Polyester Coatings on Bioglass®-Based Glass-Ceramics for Tissue Engineering. Mater. Chem. Phys. 2009, 118, 420–426. [Google Scholar] [CrossRef]
- Noszczyk, B.H.; Kowalczyk, T.; Łyżniak, M.; Zembrzycki, K.; Mikułowski, G.; Wysocki, J.; Kawiak, J.; Pojda, Z. Biocompatibility of Electrospun Human Albumin: A Pilot Study. Biofabrication 2015, 7, 015011. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for Wound Healing: Scope and Advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Kowalczyk, T. Functional Micro- and Nanofibers Obtained by Nonwoven Post-Modification. Polymers 2020, 12, 1087. [Google Scholar] [CrossRef]
- Jain, A.; Michalska, M.; Zaszczyńska, A.; Denis, P. Surface Modification of Activated Carbon with Silver Nanoparticles for Electrochemical Double Layer Capacitors. J. Energy Storage 2022, 54, 105367. [Google Scholar] [CrossRef]
- Alberti, T.; Coelho, D.S.; Voytena, A.; Pitz, H.; De Pra, M.; Mazzarino, L.; Kuhnen, S.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Nanotechnology: A Promising Tool Towards Wound Healing. Curr. Pharm. Des. 2017, 23, 3515–3528. [Google Scholar] [CrossRef]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The Safety of Nanosized Particles in Titanium Dioxide– and Zinc Oxide–Based Sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Ambrosio, A.M.A.; Borden, M.D.; Cooper, J.A. Tissue Engineering: Orthopedic Applications. Annu. Rev. Biomed. Eng. 1999, 1, 19–46. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health Economic Burden That Wounds Impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef]
- Preem, L.; Kogermann, K. Electrospun Antimicrobial Wound Dressings: Novel Strategies to Fight Against Wound Infections. In Chronic Wounds, Wound Dressings and Wound Healing; Shiffman, M.A., Low, M., Eds.; Recent Clinical Techniques, Results, and Research in Wounds; Springer International Publishing: Cham, Switzerland, 2018; Volume 6, pp. 213–253. ISBN 978-3-030-10697-3. [Google Scholar]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound Healing and Treating Wounds. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Thomas, S. Hydrocolloid Dressings in the Management of Acute Wounds: A Review of the Literature. Int. Wound J. 2008, 5, 602–613. [Google Scholar] [CrossRef]
- Ataide, J.A.; Zanchetta, B.; Santos, É.M.; Fava, A.L.M.; Alves, T.F.R.; Cefali, L.C.; Chaud, M.V.; Oliveira-Nascimento, L.; Souto, E.B.; Mazzola, P.G. Nanotechnology-Based Dressings for Wound Management. Pharmaceuticals 2022, 15, 1286. [Google Scholar] [CrossRef]
- Bužarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Gualandi, C.; Avérous, L. Nanocomposite Foams Based on Flexible Biobased Thermoplastic Polyurethane and ZnO Nanoparticles as Potential Wound Dressing Materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef]
- Weng, W.; Chi, J.; Yu, Y.; Zhang, C.; Shi, K.; Zhao, Y. Multifunctional Composite Inverse Opal Film with Multiactives for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 4567–4573. [Google Scholar] [CrossRef]
- Cui, H.; Liu, M.; Yu, W.; Cao, Y.; Zhou, H.; Yin, J.; Liu, H.; Que, S.; Wang, J.; Huang, C.; et al. Copper Peroxide-Loaded Gelatin Sponges for Wound Dressings with Antimicrobial and Accelerating Healing Properties. ACS Appl. Mater. Interfaces 2021, 13, 26800–26807. [Google Scholar] [CrossRef]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a Novel Asymmetric Wettable Chitosan-Based Sponge and Its Role in Promoting Chronic Wound Healing. Carbohydr. Polym. 2020, 227, 115296. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Q.; Cheng, H.; Yu, Q.; Zhao, W.; Zhao, C. Dually-Thermoresponsive Hydrogel with Shape Adaptability and Synergetic Bacterial Elimination in the Full Course of Wound Healing. Adv Healthc. Mater. 2022, 11, 2201049. [Google Scholar] [CrossRef]
- Liu, W.; Ou-Yang, W.; Zhang, C.; Wang, Q.; Pan, X.; Huang, P.; Zhang, C.; Li, Y.; Kong, D.; Wang, W. Synthetic Polymeric Antibacterial Hydrogel for Methicillin-Resistant Staphylococcus aureus- Infected Wound Healing: Nanoantimicrobial Self-Assembly, Drug- and Cytokine-Free Strategy. ACS Nano 2020, 14, 12905–12917. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Bera, H.; Zhang, H.; Chen, Y.; Cun, D.; Foderà, V.; Yang, M. α-Lactalbumin-Based Nanofiber Dressings Improve Burn Wound Healing and Reduce Scarring. ACS Appl. Mater. Interfaces 2020, 12, 45702–45713. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, J.; Jin, Y.; Qian, B.; Lv, W.; Zhou, Q.; Mei, E.; Neisiany, R.E.; Liu, Y.; You, Z.; et al. Breathable, Antifreezing, Mechanically Skin-like Hydrogel Textile Wound Dressings with Dual Antibacterial Mechanisms. Bioact. Mater. 2023, 21, 313–323. [Google Scholar] [CrossRef]
