Abstract
Cancer is a global health burden and is one of the leading causes of death. Photodynamic therapy (PDT) is considered an alternative approach to conventional cancer treatment. PDT utilizes a light-sensitive compound, photosensitizers (PSs), light irradiation, and molecular oxygen (O2). This generates cytotoxic reactive oxygen species (ROS), which can trigger necrosis and/ or apoptosis, leading to cancer cell death in the intended tissues. Classical photosensitizers impose limitations that hinder their clinical applications, such as long-term skin photosensitivity, hydrophobic nature, nonspecific targeting, and toxic cumulative effects. Thus, nanotechnology emerged as an unorthodox solution for improving the hydrophilicity and targeting efficiency of PSs. Among nanocarriers, mesoporous silica nanoparticles (MSNs) have gained increasing attention due to their high surface area, defined pore size and structure, ease of surface modification, stable aqueous dispersions, good biocompatibility, and optical transparency, which are vital for PDT. The advancement of integrated MSNs/PDT has led to an inspiring multimodal nanosystem for effectively treating malignancies. This review gives an overview of the main components and mechanisms of the PDT process, the effect of PDT on tumor cells, and the most recent studies that reported the benefits of incorporating PSs into silica nanoparticles and integration with PDT against different cancer cells.
1. Introduction
In recent years, cancer has been one of the leading causes of death globally, and it continues to be a primary public health burden []. The term cancer refers to a group of medical conditions originating from abnormal regulation and cell growth that lead to unregulated proliferation and neoplastic development coupled with metastatic ability [,]. In 2020, more than 19.2 million new cancer cases were diagnosed, and around 9.9 million patients died worldwide []. This number is predicted to rise by 70% by 2040 []. Many studies have reported that alcohol, advanced age, smoking, genetic mutations, hormonal imbalances, and poor lifestyle can contribute to cancer development [,]. Nowadays, different types of clinical treatments for cancer are being used, such as radiotherapy, chemotherapy, and surgery. However, these conventional treatments still show some drawbacks, such as low curative effects, poor selectivity, painful treatment procedures, toxic effects on normal cells [,], and multidrug resistance [,,]. In this context, it becomes essential to develop alternative safe treatment processes that are more effective, facile, and less invasive [].
Photodynamic therapy (PDT) has gained much attention in the past decades for treating various kinds of cancer, such as lung, skin, bladder, esophageal cancer, etc. [,,]. Photodynamic therapy is a modern, rapidly developing, and noninvasive method for diagnosing and treating cancer disease []. PDT involves using a photosensitizer (PS) that is administered locally or systemically with a nontoxic dose. Initially, the PS is nonspecifically distributed throughout the body, accumulating in the tumor tissues. The typical duration for the PS to reach its target site is reported to be between 5 min and 24 h, depending on the method of administration and type of photosensitizers. Then, the target tissue is exposed to light irradiation with a specific wavelength called the photodynamic window (600–850 nm), resulting in the formation of cytotoxic reactive oxygen species (ROS) in the presence of endogenous molecular oxygen (O2), leading to the eradication or regression of cancer cells (Figure 1) [,].
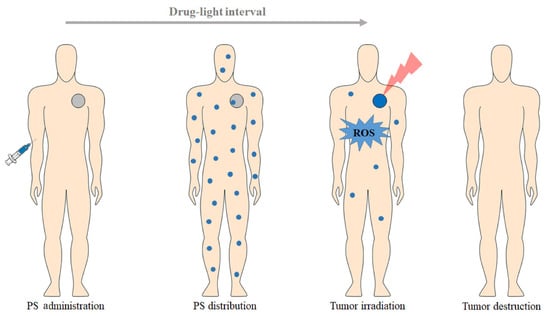
Figure 1.
A schematic overview representing the clinical application of photodynamic therapy protocol for cancer treatment [].
Photodynamic therapy has attracted significant attention, mainly in cancer treatment, owing to its spatial and temporal selectivity, where irradiation can be controlled in terms of time and position. Additionally, it is well tolerated by patients as it can be applied at the same site more than once. Moreover, it preserves fertility and does not affect pregnancy and delivery [,]. Other advantages are reported for PDT, like inhibition of drug resistance pathways, low systemic toxicity, and ease of combination with other therapeutic regimens. All these advantages make PDT a more promising alternative to conventional treatment methods like chemotherapy, radiotherapy, and surgery [,]. Like every therapeutic modality, photodynamic therapy also has some limitations. One of PDT’s major limitations is the inability to be applied to the whole body in case of metastasis. So, its utilization is limited to precancerous lesion treatment and regional malignancies. In addition, treatment with PDT mainly relies on the presence of molecular oxygen in cancerous cells, and the singlet oxygen generated during PDT has a short half-life. Another limitation is its ineffectiveness in large tumor masses and deep cancers due to the limited light penetration []. Moreover, the PSs used in PDT have several drawbacks, such as skin photosensitivity, accumulation in healthy tissues, and limited selectivity for cancer cells [,,]. Thus, photodynamic therapy has several advantages over conventional methods such as chemotherapy, radiotherapy, and surgery. The main advantages and disadvantages of PDT and other conventional therapies for cancer treatment are summarized in Table 1.

Table 1.
A summary of the advantages and disadvantages of photodynamic therapy and other conventional therapies in cancer treatment.
A solution to limit PDT’s disadvantages and make the most use of them is through designing nanoplatforms. Nanoplatforms have been designed to enhance existing photosensitizers to increase treatment efficiency by addressing the concerns of optical absorption, tumor targeting ability, and limited solubility [,]. Nanomaterials have promising optical properties that can improve the efficacy and enhance the penetration of PDT. Another use of nanomaterials is the encapsulation of photosensitizers to the nanocarriers, leading to high loading efficiencies, in addition to their surfaces, which can be decorated with versatile, functional groups to escort PSs selectively to the intended sites of action. These targeted PDT nanocarriers can have a role in reducing their side effects and improving their curative effects. Moreover, nanoparticles are stable under irradiation, unlike conventional organic PSs, which may exhibit a reduction in rates due to their instability upon irradiation exposure [].
Interestingly, some nanomaterials, such as semiconductor nanoparticles, can serve as PS carriers and, at the same time, act as reactive oxygen species (ROS) producers [,]. Among the various NPs, silica nanoparticles have been given enormous consideration due to their unique features, such as a uniform pore size and large surface area, the presence of external and internal pores, the ease of surface modification, high mechanical and thermal stability, stable aqueous dispersion, high biocompatibility and biodegradability, and a high loading capacity [,]. Herein, this review will highlight and discuss the basic principles and current challenges of using photodynamic therapy in cancer treatment and how their therapeutic significance can be improved by using silicon-based inorganic nanoparticles.
2. Photodynamic Therapy (PDT) and Photosensitizers (PSs)
Because of its efficacy and cost-effectiveness, photodynamic therapy seems to be an attractive option for cancer treatment []. It has been investigated for more than 25 years as an untraditional treatment process for cancer []. This technique integrates three components: a photosensitizer, a light source with an appropriate wavelength, and molecular oxygen inside the tissues [,]. These three main elements are summarized in Figure 2.
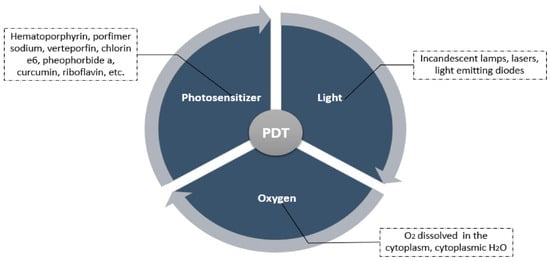
Figure 2.
A summary of the main components of photodynamic therapy [].
The light penetration into tumor cells is sophisticated; it can be scattered, reflected, or absorbed. The extent of these processes depends mainly on the light’s wavelength and the tissue type []. First, the endogenous chromophores in tissues such as myoglobin, cytochromes, and chromophores, which are responsible mainly for light absorption, can compete with photosensitizers and reduce the PDT process’s effect and reaction rate. Second, the tissue light absorption is inversely proportional to the wavelength of the light. So, the optimum reported wavelength to be used against cells ranges between 600 and 1200 nm, often called the tissue optical window [,]. Shorter wavelengths (<600 nm) do not have enough energy to produce enough reactive oxygen species and have a lower penetration depth in tissues, while longer wavelengths (>850 nm) are not sufficient to activate PSs []. Therefore, the most appropriate wavelength for PDT is between 600 and 850 nm, which is called the “phototherapeutic window” (Figure 3) [,].
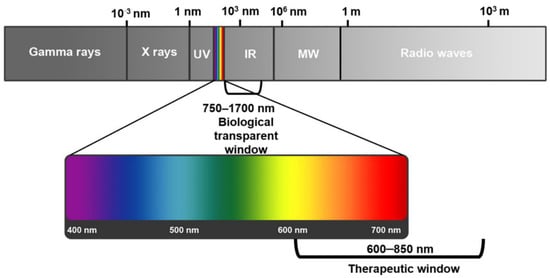
Figure 3.
Electromagnetic spectrum showing the phototherapeutic window for photodynamic therapy for cancer treatment [].
The second key component in PDT is molecular oxygen. The oxygen concentration in the tumor tissues mainly affects the PDT treatment’s effectiveness. The oxygen concentration varies between different types of tumors and even between different regions of the same tumor []. Oxygen is decisive for the production of ROS during PDT treatment. However, hypoxia is the main pathological feature of many types of solid tumors, which leads to an imbalance between oxygen delivery and oxygen consumption. Hypoxia affects the success of cancer treatment via PDT due to the absence of molecular oxygen, resulting in poor treatment outcomes [,].
Another essential component of photodynamic therapy, apart from oxygen and light, is the type of PS. A PS works by absorbing electromagnetic radiation’s visible or ultraviolet region and then transferring this radiation to the adjacent molecules with a specific efficacy []. An ideal PS should be water-soluble [], biocompatible, stable in plasma or serum solution [], easily eliminated from the human body, and inactive in the absence of light radiation [,,]. It must also possess a low photobleaching quantum yield, high intersystem crossing efficiencies [], and finally, the ability to absorb light in the long-wave part of the spectrum (600–850) nm [,].
