In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hydrodistillation of Essential Oil
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Antityrosinase Assay
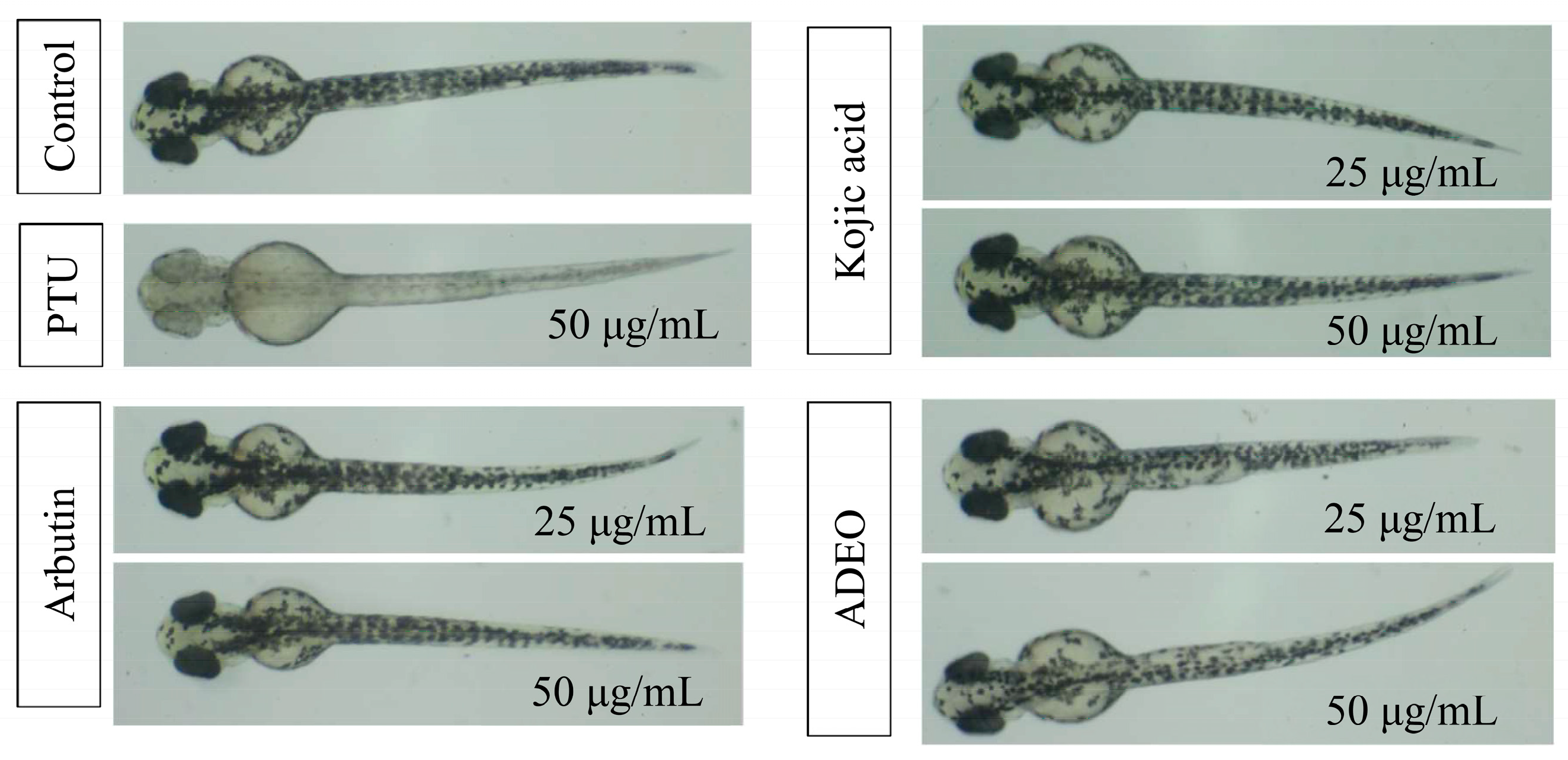
2.5. Antimelanogenesis Effect in Zebrafish Embryos
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Constituents of A. dammara Leaf Essential Oil
3.2. Tyrosinase Inhibitory Activity of A. dammara Leaf Essential Oil
3.3. Antimelanogenesis Effect of A. dammara Leaf Essential Oil in Zebrafish
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.M.; Marty, R.A.; Peters, C.F. The diterpene acids in the bled resins of three pacific kauri, Agathis vitiensis, A. lanceolata and A. macrophylla. Phytochemistry 1981, 20, 2205–2207. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Wu, M.Z.; Fookes, C.J.R.; Forster, P.I. The steam volatile oil of Wollemia nobilis and its comparison with other members of the Araucariaceae (Agathis and Araucaria). Biochem. Syst. Ecol. 2000, 28, 563–578. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; De Vita, D.; Toniolo, C.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; Guiso, M.; Nicoletti, M.; et al. Phytochemistry, chemotaxonomy, and biological activities of the Araucariaceae family—A review. Plants 2020, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Balocchi, F.; Wingfield, M.J.; Paap, T.; Ahumada, R.; Barnes, I. Pathogens of the Araucariaceae: How much do we know? Curr. For. Rep. 2022, 8, 124–147. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Verma, S.K.; Chauhan, A.; Darokar, M.P. Chemical composition and antibacterial activity of the essential oil of Kauri Pine [Agathis robusta (C. Moore ex F. Muell.) F.M. Bailey] from India. J. Wood Chem. Technol. 2016, 36, 270–277. [Google Scholar] [CrossRef]
- Chen, Z.; He, D.; Deng, J.; Zhu, J.; Mao, Q. Chemical composition and antibacterial activity of the essential oil from Agathis dammara (Lamb.) Rich fresh leaves. Nat. Prod. Res. 2015, 29, 2050–2053. [Google Scholar] [CrossRef]
- Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial potential of tropical rainforest plant extracts. Medicines 2014, 1, 32–55. [Google Scholar] [CrossRef]
- Surapuram, V.; Setzer, W.N.; McFeeters, R.L.; McFeeters, H. Antifungal activity of plant extracts against Aspergillus niger and Rhizopus stolonifer. Nat. Prod. Commun. 2014, 9, 1603–1605. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Dai, R.Y.; Leu, Y.L.; Tsai, T.Y. Effects of the melanogenic inhibitor, uracil, derived from Lactobacillus plantarum TWK10-fermented soy milk on anti-melanogenesis in B16F0 mouse melanoma cells. J. Funct. Foods 2015, 17, 314–327. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2019, 9, 1703480. [Google Scholar] [CrossRef]
