Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleuropein-Loaded Chitosan Nanoparticles
2.3. Characterization of Oleuropein Chitosan Nanoparticles
2.3.1. Determination of the Particle Size, Polydispersity Index (PDI), and Zeta Potential (ZP)
2.3.2. Determination of Entrapment Efficiency and Loading of Oleuropein in Chitosan Nanoparticles
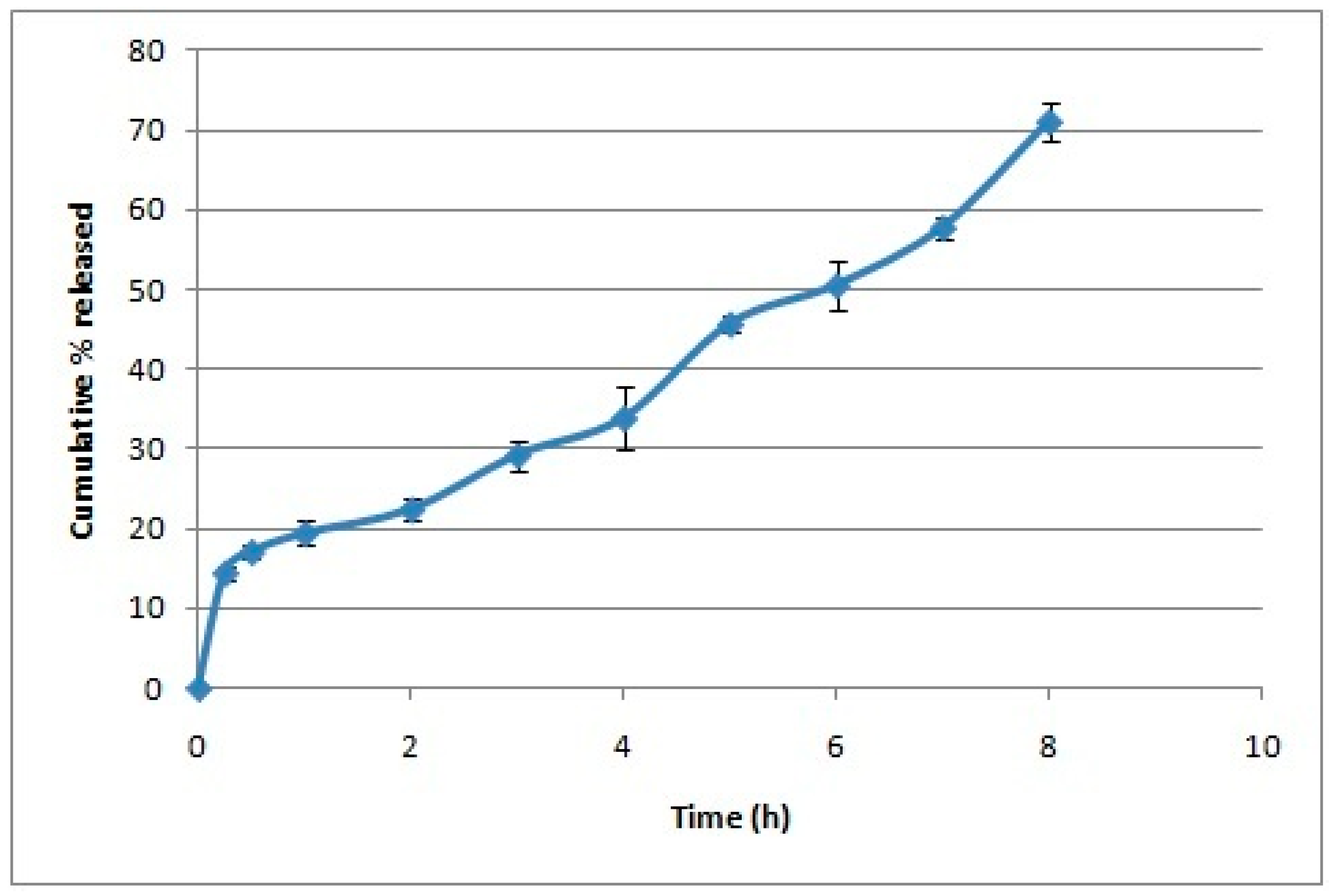
2.3.3. In Vitro Release of Oleuropein from Chitosan Nanoparticles
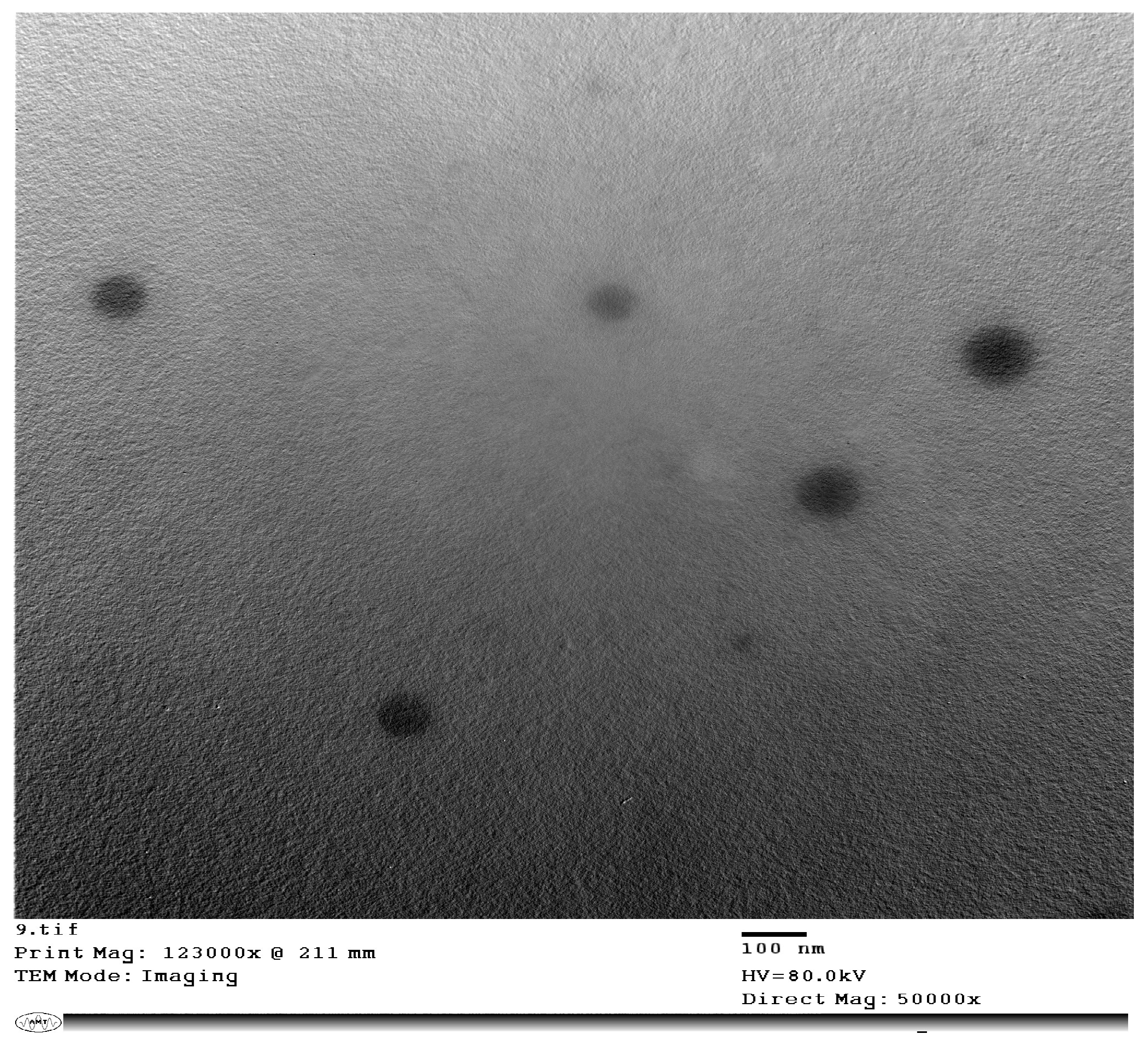
2.3.4. Transmission Electron Microscopy (TEM) Imaging of the Selected Chitosan Nanoparticles
2.3.5. In Vitro Membrane Stabilization and Anti-Inflammatory Activity
2.4. In Vivo Study
2.4.1. Animals
2.4.2. Experimental Setup
2.5. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Oleuropein-Loaded Chitosan Nanoparticles
3.2. In Vitro Anti-Inflammatory Activity
3.3. In Vivo Study
3.3.1. Effect on Serum Inflammatory Markers
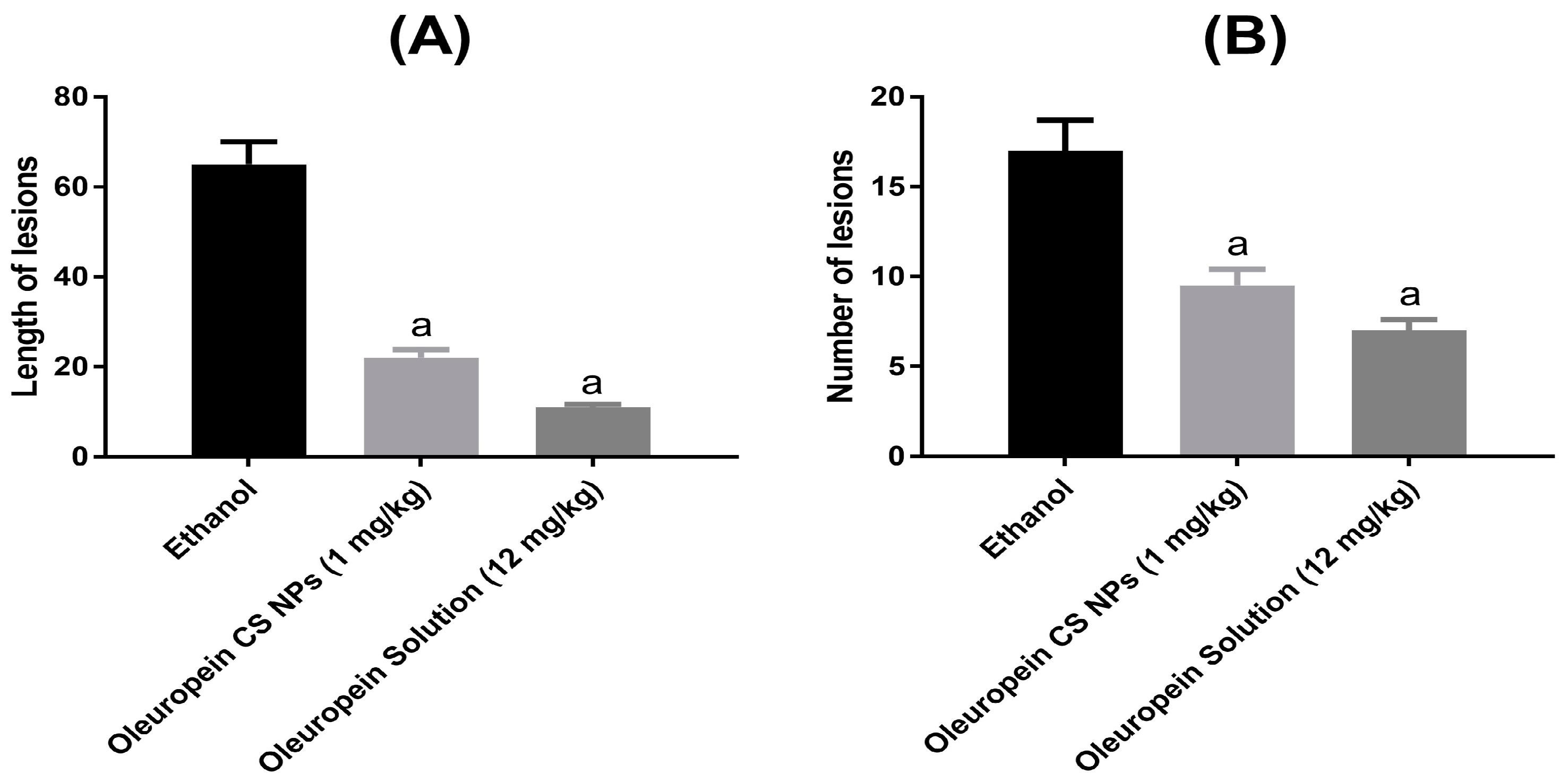
3.3.2. Effect on Ethanol-Induced Gastric Lesions
3.3.3. Levels of Gastric Prostaglandin E2 and Nitric Oxide Levels
3.3.4. Extent of Lipid Peroxidation and Antioxidant Enzymes
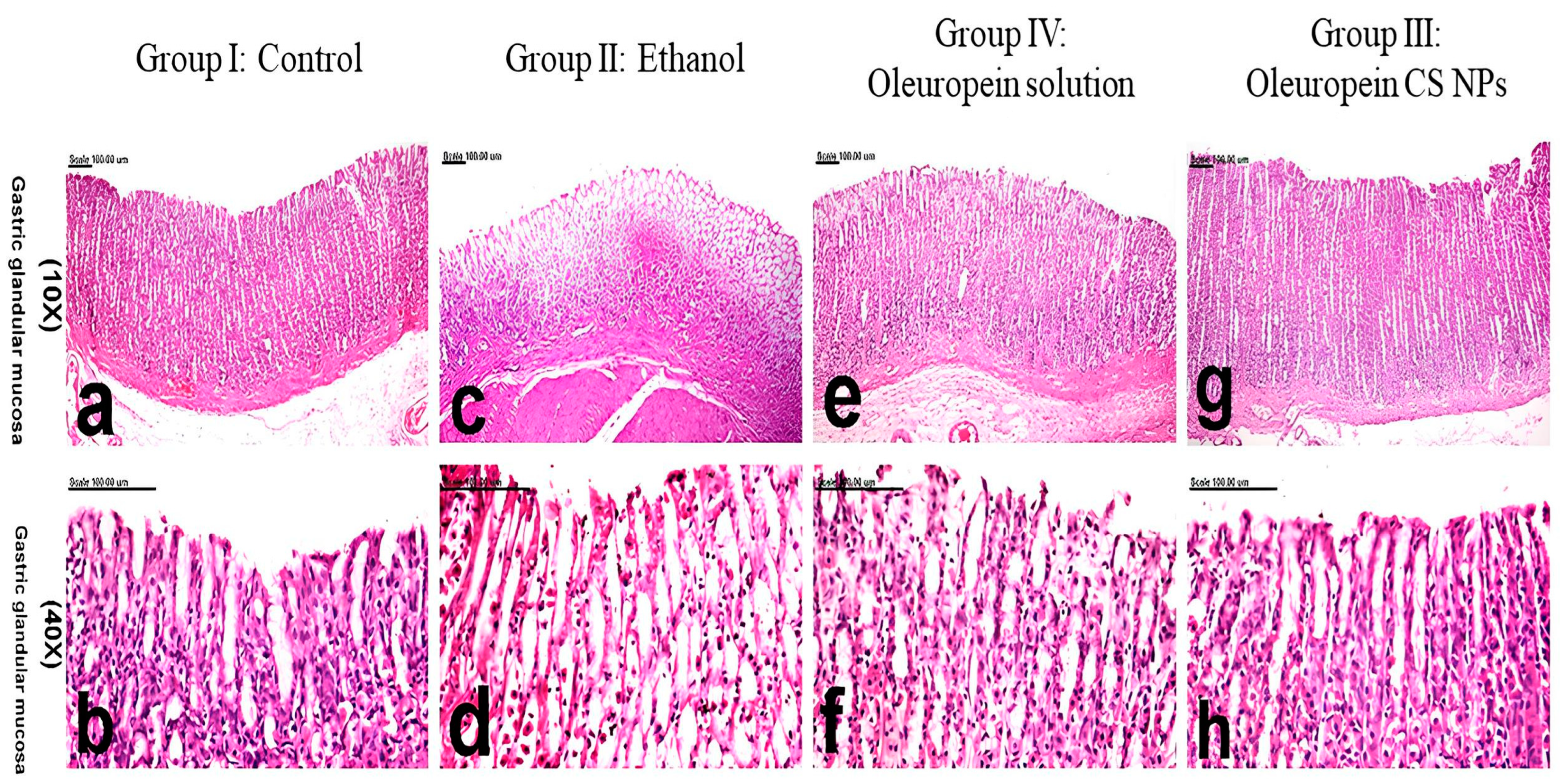
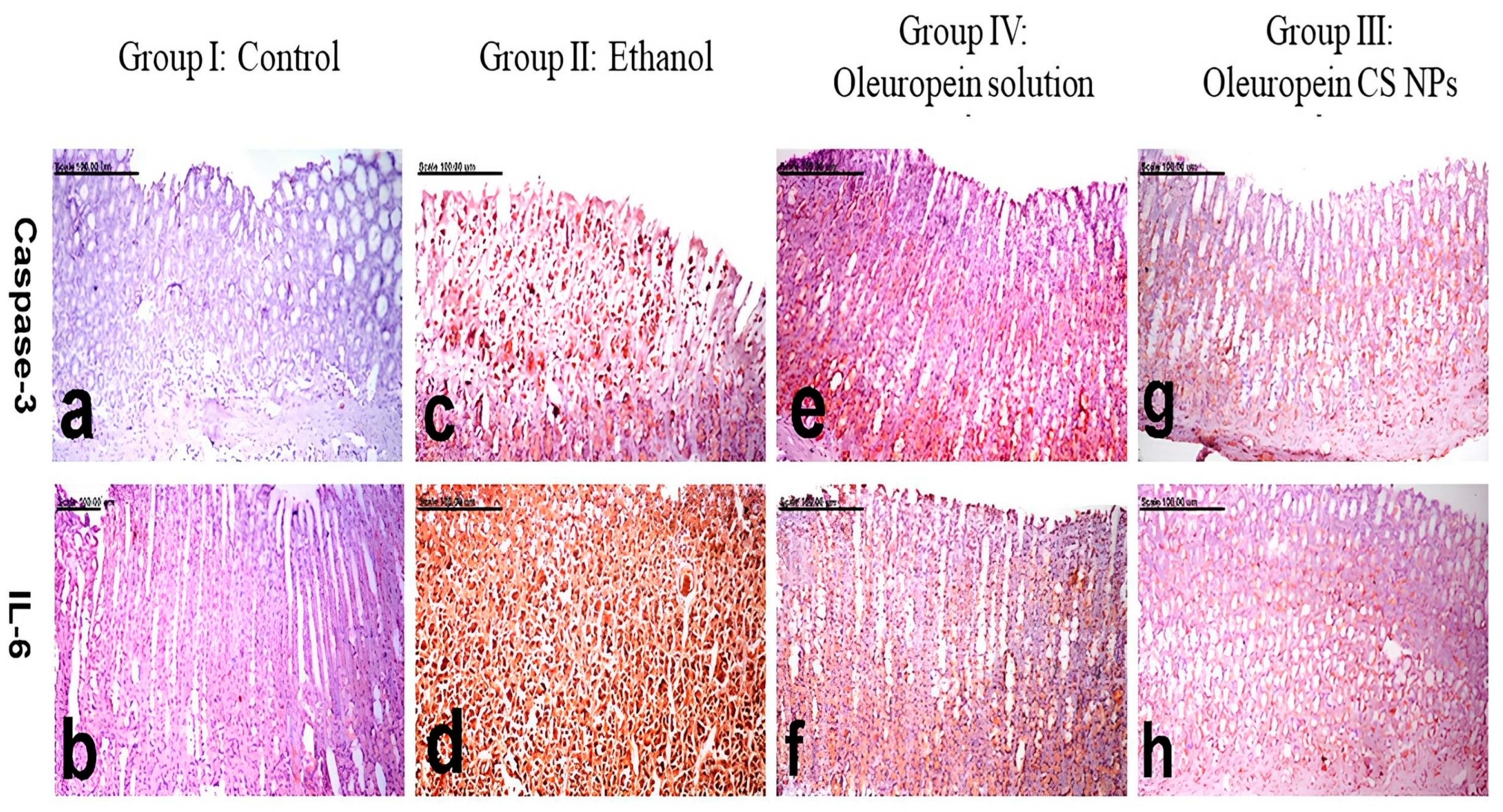
3.3.5. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari, N.; Darvishi, N.; Shohaimi, S.; Bartina, Y.; Ahmadipanah, M.; Salari, H.R.; Mohammadi, M. The Global Prevalence of Peptic Ulcer in the World: A Systematic Review and Meta-Analysis. Indian J. Surg. 2022, 84, 913–921. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Mohd, A.; Mohd, A. Role of Phenolic Compounds in Peptic Ulcer: An Overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.M.; Zaghloul, R.A.; El-Dahhan, M.S. Formulation, Optimization and Characterization of Allantoin-Loaded Chitosan Nanoparticles to Alleviate Ethanol-Induced Gastric Ulcer: In-Vitro and in-Vivo Studies. Sci. Rep. 2021, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Ristić, S.; Mitrović, D.M. Antioxidant Effect of Dry Olive (Olea europaea L.) Leaf Extract on Ethanol-Induced Gastric Lesions in Rats. Mediterr. J. Nutr. Metab. 2009, 2, 205–211. [Google Scholar] [CrossRef]
- Dragana, D.; Snežana, J.-H.; Vanja, T.; Goran, M.; Arsić, I.; Dušan, M.M. Phytochemical Analysis and Gastroprotective Activity of an Olive Leaf Extract. J. Serb. Chem. Soc. 2009, 74, 367–377. [Google Scholar]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Abdel Moneim, A.E. Olive (Olea europaea) Leaf Methanolic Extract Prevents HCl/Ethanol-Induced Gastritis in Rats by Attenuating Inflammation and Augmenting Antioxidant Enzyme Activities. Biomed. Pharmacother. 2017, 91, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.; Shady, N.H.; Ahmed, S.R.; Alnusaire, T.S.; Sayed, A.M.; Alowaiesh, B.F.; Sabouni, I.; Al-Sanea, M.M.; Mostafa, E.M.; Youssif, K.A.; et al. Antiulcer Potential of Olea europea L. Cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants 2021, 10, 644. [Google Scholar] [CrossRef]
- Alirezaei, M.; Dezfoulian, O.; Neamati, S.; Rashidipour, M.; Tanideh, N.; Kheradmand, A. Oleuropein Prevents Ethanol-Induced Gastric Ulcers via Elevation of Antioxidant Enzyme Activities in Rats. J. Physiol. Biochem. 2012, 68, 583–592. [Google Scholar] [CrossRef]