There are a variety of molecular structures of photosensitizers that are currently used in photodynamic therapy. They are divided into three generations of photosensitizers [].
The first generation of PSs comprises various forms of hematoporphyrin derivatives (HPDs), used for treating cancer in the 1950s []. Photofrin (parfimer sodium), a hematporph derivative, has been involved in treating several types of cancer, such as cervical, bladder, esophageal, lung, and brain cancers []. However, despite their wide applications, first-generation photosensitizers have several disadvantages, including skin photosensitivity [,], ineffectiveness at low doses [], instability, and nonselectivity. Another limitation of this generation is that they are excited only by short wavelengths, leading to low tissue penetration [].
The second generation of PSs was developed in the late 1980s to overcome the first generation’s drawbacks []. Typically, the second-generation PSs are macrocyclic compounds derived from porphyrin moieties such as chlorins, phthalocyanines, bacteriochlorins, benzoporphyrin, temoporfin (Foscan), talaporfin, 5-amino levulinic acid, methyl aminolevulinate, and verteporfin [,,]. In contrast to first-generation photosensitizers, these porphyrinoid compounds have faster elimination rates from the body. In addition to being more benign, they can deeply penetrate cancer tissues and absorb a spectrum ranging between 650 and 800 nm. However, they still cause tissue damage because of their higher toxicity and hydrophobic nature, which dictates the development of the third generation of PSs [,].
The development of third-generation photosensitizers mainly focuses on synthesizing structures with a higher affinity to cancer cells to facilitate cell uptake and improve the outcome of the therapeutic process []. The third generation mainly depends on modifying some available drugs with a specific agent, such as antibodies, amino acids, carbohydrates, etc., as targeting moieties. Another option is encapsulating second-generation photosensitizers in biodegradable or biocompatible nanocarriers. These modifications increase their accumulation at the target tissues [,].
Choosing an optimal photosensitizer is very important for the success of any photodynamic therapy. Despite many studies being performed using different types of photosensitizers, only a few PSs have received the U.S. Food and Drug Administration (FDA) approval for clinical use [].
The selected PSs approved by the FDA are listed in Table 2.

Table 2.
Selected FDA-approved photosensitizers for photodynamic therapy.
3. Photodynamic Therapy (PDT) Mechanisms
Photodynamic therapy combines photochemical and photophysical processes, ultimately improving therapeutic effects. Figure 4 illustrates the principle of PDT using the modified Jablonski energy diagram. Upon light absorption, the photosensitizer in the ground state (S0) is initially transformed into an excited singlet state (S1), leading to two possible processes. In the first possibility, the PS in the excited singlet state S1 is supposed to have a very short lifetime, so it returns to the ground state by emitting photon energy in the form of fluorescence emission. This type of photophysical process is used for photodynamic diagnosis (PDD) (Figure 4a) [,]. The second possibility is when the multiplication of the PS is transferred from the singlet state to the triplet state (T1) through intersystem crossing (ISC) to reach the first triplet excited state (T1). More importantly, the triplet excited state has a longer life when compared to the singlet excited state (S1) of a photosensitizer (Figure 4b) [,].
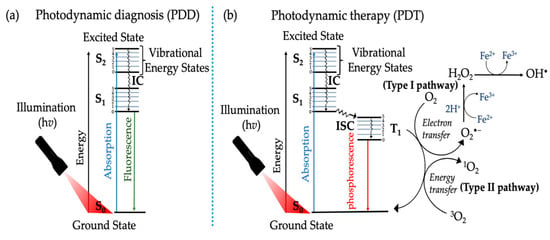
Figure 4.
Modified Jablonski energy diagram, representing the photochemical and photophysical reactions of photosensitizers in (a) photodynamic diagnosis (PDD) and (b) photodynamic therapy (PDT) [].
Photodynamic therapy can be classified into two major photochemical pathways (Type I and Type II). Both pathways lead to ROS and free radical generation upon activation with PDT process [,]. Electron transfer or/and hydrogen abstraction occurs between the substrates and photosensitizers for type I, generating free radicals. These formed radicals have a very short lifetime. They simultaneously react with molecules such as oxygen and water, producing hydrogen peroxide (H2O2), hydroxyl radical (·OH), and superoxide anion (O2−). As for the Type II process, the excitation energy is transferred to molecular oxygen (O2), forming singlet oxygen (1O2). The singlet oxygen is highly reactive and can interact with many biological substrates, eventually leading to cell death []. The photosensitizers can use either Type I, Type II, or both types of reaction instantaneously to destroy cancerous cells. The ratio between these two processes depends mainly on the oxygen concentration, substrates, photosensitizer, and the binding affinity of the photosensitizer to the substrate [,]. The therapeutic efficacy of type II PDT primarily depends on the tissue oxygen level and can only be initiated under well-oxygenated conditions. However, the hypoxic environment is a native microenvironment in solid tumors due to the rapid tumor growth and insufficient oxygen supply [].
The Effect of Photodynamic Therapy on Tumors
The destruction of tumors by photodynamic therapy is a phenomenon that has been known for about a century. There are three mechanisms by which PDT mediates tumor destruction (apoptosis and necrosis, vascular mechanism, and immunological mechanism) (Figure 5). PDT stimulates the immune system against tumor cells, while conventional treatment methods such as chemotherapy and radiotherapy mainly cause immune suppression. PDT can induce inflammation, facilitate the antitumor T lymphocytes activation, and recruit leukocytes to target areas. It is also capable of killing tumor cells directly via necrosis induction (nonprogrammed cell death) or apoptosis (programmed cell death) by (1O2). Another reported effect of PDT is a reduction in the nature of the tumor microvasculature, from which the nutrients and oxygen in the tumor tissues are derived [,]. The three main mechanisms by which PDT mediates the destruction of tumor cells are summarized in Figure 5.
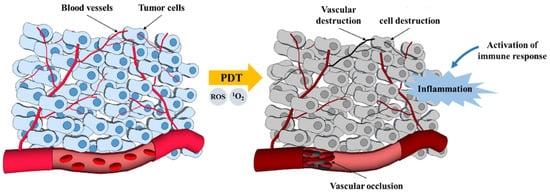
Figure 5.
PDT mechanisms for tumor destruction [].
4. Nanotechnology in Photodynamic Therapy
Current research is trying to explore new strategies to improve photodynamic therapy (PDT) and lessen its limitations. One of the promising strategies is the integration of photosensitizers with nanotechnology. This approach has become a highly effective solution to increase therapy effectiveness. In recent years, various types of nanoparticles have been developed to be used as imaging probes or drug carriers due to the following advantages [,]: First, their large surface-to-volume ratio allows for the administration of a high amount of the drug, improves its delivery, and increases the uptake concentration in the target cells [,]. Second, the smaller size of nanoparticles makes them suitable for intravenous injection and also helps them mimic the biological molecules, enabling this nanosystem to pass easily through the immune system barriers. The third advantage is their surface, which can be functionalized with specific ligands. Surface functionalization using some moieties and functional groups enhances their ability to bind to a specific receptor on the target cells, increasing their specificity [,,,]. They can also prevent the antitumor drugs’ enzymatic inactivation or degradation by the plasma components like the overexpression of drug-metabolized enzymes (DMEs) [,]. PS integration with nanomaterials can offer a broader range of wavelengths, including ultraviolet, infrared, visible, and X-ray radiation. They can also increase the solubility of hydrophobic antitumor drugs by physical loading and chemical conjugation processes [,,,]. These advantages have made nanoparticles (NPs) a promising carrier system for the photosensitizers in PDT.
Depending on the nature of the PS, different strategies can be followed for their conjugation or attachment to the nanocarriers. Some examples of these strategies are utilizing physical adsorption, covalent conjugation, hydrophobic interactions, and encapsulation. The advantages and disadvantages of loading a photosensitizer into the organic and inorganic nanocarrier mainly depend on the characteristics or properties of the nanoparticles themselves [,].
On the one hand, organic nanostructures such as micelles, liposomes, dendrimers, polymeric [,], and lipid nanoparticles are especially favorable for encapsulating hydrophobic drugs. They can also be used in drug delivery applications due to their remarkable biopharmaceutical properties, such as good bioavailability, low systemic toxicity, controlled drug release, good water solubility, and degradation resistance []. On the other hand, inorganic nanosystems such as metal oxides (iron oxide, titanium dioxide, zinc oxide, etc.), mesoporous silica, semiconductors (quantum dots, graphene), and metallic nanoparticles have also been used successfully for drug delivery. Inorganic nanosystems are distinguished by their chemical maintenance, thermal stability, good bioavailability, possibility to improve the therapeutic action of loaded drugs, and low toxicity [].
4.1. Silica Nanoparticles
The encapsulation of PSs using silica nanoparticles is one of the most attractive strategies in PDT []. Silica is one of the most abundant elements in the earth’s crust and is produced from various sources []. It is an oxide of silicon and is considered one of the most efficient carriers for drug delivery control []. In general terms, silica nanoparticles are well known for their usually higher colloidal stability, biocompatibility, and ability to produce 1O2, as well as their functionalization properties [,]. Silica nanoparticles can be classified into three different types: (1) Stöber silica nanoparticles (nonporous) without porous structure, (2) organically modified silica (Oromosil), an organophilic core produced by the combination of different silica sources, and (3) mesoporous silica nanoparticles (MSNs) with a porous structure (pore size: 2–50 nm). These different types of silica nanoparticles are differentiated based on their structures or their core nature []. Among these three types, mesoporous silica nanoparticles (MSNs) are considered suitable candidates in photodynamic therapy. MSNs possess several interesting properties that make them one of the promising nanocarriers for the effective delivery of PSs in PDT. These features include the (i) ease of functionalization with targeting ligands, (ii) outstanding biocompatibility, (iii) large surface areas (>1000 m2/g), (iv) high loading efficiencies of different therapeutic agents, (v) tunable particle sizes and morphologies [,], and (iv) optical transparency, which are crucial for PDT [,,,,]. In addition, MSNs have been reported to address some of the challenges accompanying the use of PSs in PDT, such as poor selectivity to tumor tissues, hydrophobicity, and long-term accumulation in healthy tissues. In the coming sections, we will review the most recent studies that utilized MSNs as a promising nanocarrier for PSs, aiming at improving PDT.
