In Vitro and In Vivo Evaluation of Inhalable Ciprofloxacin Sustained Release Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Preparation
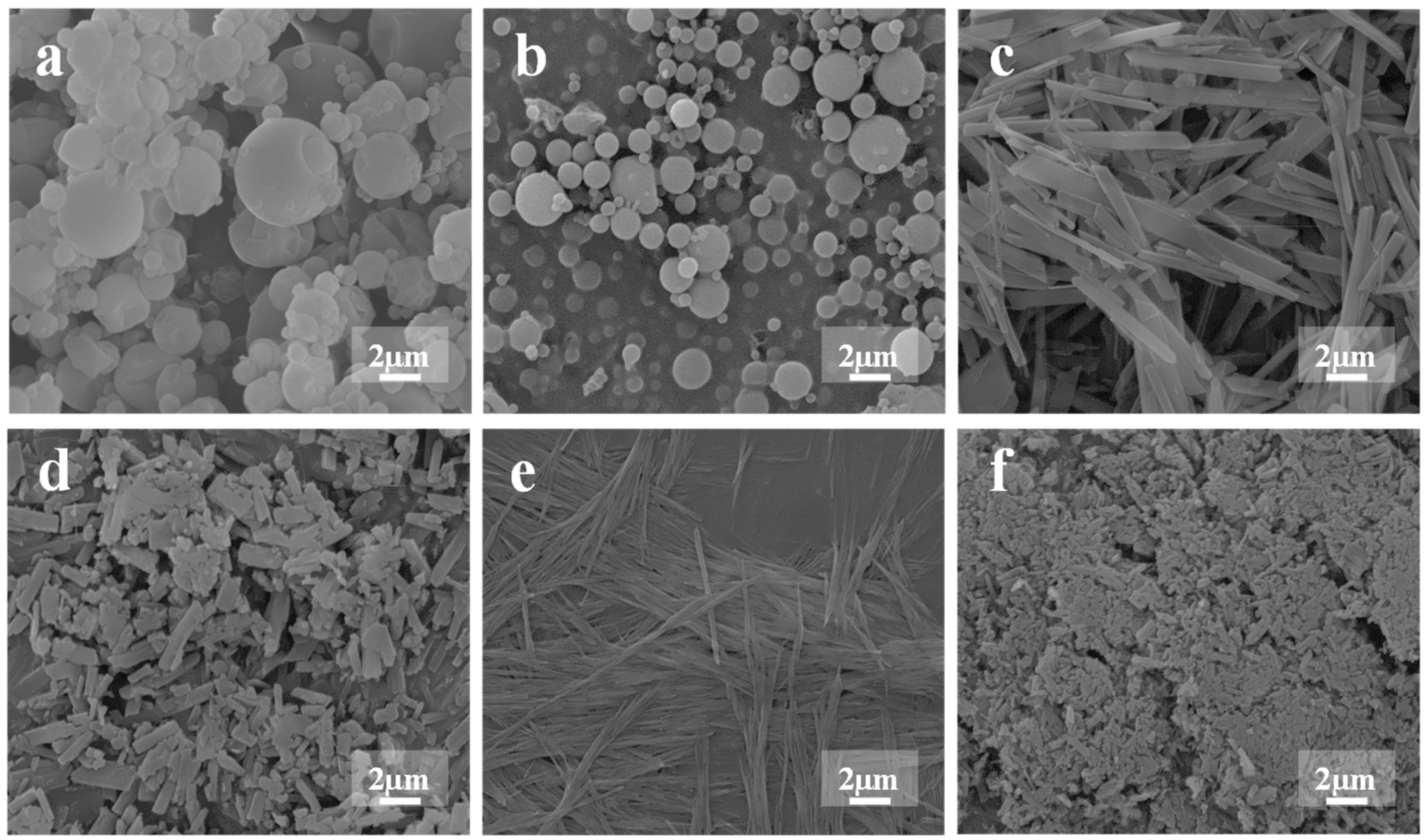
2.3. Powder Morphology
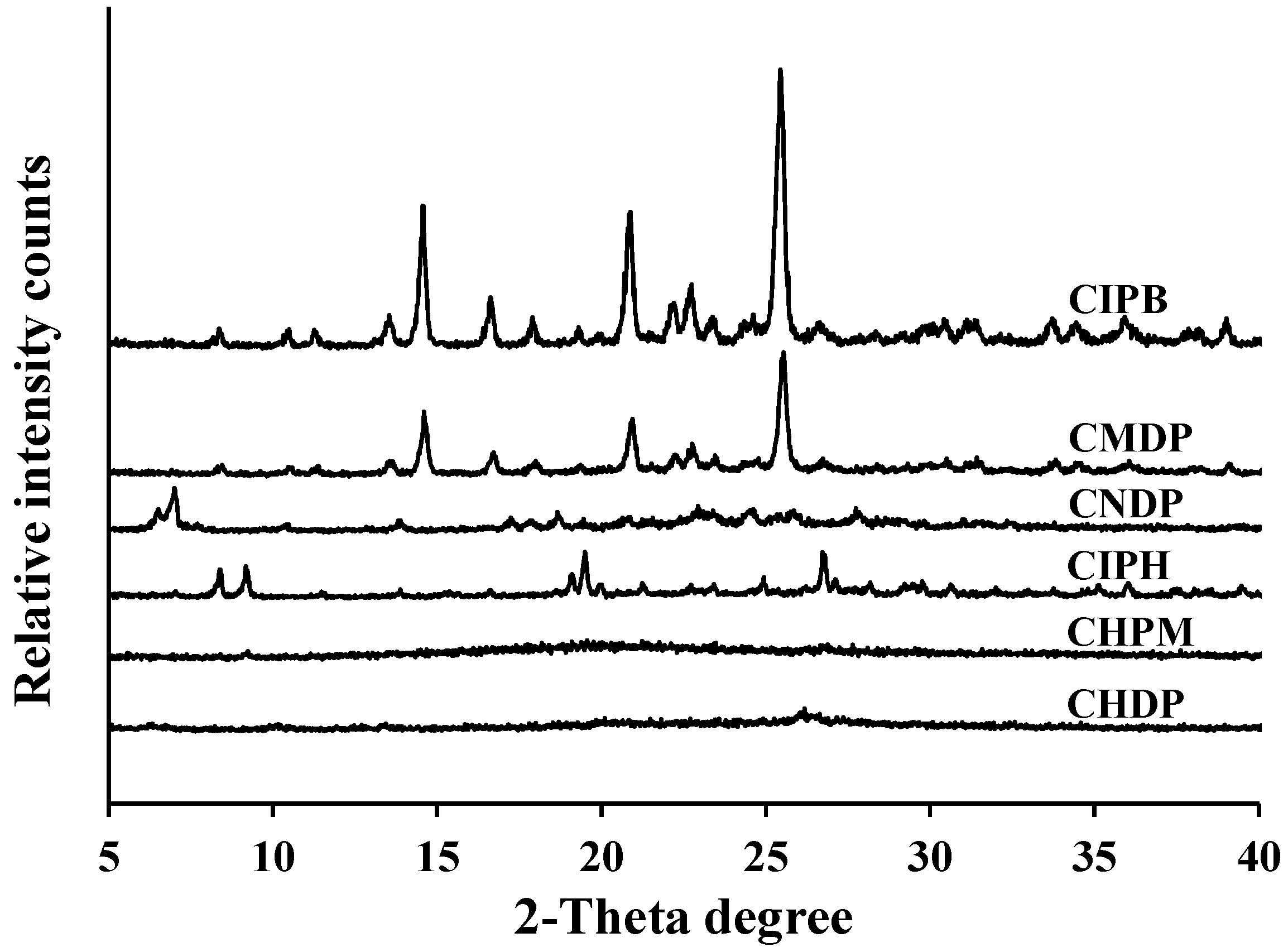
2.4. Powder X-ray Diffraction
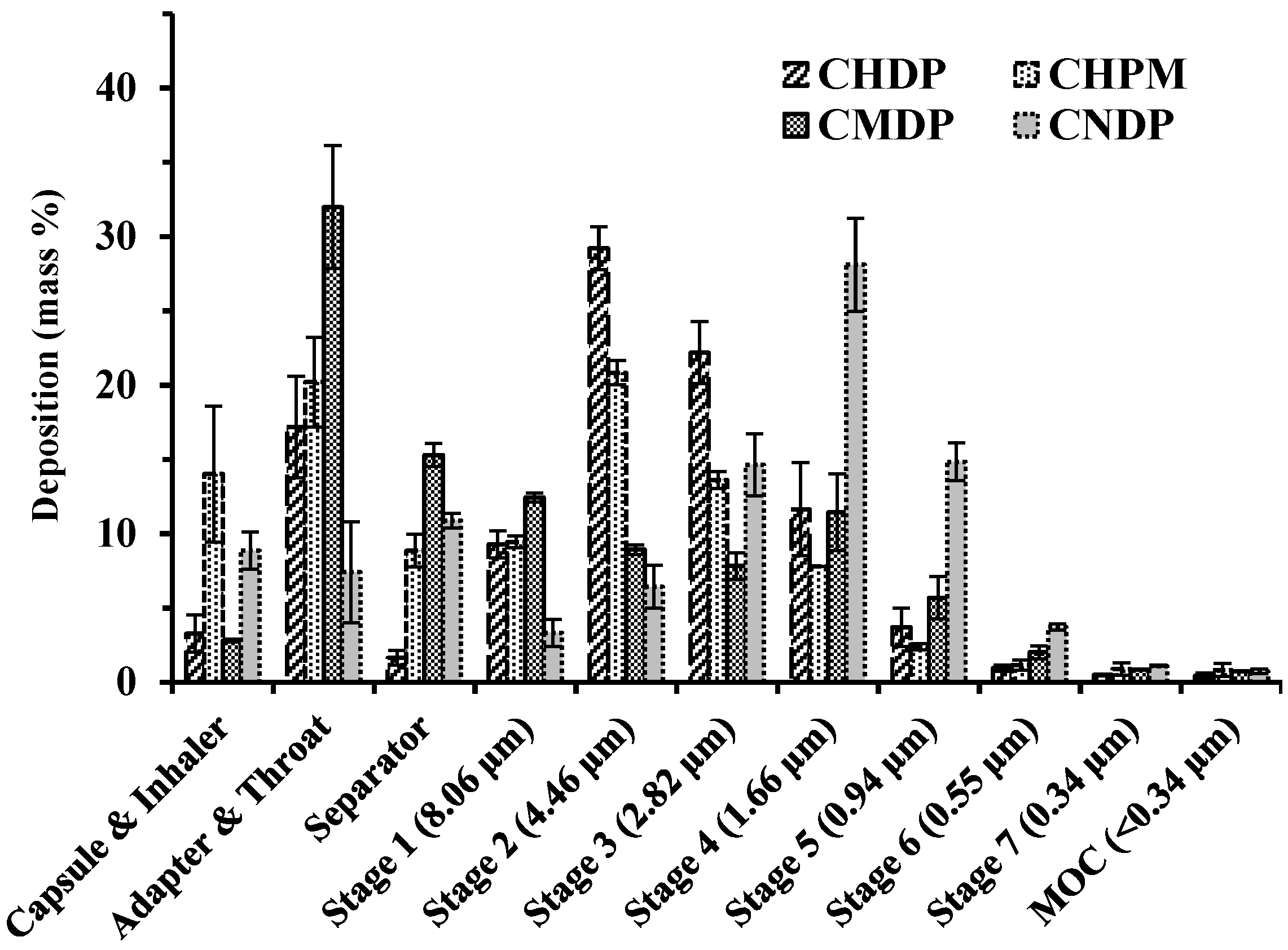
2.5. Aerosol Performance
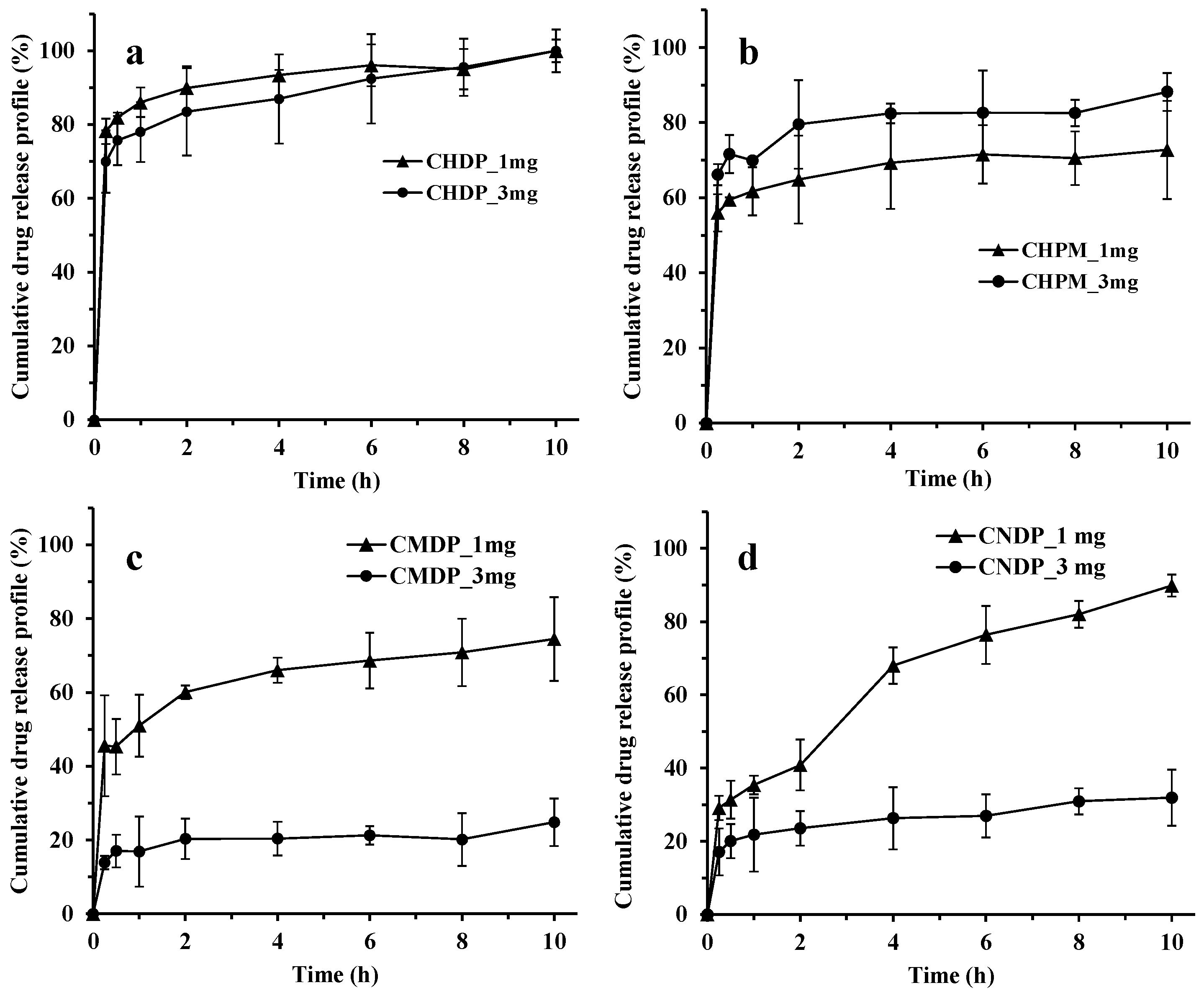
2.6. Drug Release Study
2.7. Pharmacokinetic Study in Rats
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Aerosol Performance
3.3. In Vitro Drug Release Profiles
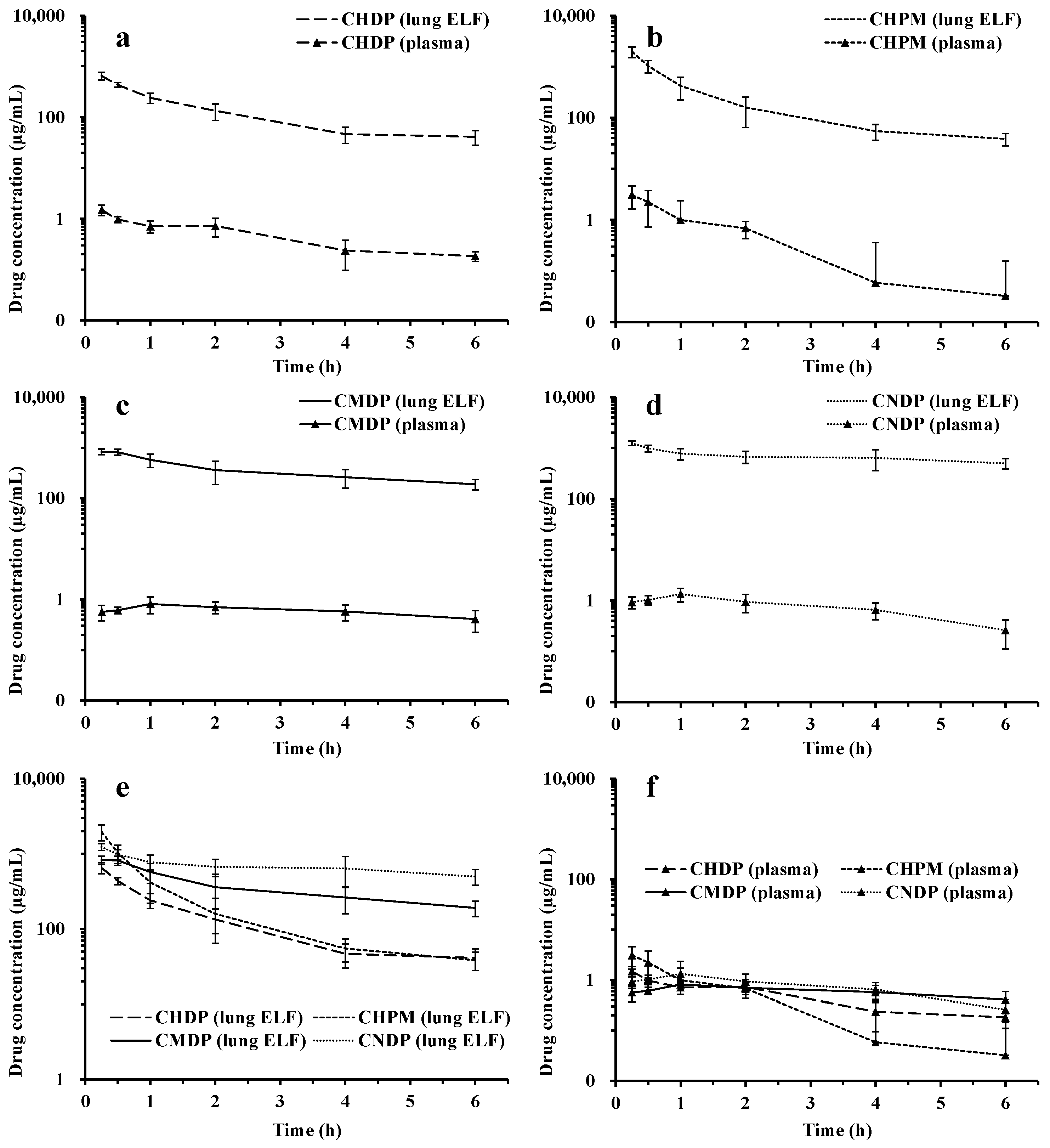
3.4. In Vivo Lung Distribution
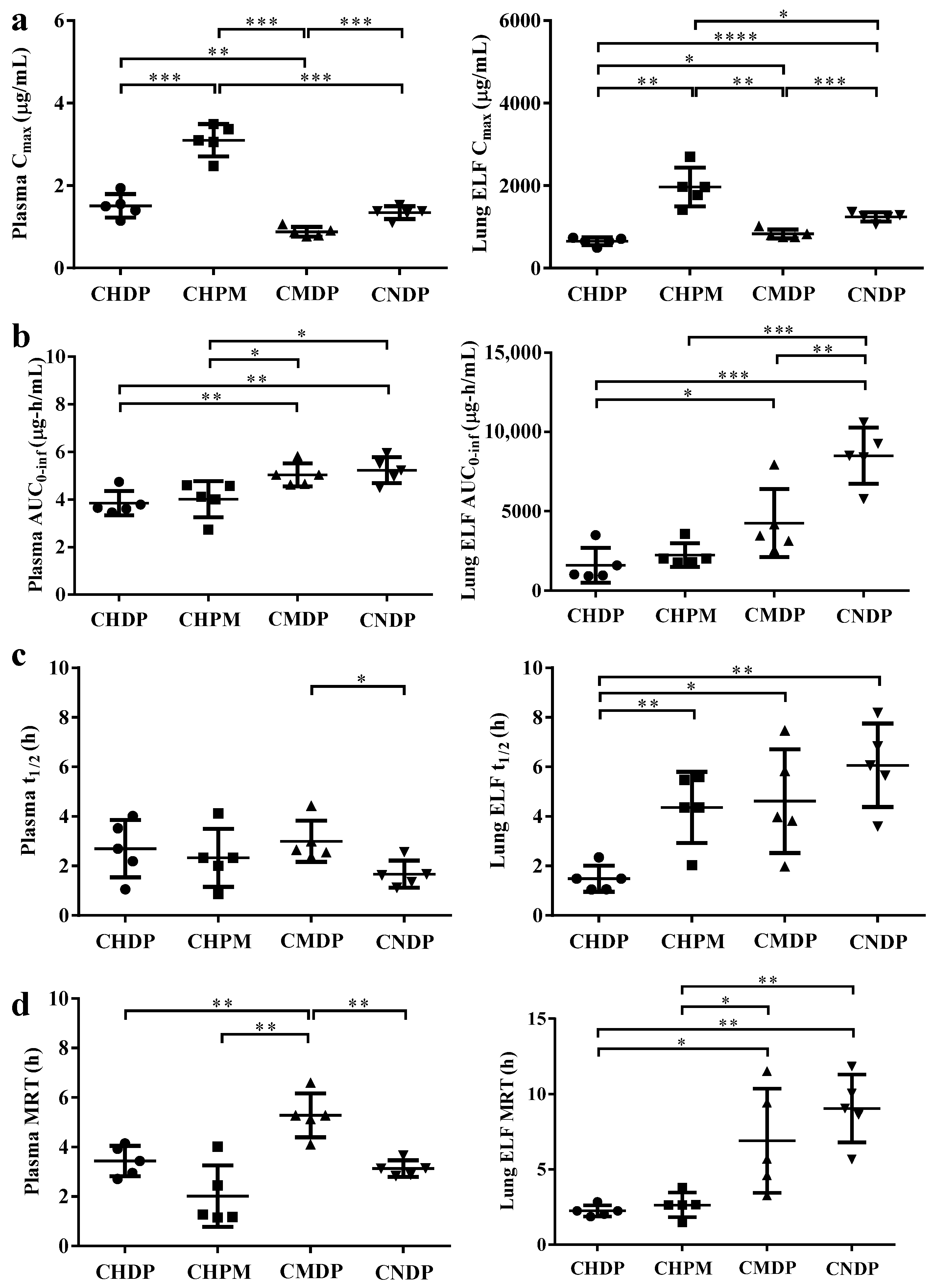
3.5. In Vivo Pharmacokinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliver, A.; Cantón, R.; Campo, P.; Baquero, F.; Blázquez, J. High Frequency of Hypermutable Pseudomonas aeruginosa in Cystic Fibrosis Lung Infection. Science 2000, 288, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.G.; Olveira, C.; Girón, R.; Máiz, L.; Sibila, O.; Golpe, R.; Menéndez, R.; Rodríguez, J.; Barreiro, E.; Hermosa, J.L.R.; et al. Impact of Chronic Bronchial Infection by Staphylococcus aureus on Bronchiectasis. J. Clin. Med. 2022, 11, 3960. [Google Scholar] [CrossRef]
