Applications of Curcumin and Its Nanoforms in the Treatment of Cancer
Abstract
:1. Introduction
2. Methodology Used
3. Curcumin and Its Properties
3.1. Bioavailability
3.1.1. Absorption
3.1.2. Distribution
3.2. Physical and Chemical Properties of Curcumin
3.3. Anti-Cancer Activity of Curcumin
4. Nanoforms of Curcumin
4.1. Technologies Used to Fabricate Nanocurcumin
4.2. Activities of Curcumin Nanoforms
5. Types of Nanocarriers Used for Curcumin Delivery
5.1. Protein-Based Encapsulation of Curcumin for Delivery
5.2. Polysaccharide-Based Encapsulation of Curcumin for Delivery
5.3. Self-Assembled Molecules and Ligands as Carriers for Delivery of Curcumin
5.3.1. Metal–Organic Framework (MOF)
5.3.2. Covalent Organic Frameworks (COFs)
6. Curcumin-Loaded Nanoparticles as Therapeutic Agents for Drug Targets
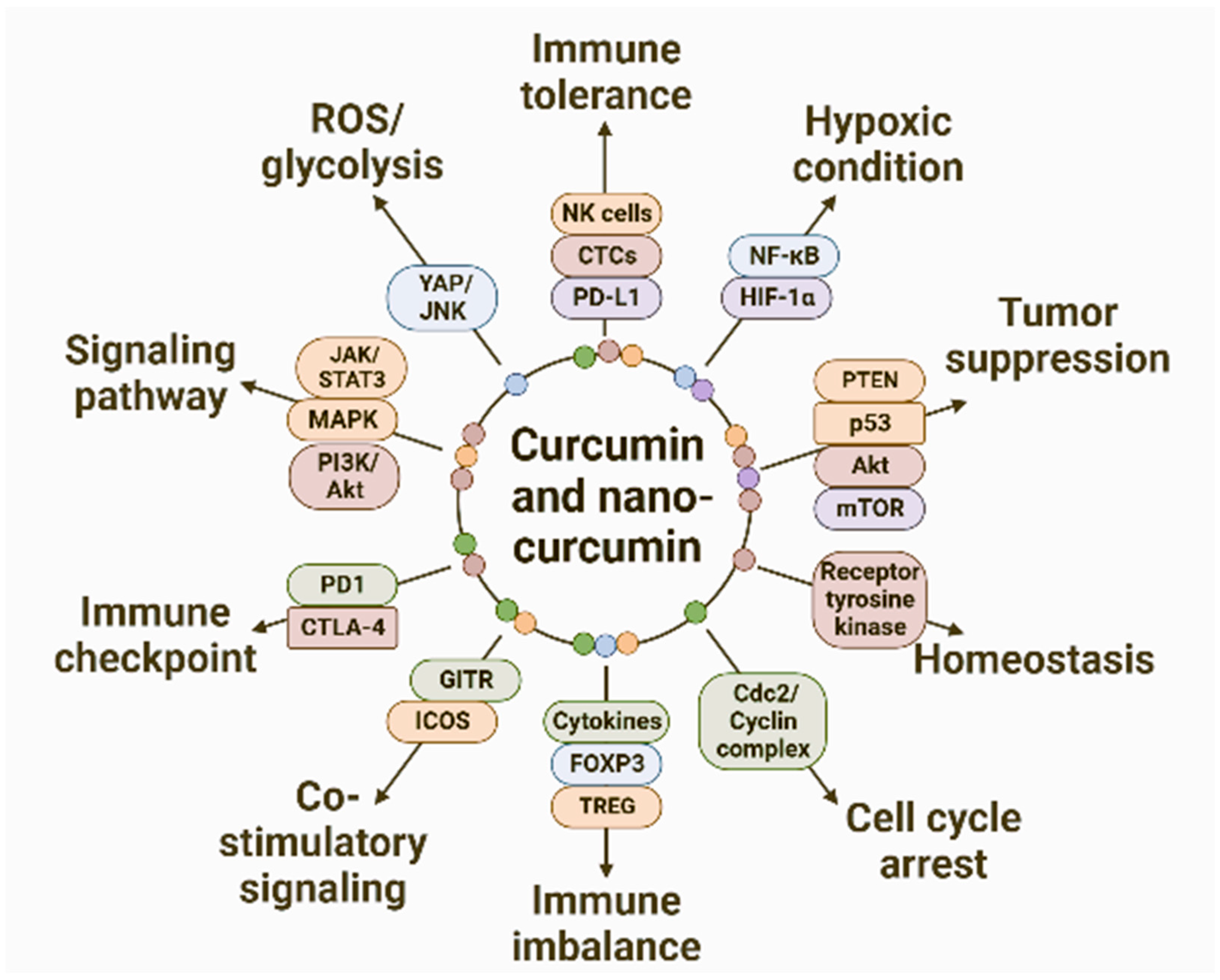
7. Anti-Cancer Mechanisms of Curcumin
7.1. ROS-Mediated Apoptosis Induced by Nanocurcumin
7.2. Influence of Curcumin and Nanocurcumin on Various Signaling Pathways
7.3. Curcumin and Cell Cycle Pathways
7.4. Curcumin and Immunomodulation
7.4.1. Immunomodulatory Activity of Curcumin and Nanocurcumin
7.4.2. Role of Curcumin and Nanocurcumin in Immune Tolerance, Co-Stimulation, and Co-Inhibition
7.4.3. Immune Checkpoint Modulation by Curcumin and Nanocurcumin
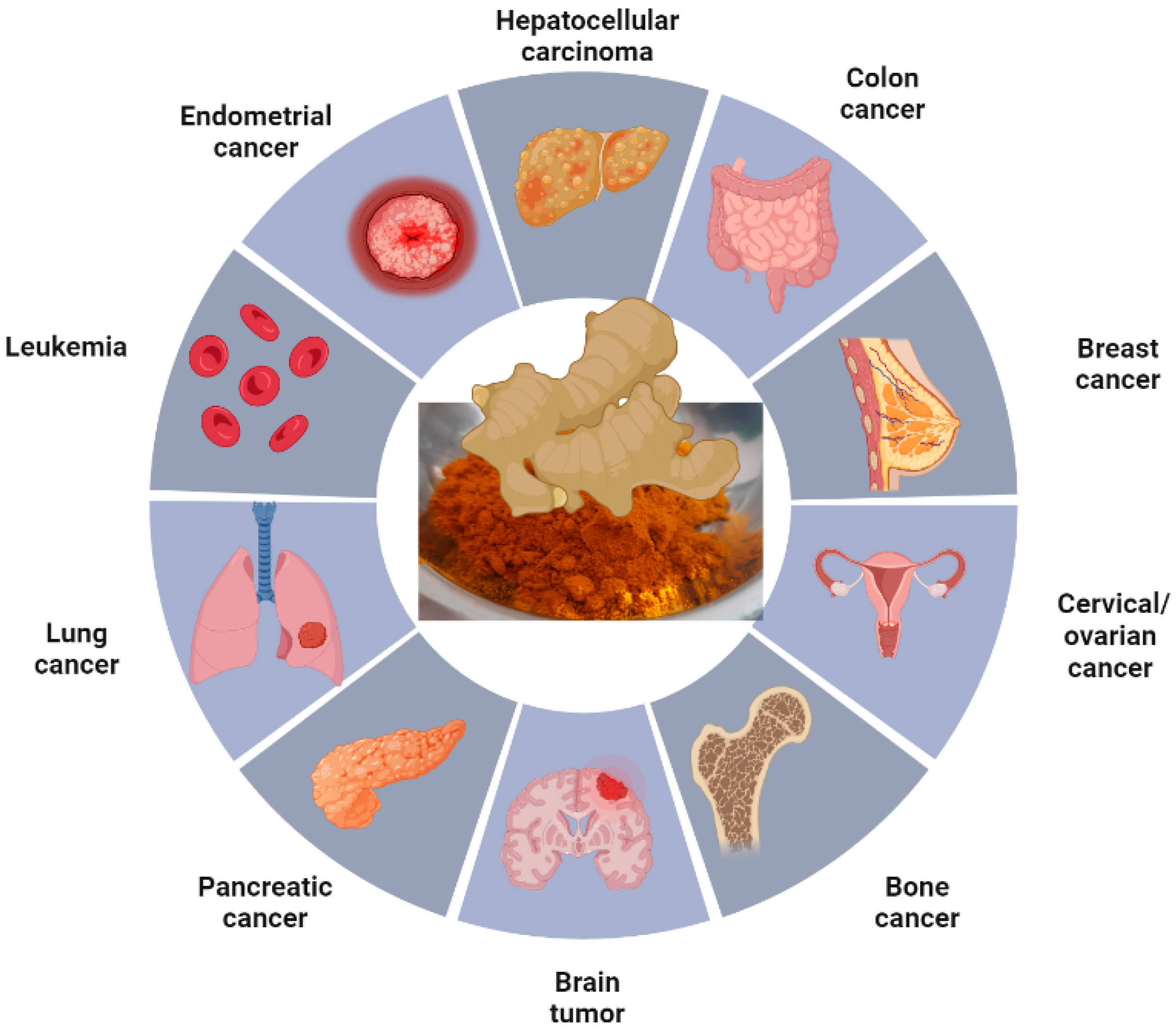
8. Application of Various Nanoforms of Curcumin in Different Types of Cancer
9. Route of Administration of Curcumin and Its Nanoform
9.1. Non-Invasive Modes of Delivery
9.1.1. Topical Application
9.1.2. Oral Administration
9.1.3. Nasal Administration
9.1.4. Pulmonary Route
9.2. Invasive Modes of Delivery
9.2.1. Intravenous
9.2.2. Intra-Arterial
10. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Gopinath, H.; Karthikeyan, K. Turmeric: A condiment, cosmetic and cure. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 16. [Google Scholar] [CrossRef]
- Garodia, P.; Girisa, S.; Rana, V.; Kunnumakkara, A.B.; Aggarwal, B.B. Lessons to be learnt from ayurveda: Nutraceuticals and cosmeceuticals from ayurveda herbs. In Ayurveda in the New Millennium; CRC Press: Boca Raton, FL, USA, 2020; pp. 199–222. [Google Scholar]
- Badmanaban, R.; Saha, D.; Sen, D.J.; Biswas, A.; Mandal, S.; Basak, S. Turmeric: A holistic Solution for Biochemical malfunction. Res. J. Pharm. Technol. 2021, 14, 5540–5550. [Google Scholar]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Gupta, J.; Gupta, R. Miracles of herbal phytomedicines in treatment of skin disorders: Natural healthcare perspective. Infect. Disord. Drug Targets 2021, 21, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Toden, S.; Goel, A. The holy grail of curcumin and its efficacy in various diseases: Is bioavailability truly a big concern? J. Restor. Med. 2017, 6, 27. