Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Polymorphs of GABA and Gabapentin
3.2. Monohydrate of Gabapentin (I-2)
3.3. Multi-Component Systems with Fumaric Acid (3)
3.4. Multi-Component Systems with Succinic Acid (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rosa, C.; Scoti, M.; Di Girolamo, R.; Ballesteros, O.R.; Auriemma, F.; Malafronte, A. Polymorphism in polymers: A tool to tailor material’s properties. Polym. Cryst. 2020, 3, 10101. [Google Scholar] [CrossRef]
- Shi, W.; Lee, W.S.V.; Xue, J. Recent Development of Mn-based Oxides as Zinc-Ion Battery Cathode. ChemSusChem 2021, 14, 1634–1658. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Shi, H.; An, Z.; Huang, W. Manipulating the Ultralong Organic Phosphorescence of Small Molecular Crystals. Chemistry 2020, 26, 4437–4448. [Google Scholar] [CrossRef] [PubMed]
- Bennion, J.C.; Matzger, A.J. Development and Evolution of Energetic Cocrystals. Acc. Chem. Res. 2021, 54, 1699–1710. [Google Scholar] [CrossRef]
- Bu, R.; Li, H.; Zhang, C. Polymorphic Transition in Traditional Energetic Materials: Influencing Factors and Effects on Structure, Property, and Performance. Cryst. Growth Des. 2020, 20, 3561–3576. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Bernstein, J. Disappearing Polymorphs. Acc. Chem. Res. 1995, 28, 193–200. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Lancaster, R.W.; Bernstein, J. Disappearing polymorphs revisited. Angew. Chem. Int. Ed. Engl. 2015, 54, 6972–6993. [Google Scholar] [CrossRef]
- Chistyakov, D.; Sergeev, G. The Polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, R.; Thakur, T.S. Crystal Polymorphism in Pharmaceutical Science. In Comprehensive Supramolecular Chemistry II; Wilson, A., Jayawickramarajah, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 283–309. [Google Scholar]
- Cruz, P.C.; Rocha, F.A.; Ferreira, A.M. Application of Selective Crystallization Methods to Isolate the Metastable Polymorphs of Paracetamol: A Review. Org. Process Res. Dev. 2019, 23, 2592–2607. [Google Scholar] [CrossRef]