- Hasan, S.; Hasan, M.A.; Hassan, M.U.; Amin, M.; Javed, T.; Fatima, L. Biopolymers in Diabetic Wound Care Management: A Potential Substitute to Traditional Dressings. Eur. Polym. J. 2023, 189, 111979. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable Wound Dressing Based on Carboxymethyl Chitosan Triple-Network Hydrogel for Effective Wound Antibacterial and Hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Orlov, A.; Gefen, A. Differences in Prophylactic Performance across Wound Dressing Types Used to Protect from Device-related Pressure Ulcers Caused by a Continuous Positive Airway Pressure Mask. Int. Wound J. 2023, 20, 942–960. [Google Scholar] [CrossRef]
- Lan, Z.; Kar, R.; Chwatko, M.; Shoga, E.; Cosgriff-Hernandez, E. High Porosity PEG -based Hydrogel Foams with Self-tuning Moisture Balance as Chronic Wound Dressings. J. Biomed. Mater. Res. 2023, 111, 465–477. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Wu, B.; Zou, K.; Chen, H. Efficacy of Different Types of Dressings on Pressure Injuries: Systematic Review and Network Meta-Analysis. Nurs. Open 2023, 10, 5857–5867. [Google Scholar] [CrossRef]
- Han, S. Innovations and Advances in Wound Healing, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-46586-8. [Google Scholar]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; El-Naggar, M.E. Traditional and Modern Wound Dressings—Characteristics of Ideal Wound Dressings. In Antimicrobial Dressings; Elsevier: Amsterdam, The Netherlands, 2023; pp. 21–42. ISBN 978-0-323-95074-9. [Google Scholar]
- Alka, A.; Verma, A.; Mishra, N.; Singh, N.; Singh, P.; Nisha, R.; Pal, R.R.; Saraf, S.A. Polymeric Gel Scaffolds and Biomimetic Environments for Wound Healing. Curr. Pharm. Des. 2023, 29, 3221–3239. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Bhoyar, S.D.; Malhotra, K.; Madke, B. Dressing Materials: A Comprehensive Review. J. Cutan. Aesthetic Surg. 2023, 16, 81–89. [Google Scholar] [CrossRef]
- Malekzadeh, H.; Tirmizi, Z.; Arellano, J.A.; Egro, F.M.; Ejaz, A. Application of Adipose-Tissue Derived Products for Burn Wound Healing. Pharmaceuticals 2023, 16, 1302. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo De Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a Topical Treatment for Wounds. Cochrane Database Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef]
- Millikan, L.E. Wound Healing and Dermatologic Dressings. Clin. Dermatol. 1987, 5, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; Ribeiro De Castro, M.C. New Dressings, Including Tissue-Engineered Living Skin. Clin. Dermatol. 2002, 20, 715–723. [Google Scholar] [CrossRef]
- Martin, L.; Wilson, C.G.; Koosha, F.; Tetley, L.; Gray, A.I.; Senel, S.; Uchegbu, I.F. The Release of Model Macromolecules May Be Controlled by the Hydrophobicity of Palmitoyl Glycol Chitosan Hydrogels. J. Control. Release 2002, 80, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMed 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Collagen-Based Biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382. [Google Scholar] [CrossRef]
- Doillon, C.J.; Silver, F.H. Collagen-Based Wound Dressing: Effects of Hyaluronic Acid and Firponectin on Wound Healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable Chitosan as a Dressing for Wound Occlusion and Accelerator in Healing Process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-Based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-Art. Curr. Drug Targets 2018, 19, 527–550. [Google Scholar] [CrossRef]
- Panduranga Rao, K. Recent Developments of Collagen-Based Materials for Medical Applications and Drug Delivery Systems. J. Biomater. Sci. Polym. Ed. 1996, 7, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Boyce, S.T. Engineered Skin Substitutes: Practices and Potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef]