4.2. MSNs in Photodynamic Therapy
Photosensitizer subcellular localization uptake can be classified into either active or passive targeting processes (Figure 6).
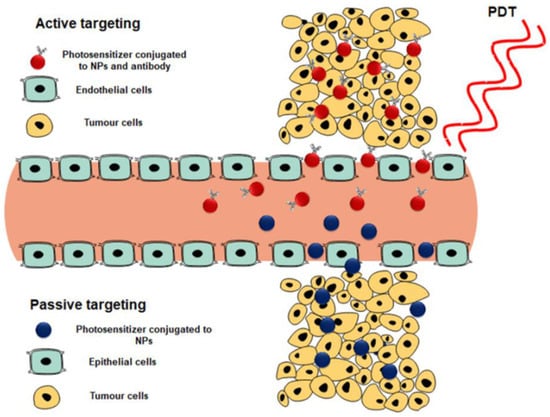
Figure 6.
Active and passive targeting of photosensitizers to generate single oxygen and reactive oxygen species for tumor destruction [].
Active targeting is achieved by decorating the surface of the photosensitizer-loaded nanocarrier system with a specific targeting ligand. This functional decoration can be via peptides, antibodies, folic acid, aptamers, carbohydrates, or small ligands, which are overexpressed only on cancer cells. As a result, the PS uptake in these cells is actively internalized and specifically enhanced []. On the other hand, passive targeting can occur via the permeability and retention effect (EPR), causing a leaky vasculature on tumor tissues [,].
This naturally occurring process utilizes the difference in pathophysiological and anatomical abnormalities between cancer tissue and normal cells. The active targeting of nanoparticles provides a more selective absorption of photosensitizers with an increased concentration accumulation [].
4.3. Photosensitizers Loaded on MSNs
Two main groups of silica nanocarriers can be primarily found in this review. On the one hand, silica nanoparticles are often used as a coating for other nanostructures. The second type is silica-based nanomaterials that are involved in the photosensitizer’s incorporation, making this type the fourth generation of photosensitizers []. In this review, we have focused on recent work on the applications of silica nanoparticles as photosensitizer vehicles for photodynamic therapy.
The following sections show a more profound view of the most representative examples of loading PSs onto silica nanoparticles. These sections are divided according to the type of photosensitizer used.
4.3.1. Porphyrin Photosensitizer
Porphyrins are macrocyclic pigments, commonly occurring in nature. They are often known as pigments of life. Porphyrin’s name comes from porphyra, a Greek word that means purple, because porphyrins are usually red or bright purple. Porphyrins are widely used in PDT owing to the unique photosensitive porphyrins []. In fact, several studies were carried out with porphyrins as photosensitizers in PDT. First, Elisa Bouffard et al. developed highly efficient targeting molecules of mesoporous silica nanoparticles (MSNs), carrying porphyrin as the photosensitizer for photodynamic therapy targeting prostate cancer. The surface of NPs is decorated with diamino-side carboxylate, which can specifically bind to mannose-6-phosphate receptors as it is overexpressed in prostate cancer. This targeted PS via porphyrin leads to a higher endocytosis of tumor cells []. Dina Aggad et al. reported ethylene-based periodic mesoporous organosilica nanoparticles (PMOs) for PDT and the autonomous delivery of gemcitabine hydrochloride, an FDA-approved chemotherapeutic drug, in cancer cells. Depending on the nature of the photosensitizer (tetrasiylated porphyrins or monosilylated porphyrin) and its aggregation state, they could form two-photon PDT. The synergistic effect of two-photon irradiation with gemcitabine raised the percentage of cell death by about 20% compared to the delivery process without irradiation []. Si Li et al. were also able to design mesoporous silica nanoparticles (MSNs) as a nanocarrier composed of silica nanoparticles (SiNPs), 5,10,15,20-tetrakis (1-methyl 4-pyridinio), and porphyrin tetra (p-toluenesulfonate) (TMPyP) photosensitizer, and this was further decorated with folic acid (FA). Under light irradiation near-infrared light (NIR = 655 nm), the embedded TMPyP could generate singlet oxygen. Additionally, the doxorubicin (DOX) anticancer drug could be loaded onto them for chemotherapeutic purposes. In vitro cytotoxicity assay shows that the viability of MCF-7 breast cancer cells, the most common type of cancer among women, treated with MSN@SiNP@TMPyP-FA/DOX under NIR irradiation was lowered by 30% []. In a different study, the periodic mesoporous organosilica nanoparticles (PMOs) encapsulated with protoporphyrin IX (PpIX) photosensitizer molecules showed a significant PDT effect in colon carcinoma (HT-29) and Esherichia coli (E. coli), a Gram-negative bacterial strain. These nanoparticle frameworks were beneficial for in vitro PDT on human colon cancer cells []. Jiefei Wang et al. managed to develop new nanoparticles by assembling porphyrin as the core surrounded by convenient functionalization. The designed nanoparticles improved their biocompatibility with the cancer cells and their ability to generate lethal singlet oxygen 1O2 []. Also, silica was reported to be used in coating gold nanorods (AuNRs), conjugated with 4-carboxyphenyl porphyrin (TCPP), to form AuNR@SiO2-TCCP where AuNRs act as a photothermal agent, while TCPP acts as a photosensitizer. Like the ones developed by Wang et al. [], this prepared nanoparticles possess good biocompatibility and can generate single oxygen efficiently. The results illustrated that the AuNR@SiO2-TCPP showed a high synergistic effect in photodynamic and photothermal therapy against cancer cells in both in vivo and in vitro studies []. Rod-like nanomaterials were also studied in different studies where rod-like mesoporous silica nanoparticles coated with gold nanoshell and modified with ultrasmall gadolinium (Gd)-chelated 5,10,15,20 tetrakis (4-sulfonat-o-phenyl) porphyrin (TPPS4) photosensitizers were designed. This designed nanostructure could control PTT/PDT combined anticancer therapy. Also, nanosheets were used, where gold nanosheet was found to act as a perfect PTT agent, and a TPPS4(Gd) photosensitizer was able to generate singlet oxygen 1O2 with high efficiency [].
4.3.2. Phthalocyanines Photosensitizers
Phthalocyanines are a class of promising second-generation photosensitizers that exhibit higher singlet oxygen quantum yields and have strong absorption in the near-infrared region (NIR) [].
A study conducted by Ozge Er et al. described the loading of zinc phthalocyanine (ZnPc) onto mesoporous silica nanoparticles (MSNPs) for in vivo and intracellular PDT against pancreatic cancer cells. The histopathology studies revealed that the necrosis of tumor cells was higher in the treated group than in the control group. The surface modification of MSNPs using polyethylene glycol (PEG) led to an increase in the blood circulation time, a decrease in the extraction rate of these nanophotosensitizers, and a decrease in the labeling process []. In another study, a functionalized zinc (II) phthalocyanine (ZnPc) was conjugated with stellate mesoporous silica nanoparticles (SMSNs) through the formation of an acid-sensitive hydrazone bond. This nanosystem (SMSN-ZnPc) was activated inside tumor cells, and ROS were generated efficiently against Hela cells. It can also exhibit efficient tumor growth inhibition with negligible systemic toxicity []. Özge Er et al. also developed mesoporous silica nanoparticles loaded with zinc (II) 2,3,9,10,16,17,23,24-octa (tert-butyl phenoxy phthalocyaninato (2-)-N29,N30,N31,N32 (ZnPcOBP) as a photosensitizer to determine the production of singlet oxygen and achieve in vitro PDT against pancreatic cancer cells. When ZnPcOBP was incorporated in silica nanoparticles, it showed a high phototoxic effect, which was enhanced by cetuximab. Cetuximab is a monoclonal antibody that mainly targets the epidermal growth factor receptor (EGFR). So, the imidazole was an excellent vehicle for the selective delivery of ZnPcOBP to pancreatic cancer cells (ASPC-1, PANC-1, MATpaca-2) in vitro []. Yiming Zhou et al. reported ultrasmall PH-responsive silicon phthalocyanine nanomicelles (PSN0), which were designed for selective PDT against tumors, with minimum damage to normal tissues. The results of in vivo studies declared that PSN, as a pH-responsive photosensitizer, has broad applications depending on the selectivity of the PDT against tumor cells. It also reported that the tumor cells were eliminated without recurrence []. A study conducted by Tingting Shen et al. aimed to improve ROS production in specific H1299 tumor locations via loading gold nanoparticles (AuNPs) onto the surface of mesoporous silica-coated upconversion nanoparticles. This nanosystem was loaded into the silica shell with a photosensitizer (silica phthalocyanine dihydroxide) and small molecules DC50 (C17H14BrF2N3OS). This nanoplatform exhibited a specific cytotoxic effect by expressing Atox1 and CCS proteins after internalization by specific tumor cells []. Burcu GÜleryÜz et al. developed a (MC540/ZnPc-UCNP@Au) nanoplatform through the synthesis of upconversion nanoparticles (UCNP) to convert near-infrared light into multiple visible wavelengths, coated with porous silica nanoparticles and then uploaded with a dual photosensitizer merocyanine 540 (MC540), zinc phthalocyanine (ZnPc), and gold (Au) functionalization to enhance PDT treatment against prostate cancer cell (PC3). MC540/ZnPc-UCNP@Au nanoplatforms could transform near-infrared (980 nm) to visible (540 and 660 nm) to activate PSs. In addition, this nanoplatform allows for treatment of a deep-seated prostate cancer cell due to the high penetration property of NIR light [].