- Nugrahani, I.; Parwati, R.D. Challenges and Progress in Nonsteroidal Anti-Inflammatory Drugs Co-Crystal Development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- Jia, S.; Gao, Z.; Tian, N.; Li, Z.; Gong, J.; Wang, J.; Rohani, S. Review of melt crystallization in the pharmaceutical field, towards crystal engineering and continuous process development. Chem. Eng. Res. Des. 2021, 166, 268–280. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization control via membrane distillation-crystallization: A review. Desalination 2021, 519, 115315. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Qiao, B.; Wang, Y. Review of Liquid–Liquid Phase Separation in Crystallization: From Fundamentals to Application. Cryst. Growth Des. 2021, 21, 7306–7325. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions**. Chem. Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef]
- Higashi, K.; Ueda, K.; Moribe, K. Recent progress of structural study of polymorphic pharmaceutical drugs. Adv. Drug Deliv. Rev. 2017, 117, 71–85. [Google Scholar] [CrossRef]
- Chethan, B.S.; Lokanath, N.K. Study of the crystal structure, H-bonding and noncovalent interactions of novel cocrystal by systematic computational search approach. J. Mol. Struct. 2022, 1251, 131936. [Google Scholar] [CrossRef]
- Cappuccino, C.; Cusack, D.; Flanagan, J.; Harrison, C.; Holohan, C.; Lestari, M.; Walsh, G.; Lusi, M. How Many Cocrystals Are We Missing? Assessing Two Crystal Engineering Approaches to Pharmaceutical Cocrystal Screening. Cryst. Growth Des. 2022, 22, 1390–1397. [Google Scholar] [CrossRef]
- Dhibar, M.; Chakraborty, S.; Basak, S. Assessment of Effects of Solvents on Cocrystallization by Computational Simulation Approach. Curr. Drug. Deliv. 2021, 18, 44–53. [Google Scholar] [CrossRef]
- Ali, A.; Kuznetsov, A.; Khan, M.U.; Tahir, M.N.; Ashfaq, M.; Raza, A.R.; Muhammad, S. 2-Amino-6-methylpyridine based co-crystal salt formation using succinic acid: Single-crystal analysis and computational exploration. J. Mol. Struct. 2021, 1230, 129893. [Google Scholar] [CrossRef]
- Barbas, R.; Font-Bardia, M.; Frontera, A.; Prohens, R. Polymorphism in the 1/1 Pterostilbene/Picolinic Acid Cocrystal. Cryst. Growth Des. 2022, 22, 590–597. [Google Scholar] [CrossRef]