- Zhang, L.; Bera, H.; Wang, H.; Wang, J.; Guo, Y.; Shi, C.; Cun, D.; Moser, C.; Høiby, N.; Yang, M. Combination and nanotechnology based pharmaceutical strategies for combating respiratory bacterial biofilm infections. Int. J. Pharm. 2022, 616, 121507. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Heltshe, S.L.; Hilliard, J.B.; Konstan, M.W. Pseudomonas aeruginosa antimicrobial susceptibility test (AST) results and pulmonary exacerbation treatment responses in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Brillault, J.; De Castro, W.V.; Couet, W. Relative Contributions of Active Mediated Transport and Passive Diffusion of Fluoroquinolones with Various Lipophilicities in a Calu-3 Lung Epithelial Cell Model. Antimicrob. Agents Chemother. 2010, 54, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Hastedt, J.E.; Bäckman, P.; Clark, A.R.; Doub, W.; Hickey, A.; Hochhaus, G.; Kuehl, P.J.; Lehr, C.-M.; Mauser, P.; McConville, J.; et al. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP Workshop March 16–17th, 2015 in Baltimore, MD. AAPS Open 2016, 2, 1. [Google Scholar] [CrossRef]
- Ho, D.-K.; Nichols, B.L.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, A.V.L.; Brillault, J.; Grégoire, N.; Lamarche, I.; Gobin, P.; Couet, W.; Marchand, S. Biopharmaceutical Characterization of Nebulized Antimicrobial Agents in Rats: 1. Ciprofloxacin, Moxifloxacin, and Grepafloxacin. Antimicrob. Agents Chemother. 2014, 58, 3942–3949. [Google Scholar] [CrossRef]
- Shi, C.; Ignjatović, J.; Wang, J.; Guo, Y.; Zhang, L.; Cvijić, S.; Cun, D.; Yang, M. Evaluating the pharmacokinetics of intrapulmonary administered ciprofloxacin solution for respiratory infections using in vivo and in silico PBPK rat model studies. Chin. Chem. Lett. 2023, 34, 107463. [Google Scholar] [CrossRef]
- Mantero, M.; Gramegna, A.; Pizzamiglio, G.; D’adda, A.; Tarsia, P.; Blasi, F. Once daily aerosolised tobramycin in adult patients with cystic fibrosis in the management of Pseudomonas aeruginosa chronic infection. Multidiscip. Respir. Med. 2017, 12, 2. [Google Scholar] [CrossRef]
- Wan, F.; Nylander, T.; Klodzinska, S.N.; Foged, C.; Yang, M.; Baldursdottir, S.G.; Nielsen, H.M. Lipid Shell-Enveloped Polymeric Nanoparticles with High Integrity of Lipid Shells Improve Mucus Penetration and Interaction with Cystic Fibrosis-Related Bacterial Biofilms. ACS Appl. Mater. Interfaces 2018, 10, 10678–10687. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bera, H.; Shi, C.; Zhang, L.; Cun, D.; Yang, M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm. Sin. B 2021, 11, 2565–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.T.; Leung, S.S.Y.; Tang, P.; Parumasivam, T.; Loh, Z.H.; Chan, H.-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv. Drug Deliv. Rev. 2015, 85, 83–99. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery strategies for sustained drug release in the lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef]
- Leng, D.; Thanki, K.; Foged, C.; Yang, M. Formulating Inhalable Dry Powders Using Two-Fluid and Three-Fluid Nozzle Spray Drying. Pharm. Res. 2018, 35, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Le, Y.; Liu, H.; Hu, T.; Shen, Z.; Yun, J.; Chen, J.-F. Preparation of microsized spherical aggregates of ultrafine ciprofloxacin particles for dry powder inhalation (DPI). Powder Technol. 2009, 194, 81–86. [Google Scholar] [CrossRef]
- El-Gendy, N.; Desai, V.; Berkland, C. Agglomerates of Ciprofloxacin Nanoparticles Yield Fine Dry Powder Aerosols. J. Pharm. Innov. 2010, 5, 79–87. [Google Scholar] [CrossRef]
- Shi, C.; Ignjatović, J.; Liu, T.; Han, M.; Cun, D.; Đuriš, J.; Yang, M.; Cvijić, S. In vitro-in vivo-in silico approach in the development of inhaled drug products: Nanocrystal-based formulations with budesonide as a model drug. Asian J. Pharm. Sci. 2021, 16, 350–362. [Google Scholar] [CrossRef]
- Velaga, S.P.; Djuris, J.; Cvijic, S.; Rozou, S.; Russo, P.; Colombo, G.; Rossi, A. Dry powder inhalers: An overview of the in vitro dissolution methodologies and their correlation with the biopharmaceutical aspects of the drug products. Eur. J. Pharm. Sci. 2018, 113, 18–28. [Google Scholar] [CrossRef]
- Neckel, U.; Joukhadar, C.; Frossard, M.; Jäger, W.; Müller, M.; Mayer, B.X. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 2002, 463, 199–206. [Google Scholar] [CrossRef]
- Yapa, S.W.S.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.H.; et al. Pulmonary and Systemic Pharmacokinetics of Inhaled and Intravenous Colistin Methanesulfonate in Cystic Fibrosis Patients: Targeting Advantage of Inhalational Administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection. Drug Deliv. 2020, 27, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Zeng, L.; Mangal, S.; Nie, H.; Rowles, M.R.; Guo, R.; Han, Y.; Park, J.H.; Zhou, Q. Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations. Pharm. Res. 2018, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Development and characterization of a spray-dried inhalable ciprofloxacin-quercetin co-amorphous system. Int. J. Pharm. 2022, 618, 121657. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Ahn, P.; Park, H.; Bhujbal, S.; Zemlyanov, D.; Cavallaro, A.-A.; Mangal, S.; Li, J.; Zhou, Q.T. Improved Physical Stability and Aerosolization of Inhalable Amorphous Ciprofloxacin Powder Formulations by Incorporating Synergistic Colistin. Mol. Pharm. 2018, 15, 4004–4020. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, L.; Su, J.; Sun, Y.; Zhang, L.; Li, J.; Beck-Broichsitter, M.; Muenster, U.; Chen, L.; Mao, S. Engineering large porous microparticles with tailored porosity and sustained drug release behavior for inhalation. Eur. J. Pharm. Biopharm. 2020, 155, 139–146. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Li, J.; Beck-Broichsitter, M.; Muenster, U.; Wang, X.; Zhao, J.; Mao, S. Optimization of budesonide-loaded large-porous microparticles for inhalation using quality by design approach. J. Drug Deliv. Sci. Technol. 2019, 53, 101140. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Cun, D.; Zhang, C.; Bera, H.; Yang, M. Particle engineering principles and technologies for pharmaceutical biologics. Adv. Drug Deliv. Rev. 2021, 174, 140–167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Xu, E.-Y.; Moehwald, M.; Chen, L.; Zhang, X.; Mao, S. Inhalable PLGA microspheres: Tunable lung retention and systemic exposure via polyethylene glycol modification. Acta Biomater. 2021, 123, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef] [PubMed]
| Formulation | EF (%) | MMAD (µm) | GSD | FPF (%) |
|---|---|---|---|---|
| CHDP | 97.15 ± 0.17 | 4.42 ± 0.32 | 1.80 ± 0.01 | 46.98 ± 6.17 |
| CHPM | 96.07 ± 0.42 | 4.53 ± 0.01 | 1.84 ± 0.20 | 37.39 ± 2.55 * |
| CMDP | 96.68 ± 0.19 | 3.83 ± 0.47 | 2.78 ± 0.13 * | 30.95 ± 5.01 ** |
| CNDP | 98.18 ± 0.17 | 2.24 ± 0.01 ** | 1.65 ± 0.03 | 71.81 ± 2.11 *** |
| Region/Deposition | CHDP (%) | CHPM (%) | CMDP (%) | CNDP (%) |
|---|---|---|---|---|
| Trachea | 7.75 ± 2.96 | 4.76 ± 0.78 | 9.41 ± 8.87 | 12.03 ± 1.63 |
| Bronchi | 41.34 ± 14.11 | 43.60 ± 8.63 | 49.76 ± 18.60 | 20.91 ± 2.72 |
| Alveoli | 50.91 ± 13.15 | 51.64 ± 7.2 | 40.83 ± 12.16 | 67.07 ± 9.22 |
| Formulation | AUCEPR | AppELF | PTI |
|---|---|---|---|
| i.v. injection # | 1.30 | NA | NA |
| i.t. solution # | 5.71 | 4.76 | 4.39 |
| i.t. CHDP | 278.21 | 153.30 | 213.65 |