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef] [PubMed]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of curcumin in skin disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Patel, P.K.; Sahu, J.; Sahu, L.; Prajapati, N.K.; Dubey, B. Aegle marmelos: A review on its medicinal properties. Int. J. Pharm. Phytopharm. Res. 2012, 1, 332–341. [Google Scholar]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and curcumin: From traditional to modern medicine. In Studies on Biomarkers and New Targets in Aging Research in Iran: Focus on Turmeric and Curcumin; Springer: Cham, Switzerland, 2021; pp. 15–39. [Google Scholar]
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-drug Resistance (MDR): Reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-C.; Chueh, F.-S.; Hsiao, Y.-T.; Cheng, Z.-Y.; Lien, J.-C.; Liu, K.-C.; Peng, S.-F.; Chung, J.-G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Wang, L.-J.; Rajesh, R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: Colocalization of ALDH and CD44. Mater. Sci. Eng. C 2019, 102, 362–372. [Google Scholar] [CrossRef]
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Motlagh, N.S.H.; Naghib, S.M.; Haghiralsadat, F.; Jaliani, H.Z.; Moradi, A. A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment. Sci. Rep. 2022, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-based nanoformulations: A promising adjuvant towards cancer treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef]
- Alipour, M.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Sharifi, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. PTR 2022, 36, 1156–1181. [Google Scholar]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Gencer, M.Z.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Nirachonkul, W.; Ogonoki, S.; Thumvijit, T.; Chiampanichayakul, S.; Panyajai, P.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S. CD123-targeted nano-curcumin molecule enhances cytotoxic efficacy in leukemic stem cells. Nanomaterials 2021, 11, 2974. [Google Scholar] [CrossRef]
- Idoudi, S.; Bedhiafi, T.; Hijji, Y.M.; Billa, N. Curcumin and derivatives in nanoformulations with therapeutic potential on colorectal cancer. AAPS PharmSciTech 2022, 23, 115. [Google Scholar] [CrossRef] [PubMed]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Xu, J.; Zhao, R.; Xiong, C.; Habu, J.; Wang, Y.; Luo, X. Global publication trends and research hotspots of curcumin application in tumor: A 20-year bibliometric approach. Front. Oncol. 2022, 12, 1033683. [Google Scholar] [CrossRef]