- Roselló, Y.; Benito, M.; Barceló-Oliver, M.; Frontera, A.; Molins, E. 1-Ethyluracil, a New Scaffold for Preparing Multicomponent Forms: Synthesis, Characterization, and Computational Studies. Cryst. Growth Des. 2021, 21, 4857–4870. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, B.; Ji, W.-J.; Guo, C.-Y.; Hong, M.; Qi, M.-H.; Ren, G.-B. Insight into the Formation of Cocrystals of Flavonoids and 4,4′-Vinylenedipyridine: Heteromolecular Hydrogen Bonds, Molar Ratio, and Structural Analysis. Cryst. Growth Des. 2021, 21, 2720–2733. [Google Scholar] [CrossRef]
- Boldyreva, E. Glycine: The Gift that Keeps on Giving. Isr. J. Chem. 2021, 61, 828–850. [Google Scholar] [CrossRef]
- Bhat, M.; Dharmaprakash, S. Growth of nonlinear optical γ-glycine crystals. J. Cryst. Growth 2002, 236, 376–380. [Google Scholar] [CrossRef]
- Dawson, A.; Allan, D.R.; Belmonte, S.A.; Clark, S.J.; David, W.I.F.; McGregor, P.A.; Parsons, S.; Pulham, C.R.; Sawyer, L. Effect of High Pressure on the Crystal Structures of Polymorphs of Glycine. Cryst. Growth Des. 2005, 5, 1415–1427. [Google Scholar] [CrossRef]
- Kim, K.; Centrone, A.; Hatton, T.A.; Myerson, A.S. Polymorphism control of nanosized glycine crystals on engineered surfaces. CrystEngComm 2011, 13, 1127–1131. [Google Scholar] [CrossRef]
- Li, L.; Rodríguez-Hornedo, N. Growth kinetics and mechanism of glycine crystals. J. Cryst. Growth 1992, 121, 33–38. [Google Scholar] [CrossRef]
- Srinivasan, K. Crystal growth of α and γ glycine polymorphs and their polymorphic phase transformations. J. Cryst. Growth 2008, 311, 156–162. [Google Scholar] [CrossRef]
- Towler, C.S.; Davey, R.J.; Lancaster, R.W.; Price, C.J. Impact of molecular speciation on crystal nucleation in polymorphic systems: The conundrum of gamma glycine and molecular ‘self poisoning’. J. Am. Chem. Soc. 2004, 126, 13347–13353. [Google Scholar] [CrossRef]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Ham, S.; Bhatia, S.K.; Gurav, R.; Choi, Y.-K.; Jeon, J.-M.; Yoon, J.-J.; Choi, K.-Y.; Ahn, J.; Kim, H.T.; Yang, Y.-H. Gamma aminobutyric acid (GABA) production in Escherichia coli with pyridoxal kinase (pdxY) based regeneration system. Enzyme Microb. Technol. 2022, 155, 109994. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Tomita, K.; Higashi, H.; Fujiwara, T. Crystal and Molecular Structure of ω-Amino Acids, ω-Amino Sulfonic Acids and Their Derivatives. IV. The Crystal and Molecular Structure of γ-Aminobutyric Acid (GABA), a Nervous Inhibitory Transmitter. Bull. Chem. Soc. Jpn. 1973, 46, 2199–2204. [Google Scholar] [CrossRef]