| i.t. CHPM | 427.03 | 285.05 | 327.94 |
| i.t. CMDP | 503.22 | 401.18 | 386.45 |
| i.t. CNDP | 881.81 | 737.16 | 677.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Guo, K.; Zhang, L.; Guo, Y.; Feng, Y.; Cvijić, S.; Cun, D.; Yang, M. In Vitro and In Vivo Evaluation of Inhalable Ciprofloxacin Sustained Release Formulations. Pharmaceutics 2023, 15, 2287. https://doi.org/10.3390/pharmaceutics15092287
Shi C, Guo K, Zhang L, Guo Y, Feng Y, Cvijić S, Cun D, Yang M. In Vitro and In Vivo Evaluation of Inhalable Ciprofloxacin Sustained Release Formulations. Pharmaceutics. 2023; 15(9):2287. https://doi.org/10.3390/pharmaceutics15092287
Chicago/Turabian StyleShi, Changzhi, Kewei Guo, Li Zhang, Yi Guo, Yu Feng, Sandra Cvijić, Dongmei Cun, and Mingshi Yang. 2023. "In Vitro and In Vivo Evaluation of Inhalable Ciprofloxacin Sustained Release Formulations" Pharmaceutics 15, no. 9: 2287. https://doi.org/10.3390/pharmaceutics15092287
APA StyleShi, C., Guo, K., Zhang, L., Guo, Y., Feng, Y., Cvijić, S., Cun, D., & Yang, M. (2023). In Vitro and In Vivo Evaluation of Inhalable Ciprofloxacin Sustained Release Formulations. Pharmaceutics, 15(9), 2287. https://doi.org/10.3390/pharmaceutics15092287