- Mundekkad, D.; Kameshwari, G.; Karchalkar, P.; Koti, R. The catalytic and ROS-scavenging activities of green synthesized, antiferromagnetic α-Fe2O3 nanoparticle with a prismatic octahedron morphology from pomegranate rind extract. Nanotechnology 2021, 33, 045706. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano encapsulated curcumin: And its potential for biomedical applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.; Najda, A.; Alanazi, I.S.; Germoush, M.O. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, W.; Li, Z.; Liu, B.; Hu, Y.; Chen, S.; de Vries, R.; Yuan, Y.; Quintero, L.E.E.; Hou, G. The stability and bioavailability of curcumin loaded α-lactalbumin nanocarriers formulated in functional dairy drink. Food Hydrocoll. 2022, 131, 107807. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Rao, Z.; Hu, J.; Wang, Q.; Sun, Y.; Lei, X.; Zhao, J.; Zeng, K.; Xu, Z. Study on the stability and oral bioavailability of curcumin loaded (-)-epigallocatechin-3-gallate/poly (N-vinylpyrrolidone) nanoparticles based on hydrogen bonding-driven self-assembly. Food Chem. 2022, 378, 132091. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, N.; Wang, F.; Xie, C.; Ye, R.; Tang, H.; Zhang, H.; Liu, Y. Preparation and characterization of curcumin/β-cyclodextrin nanoparticles by nanoprecipitation to improve the stability and bioavailability of curcumin. LWT 2022, 171, 114149. [Google Scholar] [CrossRef]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of papilloma virus-associated cell lines to photodynamic therapy with curcumin-loaded liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, L.; Cao, C.; Yu, Q.; Chen, L.; Liu, J. Curcumin-loaded redox response of self-assembled micelles for enhanced antitumor and anti-inflammation efficacy. Int. J. Nanomed. 2017, 12, 2489. [Google Scholar] [CrossRef]
- Kayani, Z.; Vais, R.D.; Soratijahromi, E.; Mohammadi, S.; Sattarahmady, N. Curcumin-gold-polyethylene glycol nanoparticles as a nanosensitizer for photothermal and sonodynamic therapies: In vitro and animal model studies. Photodiagnosis Photodyn. Ther. 2021, 33, 102139. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin nanoformulations with metal oxide nanomaterials for biomedical applications. Nanomaterials 2021, 11, 460. [Google Scholar] [CrossRef]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-responsive, plasmonic nanogel for dual delivery of curcumin and photothermal therapy for cancer treatment. Front. Chem. 2021, 8, 602941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, Y.; Tan, H.; Li, M.; Li, W. Curcumin nanocrystallites are an ideal nanoplatform for cancer chemotherapy. Front Nanosci. Nanotech 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Olotu, F.; Agoni, C.; Soremekun, O.; Soliman, M.E. An update on the pharmacological usage of curcumin: Has it failed in the drug discovery pipeline? Cell Biochem. Biophys. 2020, 78, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Bolger, G.T.; Licollari, A.; Tan, A.; Greil, R.; Vcelar, B.