- Mian, M.; Beghe, F.; Mian, E. Collagen as a Pharmacological Approach in Wound Healing. Int. J. Tissue React. 1992, 14, 1–9. [Google Scholar] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical Formulations and Wound Healing Applications of Chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The Use of Hyaluronic Acid Based Dressings to Treat Burns: A Review. Burn Trauma 2014, 2, 162. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Z.; Mao, X.; Cheng, J.; Huang, L.; Tang, J. Advances in Wound Dressing Based on Electrospinning Nanofibers. J. Appl. Polym. Sci. 2024, 141, e54746. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L. Electrospun Nanofiber Membranes with Various Structures for Wound Dressing. Materials 2023, 16, 6021. [Google Scholar] [CrossRef]
- Sethuram, L.; Thomas, J. Synthesis, Fabrication and Biosafety Profiles of Biobased Microemulsions Reinforced Electrospun Nanofibers for Wound Dressing Applications. Nano-Struct. Nano-Objects 2023, 33, 100940. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mirhaj, M.; Salehi, S.; Varshosaz, J.; Labbaf, S.; Golshirazi, A.; Kazemi, N.; Haghighi, V. Coaxial Electrospun Angiogenic Nanofiber Wound Dressing Containing Advanced Platelet Rich-Fibrin. Int. J. Biol. Macromol. 2022, 222, 1605–1618. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Zhai, Y.; Chen, S. Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application. Pharmaceutics 2023, 15, 2285. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, H.; Shafiee, P.; Darbandi, A.; Yusuf, M.; Mahmoudi, S.; Goudarzi, Z.M.; Mirzamohammadi, S. Application of Polyoxometalate-Based Composites for Sensor Systems: A Review. J. Compos. Compd. 2021, 3, 129–139. [Google Scholar] [CrossRef]
- Amarjargal, A.; Moazzami Goudarzi, Z.; Cegielska, O.; Gradys, A.; Kolbuk, D.; Kalaska, B.; Ruszczyńska, A.; Sajkiewicz, P. A Facile One-Stone-Two-Birds Strategy for Fabricating Multifunctional 3D Nanofibrous Scaffolds. Biomater. Sci. 2023, 11, 5502–5516. [Google Scholar] [CrossRef]
- Moazzami Goudarzi, Z.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M.; Enayati, M.S. Structural and Mechanical Properties of Fibrous Poly (Caprolactone)/Gelatin Nanocomposite Incorporated with Cellulose Nanofibers. Polym. Bull. 2020, 77, 717–740. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, H.; Zheng, Y.; Yao, Y.; Li, X.; Yu, J.; Ding, B. Nanofibrous Hydrogels Embedded with Phase-Change Materials: Temperature-Responsive Dressings for Accelerating Skin Wound Healing. Compos. Commun. 2021, 25, 100752. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F.; Czerkies, M.; Kołbuk, D. Structure and Properties of Polycaprolactone/Chitosan Nonwovens Tailored by Solvent Systems. Biomed. Mater. 2017, 12, 015020. [Google Scholar] [CrossRef] [PubMed]
- Kasoju, N.; Ye, H. (Eds.) Biomedical Applications of Electrospinning and Electrospraying; Woodhead Publishing Series in Biomaterials; Woodhead Publishing, an Imprint of Elsevier: Duxford, UK, 2021; ISBN 978-0-12-822476-2. [Google Scholar]
- Moazzami Goudarzi, Z.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M. An Investigation into Influence of Acetylated Cellulose Nanofibers on Properties of PCL/Gelatin Electrospun Nanofibrous Scaffold for Soft Tissue Engineering. Polymer 2021, 213, 123313. [Google Scholar] [CrossRef]
- Zolfagharlou Kouhi, M.; Behzad, T.; Ghasemi-Mobarakeh, L.; Allafchian, A.; Moazzami Goudarzi, Z.; Enayati, M.S. Proceeding toward the Development of Poly(ε-Caprolactone)/Cellulose Microfibrils Electrospun Biocomposites Using a Novel Ternary Solvent System. J. Text. Inst. 2020, 111, 249–259. [Google Scholar] [CrossRef]
- Amarjargal, A.; Brunelli, M.; Fortunato, G.; Spano, F.; Kim, C.S.; Rossi, R.M. On-Demand Drug Release from Tailored Blended Electrospun Nanofibers. J. Drug Deliv. Sci. Technol. 2019, 52, 8–14. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Januário, A.P.; Félix, R.; Alves, N.; Lemos, M.F.L.; Dias, J.R. Multifunctional Gelatin/Chitosan Electrospun Wound Dressing Dopped with Undaria Pinnatifida Phlorotannin-Enriched Extract for Skin Regeneration. Pharmaceutics 2021, 13, 2152. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-Drug Delivery of Ag-Chitosan Nanoparticles and Phenytoin via Core-Shell PVA/PCL Electrospun Nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Del Valle, L.; Yousefzade, O.; Macovez, R.; Franco, L.; Puiggalí, J. Chloramphenicol Loaded Polylactide Melt Electrospun Scaffolds for Biomedical Applications. Int. J. Pharm. 2021, 606, 120897. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, N.; Pezeshki-Modaress, M.; Najmoddin, N. Wet-Electrospinning of Nanofibrous Magnetic Composite 3-D Scaffolds for Enhanced Stem Cells Neural Differentiation. Chem. Eng. Sci. 2022, 264, 118144. [Google Scholar] [CrossRef]