- Koc, K.; Cerig, S.; Ucar, S.; Colak, S.; Bakir, M.; Erol, H.S.; Yildirim, S.; Hosseinigouzdagani, M.; Simsek Ozek, N.; Aysin, F.; et al. Gastroprotective Effects of Oleuropein and Thymol on Indomethacin-Induced Gastric Ulcer in Sprague-Dawley Rats. Drug Chem. Toxicol. 2020, 43, 441–453. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan Nanoparticles Making Their Way to Clinical Practice: A Feasibility Study on Their Topical Use for Acne Treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- El-Safy, S.; Tammam, S.N.; Abdel-Halim, M.; Ali, M.E.; Youshia, J.; Shetab Boushehri, M.A.; Lamprecht, A.; Mansour, S. Collagenase Loaded Chitosan Nanoparticles for Digestion of the Collagenous Scar in Liver Fibrosis: The Effect of Chitosan Intrinsic Collagen Binding on the Success of Targeting. Eur. J. Pharm. Biopharm. 2020, 148, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.M.F.; Youshia, J.; Fahmy, S.F.; George, M.Y. Nose to Brain Delivery of Naringin-Loaded Chitosan Nanoparticles for Potential Use in Oxaliplatin-Induced Chemobrain in Rats: Impact on Oxidative Stress, cGAS/STING and HMGB1/RAGE/TLR2/MYD88 Inflammatory Axes. Expert Opin. Drug Deliv. 2023, 20, 1859–1873. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.-A.; El-Sabbagh, H.M. In Vitro-in Vivo Evaluation of Chitosan-PLGA Nanoparticles for Potentiated Gastric Retention and Anti-Ulcer Activity of Diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shi, Y.; Wang, H.; Chun, C.; Chen, L.; Wang, K.; Lu, Z.; Zhao, Y.; Li, X. Protective Effects of Chitosan-Bilirubin Nanoparticles Against Ethanol-Induced Gastric Ulcers. Int. J. Nanomed. 2021, 16, 8235–8250. [Google Scholar] [CrossRef] [PubMed]