- Dobson, A.J.; Gerkin, R.E. gamma-Aminobutyric acid: A novel tetragonal phase. Acta Crystallogr. C 1996, 52, 3075–3078. [Google Scholar] [CrossRef]
- Wang, L.; Sun, G.; Zhang, K.; Yao, M.; Jin, Y.; Zhang, P.; Wu, S.; Gong, J. Green Mechanochemical Strategy for the Discovery and Selective Preparation of Polymorphs of Active Pharmaceutical Ingredient γ-Aminobutyric Acid (GABA). ACS Sustain. Chem. Eng. 2020, 8, 16781–16790. [Google Scholar] [CrossRef]
- Vamecq, J.; Feutelais, Y.; Maurois, P.; Sghaier, M.; Dichi, E.; German-Fattal, M.; Herrenknecht, C.; Gressens, P.; Cecchelli, R.; Dehouck, L.; et al. Engineering a GABA endowed with pharmacological CNS activity when given by an extracerebral route. Med. Chem. Res. 2009, 18, 255–267. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Du, S.; Xu, S.; Shi, P.; Songgu Wu, S.; Gong, J. Surprising Effect of Carbon Chain Length on Inducing Ability of Additives: Elusive Form-II of γ-Aminobutyric Acid (GABA) Induced by Sodium Carboxylate Additives. Cryst. Growth Des. 2019, 19, 3825–3833. [Google Scholar] [CrossRef]
- Lamkowski, L.; Komisarek, D.; Merz, K. GABA-Controlled Synthesis of the Metastable Polymorphic Form and Crystallization Behavior with a Chiral Malic Acid. Cryst. Growth Des. 2022, 22, 356–362. [Google Scholar] [CrossRef]
- Malapile, R.J.; Nyamayaro, K.; Nassimbeni, L.R.; Báthori, N.B. Multicomponent crystals of baclofen with acids and bases—Conformational flexibility and synthon versatility. CrystEngComm 2021, 23, 91–99. [Google Scholar] [CrossRef]
- Mirza, S.; Miroshnyk, I.; Rantanen, J.; Aaltonen, J.; Harjula, P.; Kiljunen, E.; Heinämäki, J.; Yliruusi, J. Solid-state properties and relationship between anhydrate and monohydrate of baclofen. J. Pharm. Sci. 2007, 96, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Herbst, M.; Komisarek, D.; Strothmann, T.; Vasylyeva, V. A Lection in Humbleness: Crystallization of Chiral and Zwitterionic APIs Baclofen and Phenibut. Crystals 2022, 12, 1393. [Google Scholar] [CrossRef]