- Thitiwongsawet, P.; Wisesanupong, B.; Pukkanasut, S. Electrospun Gelatin Fiber Bundles by Self-Bundling Electrospinning. Adv. Mater. Res. 2015, 1105, 190–194. [Google Scholar] [CrossRef]
- Avossa, J.; Pota, G.; Vitiello, G.; Macagnano, A.; Zanfardino, A.; Di Napoli, M.; Pezzella, A.; D’Errico, G.; Varcamonti, M.; Luciani, G. Multifunctional Mats by Antimicrobial Nanoparticles Decoration for Bioinspired Smart Wound Dressing Solutions. Mater. Sci. Eng. C 2021, 123, 111954. [Google Scholar] [CrossRef]
- Komur, B.; Bayrak, F.; Ekren, N.; Eroglu, M.S.; Oktar, F.N.; Sinirlioglu, Z.A.; Yucel, S.; Guler, O.; Gunduz, O. Starch/PCL Composite Nanofibers by Co-Axial Electrospinning Technique for Biomedical Applications. Biomed. Eng. Online 2017, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate Polymer-Based Silver Nanocomposites: Recent Progress in the Antimicrobial Wound Dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef]
- Paduraru, A.; Ghitulica, C.; Trusca, R.; Surdu, V.A.; Neacsu, I.A.; Holban, A.M.; Birca, A.C.; Iordache, F.; Vasile, B.S. Antimicrobial Wound Dressings as Potential Materials for Skin Tissue Regeneration. Materials 2019, 12, 1859. [Google Scholar] [CrossRef]
- Qu, X.; Liu, H.; Zhang, C.; Lei, Y.; Lei, M.; Xu, M.; Jin, D.; Li, P.; Yin, M.; Payne, G.F.; et al. Electrofabrication of Functional Materials: Chloramine-Based Antimicrobial Film for Infectious Wound Treatment. Acta Biomater. 2018, 73, 190–203. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart Wound Dressing for Advanced Wound Management: Real-Time Monitoring and on-Demand Treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lun, X.; Sheng, H.; Yan, A. Effects of Wound Dressing Based on the Combination of Silver@curcumin Nanoparticles and Electrospun Chitosan Nanofibers on Wound Healing. Bioengineered 2022, 13, 4328–4339. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Liu, Z.; Wei, S.; Su, X.; Zhang, G. The Facile Fabrication of Wound Compatible Anti-Microbial Nanoparticles Encapsulated Collagenous Chitosan Matrices for Effective Inhibition of Poly-Microbial Infections and Wound Repairing in Burn Injury Care: Exhaustive in Vivo Evaluations. J. Photochem. Photobiol. B Biol. 2019, 197, 111539. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Fang, J.; Yao, G.; Zhao, H.; Dodson, M.; Wang, X. In Vivo Wound Healing and Antibacterial Performances of Electrospun Nanofibre Membranes. J. Biomed. Mater. Res. 2010, 94A, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, L.; Zhang, S.; Chen, T.; Yang, J.; Long, S.; Wang, X. Electrospun Antibacterial Nanofibers for Wound Dressings and Tissue Medicinal Fields: A Review. J. Innov. Opt. Health Sci. 2020, 13, 2030012. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic Wound Infection: Facts and Controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-Responsive Electrospun Nanofibers for Drug Delivery, Cancer Therapy, Wound Dressing, and Tissue Engineering. J. Nanobiotechnol. 2023, 21, 237. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun Nanofibers for Drug Delivery Applications: Methods and Mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- Scaffaro, R.; Settanni, L.; Gulino, E.F. Release Profiles of Carvacrol or Chlorhexidine of PLA/Graphene Nanoplatelets Membranes Prepared Using Electrospinning and Solution Blow Spinning: A Comparative Study. Molecules 2023, 28, 1967. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-Biofunction of Antimicrobial Peptide-Immobilized Silk Fibroin Nanofiber Membrane: Implications for Wound Healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Parisien, A.; Allain, B.; Zhang, J.; Mandeville, R.; Lan, C.Q. Novel Alternatives to Antibiotics: Bacteriophages, Bacterial Cell Wall Hydrolases, and Antimicrobial Peptides. J. Appl. Microbiol. 2007, 104, 1–13. [Google Scholar] [CrossRef]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Nguyen, T.T.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The Human Cathelicidin LL-37 Host Defense Peptide Upregulates Tight Junction-Related Proteins and Increases Human Epidermal Keratinocyte Barrier Function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef]