- Perumcherry Raman, S.; Dara, P.K.; Vijayan, D.K.; Chatterjee, N.S.; Raghavankutty, M.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Anti-Ulcerogenic Potential of Anthocyanin-Loaded Chitosan Nanoparticles against Alcohol-HCl Induced Gastric Ulcer in Rats. Nat. Prod. Res. 2022, 36, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, I.; Taheri, R.A. Preparation, characterization and release behavior of chitosan-coated nanoliposomes (chitosomes) containing olive leaf extract optimized by response surface methodology. J. Food Sci. Technol. 2021, 58, 3430–3443. [Google Scholar] [CrossRef]
- Sotoudeheian, M.; Hoseini, S.; Mirahmadi, S.M.S.; Farahmandian, N.; Pazoki-Toroudi, H. Oleuropein as a Therapeutic Agent for Non-alcoholic Fatty Liver Disease During Hepatitis C. Rev. Bras. Farmacogn. 2023, 33, 688–695. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- El-Gogary, R.I.; Ragai, M.H.; Moftah, N.; Nasr, M. Oleuropein as a novel topical antipsoriatic nutraceutical: Formulation in microemulsion nanocarrier and exploratory clinical appraisal. Expert Opin. Drug Deliv. 2021, 18, 1523–1532. [Google Scholar] [CrossRef]
- Abu-Azzam, O.; Nasr, M. In Vitro Anti-Inflammatory Potential of Phloretin Microemulsion as a New Formulation for Prospective Treatment of Vaginitis. Pharm. Dev. Technol. 2020, 25, 930–935. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical Composition, Antioxidative and Anti-Inflammatory Activity of Extracts Prepared from Aerial Parts of Oenothera biennis L. and Oenothera Paradoxa Hudziok Obtained after Seeds Cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane Stabilization as a Mechanism of the Anti-Inflammatory Activity of Methanol Extract of Garden Egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Simões, S.; Lopes, R.; Campos, M.C.D.; Marruz, M.J.; da Cruz, M.E.M.; Corvo, L. Animal Models of Acute Gastric Mucosal Injury: Macroscopic and Microscopic Evaluation. Anim. Model Exp. Med. 2019, 2, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.-R.; Li, H.; Wang, S.-W. Gastroprotective Effect of Gallic Acid against Ethanol-Induced Gastric Ulcer in Rats: Involvement of the Nrf2/HO-1 Signaling and Anti-Apoptosis Role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- Góth, L. A Simple Method for Determination of Serum Catalase Activity and Revision of Reference Range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Singh, S.; Khajuria, A.; Taneja, S.C.; Khajuria, R.K.; Singh, J.; Johri, R.K.; Qazi, G.N. The Gastric Ulcer Protective Effect of Boswellic Acids, a Leukotriene Inhibitor from Boswellia Serrata, in Rats. Phytomedicine 2008, 15, 408–415. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Q.; Xu, N.; Cai, J.; Luo, D.; Zhang, Q.; Su, Z.; Gao, C.; Liu, Y. Antioxidative and Anti-Inflammatory Effects of Water Extract of Acrostichum aureum Linn. against Ethanol-Induced Gastric Ulcer in Rats. Evid. Based Complement Altern. Med. 2018, 2018, 3585394. [Google Scholar] [CrossRef]