Another commonly used second-generation PS is phthalocyanine (Pc), which was re-formulated, forming benzyl ester dendrimer silicon phthalocyanine (D-SiPc). In order to improve the cell permeability of D-SiPc, it was loaded on amphiphilic block copolymer (methoxy polyethylene glycol-polylactic acid, MPEG500-PLA300) to form polymeric nanoparticles. These polymeric nanoparticles exhibited a high photo-cytotoxic effect against U251 glioma cells under laser irradiation []. Xiuqin Chen et al. developed a cholesterol silicon (IV) phthalocyanine (Chol-Pc) photosensitizer based on DSPE@Chol-Pc nanoparticles, using DSPE-PEG500 as a nanocarrier. This nanosystem could effectively produce reactive oxygen species (ROS) and exhibited a phototoxic effect on (MCF-7) breast cancer cells, leading to cell death by destroying the cholesterol-rich membrane. DSPE@Chol-Pc is mainly distributed in rich cholesterol cells, especially in the Golgi apparatus of breast cancer cells [].
4.3.3. Chlorin e6 Photosensitizer
Chlorin e6 (Ce6) is an FDA-approved second-generation photosensitizer that meets the desired clinical properties for photodynamic therapy. It is characterized by a high reactive oxygen species generation ability and high anticancer potency against many types of cancer cells. The major drawback of Ce6 is its hydrophobicity, leading to its rapid clearance from the circulatory system and poor biodistribution. Several nanosystems have been designed to overcome this drawback and enhance Ce6 bioavailability [,]. Xuemel Wang et al. reported redox nanocarriers (RN), which are prepared using hollow mesoporous silica nanospheres decorated with a redox polymer ligand. This designed structure is characterized by high biocompatibility and low in vitro cytotoxicity. This nanocarrier was loaded with catalase and metformin, which is responsible for inhibiting the mitochondrial respiration of cancer cells, reducing the activity of tumor cells, and increasing the concentration of oxygen in PDT, upon its linkage with chlorine e6 photosensitizer []. In another study, MSNs have been investigated for controlling drug delivery to the target site. This system consists of two main constituents: a gadolinium complex (Gd-DOTA) that acts as the ROS gatekeeper and polyethylene glycol (PEG) conjugated with Ce6, which acts as the ROS generator. Both constituents are important for magnetic resonance imaging to guide photodynamic chemotherapy. It also contains doxorubicin (Dox), a chosen anticancer drug that is responsible for maintaining the structural integrity and enhancing the in vitro imaging signal. DOX-R-MSNs, under 660 nm laser irradiation, exhibit a rapid DOX release at the tumor site, effectively inhibiting tumor growth []. Xiaoli Cai et al. developed promising three-dimensional dendritic mesoporous silica nanoparticles to deliver a novel hydrophobic photosensitizer (Chlorin e6) to lung cancer cells (A549). The nanoenzymes (platinum (Pt) nanoparticles) were immobilized into the channels of the nanosystem to catalyze the conversion process of hydrogen peroxide (H2O2) to oxygen intracellularly. Also, the surface was decorated with triphenylphosphine (TPP) to target the mitochondria. MTT assays showed that more than 80% of the cancer cells died after treatment with Pt-DMSN-TPP/Ce6 nanoparticles irradiated with 660 nm []. Qi Sun et al. first reported that gold (Au) nanorods were capped to chlorine e6-doped mesoporous silica nanorods for the single wavelength of NIR light-triggered combined phototherapy (photodynamic therapy (PDT) and photothermal therapy (PTT)). This single wavelength of light and the rod shape of the nanosystem for the combined phototherapy had the following remarkable features: (1) the effect of PDT and PTT could be achieved under a single wavelength of near-infrared light (660 nm), which makes the therapeutical process feasible and simple and (2) chlorin e6 doped into a mesoporous nanorod without being released from the nanocarrier during delivery. AuNRs-Ce6-MSNRs are not only able to produce single oxygen for PDT based on chlorin e6 after the uncapping of gold nanorods under the single NIR irradiation, but they are also able to generate heat to perform the PTT effect based on AuNRs []. In another study, the photosensitizer chlorin e6 was covalently attached to the external and internal surfaces of MSNs. Then, the nanoparticles were anchored by triphenyl phosphonium for selective mitochondrial targeting. When the nanophotosensitizer was irradiated with a laser (655 nm, 0.1 W.cm−2), it generated a large amount of ROS in the mitochondria, leading to mitochondrial dysfunction and cell apoptosis []. Zhen Lu Yong et al. reported a multifunctional and safe oxygen-evolving nanoplatform composed of Prussian blue core and chlorin e6-anchored periodic mesoporous organosilica shell (PB@PMO-Ce6). The Prussian blue (PB) can catalyze hydrogen peroxide (H2O2) to generate molecular oxygen (O2), and the chlorin e6, upon laser irradiation, becomes able to transform the produced molecular oxygen O2 to a more reactive oxygen species (ROS). The histopathological analysis showed that this nanoplatform could elevate singlet oxygen to effectively inhibit the growth of tumor cells without obvious damage to major organs []. Sanghyo Park et al. developed MSNs that are conjugated with hyaluronic acid (HA) for specific cancer cell targeting. Also, the prepared HA-MSNs exhibited a high drug-loading capacity and sustained drug release. To enhance the anticancer effect of PDT, chlorin e6 (Ce6) and doxorubicin (DOX) were loaded in HA-MSNs to form a (DOX/Ce6/HA-MSNs) nanoplatform, which exhibited highly effective cytotoxicity on squamous cell carcinoma 7 (SCC7) compared to the corresponding free drug []. A smart multifunctional nanoplatform was designed by coating core–shell composite mesoporous silica-encapsulated upconversion nanoparticles and chlorin e6 (Ce6) with degradable calcium phosphate, and then this system was loaded with doxorubicin (DOX). This nanoplatform has the synergistic effect of ROS, which mediates drug release to achieve a high therapeutic effect. Based on this experiment’s results, these nanoplatforms conjugated with chlorin e6 photosensitizer can significantly enhance the cellular uptake and the photodynamic therapy response in both in vivo and in vitro processes. Moreover, it improved the selectivity of nanosystems towards cancer cells []. Additionally, hollow mesoporous silica nanospheres were loaded with doxorubicin (DOX) and chlorine e6 (Ce6). Afterwards, bovine serum albumin (BSA) and integrated manganese dioxide (MnO2) formed a BA-MnO2 nanoparticle that was anchored to the surface of a loaded hollow mesoporous silica nanoparticle through the formation of disulfide bonds to form (BSA-MnO2@HMSNs-DOXCe6, BMHDC). Such a nanoplatform exhibited a loading capacity effect (36% of Ce6 and 14% of DOX) and pH sensitivity via Ce6 and DOX behavior through the breakdown of disulfide bonds []. A different study successfully developed hollow MSNs co-loaded with manganese oxide NPs and chlorine e6 for tumor magnetic resonance imaging (MRT) and in vivo PDT. This universal “on/off” switching strategy was found to be able to control the loading amount of Ce6 and Mn. The results have shown that the increase in O2 concentration improved PDT efficiency and exhibited effective tumor inhibition, reducing the side effects on normal cells []. Jiaxing Yang et al. developed silica nanoparticles decorated with cell membrane (CM) derived from SGC7901 cells to specifically target gastric cancer (homogenous SGC7901) in vivo and in vitro. The designed CM/SLN/Ce6 diameter was about 115.6 nm, with a surface charge of −30.4 mv []. Another recent study reported successfully fabricated upconversion nanoparticles (UCNPs) with a dual nature (ROS and pH) to utilize chlorin e6 (Ce6) and doxorubicin (DOX). The DOX and Ce6 were conjugated in a 1:1 (w:w) ratio and then loaded onto the surface of UCNPs@mesoporous silica nanoparticles with a diameter of 85.63 ± 9.87 nm. Ce6 controlled the release of DOX under NIR laser irradiation at 980 nm. A cytotoxic study showed that this modified nanosystem could successfully deliver DOX and Ce6 at a specific tumor site, causing cell death []. Xiang Long Tang et al. developed a Fe3O4@mSiO2(DOX)@HSA(Ce6) nanoplatform where doxorubicin (DOX) molecules were loaded onto Fe3O4/mSiO2 to form Fe3O4 mSiO2(DOX), and then polydopamine (PDA) was coated onto Fe3O4/mSiO2(DOX) to obtain PDA-coated Fe3O4/mSiO2(DOX). Human serum albumin (HAS) was conjugated to the other surface of the nanosystem to increase the blood circulation time and biocompatibility and act as a vehicle for the chlorin e6 photosensitizer []. Hu et al. developed upconversion nanoparticles (UCNPs) that encapsulated chlorin e6 (Ce6) and the glyican-3 antibody (GPC3), which is overexpressed in hepatocellular carcinoma cells. This nanoplatform had great anticancer potential and was strongly expected to be used in liver cancer treatment [].