- Tasneem, R.; Khan, H.M.S.; Rasool, F.; Khan, K.U.R.; Umair, M.; Esatbeyoglu, T.; Korma, S.A. Development of phytocosmeceutical microemulgel containing flaxseed extract and its in vitro and in vivo characterization. Pharmaceutics 2022, 14, 1656. [Google Scholar] [CrossRef]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale roscoe, attenuates α-MSH-induced melanogenesis in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in biomedical functions of natural whitening substances in the treatment of skin pigmentation diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, H.; Xue, X.; Chen, Y.; He, Z.; Yu, Z.; Zhang, L.; Miao, X. Dual antimelanogenic effect of nicotinamide-stabilized phloretin nanocrystals in larval zebrafish. Pharmaceutics 2022, 14, 1825. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, I.H.; Huang, X.Z.; Chen, H.J.; Chang, S.T.; Chang, M.L.; Ho, Y.T.; Chang, H.T. Antimelanogenesis effects of leaf extract and phytochemicals from ceylon olive (Elaeocarpus serratus) in zebrafish model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Dong, Q.; Li, J.; Li, F.; Michniak-Kohn, B.B.; Zhao, D.; Ho, C.-T.; Huang, Q. Anti-melanogenic mechanism of tetrahydrocurcumin and enhancing its topical delivery efficacy using a lecithin-based nanoemulsion. Pharmaceutics 2021, 13, 1185. [Google Scholar] [CrossRef] [PubMed]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, 241–246. [Google Scholar] [CrossRef]
- Ferreira, A.M.; de Souza, A.A.; Koga, R.d.C.R.; Sena, I.d.S.; Matos, M.d.J.S.; Tomazi, R.; Ferreira, I.M.; Carvalho, J.C.T. Anti-melanogenic potential of natural and synthetic substances: Application in zebrafish model. Molecules 2023, 28, 1053. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.Y.; Jang, S.K.; Kim, K.J.; Park, M.J. Inhibition of melanogenesis by essential oils from the Citrus cultivars peels. Int. J. Mol. Sci. 2023, 24, 4207. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cha, B.J.; Lee, Y.S.; Kim, G.S.; Noh, H.J.; Kim, S.Y.; Kang, H.C.; Kim, J.H.; Baek, N.I. The potential of minor ginsenosides isolated from the leaves of Panax ginseng as inhibitors of melanogenesis. Int. J. Mol. Sci. 2015, 16, 1677–1690. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.G.; Lee, Y.G.; Kim, J.H.; Lee, J.W.; Choi, B.R.; Jang, I.B.; Kim, G.S.; Baek, N.I. Isolation and quantification of ginsenoside rh23, a new anti-melanogenic compound from the leaves of Panax ginseng. Molecules 2018, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, J.S.; Jeong, Y.T.; Byun, G.H.; Kim, J.H. Melanogenesis inhibition activity of floralginsenoside A from Panax ginseng berry. J. Ginseng Res. 2017, 41, 602–607. [Google Scholar] [CrossRef]