- Yahia, H.; Hassan, A.; El-Ansary, M.R.; Al-Shorbagy, M.Y.; El-Yamany, M.F. IL-6/STAT3 and Adipokine Modulation Using Tocilizumab in Rats with Fructose-Induced Metabolic Syndrome. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2021, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, M. A Review of the Most Common in Vivo Models of Stomach Ulcers and Natural and Synthetic Anti-Ulcer Compounds: A Comparative Systematic Study. Phytomed. Plus 2022, 2, 100264. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Björne, H.; Petersson, J.; Phillipson, M.; Weitzberg, E.; Holm, L.; Lundberg, J.O. Nitrite in Saliva Increases Gastric Mucosal Blood Flow and Mucus Thickness. J. Clin. Investig. 2004, 113, 106–114. [Google Scholar] [CrossRef]
- Ohta, Y.; Nishida, K. L-Arginine Protects against Stress-Induced Gastric Mucosal Lesions by Preserving Gastric Mucus. Clin. Exp. Pharmacol. Physiol. 2002, 29, 32–38. [Google Scholar] [CrossRef]
- Liang, T.-Y.; Deng, R.-M.; Li, X.; Xu, X.; Chen, G. The Role of Nitric Oxide in Peptic Ulcer: A Narrative Review. Med. Gas Res. 2021, 11, 42–45. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Akhtari, K.; Hassanzadeh, H.; Zarei, S.A.; Fakhraei, N.; Hassanzadeh, K. The Role of Structural C–H Compared with Phenolic OH Sites on the Antioxidant Activity of Oleuropein and Its Derivatives as a Great Non-Flavonoid Family of the Olive Components: A DFT Study. Food Chem. 2014, 164, 251–258. [Google Scholar] [CrossRef]
| Drug Loading (mg Drug/mg Solids) * | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| - | 156.2 ± 3.00 | 0.16 ± 0.01 | +15.2 ± 0.1 |
| 3 mg/7.5 mg | 174.3 a ± 2.4 | 0.33 a ± 0.016 | +11.2 a ± 0.2 |