- Komisarek, D.; Pallaske, M.; Vasylyeva, V. Crystal Structure and Thermal Properties of Phenibut, Phenibut H 2 O and Phenibut HCl: A Case for Phase Stability Based on Structural Considerations. Z. Anorg. Allg. Chem. 2021, 647, 984–991. [Google Scholar] [CrossRef]
- Komisarek, D.; Haj Hassani Sohi, T.; Vasylyeva, V. Co-crystals of zwitterionic GABA API’s pregabalin and phenibut: Properties and application. CrystEngComm 2022, 24, 8390–8398. [Google Scholar] [CrossRef]
- Khandavilli, U.B.R.; Lusi, M.; Frawley, P.J. Plasticity in zwitterionic drugs: The bending properties of Pregabalin and Gabapentin and their hydrates. IUCrJ 2019, 6, 630–634. [Google Scholar] [CrossRef]
- Couvrat, N.; Sanselme, M.; Poupard, M.; Bensakoun, C.; Drouin, S.H.; Schneider, J.-M.; Coquerel, G. Solid-State Overview of R-Baclofen: Relative Stability of Forms A, B and C and Characterization of a New Heterosolvate. J. Pharm. Sci. 2021, 110, 3457–3463. [Google Scholar] [CrossRef]
- Córdova-Villanueva, E.N.; Rodríguez-Ruiz, C.; Sánchez-Guadarrama, O.; Rivera-Islas, J.; Herrera-Ruiz, D.; Morales-Rojas, H.; Höpfl, H. Diastereomeric Salt Formation by the γ-Amino Acid RS -Baclofen and L -Malic Acid: Stabilization by Strong Heterosynthons Based on Hydrogen Bonds between RNH 3+ and COOH/COO—Groups. Cryst. Growth Des. 2018, 18, 7356–7367. [Google Scholar] [CrossRef]
- Samas, B.; Wang, W.; Godrej, D.B. 1:1 Cocrystal of (S)-3-(ammoniomethyl)-5-methylhexanoate and (S)-mandelic acid. Acta Crystallogr. E Struct. Rep. Online 2007, 63, o3938. [Google Scholar] [CrossRef]
- Steendam, R.R.E.; Khandavilli, U.B.R.; Keshavarz, L.; Frawley, P.J. Solution versus Crystal Hydration: The Case of γ-Amino Acid Pregabalin. Cryst. Growth Des. 2019, 19, 4483–4488. [Google Scholar] [CrossRef]
- Venu, N.; Vishweshwar, P.; Ram, T.; Surya, D.; Apurba, B. (S)-3-(Ammoniomethyl)-5-methylhexanoate (pregabalin). Acta Crystallogr. C 2007, 63, o306–o308. [Google Scholar] [CrossRef]
- Gendron, F.-X.; Mahieux, J.; Sanselme, M.; Coquerel, G. Resolution of Baclofenium Hydrogenomaleate by Using Preferential Crystallization. A First Case of Complete Solid Solution at High Temperature and a Large Miscibility Gap in the Solid State. Cryst. Growth Des. 2019, 19, 4793–4801. [Google Scholar] [CrossRef]