- Chang, H.T.; Chang, M.L.; Chen, Y.T.; Chang, S.T.; Hsu, F.L.; Wu, C.C.; Ho, C.K. Evaluation of motor coordination and anti-depressant activities of Cinnamomum osmophloeum ct. linalool leaf oil in rodent model. Molecules 2021, 26, 3037. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.R.; Chang, M.L.; Chang, S.T.; Ho, Y.T.; Chang, H.T. Cytotoxicity and apoptosis induction of 6,7-dehydroroyleanone from Taiwania cryptomerioides bark essential oil in hepatocellular carcinoma cells. Pharmaceutics 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. ISBN 978-1932633214. [Google Scholar]
- Zhang, N.; Bian, Y.; Yao, L. Essential oils of Gardenia jasminoides J. Ellis and Gardenia jasminoides f. longicarpa Z.W. Xie & M. Okada flowers: Chemical characterization and assessment of anti-inflammatory effects in alveolar macrophage. Pharmaceutics 2022, 14, 966. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, Y.Y.; Chang, S.T.; Chang, H.T. Xanthine oxidase inhibitory activity and chemical composition of Pistacia chinensis leaf essential oil. Pharmaceutics 2022, 14, 1982. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-melanogenic properties of greek plants. a novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef]
- Setoguchi, H.; Asakawa Osawa, T.; Pintaud, J.C.; Jaffré, T.; Veillon, J.M. Phylogenetic relationships within Araucariaceae based on rbcL gene sequences. Am. J. Bot. 1998, 85, 1507–1516. [Google Scholar] [CrossRef]
- Escapa, I.H.; Iglesias, A.; Wilf, P.; Catalano, S.A.; Caraballo-Ortiz, M.A.; Rubén Cúneo, N. Agathis trees of Patagonia’s Cretaceous-Paleogene death landscapes and their evolutionary significance. Am. J. Bot. 2018, 105, 1345–1368. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, T.Y.; Chang, L.Z.; Wang, H.F.; Yih, K.H.; Hsieh, W.Y.; Chang, T.M. Inhibition of melanogenesis versus antioxidant properties of essential oil extracted from leaves of Vitex negundo Linn and chemical composition analysis by GC-MS. Molecules 2012, 17, 3902–3916. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef]
- Sangthong, S.; Promputtha, I.; Pintathong, P.; Chaiwut, P. Chemical constituents, antioxidant, anti-tyrosinase, cytotoxicity, and anti-melanogenesis activities of Etlingera elatior (Jack) leaf essential oils. Molecules 2022, 27, 3469. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Ahmad, F.; Yen, K.H. Chemical compositions and biological activities of the essential oils of Beilschmiedia madang Blume (Lauraceae). Arch. Pharm. Res. 2015, 38, 485–493. [Google Scholar] [CrossRef]