| 5 mg/7.5 mg | 208.6 a,b ± 3.4 | 0.38 a,b ± 0.01 | +13.0 a,b ± 0.5 |
| 10 mg/7.5 mg | Drug precipitated | ||
| Membrane Stabilization | Lipoxygenase Inhibitory % | ||||
|---|---|---|---|---|---|
| Sample Concentration (µg/ mL) | Oleuropein Solution | Oleuropein CS NPs | Sample Concentration (µg/ mL) | Oleuropein Solution | Oleuropein CS NPs |
| 1000 | 89.32 ± 2.3 | 92.34 ± 0.11 | 125 | 81.64 ± 0.34 | 97.83 ± 1.6 |
| 500 | 80.19 ± 1.5 | 86.35 ± 3.20 | 62.5 | 74.09 ± 0.64 | 91.15 ± 2.1 |
| 250 | 72.98 ± 1.3 | 79.38 ± 0.34 | 31.25 | 61.91 ± 0.72 | 86.37 ± 0.92 |
| 125 | 68.91 ± 0.58 | 71.82 ± 0.92 | 15.63 | 50.08 ± 0.89 | 76.35 ± 1.41 |
| 62.5 | 61.80 ± 1.5 | 68.24 ± 1.73 | 7.81 | 31.25 ± 1.37 | 55.08 ± 0.58 |
| 31.25 | 56.32 ± 3.1 | 59.73 ± 2.41 | 3.9 | 24.69 ± 2.64 | 23.13 ± 2.64 |
| 15.63 | 38.94 ± 2.5 | 42.19 ± 2.03 | 1.95 | 15.62 ± 1.7 | 18.95 ± 1.7 |
| 7.81 | 21.06 ± 1.5 | 35.76 ± 1.04 | 0.98 | 7.2 ± 0.63 | 8.21 ± 2.1 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| IC50 | 25.6 | 22.6 | IC50 | 15.6 | 7.17 |
| Marker | Group I (Normal) | Group II (Ethanol) | Group III (Oleuropein CS NPs) | Group IV (Oleuropein Solution) |
|---|---|---|---|---|
| IL-1 beta (pg/mg ptn) | 5.37 ± 0.32 | 17.88 a ± 1.84 | 8.64 b ± 0.51 | 7.54 b ± 0.48 |
| TNF alpha (ng/mL) | 302.00 ± 20.60 | 667.86 a ± 25.50 | 400.13 b ± 30.70 | 377.71 b ± 18.80 |
| PGE2 (Pg/mL) | 3.19 ± 0.18 | 0.81 a ± 0.12 | 1.98 a,b ± 0.05 | 2.18 a,b ± 0.15 |
| NO (uM/mgprot) | 48.17 ± 4.30 | 23.82 a ± 1.50 | 27.58 a ± 0.91 | 32.71 a ± 3.1 |
| GSH (umol/g ptn) | 1.12 ± 0.05 | 0.61 a ± 0.02 | 0.73 a ± 0.02 | 0.85 a,b ± 0.04 |