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B. Pharmacokinetics of liposomal curcumin (Lipocurc™) infusion: Effect of co-medication in cancer patients and comparison with healthy individuals. Cancer Chemother. Pharmacol. 2019, 83, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Slika, L.; Patra, D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020, 17, 61–75. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Zhang, D.; Kanakkanthara, A. Beyond the paclitaxel and vinca alkaloids: Next generation of plant-derived microtubule-targeting agents with potential anticancer activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, M.; Li, Y.; Chen, A.; Li, G.; Zhang, J.; Hu, H.; Wang, X.; Li, S. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int. J. Nanomed. 2015, 10, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Margulis, K.; Magdassi, S.; Lee, H.S.; Macosko, C.W. Formation of curcumin nanoparticles by flash nanoprecipitation from emulsions. J. Colloid Interface Sci. 2014, 434, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Busa, P.; Lin, S.-X.; Deng, J.-P.; Mou, C.-Y.; Lee, C.-H. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J. Photochem. Photobiol. B Biol. 2017, 169, 124–133. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, H.R.; Jeon, S.; Han, D.; Park, Y.S. Curcumin attenuates acrolein-induced COX-2 expression and prostaglandin production in human umbilical vein endothelial cells. J. Lipid Atheroscler. 2020, 9, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, P.; Thangapandiyan, M.; Raja, P.; Rao, G. Pathological and Molecular Studies on Antitumor effect of Curcumin and Curcumin Solid Lipid Nanoparticles. Pak. Vet. J. 2023, 43, 315–320. [Google Scholar]
- Lakshmanan, A.; Akasov, R.A.; Sholina, N.V.; Demina, P.A.; Generalova, A.N.; Gangadharan, A.; Sardar, D.K.; Lankamsetty, K.B.; Khochenkov, D.A.; Khaydukov, E.V.; et al. Nanocurcumin-Loaded UCNPs for Cancer Theranostics: Physicochemical Properties, In Vitro Toxicity, and In Vivo Imaging Studies. Nanomaterials 2021, 11, 2234. [Google Scholar] [CrossRef]
- Rad, M.E.; Egil, A.C.; Ince, G.O.; Yuce, M.; Zarrabi, A. Optimization of curcumin loaded niosomes for drug delivery applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129921. [Google Scholar]
- Targhi, A.A.; Moammeri, A.; Jamshidifar, E.; Abbaspour, K.; Sadeghi, S.; Lamakani, L.; Akbarzadeh, I. Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: Enhanced antibacterial and anti-biofilm activities. Bioorganic Chem. 2021, 115, 105116. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Osei, E.; Fleck, A.; Darko, J.; Mutsaers, A.J.; Wettig, S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl. Nanosci. 2018, 8, 347–357. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.N.