- Suganya, P.; Jeyaprakash, K.; Mallavarapu, G.R.; Murugan, R. Comparison of the chemical composition, tyrosinase inhibitory and anti-inflammatory activities of the essential oils of Pogostemon plectranthoides from India. Ind. Crops Prod. 2015, 69, 300–307. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Lin, F.J.; Li, H.; Wu, D.T.; Zhuang, Q.G.; Li, H.B.; Geng, F.; Gan, R.Y. Recent development in zebrafish model for bioactivity and safety evaluation of natural products. Crit. Rev. Food Sci. Nutr. 2022, 62, 8646–8674. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Occhiuto, C.; Cimino, F.; Cristani, M.; Saija, A.; Molonia, M.S.; Ruberto, G.; D’Angelo, V.; Germanò, M.P.; et al. Evaluation of antioxidant, anti-inflammatory and antityrosinase potential of extracts from different aerial parts of Rhanterium suaveolens from Tunisia. Chem. Biodivers. 2021, 18, e2100316. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Lei, X.; Liao, L.; Fu, T.; Yuan, Y.; Huang, X.; Zou, L.; Liu, Y.; Ruan, R.; et al. Chemical composition and evaluation of antioxidant activities, antimicrobial, and anti-melanogenesis effect of the essential oils extracted from Dalbergia pinnata (Lour.) Prain. J. Ethnopharmacol. 2020, 254, 112731. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, J.; Li, S.; Wu, Y.; Wang, Z.; Chen, S.; Chen, H. Discovery and identification of potential anti-melanogenic active constituents of Bletilla striata by zebrafish model and molecular docking. BMC Complement. Med. Ther. 2022, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, Y.C.; Tsai, M.L.; Cheng, C.Y.; Liu, L.L.; Yen, Y.W.; Chen, W.L. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef] [PubMed]



| RT a (min) | KI b | rKI c | Constituent | M.F. | M.W. | Relative Content (%) | Identified Method d |
|---|---|---|---|---|---|---|---|
| 23.34 | 1335 | 1338 | δ-Elemene | C15H24 | 204 | 0.23 ± 0.03 | MS, KI |
| 23.84 | 1348 | 1351 | α-Cubebene | C15H24 | 204 | 1.65 ± 0.21 | MS, KI |
| 24.69 | 1369 | 1371 | α-Ylangene | C15H24 | 204 | 2.84 ± 0.39 | MS, KI |
| 24.94 | 1375 | 1376 | α-Copaene | C15H24 | 204 | 2.47 ± 0.31 | MS, KI |
| 25.40 | 1386 | 1388 | β-Cubebene | C15H24 | 204 | 0.62 ± 0.06 | MS, KI |
| 26.50 | 1416 | 1419 | β-Caryophyllene | C15H24 | 204 | 8.58 ± 0.94 | MS, KI |
| 27.68 | 1454 | 1454 | α-Caryophyllene | C15H24 | 204 | 4.37 ± 0.31 | MS, KI |
| 28.35 | 1475 | 1477 | γ-Gurjunene | C15H24 | 204 | 15.57 ± 0.49 | MS, KI |