- Chang, A.K.T.; Frias, R.R.; Alvarez, L.V.; Bigol, U.G.; Guzman, J.P.M.D. Comparative Antibacterial Activity of Commercial Chitosan and Chitosan Extracted from Auricularia sp. Biocatal. Agric. Biotechnol. 2019, 17, 189–195. [Google Scholar] [CrossRef]
- Fatahian, R.; Mirjalili, M.; Khajavi, R.; Rahimi, M.K.; Nasirizadeh, N. A Novel Hemostat and Antibacterial Nanofibrous Scaffold Based on Poly(Vinyl Alcohol)/Poly(Lactic Acid). J. Bioact. Compat. Polym. 2020, 35, 189–202. [Google Scholar] [CrossRef]
- Bandeira, M.; Chee, B.S.; Frassini, R.; Nugent, M.; Giovanela, M.; Roesch-Ely, M.; Crespo, J.D.S.; Devine, D.M. Antimicrobial PAA/PAH Electrospun Fiber Containing Green Synthesized Zinc Oxide Nanoparticles for Wound Healing. Materials 2021, 14, 2889. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.; Akram, M.; Alshemary, A.; Hussain, R. Antibacterial Polylactic Acid/Chitosan Nanofibers Decorated with Bioactive Glass. Appl. Surf. Sci. 2016, 387, 1–7. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A Multimodal Antimicrobial Platform Based on MXene for Treatment of Wound Infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
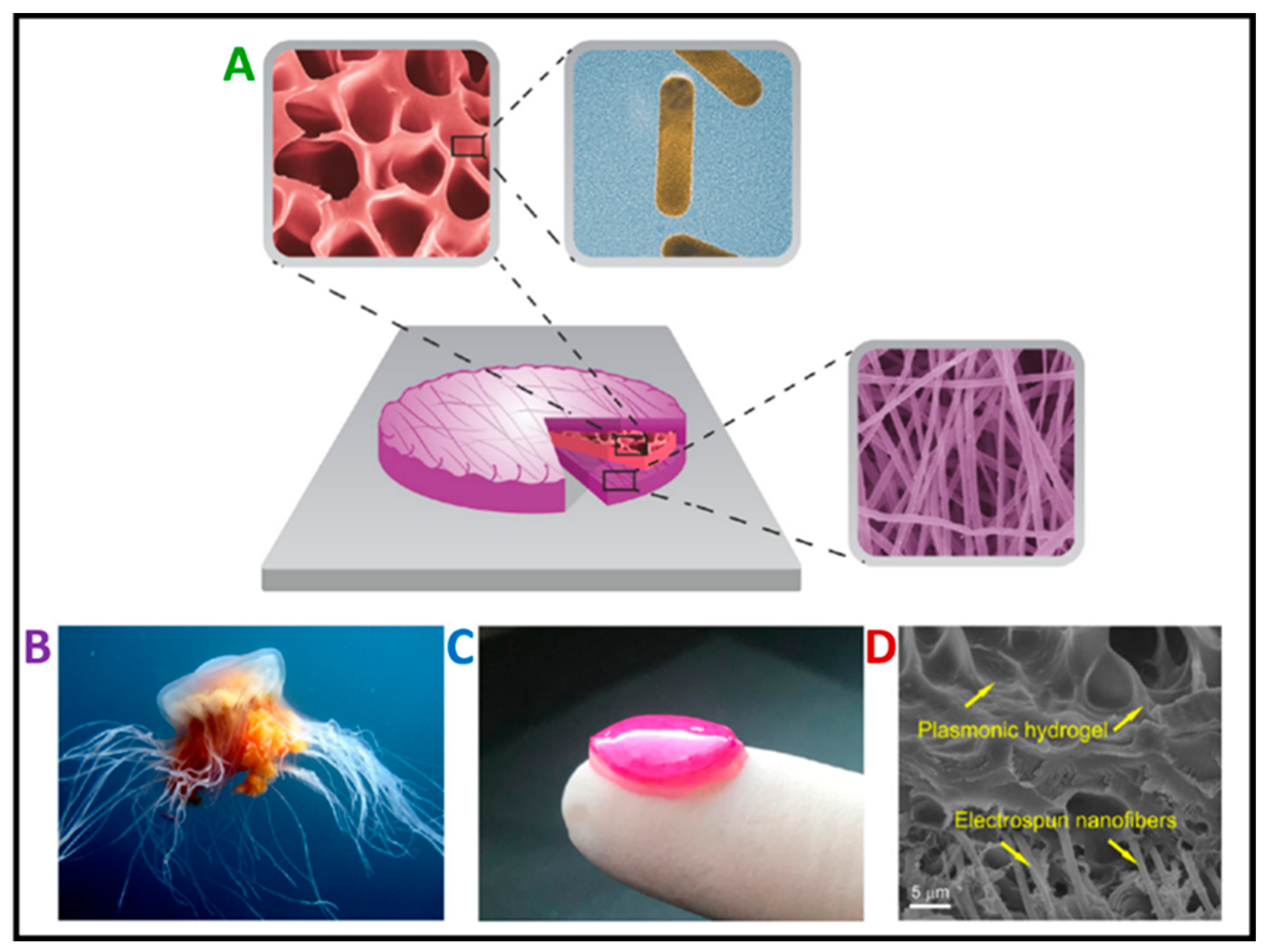
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Chung, O.H.; Park, J.S. Coaxial Electrospun Poly(Lactic Acid)/Chitosan (Core/Shell) Composite Nanofibers and Their Antibacterial Activity. Carbohydr. Polym. 2011, 86, 1799–1806. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Lakshmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Nozari, M.; Gholizadeh, M.; Zahiri Oghani, F.; Tahvildari, K. Studies on Novel Chitosan/Alginate and Chitosan/Bentonite Flexible Films Incorporated with ZnO Nano Particles for Accelerating Dermal Burn Healing: In Vivo and In Vitro Evaluation. Int. J. Biol. Macromol. 2021, 184, 235–249. [Google Scholar] [CrossRef]
- Wei, X.; Cai, J.; Lin, S.; Li, F.; Tian, F. Controlled Release of Monodisperse Silver Nanoparticles via in Situ Cross-Linked Polyvinyl Alcohol as Benign and Antibacterial Electrospun Nanofibers. Colloids Surf. B Biointerfaces 2021, 197, 111370. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.-Q. Polyvinyl Alcohol Nanofiber Based Three Phase Wound Dressings for Sustained Wound Healing Applications. Mater. Lett. 2019, 241, 168–171. [Google Scholar] [CrossRef]
- Ballesteros, C.A.S.; Correa, D.S.; Zucolotto, V. Polycaprolactone Nanofiber Mats Decorated with Photoresponsive Nanogels and Silver Nanoparticles: Slow Release for Antibacterial Control. Mater. Sci. Eng. C 2020, 107, 110334. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Radwan, H.A.; Abdelaal, S.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.R.; Hady, M.A. Polycaprolactone Based Electrospun Matrices Loaded with Ag/Hydroxyapatite as Wound Dressings: Morphology, Cell Adhesion, and Antibacterial Activity. Int. J. Pharm. 2021, 593, 120143. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel Electrospun Chitosan/Polyvinyl Alcohol/Zinc Oxide Nanofibrous Mats with Antibacterial and Antioxidant Properties for Diabetic Wound Healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe Vera-Loaded Nanofibrous Scaffold Based on Zein/Polycaprolactone/Collagen for Wound Healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive Antibacterial Bilayer PCL/Gelatin Nanofibrous Scaffold Promotes Full-Thickness Wound Healing. Int. J. Pharm. 2020, 583, 119413. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of Antibacterial Bacterial Cellulose Composite Membranes Modified with Chitosan or Chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Zhou, W.; Yang, S.; Ye, S.; Cao, F.; Liu, Y.; Xiong, Y. Preparation of a Novel Composite Nanofiber Gel-Encapsulated Human Placental Extract through Layer-by-Layer Self-Assembly. Exp. Ther. Med. 2016, 11, 1447–1452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiti, K.; Suwantong, O. The Potential Use of Curcumin-β-Cyclodextrin Inclusion Complex/Chitosan-Loaded Cellulose Sponges for the Treatment of Chronic Wound. Int. J. Biol. Macromol. 