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Lakshmi, B.A.; Kim, S.; Kim, J. Synthesis and characterization of acetyl curcumin-loaded core/shell liposome nanoparticles via an electrospray process for drug delivery, and theranostic applications. Eur. J. Pharm. Biopharm. 2019, 142, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: An in vitro evaluation. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 349–358. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; Porfire, A. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol. Rep. 2018, 70, 331–339. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano formulation approaches for curcumin delivery-a review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Zwain, T.; Taneja, N.; Zwayen, S.; Shidhaye, A.; Palshetkar, A.; Singh, K.K. Albumin nanoparticles—A versatile and a safe platform for drug delivery applications. In Nanoparticle Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–358. [Google Scholar]
- Yao, K.; Chen, W.; Song, F.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic effects of photo-irradiation and curcumin-chitosan/alginate nanoparticles on tumor necrosis factor-alpha-induced psoriasis-like proliferation of keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, S.; Ma, M.; Wang, D.; Xu, Y. Encapsulation and delivery of curcumin in cellulose nanocrystals nanoparticles using pH-driven method. LWT 2022, 155, 112863. [Google Scholar] [CrossRef]
- Liang, S.; Du, J.; Hong, Y.; Cheng, L.; Gu, Z.; Li, Z.; Li, C. Octenyl succinate anhydride debranched starch-based nanocarriers for curcumin with improved stability and antioxidant activity. Food Hydrocoll. 2023, 135, 108118. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, Y.; Luo, S.; Mu, D.; Li, X.; Zhong, X.; Jiang, S.; Zheng, Z. Encapsulation of curcumin in soluble soybean polysaccharide-coated gliadin nanoparticles: Interaction, stability, antioxidant capacity, and bioaccessibility. J. Sci. Food Agric. 2022, 102, 5121–5131. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Schulte, Z.M.; Luiz, M.T.; Bento da Silva, P.c.; Frem, R.C.; Rosi, N.L.; Chorilli, M. Breast cancer targeting of a drug delivery system through postsynthetic modification of curcumin@ N3-bio-MOF-100 via click chemistry. Inorg. Chem. 2021, 60, 11739–11744. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Li, C.; Zhang, X.; Zhou, X.; Aramesh, N.; Zhou, H.; Jia, J. Recent advances in covalent organic frameworks for cancer diagnosis and therapy. Biomater. Sci. 2021, 9, 5745–5761. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.L.; Li, W.Y.; Li, Y.A.; Dong, Y.B. Covalent organic frameworks (COFs) for cancer therapeutics. Chem. Eur. J. 2020, 26, 5583–5591. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Wu, N.; Sun, L.; Yang, W. Covalent Organic Frameworks (COFs): A Necessary Choice For Drug Delivery. ChemistrySelect 2022, 7, e202202538. [Google Scholar] [CrossRef]