4.3.4. Indocyanine Green Photosensitizers
Indocyanine green (ICG) is a near-infrared tricarbocyanine dye approved by the FDA for human clinical use. Indocyanine green is a photosensitizer that kills tumor cells by producing photothermal heat or singlet oxygen. Its limitations include rapid aqueous degradation, a short half-life time, and high aggregation rates. To address these limitations, ICG is formulated with NPs []. For instance, Yuting Hung et al. developed dendritic mesoporous organosilica nanoparticles (MONs), which can be used to encapsulate indocyanine green (ICG) photosensitizer on macromolecular catalase (CAT) to overcome hypoxia of tumor cells upon 808 nm laser irradiations. ICG can generate highly cytotoxic (1O2) singlet oxygen and ROS to release photoacoustic imaging and kill cancer cells []. In a different study, the ICG was incorporated with doxorubicin hydrochloride into hollow mesoporous silica nanoparticles and dopamine-modified hyaluronic acid (DA-HA) to act as targeting agents and gatekeepers linked to HMSNs via boronate ester bonds. In vitro cell culture experiments showed that the ID@HMSNs-B-HA nanoplatform (where ID represents both DOX and ICG) could inhibit murine mammary carcinoma cells (4T1) via a combination of PDT with chemotherapy []. Chenlu Huang et al. also reported mesoporous silica nanoparticles (MSNs) encapsulated with perfluorohexane (PFH) with indocyanine green as photosensitizer, coated with a polydopamine (PDA) layer and polyethylene glycol-folic acid. MSNs-PFH@PDA-ICG-PEG-FA had a good monodispersity, with enhanced ICG photostability and cellular uptake. Upon irradiation with 808 nm NIR, the nanocarrier produces ROS for effective PDT and generates hyperthermia to realize PTT []. Cui-E-Shi et al. designed mitochondria-targeted hollow mesoporous silica nanoparticles (THMSNs) loaded with phase change material L-menthol (LM). Meanwhile, an ICG photosensitizer and doxorubicin (DOX) were encapsulated into THMSNs. The formed THMSNs@LMDI has improved the specific accumulation in mitochondria and cellular internalization []. Another study reported mesoporous silica nanoparticles encapsulation with indocyanine green and α-tocopherol succinate to reduce innate oxygen consumption by blocking the mitochondrial respiration chain. The MSN was modified with phenyl phosphine, enhancing blood circulation and mitochondrial target specificity. This nanosystem offers a promising nanoplatform to overcome hypoxia, which is responsible for limiting PDT application []. A study conducted by Bakai Zhang et al. reported gold nanorods (AuNRs) coated with SiO2 as a first dense layer, then with mesoporous SiO2 as a second mesoporous layer, forming Au@SiO2@mSiO2 to combine photodynamic therapy and photothermal therapy for multifunction theragnostic use. This nanosystem was loaded with indocyanine green (ICG), serving as an excellent agent for PDT and PTT. By using Au@SiO2@mSiO2-ICG, the ability to kill cancer cells increases three times compared to free ICG [].
4.3.5. Other Photosensitizers
Triple-negative breast cancer (TNBC) is considered an aggressive subset of breast cancer, and currently, no effective therapeutic drug has been identified. Tao Zhang et al. developed a biomimetic nanoplatform based on a leucocyte/platelet hybrid membrane (LPHM) and large-pore dendritic mesoporous silicon nanoparticles (DLMSNs). This system was loaded with doxorubicin (DOX) and fluorescent dye (IR = 780 nm) photosensitizer to prepare an LPHM@DLMSN@DOX/IR 780 nanoplatform, which effectively suppressed tumor growth []. In a different study, Triapazamine (TPZ) was used as a photosensitizer encapsulated in mesoporous silica nanoparticles (MSNs). MSNs were decorated with polyethylene glycol and folic acid to specifically target the activated macrophage and inhibit the progression of arthritis []. Another recent study reported on verteporfin (Ver), a second-generation photosensitizer conjugated with mesoporous silica nanoparticles. The resulting Ver-MSNs are considered an efficient nanoplatform, that can reduce or inhibit melanoma growth (skin cancer). In vitro experiments used melanoma cells irradiated with red light (693 nm) to decrease cancer cell proliferation []. The advanced stage of melanoma cancer is responsible for most cancer deaths. However, the survival ratio could be more than 90% if treated in the early stage []. Ka Chen et al. designed a multifunctional drug delivery system (RB-DOX@HMSNs-N=C-HA) to realize PDT. Hollow mesoporous silica nanoparticles (HMSNs) were used as a host material to encapsulate rose Bengal (RB) photosensitizer and doxorubicin. The surface of HMSNs was modified with hyaluronic acid (HA). The drug loading capacity was 12.78% for RB and 15.3% for DOX. The in vitro cellular uptake and cytotoxicity assay proved that the RB-DOX@HMSNs-N=C-HA nanosystem could efficiently target murine mammary carcinoma cells and inhibit tumor cell viability with combined chemo-photodynamic synergistic therapy []. Gaizhen Kuong et al. reported a mesoporous silica-based drug delivery system decorated with polyethylene glycol and curcumin (Cur) as photosensitizers to solve the low bioavailability of curcumin. The results showed that MSN-PEG@Cur generated ROS upon irradiation to get effective PDT in cancer treatment []. Another study reported the synthesis of MSNs to encapsulate the Ru (II) polypyridine complex, another type of photosensitizer. The surface of MSNs was decorated with folic acid, which acts as a targeting moiety for folate receptors, overexpressed in ovarian carcinoma cells. Upon irradiation of the nanophotosensitizer at 480 or 540 nm, the conjugate was nontoxic in normal tissue while showing a phototoxic effect in ovarian carcinoma cells []. A study conducted by Yanjun Yang et al. reported diselenide mesoporous silica nanoparticles modified with polyethylene glycol. These carriers encapsulated a methylene blue photosensitizer and doxorubicin chemotherapeutic drug. Under low doses of red-light irradiation during PDT, the ROS mediates a diselenide bond, resulting in the degradation of the organosilica matrix. Both drugs were released, resulting in a chemo-photodynamic therapy for breast cancer []. Zhibin Yin et al. developed mesoporous silica nanoparticles loaded with gold nanoclusters (AuNCs) and manganese dioxide (MnO2) nanosheets, which were wrapped as switching shield shells (AuNCs@mSiO2@MnO2). Under irradiation with a 635 nm laser, the stable MnO2 shells eliminated the singlet oxygen generator (1O2) to switch off photodynamic therapy and magnetic resonance imaging. While in the acidic microenvironment of tumor cells, the MnO2 shell reacts with H2O2, resulting in the degradation of MnO2 and generation of molecular oxygen O2 to enhance the PDT effect. Also, the cell viability of MDA-MA435 (metastatic human breast cancer) cells was reduced to 4%, and the tumor cells completely disappeared []. Dongxue Guo et al. developed a mesoporous silica nanoplatform, and its surface was decorated with gold nanoparticles. To improve the stability of the nanoplatform and control the drug release, the modified polyethylene glycol was introduced to the nanosystem to act as a gatekeeper. The formed MSN-Au-PEG nanoplatform loaded with DOX anticancer drug improved its effect in PDT []. Also, mesoporous silica nanoparticles were loaded with singlet oxygen (1O2) photosensitizer and DOX, and the surface of the nanoparticles was integrated with nitric oxide (NO) photodonor (NOPO). The complete nanoconstruct (PS-MSNs/NOPD/DOX) can deliver singlet oxygen and NO under green and blue light, respectively. Also, it helps release DOX under specific physiological conditions in A375 melanoma cancer cells []. Ting Sheng Yan et al. reported the formation of a biodegradable pH-sensitive hollow mesoporous silica nanoparticle (HMSN) for co-delivery of photosensitizer pheophorbide (PA) and an antitumor agent (DOX). This nanoplatform surface was decorated with folic acid to mediate endocytosis, which could effectively avoid the side effects on normal tissues []. The surface of the MSNs was decorated with iodine BODIPY containing a disulfide bond and a carboxyl group (COOH) for PDT. Also, PEGylation was performed to improve their water solubility by forming MSN-I2BOD-PEG. The formed nanoparticles could generate more reactive oxygen species to kill tumor cells and enhance endocytosis by Hela cells under 500 nm light irradiation []. In another study, Ruth Prieto Monter et al. also designed mesoporous silica nanoparticles loaded with BODIPY as a photosensitizer, and the surface was decorated with PEG and FA. BOD-PEG-FA under 518 nm irradiation (10 J/cm−2) could improve biocompatibility and generate more singlet oxygen to kill cancer cells []. Binbin Ding et al. developed monodispersed mesoporous silica-coated upconversion nanoparticles (UCNPs) to act as carriers to a specific photosensitizer MC540 and OVA (chicken oval albumin). Also, the nanoparticle’s surface was decorated with tumor cell fragments (TFs) as tumor antigens were developed. Under 980 nm NIR irradiation, this nanosystem possesses the best synergistic immunopotentiation action, verified by a high frequency of CD4+ and CD8+ and strong Th1 and Th2 immune responses []. Julia Elistratova et al. reported silica nanoparticles decorated with luminescent hexamolybdeum cluster complexes as photosensitizers. The prepared C-SNs hybrid nanosystem showed a significant PDT effect on the MCF-7 cancer cell line by a high potential ability to generate ROS []. Cichorium pumilum (CP) is a natural photosensitizer with many useful effects in treating cancer. However, its low bioavailability and poor water solubility have confined its use. These limitations can be addressed when it is encapsulated in silicon nanoparticles. The results showed that CP-SiNPs exhibited a high efficacy compared to free CP by a rise of 49.45% in the concentration effect []. Ya-Kun Dou et al. developed a ruthenium complex loaded onto silica nanoparticles, and the surface was decorated with folic acid. This synthesized SiNPs-Ru could emit red fluorescence by two-photon excitations, effectively killing cancer cells via PDT []. A recent study reported core–shell hybrid nanoparticles, formed by encapsulated xylan carrying a 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (TPPOH). In vitro analysis showed that the formed nanosystem is more effective against HCT116 cells and HT-29 (colorectal cancer cell lines) than free TPPOH []. Finally, Xufeng Zhu et al. developed a mesoporous ruthenium nanosystem with a dual targeting function. Aptamer AS441 (Apt) and transferrin (Tf) were grafted on the surface of a mesoporous ruthenium nanoplatform (MRN) with a higher loading capacity, and [Ru (bpy)2 (tip)] was used as a photosensitizer. Both targeting ligand and capping agents enable the effective penetration of the blood–brain barrier and target glioma cancer. Also, RBT produced reactive oxygen species and induced apoptosis in cancer cells when subjected to laser irradiation [,,].
The application of silica nanoparticles as photosensitizer vehicles for photodynamic therapy is summarized in Table 3.

Table 3.