- Bennett, M.I.; Simpson, K.H. Gabapentin in the treatment of neuropathic pain. Palliat. Med. 2004, 18, 5–11. [Google Scholar] [CrossRef]
- Cheng, J.-K.; Chiou, L.-C. Mechanisms of the antinociceptive action of gabapentin. J. Pharmacol. Sci. 2006, 100, 471–486. [Google Scholar] [CrossRef]
- Nicholson, B. Gabapentin use in neuropathic pain syndromes. Acta Neurol. Scand. 2000, 101, 359–371. [Google Scholar] [CrossRef]
- Delaney, S.P.; Smith, T.M.; Korter, T.M. Conformation versus cohesion in the relative stabilities of gabapentin polymorphs. RSC Adv. 2014, 4, 855–864. [Google Scholar] [CrossRef]
- Dempah, K.E.; Barich, D.H.; Kaushal, A.M.; Zong, Z.; Desai, S.D.; Suryanarayanan, R.; Kirsch, L.; Munson, E.J. Investigating gabapentin polymorphism using solid-state NMR spectroscopy. AAPS PharmSciTech 2013, 14, 19–28. [Google Scholar] [CrossRef][Green Version]
- Lin, S.-Y.; Hsu, C.-H.; Ke, W.-T. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int. J. Pharm. 2010, 396, 83–90. [Google Scholar] [CrossRef]
- Martins, I.C.B.; Gomes, J.R.B.; Duarte, M.T.; Mafra, L. Understanding Polymorphic Control of Pharmaceuticals Using Imidazolium-Based Ionic Liquid Mixtures as Crystallization Directing Agents. Cryst. Growth Des. 2017, 17, 428–432. [Google Scholar] [CrossRef]
- Tulli, L.G.; Moridi, N.; Wang, W.; Helttunen, K.; Neuburger, M.; Vaknin, D.; Meier, W.; Shahgaldian, P. Polymorphism control of an active pharmaceutical ingredient beneath calixarene-based Langmuir monolayers. ChemComm 2014, 50, 3938–3940. [Google Scholar] [CrossRef][Green Version]
- Reece, H.A.; Levendis, D.C. Polymorphs of gabapentin. Acta Crystallogr. C 2008, 64, o105–o108. [Google Scholar] [CrossRef]
- André, V.; Fernandes, A.; Santos, P.P.; Duarte, M.T. On the Track of New Multicomponent Gabapentin Crystal Forms: Synthon Competition and pH Stability. Cryst. Growth Des. 2011, 11, 2325–2334. [Google Scholar] [CrossRef]
- Kumari, H.; Zhang, J.; Erra, L.; Barbour, L.J.; Deakyne, C.A.; Atwood, J.L. Cocrystals of gabapentin with C-alkylresorcin[4]arenes. CrystEngComm 2013, 15, 4045. [Google Scholar] [CrossRef]