2020, 164, 3250–3258. [Google Scholar] [CrossRef]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of Composite Hydroxybutyl Chitosan Sponge and Its Role in Promoting Wound Healing. Carbohydr. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an In-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and Characterization of a Novel Asymmetric 3D Printed Construct Aimed for Skin Tissue Regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Mary, D.S.; Kannan, S. Copper Incorporated Microporous Chitosan-Polyethylene Glycol Hydrogels Loaded with Naproxen for Effective Drug Release and Anti-Infection Wound Dressing. Int. J. Biol. Macromol. 2017, 95, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.C.; Rezende, T.M.B.; Franco, O.L. Nanofibers as Drug-Delivery Systems for Antimicrobial Peptides. Drug Discov. Today 2021, 26, 2064–2074. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of Electrospun Polymeric Wound Dressings with Antimicrobial Peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Rajan, M.; Ahmed, B.M.; Al-Maadeed, M.A.S.A. (Eds.) Polymer Nanocomposites in Biomedical Engineering; Lecture Notes in Bioengineering; Springer: Cham, Switerland, 2019; ISBN 978-3-030-04740-5. [Google Scholar]
- Peschel, A. How Do Bacteria Resist Human Antimicrobial Peptides? Trends Microbiol. 2002, 10, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of Antimicrobial Chitosan Based Nanofiber Dressings for Wound Healing Applications. Nanomed. J. 2018, 5, 6–14. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. Coli and S. Aureus Growth. Rev. Bras. De Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of Chitosan Based Nanoparticles and Their in Vitro Evaluation against Phytopathogenic Fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Hadisi, Z.; Bakhsheshi-Rad, H.R.; Walsh, T.; Dehghan, M.M.; Farzad-Mohajeri, S.; Gholami, H.; Diyanoush, A.; Pagan, E.; Akbari, M. In Vitro and In Vivo Evaluation of Silk Fibroin-Hardystonite-Gentamicin Nanofibrous Scaffold for Tissue Engineering Applications. Polym. Test. 2020, 91, 106698. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Mo, X.; Zhang, K.; Fan, L.; Yin, A.; He, C.; Wang, H. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-Dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Severyukhina, A.N.; Petrova, N.V.; Yashchenok, A.M.; Bratashov, D.N.; Smuda, K.; Mamonova, I.A.; Yurasov, N.A.; Puchinyan, D.M.; Georgieva, R.; Bäumler, H.; et al. Light-Induced Antibacterial Activity of Electrospun Chitosan-Based Material Containing Photosensitizer. Mater. Sci. Eng. C 2017, 70, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Leal, E.C.; Carvalho, L.; De Sousa, H.C.; Carvalho, E. Chitosan-Based Dressings Loaded with Neurotensin—An Efficient Strategy to Improve Early Diabetic Wound Healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef]
- Kirichenko, A.K.; Bolshakov, I.N.; Ali-Rizal, A.E.; Vlasov, A.A. Morphological Study of Burn Wound Healing with the Use of Collagen-Chitosan Wound Dressing. Bull. Exp. Biol. Med. 2013, 154, 692–696. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of Tannic Acid/Chitosan/Pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Ahlawat, J.; Kumar, V.; Gopinath, P. Carica Papaya Loaded Poly (Vinyl Alcohol)-Gelatin Nanofibrous Scaffold for Potential Application in Wound Dressing. Mater. Sci. Eng. C 2019, 103, 109834. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and Characterization of Electrospun Silk Fibroin Based Asymmetric Membranes for Wound Dressing Applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In Vivo Evaluation of the Wound Healing Properties of Bio-Nanofiber Chitosan/Polyvinyl Alcohol Incorporating Honey and Nepeta Dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef] [PubMed]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun Anti-Inflammatory Patch Loaded with Essential Oils for Wound Healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef]