Encapsulation of PSs into silica nanoparticles in cancer photodynamic therapy.
5. Conclusions and Future Prospects
This review described the most recent state-of-the-art studies reporting the use of integrated MSNs/PDT for improving cancer therapy. Photodynamic therapy is a breakthrough noninvasive therapy that relies mainly on the presence of molecular oxygen, light (with a specific wavelength), and PSs. Conventional PDT is limited by the depth of light penetration and the PSs’ limitations, such as nonselective targeting of the intended organs, hydrophobicity, cumulative toxicity, and poor cellular uptake. In this regard, several studies have reported the integration of nanocarriers to address the limitations of PSs and, hence, modernize PDT compared to the conventional one. MSNs set themselves apart from other types of organic and inorganic nanoparticles. This is attributed to their ease of fabrication, high loading capacities, ease of surface decoration with various functional groups, biocompatibility, and optical transparency. All the recent studies have proven that PSs combined with MSNs could show beneficial properties over free photosensitizers in a solution, such as by enhancing their selectivity and internalization in tumor cells. Additionally, silica nanoparticles can increase the solubility and stability of PSs in the physiological media and minimize or eliminate the cytotoxic effect in dark conditions. Recently, several studies have reported the application of MSNs in PDT for cancer treatment, which might revive hope for cancer patients.
Despite the endeavors in synthesizing biocompatible MSNs to serve as carriers for various PSs to improve cancer therapy, several challenges do exist that need to be addressed. Among these challenges is the shortage of enough in-depth knowledge on the in vivo biocompatibility and biodistribution of MSNs, their immunological reactions, biodegradation, bioelimination, and cumulative toxic effects. Future research should study the biosafety concerns related to the application of MSNs in cancer therapy rather than focusing only on their synthesis, functionalization, and optimization. For clinical applications, more in vivo mechanistic studies and clinical trials should be conducted to add a wealth of information to discover safe and effective cancer therapies. To date, and to the best of our knowledge, no clinical investigations involving the use of MSNs in combination with PDT in cancer therapy have been reported. The hallmark for the success of MSN-based therapies is including in vivo research and clinical trials that will be conducted in the coming years.
In conclusion, MSNs are prominent nanocarriers that supplement the conventional PDT. The hallmark for the success of MSN-based therapies is the inclusion of more in-depth in vivo studies and clinical trials in future studies.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mfouo-Tynga, I.; Dias, L.D.; Inada, N.M.; Kurachi, C. Biophysical and Biological Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.; Chota, A.; Abrahamse, H.; George, B.P. Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 10931. [Google Scholar] [CrossRef] [PubMed]
- Dhilip Kumar, S.S.; Abrahamse, H. Biocompatible Nanocarriers for Enhanced Cancer Photodynamic Therapy Applications. Pharmaceutics 2021, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. 2021, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Chota, A.; George, B.P.; Abrahamse, H. Recent Advances in Green Metallic Nanoparticles for Enhanced Drug Delivery in Photodynamic Therapy: A Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 4808. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Ritacco, I.; Al Assy, M.; Abd El-Rahman, M.K.; Fahmy, S.A.; Russo, N.; Shoeib, T.; Sicilia, E. Hydrolysis in Acidic Environment and Degradation of Satraplatin: A Joint Experimental and Theoretical Investigation. Inorg. Chem. 2017, 56, 6013–6026. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Arbeloa, T.; Martínez-Martínez, V. Photosensitizer-Mesoporous Silica Nanoparticles Combination for Enhanced Photodynamic Therapy†. Photochem. Photobiol. 2023, 99, 882–900. [Google Scholar] [CrossRef]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef]
- Abdelsalam, A.M.; Somaida, A.; Ambreen, G.; Ayoub, A.M.; Tariq, I.; Engelhardt, K.H.; Garidel, P.; Fawaz, I.; Amin, M.U.; Wojcik, M.; et al. Surface tailored zein as a novel delivery system for hypericin: Application in photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112420. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Sun, H.; Guo, D. Type I photodynamic therapy by organic–inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 15–62. [Google Scholar] [CrossRef]
- Niculescu, A.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Zhang, Z.; Liu, Z. Advances in photosensitizer-related design for photodynamic therapy. Asian J. Pharm. Sci. 2021, 16, 668–686. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.R.; Calori, I.R.; Tedesco, A.C. Photosensitizer-based metal-organic frameworks for highly effective photodynamic therapy. Mater. Sci. Eng. C 2021, 131, 112514. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur. J. Pharm. Biopharm. 2017, 126, 233–241. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Wang, S.; Mu, C.; Zhang, F.; Tang, H.; Ning, W. Acupuncture or moxibustion adjuvant chemotherapy for advanced non-small cell lung cancer: Systematic review and network meta-analysis. Medicine 2023, 102, e35000. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Si, J.; Zheng, M.; Zhou, Q.; Ge, Z. X-ray-responsive prodrugs and polymeric nanocarriers for multimodal cancer therapy. Chem. Commun. 2023, 59, 8323–8331. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, A.E.; Khachemoune, A. Photodynamic Therapy in Treating a Subset of Basal Cell Carcinoma: Strengths, Shortcomings, Comparisons with Surgical Modalities, and Potential Role as Adjunctive Therapy. Am. J. Clin. Dermatol. 2023, 2. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Kouchi, F.R.; Eixenberger, J.E.; Shirini, D.; Estrada, D.; Shirini, F. Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems. Pharmaceutics 2022, 14, 322. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Pouya Hadipour Moghaddam, S.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef]
- Makuch, S.; Dróżdż, M.; Makarec, A.; Ziólkowski, P.P.; Woźniak, M. An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. Int. J. Mol. Sci. 2022, 23, 9845. [Google Scholar] [CrossRef]
- Plenagl, N.; Duse, L.; Seitz, B.S.; Goergen, N.; Pinnapireddy, S.R.; Jedelská, J.; Brüßler, J.; Bakowsky, U. Photodynamic therapy—Hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv. 2019, 26, 23–33. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef]
- Hu, J.; Lei, Q.; Zhang, X. Recent advances in photonanomedicines for enhanced cancer photodynamic therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Amin, M.U.; Ambreen, G.; Dayyih, A.A.; Abdelsalam, A.M.; Somaida, A.; Engelhardt, K.H.; Wojcik, M.; Schäfer, J.; Bakowsky, U. Photodynamic and antiangiogenic activities of parietin liposomes in triple negative breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 134, 112543. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Azzazy, H.M.; Schaefer, J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-photosensitizers for enhanced photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102597. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Namanloo, R.A.; Aghili, S.S.; Maskani, P.; Alam, M.; Abbasi, K.; Nouri, F.; Tahmasebi, E.; Yazdanian, M.; Tebyaniyan, H. Photodynamic therapy in oral cancer: A review of clinical studies. Med. Oncol. 2023, 40, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Ali, M.Y.; Tariq, I.; Pinnapireddy, S.R.; Duse, L.; Goergen, N.; Wölk, C.; Hause, G.; Jedelská, J.; et al. Wavelength dependent photo-cytotoxicity to ovarian carcinoma cells using temoporfin loaded tetraether liposomes as efficient drug delivery system. Eur. J. Pharm. Biopharm. 2020, 150, 50–65. [Google Scholar] [CrossRef]
- Agel, M.R.; Baghdan, E.; Pinnapireddy, S.R.; Lehmann, J.; Schäfer, J.; Bakowsky, U. Curcumin loaded nanoparticles as efficient photoactive formulations against gram-positive and gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 178, 460–468. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2019, 96, 506–516. [Google Scholar] [CrossRef]
- Dayyih, A.A.; Gutberlet, B.; Preis, E.; Engelhardt, K.H.; Amin, M.U.; Abdelsalam, A.M.; Bonsu, M.A.; Bakowsky, U. Thermoresponsive Liposomes for Photo-Triggered Release of Hypericin Cyclodextrin Inclusion Complex for Efficient Antimicrobial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 31525–31540. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Hong, S.H.; Son, J.; Yoo, J.; Park, C.; Choi, Y.; Koo, H. Recent advances in nanoparticle carriers for photodynamic therapy. Quant. Imaging Med. Surg. 2018, 8, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Sedky, N.K.; Braoudaki, M.; Mahdy, N.K.; Amin, K.; Fawzy, I.M.; Efthimiadou, E.K.; Youness, R.A.; Fahmy, S.A. Box–Behnken design of thermo-responsive nano-liposomes loaded with a platinum(iv) anticancer complex: Evaluation of cytotoxicity and apoptotic pathways in triple negative breast cancer cells. Nanoscale Adv. 2023, 5, 5399–5413. [Google Scholar] [CrossRef] [PubMed]