- Soliman, I.I.; Kandil, S.M.; Abdou, E.M. Gabapentin-saccharin co-crystals with enhanced physicochemical properties and in vivo absorption formulated as oro-dispersible tablets. Pharm. Dev. Technol. 2020, 25, 227–236. [Google Scholar] [CrossRef]
- Wenger, M.; Bernstein, J. An Alternate Crystal Form of Gabapentin: A Cocrystal with Oxalic Acid. Cryst. Growth Des. 2008, 8, 1595–1598. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Huang, X.; Li, X.; Zong, S.; Wang, N.; Hao, H. Conformational Selectivity and Evolution Affected by the Desolvation Process. Cryst. Growth Des. 2022, 22, 1283–1291. [Google Scholar] [CrossRef]
- Song, I.K.; Kang, Y.K. Conformational preferences of γ-aminobutyric acid in the gas phase and in water. J. Mol. Struct. 2012, 1024, 163–169. [Google Scholar] [CrossRef]
- CrysAlisPRO, v. 171.40; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2021.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Nyman, J.; Day, G.M. Static and lattice vibrational energy differences between polymorphs. CrystEngComm 2015, 17, 5154–5165. [Google Scholar] [CrossRef]
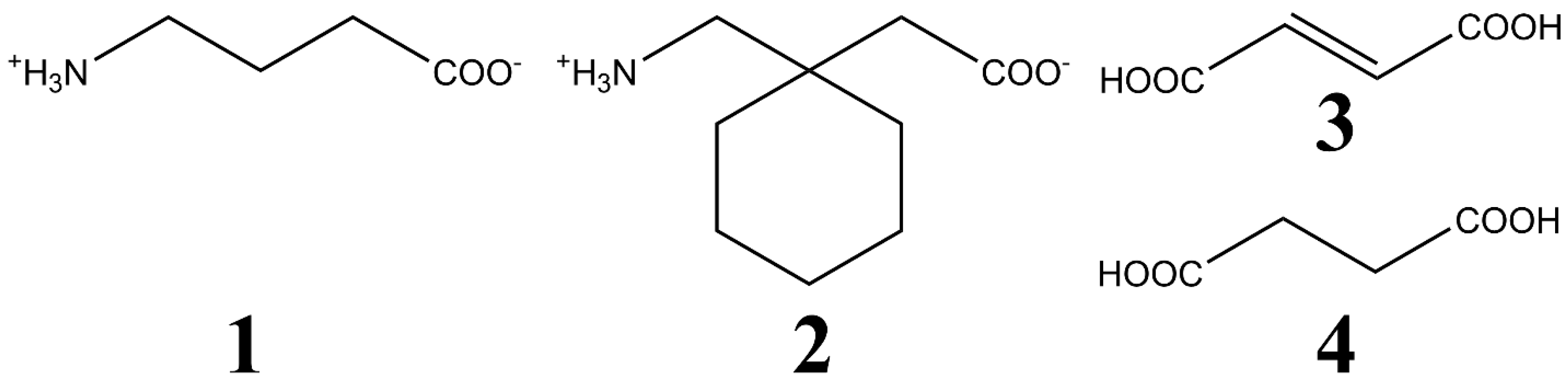
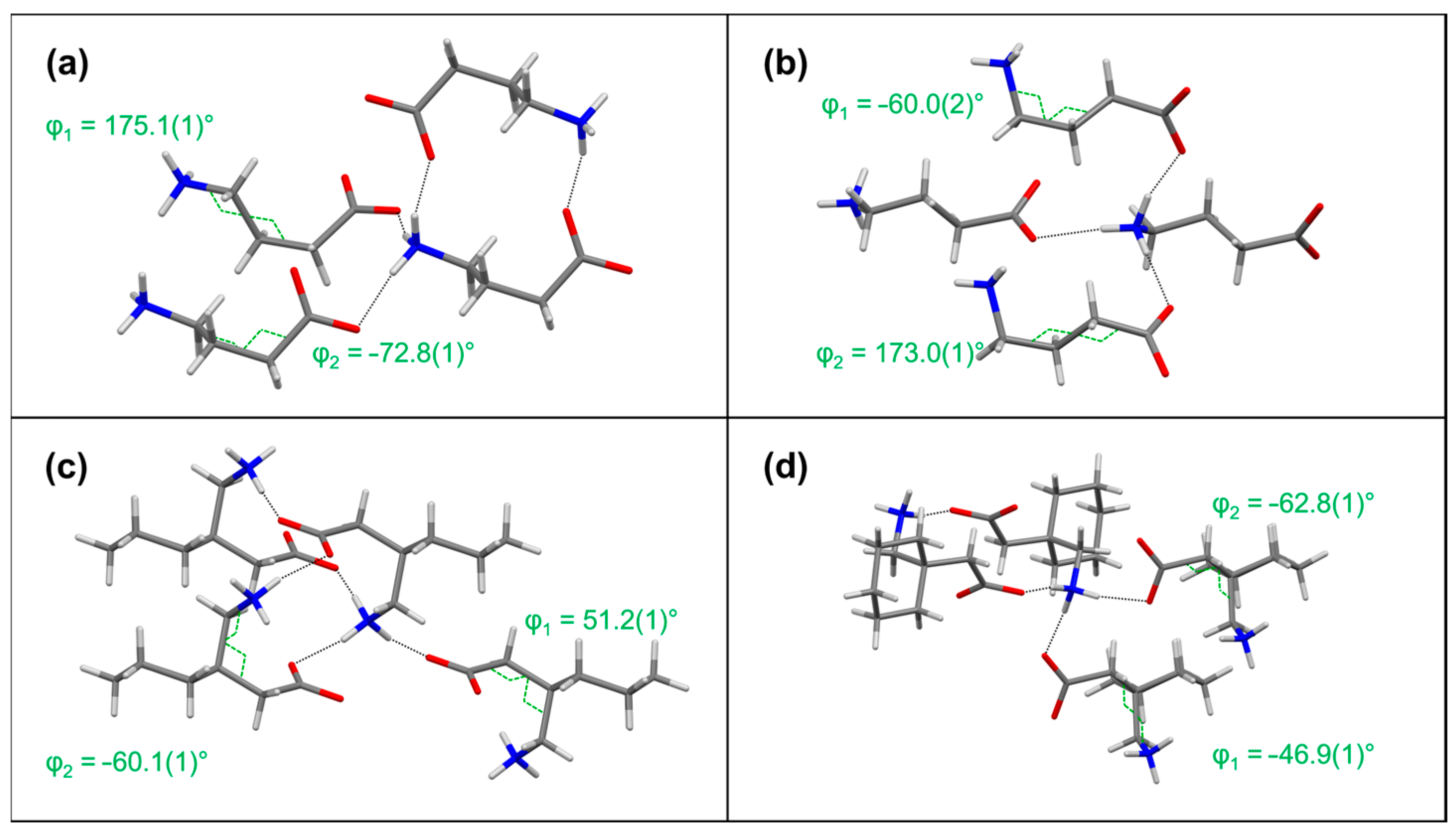

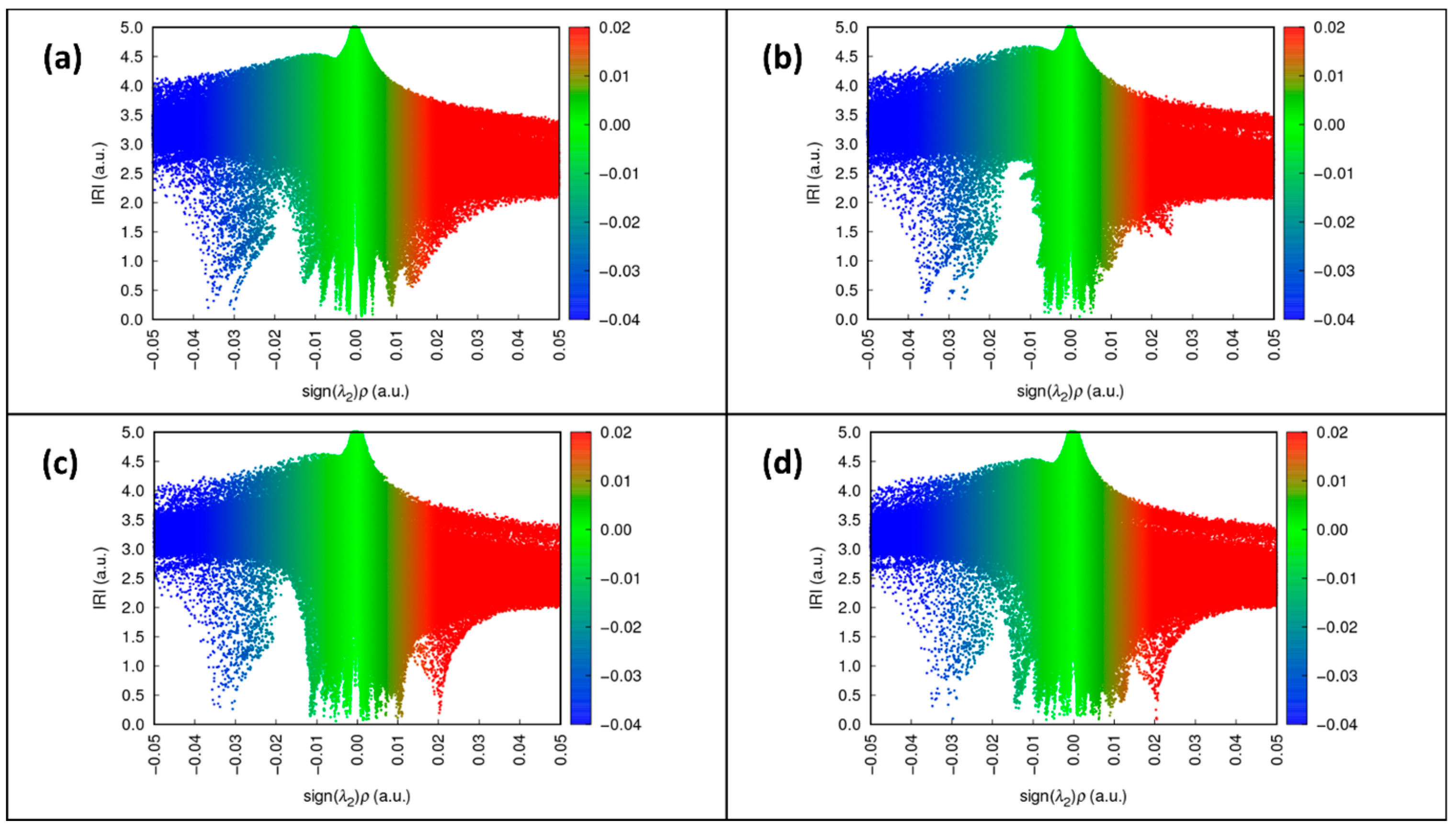
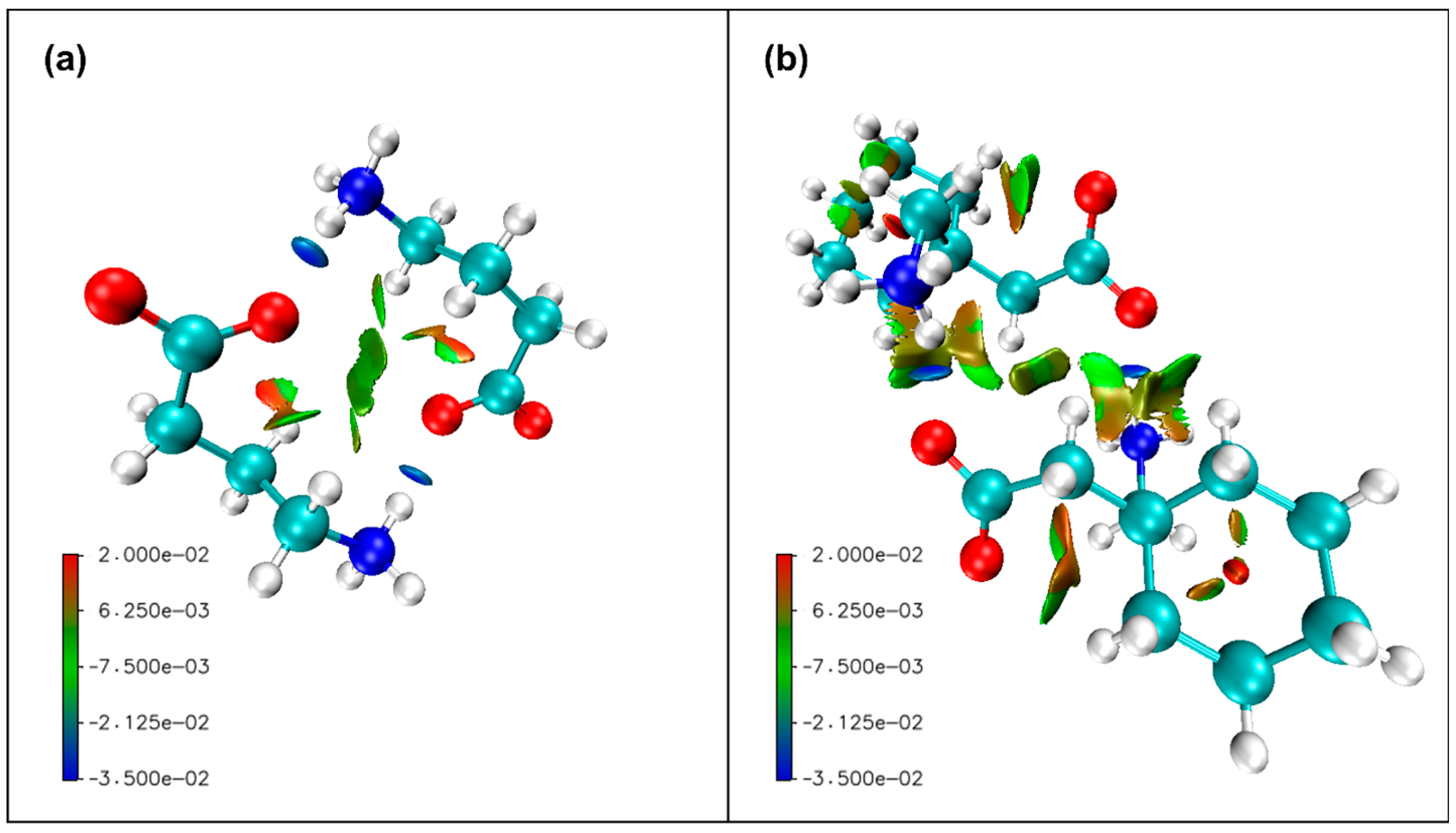
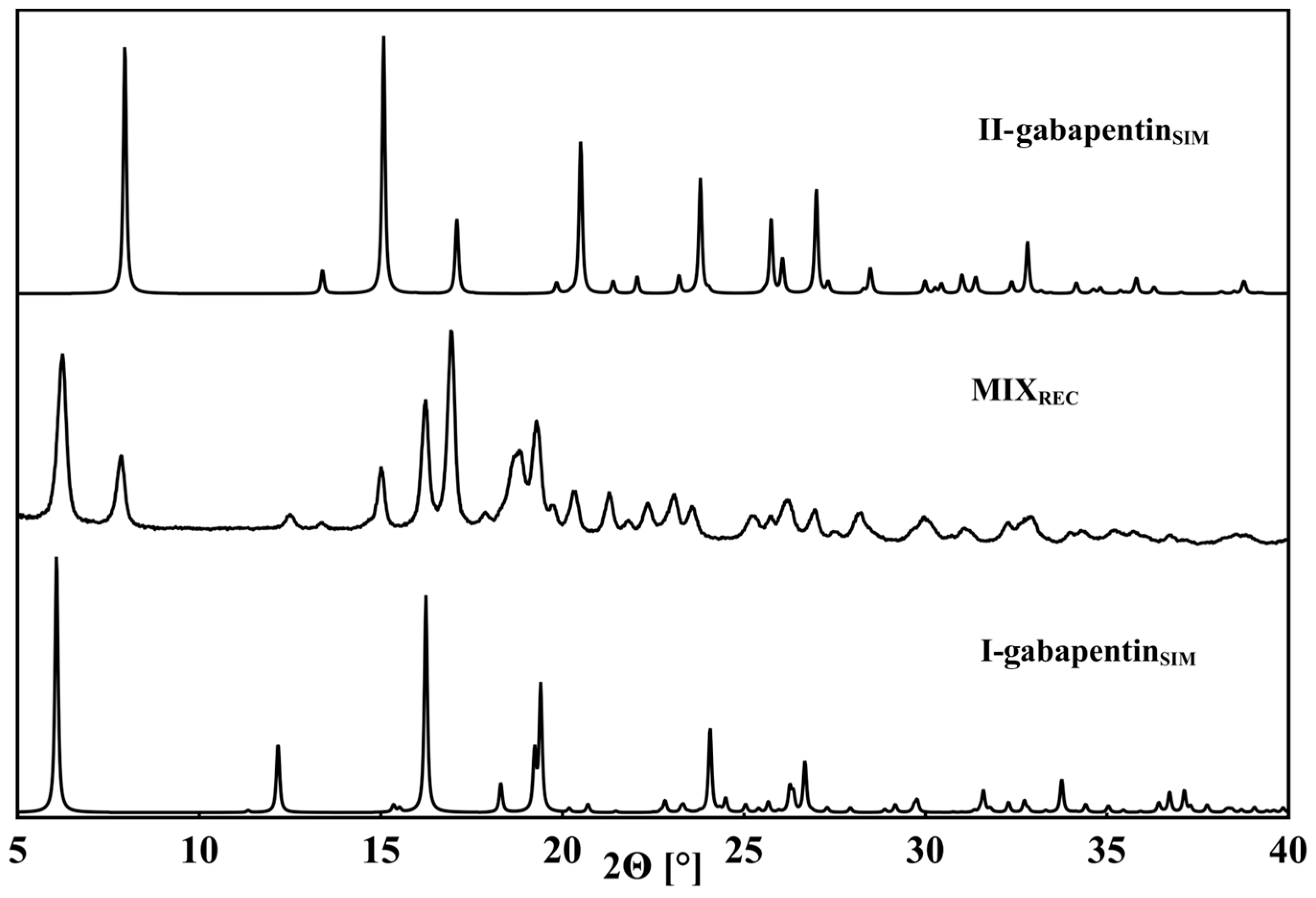
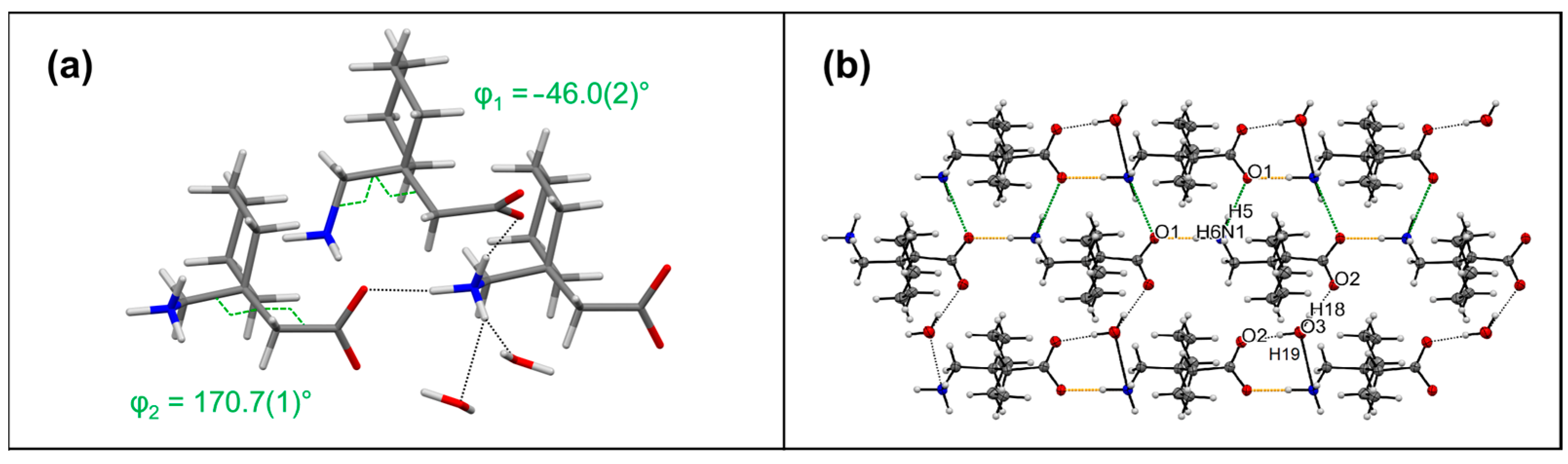


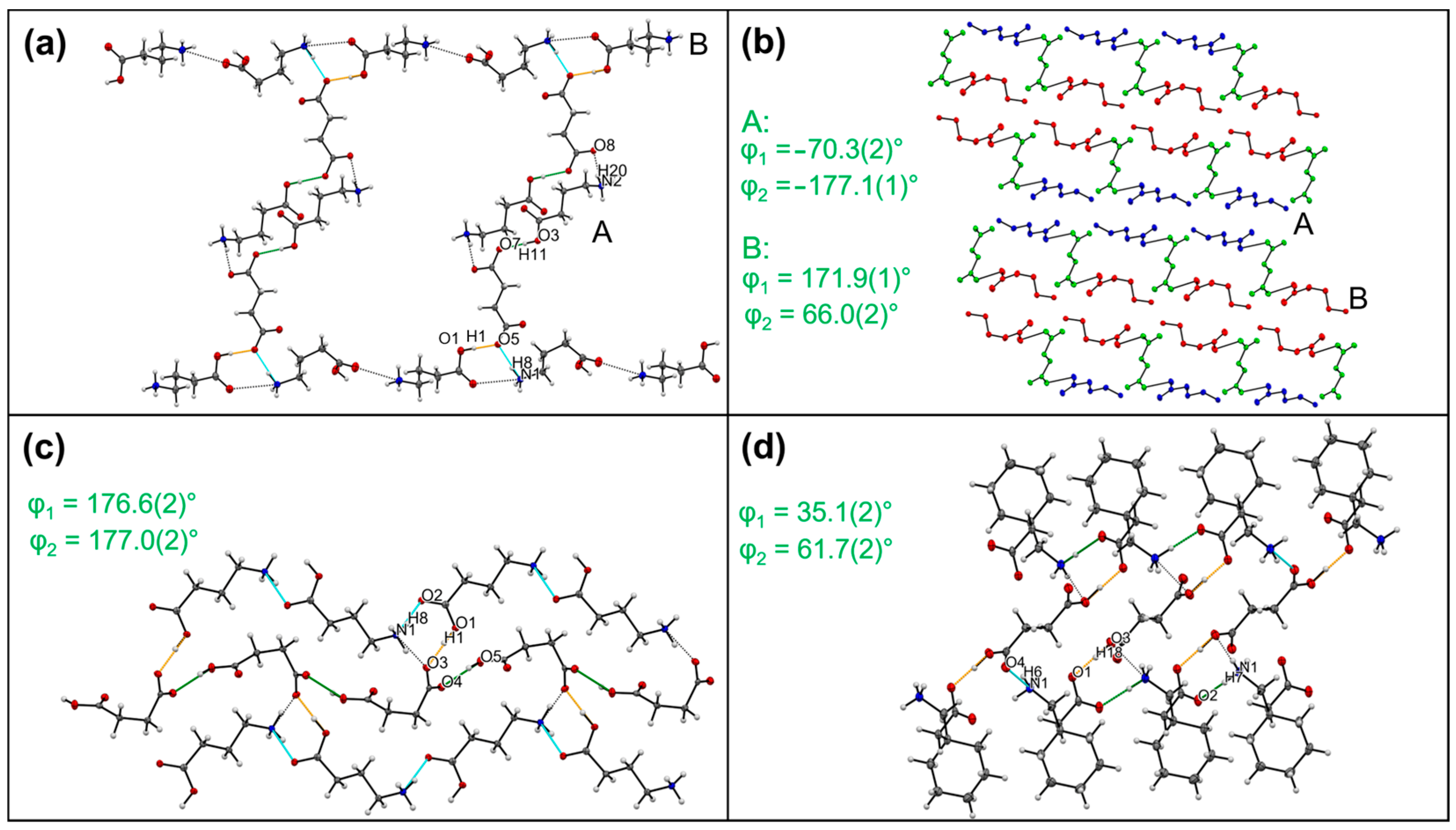
| Compound | Interaction | H…A [Å] | D…A [Å] | D-H…A [°] | Ebond [kJ mol−1] |
|---|---|---|---|---|---|
| N1-H8…O1 | 1.81 (1) | 2.760 (3) | 168 (5) | −55.37 | |
| I-1 | N1-H7…O1 | 1.82 (0) | 2.734 (2) | 169 (3) | −54.83 |
| N1-H9…O2 | 1.85 (3) | 2.755 (7) | 163 (1) | −47.52 | |
| N1-H8…O1 | 1.82 (2) | 2.753 (3) | 164 (8) | −56.37 | |
| II-1 | N1-H9…O2 | 1.90 (2) | 2.743 (9) | 179 (4) | −46.88 |
| N1-H7…O1 | 1.94 (3) | 2.790 (1) | 172 (2) | −42.87 | |
| N1-H5…O1 | 1.80 (2) | 2.753 (5) | 168 (0) | −55.48 | |
| II-2 | N1-H7…O2 | 1.83 (8) | 2.746 (7) | 168 (2) | −52.97 |
| N1-H6…O1 | 1.88 (2) | 2.778 (3) | 158 (6) | −48.73 | |
| N1-H7…O2 | 1.81 (6) | 2.733 (8) | 164 (4) | −54.65 | |
| IV-2 | N1-H5…O1 | 1.83 (0) | 2.769 (2) | 168 (2) | −53.87 |
| N1-H6…O1 | 1.87 (9) | 2.796 (2) | 164 (1) | −47.05 | |
| N1-H6…O1 | 1.73 (2) | 2.752 (2) | 173 (2) | −66.71 | |
| I-2 | N1-H5…O1 | 1.88 (3) | 2.842 (2) | 166 (2) | −51.46 |