- Mouro, C.; Simões, M.; Gouveia, I.C. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraipandy, N.; Kiran, M.S.; Fathima, N.N. Anti-Oxidant Enriched Hybrid Nanofibers: Effect on Mechanical Stability and Biocompatibility. Int. J. Biol. Macromol. 2018, 117, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Barati, A.; Arjomandzadegan, M.; Vatankhah, E. Nanofibrous Cellulose Acetate/Gelatin Wound Dressing Endowed with Antibacterial and Healing Efficacy Using Nanoemulsion of Zataria Multiflora. Int. J. Biol. Macromol. 2020, 162, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The Biomedical Potential of Cellulose Acetate/Polyurethane Nanofibrous Mats Containing Reduced Graphene Oxide/Silver Nanocomposites and Curcumin: Antimicrobial Performance and Cutaneous Wound Healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef]
- Long, L.; Liu, W.; Hu, C.; Yang, L.; Wang, Y. Construction of Multifunctional Wound Dressings with Their Application in Chronic Wound Treatment. Biomater. Sci. 2022, 10, 4058–4076. [Google Scholar] [CrossRef]
- Ren, X.; Hu, Y.; Chang, L.; Xu, S.; Mei, X.; Chen, Z. Electrospinning of Antibacterial and Anti-Inflammatory Ag@hesperidin Core-Shell Nanoparticles into Nanofibers Used for Promoting Infected Wound Healing. Regen. Biomater. 2022, 9, rbac012. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Labra-Vázquez, P.; Del Valle, L.J.; Puiggalí, J.; García-Arrazola, R.; Montiel, C.; Gimeno, M. Development of an Antimicrobial and Antioxidant Hydrogel/Nano-Electrospun Wound Dressing. RSC Adv. 2020, 10, 30508–30518. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Pan, M.; Xue, F.; Lv, F.; Ke, Q.; Xu, H. Nanofiber/Hydrogel Core–Shell Scaffolds with Three-Dimensional Multilayer Patterned Structure for Accelerating Diabetic Wound Healing. J. Nanobiotechnol. 2022, 20, 28. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Seidi, F.; Wang, Y.; Zheng, L.; Jin, Y.; Xiao, H. Injectable Chitosan Hydrogels Tailored with Antibacterial and Antioxidant Dual Functions for Regenerative Wound Healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive Wound Dressings: Advances and Prospects. Nanoscale Horiz. 2023, 8, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Zandi, N.; Dolatyar, B.; Lotfi, R.; Shallageh, Y.; Shokrgozar, M.A.; Tamjid, E.; Annabi, N.; Simchi, A. Biomimetic Nanoengineered Scaffold for Enhanced Full-Thickness Cutaneous Wound Healing. Acta Biomater. 2021, 124, 191–204. [Google Scholar] [CrossRef]
- Xu, M.; Li, Q.; Fang, Z.; Jin, M.; Zeng, Q.; Huang, G.; Jia, Y.-G.; Wang, L.; Chen, Y. Conductive and Antimicrobial Macroporous Nanocomposite Hydrogels Generated from Air-in-Water Pickering Emulsions for Neural Stem Cell Differentiation and Skin Wound Healing. Biomater. Sci. 2020, 8, 6957–6968. [Google Scholar] [CrossRef]
- Zhang, E.; Guo, Q.; Ji, F.; Tian, X.; Cui, J.; Song, Y.; Sun, H.; Li, J.; Yao, F. Thermoresponsive Polysaccharide-Based Composite Hydrogel with Antibacterial and Healing-Promoting Activities for Preventing Recurrent Adhesion after Adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef]
- Niyompanich, J.; Chuysinuan, P.; Pavasant, P.; Supaphol, P. Development of Thermoresponsive Poloxamer in Situ Gel Loaded with Gentamicin Sulfate for Cavity Wounds. J. Polym. Res. 2021, 28, 128. [Google Scholar] [CrossRef]
- Kamlungmak, S.; Rugmai, S.; Tinpun, K.; Nakpheng, T.; Srichana, T. Phase Behavior, In Vitro Drug Release, and Antibacterial Activity of Thermoresponsive Poloxamer–Polyvinyl Alcohol Hydrogel-loaded Mupirocin Nanoparticles. J. Appl. Polym. Sci. 2020, 137, 49325. [Google Scholar] [CrossRef]
- Liu, X.; Gan, H.; Hu, C.; Sun, W.; Zhu, X.; Meng, Z.; Gu, R.; Wu, Z.; Dou, G. Silver Sulfadiazine Nanosuspension-Loaded Thermosensitive Hydrogel as a Topical Antibacterial Agent. Int. J. Nanomed. 2018, 14, 289–300. [Google Scholar] [CrossRef]
- Mahdieh, Z.; Postma, B.; Herritt, L.A.; Hamilton, R.F.; Harkema, J.R.; Holian, A. Hyperspectral Microscopy of Subcutaneously Released Silver Nanoparticles Reveals Sex Differences in Drug Distribution. Micron 2022, 153, 103193. [Google Scholar] [CrossRef]
- Gong, X.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Thermochromic Hydrogel-Functionalized Textiles for Synchronous Visual Monitoring of On-Demand In Vitro Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 51225–51235. [Google Scholar] [CrossRef] [PubMed]
- Radhakumary, C.; Antonty, M.; Sreenivasan, K. Drug Loaded Thermoresponsive and Cytocompatible Chitosan Based Hydrogel as a Potential Wound Dressing. Carbohydr. Polym. 2011, 83, 705–713. [Google Scholar] [CrossRef]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.; De Araujo Lima, B.; Do Nascimento, M.; Lombello, C.; Brocchi, M.; Seabra, A. Biocompatible and Antibacterial Nitric Oxide-Releasing Pluronic F-127/Chitosan Hydrogel for Topical Applications. Polymers 2018, 10, 452. [Google Scholar] [CrossRef]