| Catalase (U/g.tissue) | 363.78 ± 21.50 | 41.49 a ± 3.70 | 157.87 a,b ± 15.50 | 264.67 a,b,c ± 10.40 |
| TBARS (nmol/mg ptn) | 92.90 ± 0.97 | 150.32 a ± 3.50 | 119.36 a,b ± 1.60 | 115.74 a,b ± 2.90 |
| Group | Pathologic Score |
|---|---|
| Group I (Control) | 0.30 ± 0.21 |
| Group II (Ethanol-induced ulcer pretreated with physiological saline) | 5.70 a ± 0.21 |
| Group III (Ethanol-induced ulcer pretreated with oleuropein CS NPs) | 0.40 b,d ± 0.12 |
| Group IV (Ethanol-induced ulcer pretreated with oleuropein solution) | 1.50 a,b ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Allah, H.; Youshia, J.; Abdel Jaleel, G.A.; Hassan, A.; El Madani, M.; Nasr, M. Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept. Pharmaceutics 2024, 16, 153. https://doi.org/10.3390/pharmaceutics16010153
Abd-Allah H, Youshia J, Abdel Jaleel GA, Hassan A, El Madani M, Nasr M. Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept. Pharmaceutics. 2024; 16(1):153. https://doi.org/10.3390/pharmaceutics16010153
Chicago/Turabian StyleAbd-Allah, Hend, John Youshia, Gehad A. Abdel Jaleel, Azza Hassan, Mevidette El Madani, and Maha Nasr. 2024. "Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept" Pharmaceutics 16, no. 1: 153. https://doi.org/10.3390/pharmaceutics16010153
APA StyleAbd-Allah, H., Youshia, J., Abdel Jaleel, G. A., Hassan, A., El Madani, M., & Nasr, M. (2024). Gastroprotective Chitosan Nanoparticles Loaded with Oleuropein: An In Vivo Proof of Concept. Pharmaceutics, 16(1), 153. https://doi.org/10.3390/pharmaceutics16010153