| 28.50 | 1479 | 1481 | Germacrene D | C15H24 | 204 | 8.53 ± 0.20 | MS, KI |
| 29.03 | 1495 | 1500 | α-Muurolene | C15H24 | 204 | 1.71 ± 0.01 | MS, KI |
| 29.46 | 1510 | 1513 | γ-Cadinene | C15H24 | 204 | 5.33 ± 0.16 | MS, KI |
| 29.64 | 1517 | 1522 | δ-Cadinene | C15H24 | 204 | 16.12 ± 0.53 | MS, KI |
| 30.00 | 1531 | 1534 | trans-1,4-Cadinadiene | C15H24 | 204 | 0.58 ± 0.03 | MS, KI |
| 30.12 | 1535 | 1538 | α-Cadinene | C15H24 | 204 | 0.46 ± 0.03 | MS, KI |
| 30.24 | 1540 | 1545 | α-Calacorene | C15H20 | 200 | 0.25 ± 0.02 | MS, KI |
| 30.81 | 1560 | 1565 | β-Calacorene | C15H20 | 200 | 0.10 ± 0.01 | MS, KI |
| 32.54 | 1626 | 1628 | 1-epi-Cubenol | C15H26O | 222 | 0.41 ± 0.03 | MS, KI |
| 32.91 | 1641 | 1646 | δ-Cadinol | C15H26O | 222 | 1.31 ± 0.09 | MS, KI |
| 33.23 | 1654 | 1654 | α-Cadinol | C15H26O | 222 | 1.39 ± 0.10 | MS, KI |
| 38.69 | 1898 | 1896 | Rimuene | C20H36 | 276 | 1.21 ± 0.14 | MS, KI |
| 41.63 | 2047 | 2043 | 16-Kaurene | C20H32 | 272 | 12.43 ± 1.32 | MS, KI |
| Sesquiterpene Hydrocarbons | 69.35 ± 2.18 | ||||||
| Oxygenated Sesquiterpenes | 3.10 ± 0.21 | ||||||
| Diterpene Hydrocarbons | 13.64 ± 1.46 | ||||||
| Total Identified | 86.09 ± 0.51 | ||||||
| Specimen | IC50 (μg/mL) | |
|---|---|---|
| L-Tyrosine as the Substrate | L-DOPA as the Substrate | |
| Leaf essential oil | - * | 690.02 ± 18.85 |
| Kojic acid ** | 2.46 ± 0.06 | 6.74 ± 0.09 |
| Specimen | Concentration (μg/mL) | Inhibition (%) |
|---|---|---|
| PTU | 50 | 98.23 ± 1.01 d |
| Arbutin | 25 | 19.62 ± 9.10 a,b |
| 50 | 21.49 ± 5.98 a,b | |
| Kojic acid | 25 | 18.53 ± 3.56 a |
| 50 | 20.44 ± 7.27 a,b | |
| Leaf essential oil | 12.5 | 21.03 ± 10.02 a,b |
| 25 | 37.36 ± 9.79 b,c | |
| 50 | 43.48 ± 7.30 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.-T.; Liu, I.-H.; Chang, S.-T.; Wang, S.-Y.; Chang, H.-T. In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara. Pharmaceutics 2023, 15, 2269. https://doi.org/10.3390/pharmaceutics15092269
Ho Y-T, Liu I-H, Chang S-T, Wang S-Y, Chang H-T. In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara. Pharmaceutics. 2023; 15(9):2269. https://doi.org/10.3390/pharmaceutics15092269
Chicago/Turabian StyleHo, Yu-Tung, I-Hsuan Liu, Shang-Tzen Chang, Sheng-Yang Wang, and Hui-Ting Chang. 2023. "In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara" Pharmaceutics 15, no. 9: 2269. https://doi.org/10.3390/pharmaceutics15092269
APA StyleHo, Y.-T., Liu, I.-H., Chang, S.-T., Wang, S.-Y., & Chang, H.-T. (2023). In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara. Pharmaceutics, 15(9), 2269. https://doi.org/10.3390/pharmaceutics15092269