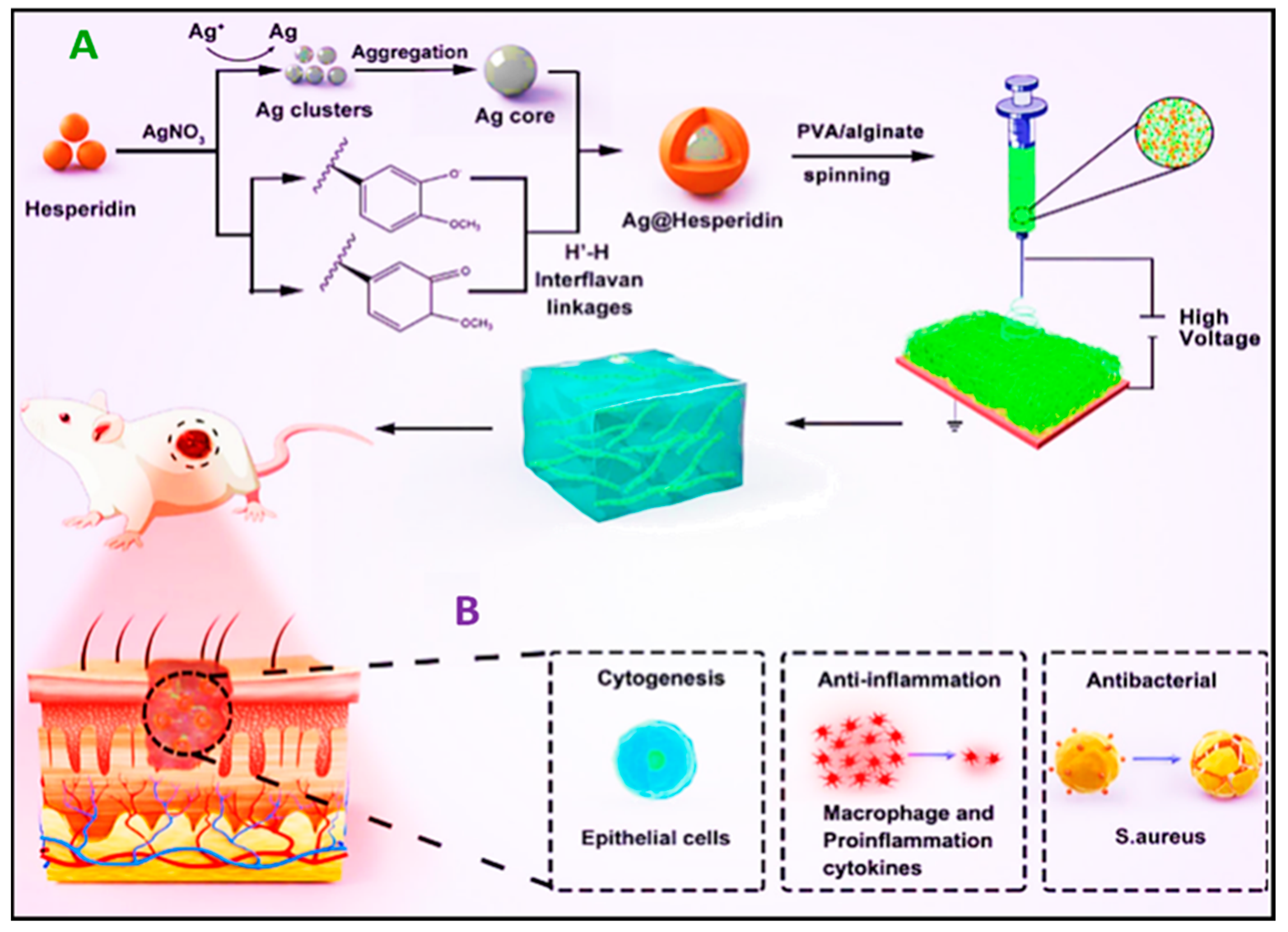
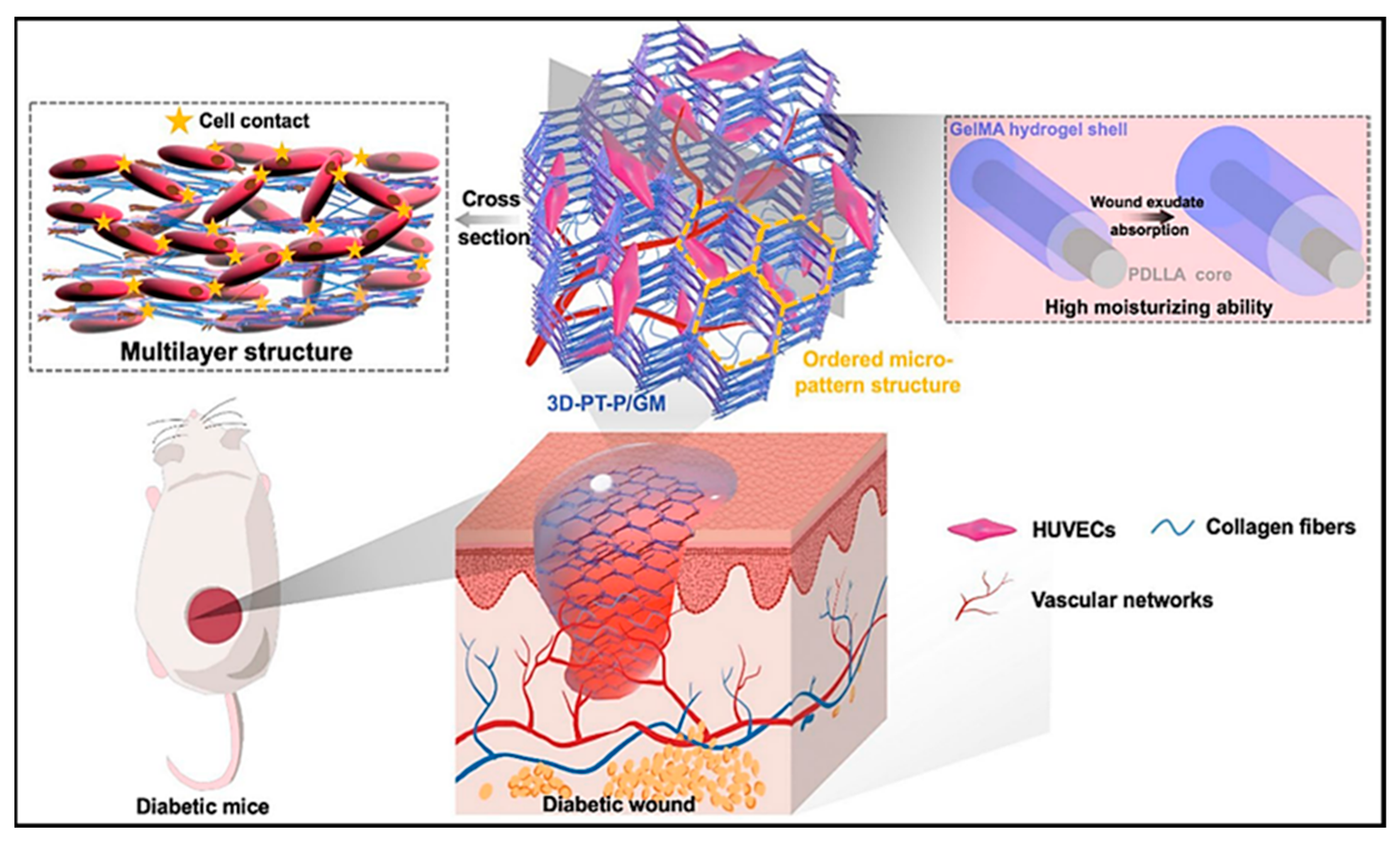

| Wound Dressing Type | Wound Type | Advantages | Disadvantages | Examples | Ref. |
|---|---|---|---|---|---|
| Traditional wound dressings | |||||
| Tulle | Shallow wounds | Does not adhere to the wound | Insufficient, often additional dressing required | Paratulle, Jelonet, Bactigras | [41] |
| Gauze | Minor clean wounds | Cheap, used as cover | Frequent changing, may adhere to the wound | Xeroform | [42] |
| Interactive and bioactive wound dressings | |||||
| Hydrogels | Dry wounds, wounds with low-to-medium exudate, necrotic wounds, burn wounds | Maintains moisture, allows vapor and oxygen exchange, does not react with tissue | Infections of the skin, mechanically weak | Intrasite Hydrosorb, Transigel, Curafil, FlexiGel, Aquaform Vigilon, Curasol | [43] |
| Semi-permeable films | Shallow wounds | Flexible, conformable, allow gas exchange | Minimal adhesion, skin maceration, dryness | Opsite, DuoDERM, Bioclusive, Mefilm, Transeal, Tegaderm, Omniderm | [44] |
| Semi-permeable foams | Moderate wounds, heavy wounds | Highly absorbent | Dryness | Lyofoam, Mepilex, Allevyn Curafoam, Polymem, | [45] |
| Hydrocolloids | Minor burns, light wounds, moderate exuding wounds | Absorption and debridement of wound exudates, permeable to water vapor, fluid exchange | Not intended to be used for heavy wounds | Granuflex, Tegasorb, DuoDERM, Replicare, NuDerm, Tegasorb | [46] |
| Hydrofibres | Burns, medium wounds, heavy wounds | Highly absorbent | Secondary dressing | Aquacel | [47] |
| Material | Method of Synthesis | Process Parametres | Properties Investigated | Ref. |
|---|---|---|---|---|
| Chitosan/PEO/semelil | Electrospinning | V—10–15 kV FR—0.45–0.60 mL/h T-C-D—10–15 cm. | Semelil release | [153] |
| PCL/PVA/curcumin | Forcespinning | Biocompatibility, anti-bacterial property, absorption | [154] | |
| Chitosan/PVA/Nepeta dschuparensis/honey | Electrospinning | V—17 kV FR—0.5 mL/h | In vivo properties, biocompatibility, biodegrability | [155] |
| PCL–silk fibroin/silk fibroin–hyaluric acid–Thymol | Electrospinning | V—30 kV FR—2.3 mL/h, 12 cm | Biocompatibility, biodegrability, antibacterial property | [156] |
| Polycaprolactone/tyrosol/Thymol | Electrospinning | V—28 kV FR—2.3 mL/h, T-C-D—12 cm | Antibacterial property | [154] |
| Cellulose acetate/β-cyclodextrin/Thymol | Electrospinning | V—+6.62 to +10.22 kV FR—1.0 mL/h | Antibacterial activity, drug release | [157] |
| PCL/PVA/Eugenol/Chitosan | Electrospinning | V—75.0 kV FR—13 cm | Release Eugenol, biocompatible, non-toxic, antibacterial properties | [158] |
| Silk fibroin/fenugreek/collagen | Electrospinning | V—25 kV FR—0.5 mL/h, T-C-D—10 cm | Biocompatible, wound healing, antioxidant property | [159] |
| PCL/lawsone/gelatin | Electrospinning | 15 kV 1.19 mL h−1 14 cm | Wound healing, antibacterial properties, biocompatible, healing | [160] |
| Polyurethane/silver/cellulose acetate/graphene oxide/curcumin | Electrospinning | 17 kV 0.4 mL/h. 15 cm | Biocompatible, promote wound healing, antibacterial property | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazzami Goudarzi, Z.; Zaszczyńska, A.; Kowalczyk, T.; Sajkiewicz, P. Electrospun Antimicrobial Drug Delivery Systems and Hydrogels Used for Wound Dressings. Pharmaceutics 2024, 16, 93. https://doi.org/10.3390/pharmaceutics16010093
Moazzami Goudarzi Z, Zaszczyńska A, Kowalczyk T, Sajkiewicz P. Electrospun Antimicrobial Drug Delivery Systems and Hydrogels Used for Wound Dressings. Pharmaceutics. 2024; 16(1):93. https://doi.org/10.3390/pharmaceutics16010093
Chicago/Turabian StyleMoazzami Goudarzi, Zahra, Angelika Zaszczyńska, Tomasz Kowalczyk, and Paweł Sajkiewicz. 2024. "Electrospun Antimicrobial Drug Delivery Systems and Hydrogels Used for Wound Dressings" Pharmaceutics 16, no. 1: 93. https://doi.org/10.3390/pharmaceutics16010093
APA StyleMoazzami Goudarzi, Z., Zaszczyńska, A., Kowalczyk, T., & Sajkiewicz, P. (2024). Electrospun Antimicrobial Drug Delivery Systems and Hydrogels Used for Wound Dressings. Pharmaceutics, 16(1), 93. https://doi.org/10.3390/pharmaceutics16010093