- Yasir Ali, M.; Tariq, I.; Farhan Sohail, M.; Umair Amin, M.; Ali, S.; Reddy Pinnapireddy, S.; Ali, A.; Schäfer, J.; Bakowsky, U. Selective Anti-ErbB3 Aptamer Modified Sorafenib Microparticles: In Vitro and In Vivo Toxicity Assessment. Eur. J. Pharm. Biopharm. 2019, 145, 42–53. [Google Scholar] [CrossRef]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Sedky, N.K.; Abdel-Kader, N.M.; Issa, M.Y.; Abdelhady, M.M.; Shamma, S.N.; Bakowsky, U.; Fahmy, S.A. Co-Delivery of Ylang Ylang Oil of Cananga odorata and Oxaliplatin Using Intelligent pH-Sensitive Lipid-Based Nanovesicles for the Effective Treatment of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8392. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Sedky, N.K.; Ramzy, A.; Abdelhady, M.M.; Alabrahim, O.A.; Shamma, S.N.; Azzazy, H.M. Green extraction of essential oils from Pistacia lentiscus resins: Encapsulation into Niosomes showed improved preferential cytotoxic and apoptotic effects against breast and ovarian cancer cells. J. Drug Deliv. Sci. Technol. 2023, 87, 104820. [Google Scholar] [CrossRef]
- Youness, R.A.; Al-mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics 2022, 14, 142. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Mamdouh, W. Garlic oil–loaded PLGA nanoparticles with controllable size and shape and enhanced antibacterial activities. J. Appl. Polym. Sci. 2018, 135, 46133. [Google Scholar] [CrossRef]
- Amin, M.U.; Ali, S.; Tariq, I.; Ali, M.Y.; Pinnapreddy, S.R.; Preis, E.; Wölk, C.; Harvey, R.D.; Hause, G.; Brüßler, J.; et al. Ultrasound-Responsive Smart Drug Delivery System of Lipid Coated Mesoporous Silica Nanoparticles. Pharmaceutics 2021, 13, 1396. [Google Scholar] [CrossRef]
- Silvestre, A.L.; di Filippo, L.D.; Besegato, J.F.; de Annunzio, S.R.; Almeida Furquim de Camargo, B.; de Melo, P.B.; Rastelli, A.N.; Fontana, C.R.; Chorilli, M. Current applications of drug delivery nanosystems associated with antimicrobial photodynamic therapy for oral infections. Int. J. Pharm. 2020, 592, 120078. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Aly, A.A.; Bräse, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706–13726. [Google Scholar] [CrossRef]
- Umair Amin, M.; Ali, S.; Yasir Ali, M.; Tariq, I.; Nasrullah, U.; Reddy Pinnapreddy, S.; Wölk, C.; Bakowsky, U.; Brüßler, J. Enhanced Efficacy and Drug Delivery with Lipid Coated Mesoporous Silica Nanoparticles in Cancer Therapy. Eur. J. Pharm. Biopharm. 2021, 165, 31–40. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Lu, J.; Lin, Y.; Feng, S.; Luo, X.; Di, D.; Wang, S.; Zhao, Q. Recent trends of mesoporous silica-based nanoplatforms for nanodynamic therapies. Coord. Chem. Rev. 2022, 15, 214687. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef] [PubMed]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, J.; Li, J.; Gao, Y.; Mao, Y.; Zhao, Q.; Wang, S. Current trends in smart mesoporous silica-based nanovehicles for photoactivated cancer therapy. J. Control. Release 2021, 339, 445–472. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, C.; Nakamura, J.; Nakamura, M. Development of Non-Porous Silica Nanoparticles towards Cancer Photo-Theranostics. Biomedicines 2021, 9, 73. [Google Scholar] [CrossRef]
- Fernandes, N.B.; Nayak, Y.; Garg, S.; Nayak, U.Y. Multifunctional engineered mesoporous silica/inorganic material hybrid nanoparticles: Theranostic perspectives. Coord. Chem. Rev. 2023, 478, 214977. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ramzy, A.; Mandour, A.A.; Nasr, S.M.; Abdelnaser, A.; Bakowsky, U.; Azzazy, H.M. PEGylated Chitosan Nanoparticles Encapsulating Ascorbic Acid and Oxaliplatin Exhibit Dramatic Apoptotic Effects against Breast Cancer Cells. Pharmaceutics 2022, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, H.; Liu, Z.; Yang, L.; Zhang, C.; He, J.; Ai, W.; Liu, Y. Enhanced Photodynamic Therapy by Improved Light Energy Capture Efficiency of Porphyrin Photosensitizers. Curr. Treat. Options Oncol. 2023, 24, 1274–1292. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, E.; Mauriello Jimenez, C.; El Cheikh, K.; Maynadier, M.; Basile, I.; Raehm, L.; Nguyen, C.; Gary-Bobo, M.; Garcia, M.; Durand, J.; et al. Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor. Int. J. Mol. Sci. 2019, 20, 2809. [Google Scholar] [CrossRef] [PubMed]
- Aggad, D.; Jimenez, C.M.; Dib, S.; Croissant, J.G.; Lichon, L.; Laurencin, D.; Richeter, S.; Maynadier, M.; Alsaiari, S.K.; Boufatit, M.; et al. Gemcitabine Delivery and Photodynamic Therapy in Cancer Cells via Porphyrin-Ethylene-Based Periodic Mesoporous Organosilica Nanoparticles. Mesoporous Nanopart. 2018, 4, 46–51. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.; Li, W.; Zhang, Y. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Kumar Kankala, R.; Busa, P.; Lee, C. Hydrophobicity-Tuned Periodic Mesoporous Organo-Silica Nanoparticles for Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 2586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Y.; Wang, X.; Yang, W.; Bai, F.; Zhang, B.; Alarid, L.; Bian, K.; Fan, H. pH-Dependent Assembly of Porphyrin-Silica Nanocomposites and Their Application in Targeted Photodynamic Therapy. Nano Lett. 2017, 17, 6916–6921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, H.; Zhao, J.; Cheng, M.; Sun, S. Synthesis of porphyrin-conjugated silica-coated Au nanorods for synergistic photothermal therapy and photodynamic therapy of tumor. Nanotechnology 2019, 30, 265102. [Google Scholar] [CrossRef]
- Yang, S.; You, Q.; Yang, L.; Li, P.; Lu, Q.; Wang, S.; Tan, F.; Ji, Y.; Li, N. Rodlike MSN@Au Nanohybrid-Modified Supermolecular Photosensitizer for NIRF/MSOT/CT/MR Quadmodal Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 6777–6788. [Google Scholar] [CrossRef]
- Er, O.; Tunçel, A.; Ocakoğlu, K.; Ince, M.; Kolatan, E.H.; Yılmaz, O.; Aktaş, S.; Yurt, F. Radiolabeling, in vitro Cell Uptake and in vivo Photodynamic Therapy Potential of Targeted Mesoporous Silica Nanoparticles Contains Zinc Phthalocyanine. Mol. Pharm. 2020, 17, 2648–2659. [Google Scholar] [CrossRef]
- Lin, A.; Li, S.; Xu, C.; Li, X.; Zheng, B.; Gu, J.; Ke, M.; Huang, J. A pH-responsive stellate mesoporous silica based nanophotosensitizer for in vivo cancer diagnosis and targeted photodynamic therapy. Biomater. Sci. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Er, Ö.; Colak, S.G.; Ocakoğlu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, M.L.; Yurt, F.; Nonell, S. Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules 2018, 23, 2749. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, W.; Wang, M.; Li, R.; Yue, X.; Dai, Z. Ultrasmall pH-responsive silicon phthalocyanine micelle for selective photodynamic therapy against tumor. J. Innov. Opt. Health Sci. 2022, 6, 2250035. [Google Scholar] [CrossRef]
- Shen, T.; Hu, X.; Liu, Y.; Zhang, Y.; Chen, K.; Xie, S.; Ke, G.; Song, G.; Zhang, X. Specific Core-Satellite Nanocarriers for Enhanced Intracellular ROS Generation and Synergistic Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5403–5412. [Google Scholar] [CrossRef] [PubMed]
- Güleryüz, B.; Ünal, U.O.; Gülsoy, M. Near Infrared Light Activated Upconversion Nanoparticles (UCNP) based Photodynamic Therapy of Prostate Cancers: An in vitro Study. Photodiagnosis Photodyn. Ther. 2021, 36, 102616. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, X.Q.; Wang, Y.; Guo, Q.; Ye, Q.; Guo, R.; Xiao, S.; Ye, Q.; Huang, Y.; Peng, Y. Benzyl ester dendrimer silicon phthalocyanine based polymeric nanoparticle for in vitro photodynamic therapy of glioma. J. Lumin. 2019, 207, 597–601. [Google Scholar] [CrossRef]
- Chen, X.Q.; Guo, Q.; Dong, S.; Chen, J.; Xie, S.; Ma, D.; Chen, L.; Yang, H.; Huang, Y.; Peng, Y. Distribution, Trafficking, and In Vitro Photodynamic Therapy Efficacy of Cholesterol Silicon(IV) Phthalocyanine and Its Nanoparticles in Breast Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 5976–5984. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Nematallah, K.A.; Mahdy, N.K.; El-Askary, H.I.; Meselhy, M.R.; El-Said Azzazy, H.M. Enhanced Antioxidant, Antiviral, and Anticancer Activities of the Extract of Fermented Egyptian Rice Bran Complexed with Hydroxypropyl-β-cyclodextrin. ACS Omega 2022, 7, 19545–19554. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Yu, B.; Zhang, X.; Shen, Y.; Cong, H. Tumor microenvironment-responsive polymer with chlorin e6 to interface hollow mesoporous silica nanoparticles-loaded oxygen supply factor for boosted photodynamic therapy. Nanotechnology 2020, 31, 305709. [Google Scholar] [CrossRef]