| O3-H19…O2 | 1.82 (3) | 2.752 (2) | 176 (2) | −49.12 |
| Compound | Interaction | H…A [Å] | D…A [Å] | D-H…A [°] | Ebond [kJ mol−1] |
|---|---|---|---|---|---|
| O1-H1…O3 | 1.72 (2) | 2.606 (7) | 179 (2) | −65.80 | |
| 1-3 | N1-H8…O3 | 1.89 (9) | 2.785 (9) | 163 (6) | −48.84 |
| N1-H9…O4 | 1.91 (0) | 2.785 (1) | 164 (3) | −49.63 | |
| O1-H1…O3 | 1.68 (3) | 2.644 (3) | 174 (2) | −73.09 | |
| 2-3a | N1-H6…O4 | 1.81 (2) | 2.761 (0) | 174 (6) | −58.11 |
| N1-H7…O4 | 1.84 (2) | 2.746 (4) | 162 (2) | −53.54 | |
| O11-H42…O10 | 1.50 (2) | 2.525 (5) | 178 (3) | −110.63 | |
| 2-3b | O1-H1…O5 | 1.51 (2) | 2.507 (0) | 175 (2) | −111.08 |
| O7-H39…O6 | 1.65 (2) | 2.559 (0) | 170 (5) | −76.81 | |
| O1-H1…O5 | 1.62 (3) | 2.543 (6) | 173 (2) | −84.68 | |
| 1-4a | O3-H11…O7 | 1.72 (3) | 2.581 (3) | 178 (2) | −64.86 |
| N1-H8…O5 | 1.84 (2) | 2.783 (8) | 173 (2) | −52.26 | |
| O1-H1…O3 | 1.52 (3) | 2.455 (2) | 175 (4) | −104.67 | |
| 1-4b | O5-H11…O4 | 1.78 (4) | 2.583 (2) | 163 (3) | −52.74 |
| N1-H8…O2 | 1.95 (3) | 2.861 (3) | 161 (3) | −38.57 | |
| O3-H18…O1 | 1.50 (3) | 2.522 (0) | 175 (2) | −111.50 | |
| 2-4 | N1-H7…O2 | 1.79 (3) | 2.734 (3) | 175 (2) | −56.03 |
| N1-H6…O4 | 1.94 (2) | 2.776 (0) | 155 (2) | −38.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komisarek, D.; Demirbas, F.; Haj Hassani Sohi, T.; Merz, K.; Schauerte, C.; Vasylyeva, V. Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin. Pharmaceutics 2023, 15, 2299. https://doi.org/10.3390/pharmaceutics15092299
Komisarek D, Demirbas F, Haj Hassani Sohi T, Merz K, Schauerte C, Vasylyeva V. Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin. Pharmaceutics. 2023; 15(9):2299. https://doi.org/10.3390/pharmaceutics15092299
Chicago/Turabian StyleKomisarek, Daniel, Fulya Demirbas, Takin Haj Hassani Sohi, Klaus Merz, Carsten Schauerte, and Vera Vasylyeva. 2023. "Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin" Pharmaceutics 15, no. 9: 2299. https://doi.org/10.3390/pharmaceutics15092299
APA StyleKomisarek, D., Demirbas, F., Haj Hassani Sohi, T., Merz, K., Schauerte, C., & Vasylyeva, V. (2023). Polymorphism and Multi-Component Crystal Formation of GABA and Gabapentin. Pharmaceutics, 15(9), 2299. https://doi.org/10.3390/pharmaceutics15092299