- Rao, V.; Han, H.S.; Lee, H.; Nguyen, V.Q.; Jeon, S.; Jung, D.; Lee, J.; Yi, G.; Park, J.H. ROS-responsive mesoporous silica nanoparticles for MR imaging-guided photodynamically maneuvered chemotherapy. Nanoscale 2018, 10, 9616–9627. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Song, Y.; Liu, D.; Yan, H.; Li, H.; Du, D.; Zhu, C.; Lin, Y. Integrating in situ formation of nanozymes with three-dimensional dendritic mesoporous silica nanospheres for hypoxia-overcoming photodynamic therapy. Nanoscale 2018, 10, 22937–22945. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; You, Q.; Pang, X.; Tan, X.; Wang, J.; Liu, L.; Guo, F.; Tan, F.; Li, N. A photoresponsive and rod-shape nanocarrier: Single wavelength of light triggered photothermal and photodynamic therapy based on AuNRs-capped & Ce6-doped mesoporous silica nanorods. Biomaterials 2017, 122, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, P.; Huang, Y.; Lu, X.; Chang, Q.; Pan, W.; Li, N.; Tang, B. Boosting the photodynamic therapy efficiency with a mitochondria-targeted nanophotosensitizer. Chin. Chem. Lett. 2019, 30, 1293–1296. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, W.; Wang, Q.; Zhao, Y.; Zhang, Y.; Tian, Y.; Tang, Y.; Wang, S.; Liu, Y.; Ni, Q.; et al. Oxygen-Evolving Mesoporous Organosilica Coated Prussian Blue Nanoplatform for Highly Efficient Photodynamic Therapy of Tumors. Adv. Sci. 2018, 5, 1700847. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, H.; Jeong, S.Y.; Yi, B.G.; Park, K.; Key, J. Hyaluronic Acid-Conjugated Mesoporous Silica Nanoparticles Loaded with Dual Anticancer Agents for Chemophotodynamic Cancer Therapy. J. Nanomater. 2019, 2019, 3481397. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Dong, S.; Gai, S.; Dong, Y.; Yang, D.; Dai, Y.; He, F.; Yang, P. Degradable Calcium Phosphate-Coated Upconversion Nanoparticles for Highly Efficient Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 47659–47670. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Q.; Yang, G.; Xiao, X.; Li, L.; Yu, T. Albumin-MnO2 gated hollow mesoporous silica nanosystem for modulating tumor hypoxia and synergetic therapy of cervical carcinoma. Colloids Surf. B Biointerfaces 2019, 179, 250–259. [Google Scholar] [CrossRef]
- Du, W.; Liu, T.; Xue, F.; Chen, Y.; Chen, Q.; Luo, Y.; Cai, X.; Ma, M.; Chen, H. Confined nanoparticles growth within hollow mesoporous nanoreactors for highly efficient MRI-guided photodynamic therapy. Chem. Eng. J. 2020, 379, 122251. [Google Scholar] [CrossRef]
- Yang, J.; Teng, Y.; Fu, Y.; Zhang, C. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int. J. Nanomed. 2019, 14, 5061–5071. [Google Scholar] [CrossRef]
- Rafique, R.; Gul, A.R.; Lee, I.G.; Baek, S.H.; Kailasa, S.K.; Iqbal, N.; Cho, E.J.; Lee, M.; Park, T.J. Photo-induced reactions for disassembling of coloaded photosensitizer and drug molecules from upconversion-mesoporous silica nanoparticles: An effective synergistic cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110545. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jing, F.; Lin, B.; Cui, S.; Yu, R.; Shen, X.; Wang, T. pH-Responsive Magnetic Mesoporous Silica-Based Nanoplatform for Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef]
- Hu, J.; Shi, J.; Gao, Y.; Yang, W.; Liu, P.; Liu, Q.; He, F.; Wang, C.; Li, T.; Xie, R.; et al. 808 nm Near-Infrared Light-Excited UCNPs@mSiO2-Ce6-GPC3 Nanocomposites For Photodynamic Therapy In Liver Cancer. Int. J. Nanomed. 2019, 14, 10009–10021. [Google Scholar] [CrossRef] [PubMed]
- Gowsalya, K.; Yasothamani, V.; Vivek, R. Emerging indocyanine green-integrated nanocarriers for multimodal cancer therapy: A review. Nanoscale Adv. 2021, 3, 3332–3352. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, K.B.; Si, Y.; Shan, C.; Guo, H.; Chen, M.; Wu, L. Dendritic organosilica nanospheres with large mesopores as multi-guests vehicle for photoacoustic/ultrasound imaging-guided photodynamic therapy. J. Colloid Interface Sci. 2020, 583, 166–177. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic Acid-Functionalized Hollow Mesoporous Silica Nanoparticles as pH-Sensitive Nanocarriers for Cancer Chemo-Photodynamic Therapy. Langmuir ACS J. Surf. Colloids 2021, 37, 2619–2628. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Guo, Q.; Zhang, L.; Fan, F.; Qin, Y.; Wang, H.; Zhou, S.; Ou-Yang, W.; Sun, H.; et al. A Dual-Model Imaging Theragnostic System Based on Mesoporous Silica Nanoparticles for Enhanced Cancer Phototherapy. Adv. Healthc. Mater. 2019, 8, e1900840. [Google Scholar] [CrossRef]
- Shi, C.; You, C.; Pan, L. Facile formulation of near-infrared light-triggered hollow mesoporous silica nanoparticles based on mitochondria targeting for on-demand chemo/photothermal/photodynamic therapy. Nanotechnology 2019, 30, 325102. [Google Scholar] [CrossRef]
- Dong, P.; Hu, J.; Yu, S.; Zhou, Y.; Shi, T.; Zhao, Y.; Wang, X.; Liu, X. A Mitochondrial Oxidative Stress Amplifier to Overcome Hypoxia Resistance for Enhanced Photodynamic Therapy. Small Methods 2021, 5, e2100581. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, L.; Chu, Z. Development of indocyanine green loaded Au@Silica core shell nanoparticles for plasmonic enhanced light triggered therapy. J. Photochem. Photobiol. A Chem. 2019, 375, 244–251. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Zhang, X.; Hou, Y.; Meng, X.; Li, G.; Xu, F.; Teng, L.; Qi, Y.; Sun, F.; et al. Folate receptor-targeting semiconducting polymer dots hybrid mesoporous silica nanoparticles against rheumatoid arthritis through synergistic photothermal therapy, photodynamic therapy, and chemotherapy. Int. J. Pharm. 2021, 607, 120947. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Miletto, I.; Gianotti, E.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renó, F. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2019, 197, 111533. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Ramzy, A.; Sawy, A.M.; Nabil, M.; Gad, M.Z.; El-Shazly, M.; Aboul-Soud, M.A.; Azzazy, H.M. Ozonated Olive Oil: Enhanced Cutaneous Delivery via Niosomal Nanovesicles for Melanoma Treatment. Antioxidants 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, C.; Liu, Z.; Zhou, Y.; Xu, Q.; Li, C.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2020, 194, 111166. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Karges, J.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S.; Gasser, G. Ru(II) Polypyridine Complex-Functionalized Mesoporous Silica Nanoparticles as Photosensitizers for Cancer Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4394–4405. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, F.; Xu, N.; Yao, Q.; Wang, R.; Xie, X.; Zhang, F.; He, Y.; Shao, D.; Dong, W.; et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials 2022, 281, 121368. [Google Scholar] [CrossRef]
- Yin, Z.; Ji, Q.; Wu, D.; Li, Z.; Fan, M.; Zhang, H.; Zhao, X.; Wu, A.; Cheng, L.; Zeng, L. H2O2-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for “Off/On” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 14928–14937. [Google Scholar] [CrossRef]
- Guo, D.; Bao, Y.; Zhang, Y.; Yang, H.; Chen, L. Reduction-responsive Au decorated mesoporous silica-based nanoplatform for photodynamic-chemotherapy. Microporous Mesoporous Mater. 2020, 292, 109729. [Google Scholar] [CrossRef]
- Tessaro, A.L.; Fraix, A.; Pedrozo da Silva, A.C.; Gazzano, E.; Riganti, C.; Sortino, S. “Three-Bullets” Loaded Mesoporous Silica Nanoparticles for Combined Photo/Chemotherapy †. Nanomaterials 2019, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; He, J.; Liu, R.; Liu, Z.; Cheng, J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr. Polym. 2020, 231, 115706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, N.; Chen, L.; Xie, Z. Reduction responsive BODIPY decorated mesoporous silica nanoscale platforms for photodynamic therapy. Microporous Mesoporous Mater. 2021, 311, 110689. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Shao, S.; Yu, C.; Teng, B.; Wang, M.; Cheng, Z.; Wong, K.; Ma, P.; Lin, J. Large-Pore Mesoporous-Silica-Coated Upconversion Nanoparticles as Multifunctional Immunoadjuvants with Ultrahigh Photosensitizer and Antigen Loading Efficiency for Improved Cancer Photodynamic Immunotherapy. Adv. Mater. 2018, 30, e1802479. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, J.G.; Mukhametshina, A.R.; Kholin, K.V.; Nizameev, I.R.; Mikhailov, M.A.; Sokolov, M.N.; Khairullin, R.; Miftakhova, R.; Shammas, G.; Kadirov, M.K.; et al. Interfacial uploading of luminescent hexamolybdenum cluster units onto amino-decorated silica nanoparticles as new design of nanomaterial for cellular imaging and photodynamic therapy. J. Colloid Interface Sci. 2019, 538, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Makhadmeh, G.N.; Abuelsamen, A.; Al-Akhras, M.H.; Aziz, A.B. Silica Nanoparticles Encapsulated Cichorium Pumilum as Promising Photosensitizer for Osteosarcoma Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102801. [Google Scholar] [CrossRef]
- Dou, Y.; Shang, Y.; He, X.; Li, W.; Li, Y.; Zhang, Y. Preparation of a Ruthenium-Complex-Functionalized Two-Photon-Excited Red Fluorescence Silicon Nanoparticle Composite for Targeted Fluorescence Imaging and Photodynamic Therapy in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 13954–13963. [Google Scholar] [CrossRef]
- Bouramtane, S.; Bretin, L.; Pinon, A.; Léger, D.Y.; Liagre, B.; Richard, L.; Brégier, F.; Sol, V.; Chaleix, V. Porphyrin-xylan-coated silica nanoparticles for anticancer photodynamic therapy. Carbohydr. Polym. 2019, 213, 168–175. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, H.; Liu, Y.; Wen, Y.; Wei, C.; Yu, Q.; Liu, J. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157. [Google Scholar] [CrossRef]
- Shabbir; Maryam; Sajid, A.; Hamid, I.; Sharif, A.; Akhtar, M.F.; Raza, M.; Ahmed, S.; Peerzada, S.; Amin, M.U. Influence of different formulation variables on the performance of transdermal drug delivery system containing tizanidine hydrochloride: In Vitro and Ex Vivo evaluations. Braz. J. Pharm. Sci. 2019, 54, e00130. [Google Scholar] [CrossRef]
- Shabbir, M.; Ali, S.; Shahid, N.; Rehman, K.; Amin, U.; Raza, M. Formulation considerations and factors affecting transdermal drug delivery system—A review. Int. J. Pharm. Integr. Life Sci. 2014, 2, 20–35. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).