- Ali, I.; Ahmed, S.B.; Elhaj, B.M.; Ali, H.S.; Alsubaie, A.; Almalki, A.S. Enhanced anticancer activities of curcumin-loaded green gum acacia-based silver nanoparticles against melanoma and breast cancer cells. Appl. Nanosci. 2021, 11, 2679–2687. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C 2020, 112, 110853. [Google Scholar] [CrossRef]
- Mittal, L.; Camarillo, I.G.; Varadarajan, G.S.; Srinivasan, H.; Aryal, U.K.; Sundararajan, R. High-throughput, label-free quantitative proteomic studies of the anticancer effects of electrical pulses with turmeric silver nanoparticles: An in vitro model study. Sci. Rep. 2020, 10, 7258. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Q.; Guo, Q.; Huang, J.; Liu, L.; Shen, X.; Peng, J. AW/O emulsion mediated film dispersion method for curcumin encapsulated pH-sensitive liposomes in the colon tumor treatment. Drug Dev. Ind. Pharm. 2019, 45, 282–291. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Abdelfatah, E.A.; Khalil, W.A. Antitumor activity of curcumin-green synthesized gold nanoparticles: In vitro study. BioNanoScience 2019, 9, 813–820. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Y.; Wei, Y.; Gao, Y.; Jiang, W.; Wang, G.; Wang, D. Facile synthetic nano-curcumin encapsulated Bio-fabricated nanoparticles induces ROS-mediated apoptosis and migration blocking of human lung cancer cells. Process Biochem. 2020, 95, 91–98. [Google Scholar] [CrossRef]
- Balasubramanyam, M.; Koteswari, A.A.; Kumar, R.S.; Monickaraj, S.F.; Maheswari, J.U.; Mohan, V. Curcumin-induced inhibition of cellular reactive oxygen species generation: Novel therapeutic implications. J. Biosci. 2003, 28, 715–721. [Google Scholar] [CrossRef]
- Yang, K.; Liao, Z.; Wu, Y.; Li, M.; Guo, T.; Lin, J.; Li, Y.; Hu, C. Curcumin and Glu-GNPs induce radiosensitivity against breast cancer stem-like cells. BioMed Res. Int. 2020, 2020, 3189217. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, L.; Chen, S.; Zheng, B.; Chen, H.; Zeng, T.; Sun, H.; Zhong, S.; Wu, W.; Lin, X. The curcumin analogue WZ35 affects glycolysis inhibition of gastric cancer cells through ROS-YAP-JNK pathway. Food Chem. Toxicol. 2020, 137, 111131. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.; Fathima, H.; Prabhu, K.S.; Siveen, K.S.; Kulinski, M.; Azizi, F.; Dermime, S.; Ahmad, A. Curcumin-mediated apoptotic cell death in papillary thyroid cancer and cancer stem-like cells through targeting of the JAK/STAT3 signaling pathway. Int. J. Mol. Sci. 2020, 21, 438. [Google Scholar] [CrossRef]
- Beni, F.A.; Kazemi, M.; Dianat-Moghadam, H.; Behjati, M. MicroRNAs regulating Wnt signaling pathway in colorectal cancer: Biological implications and clinical potentials. Funct. Integr. Genom. 2022, 22, 1073–1088. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020, 689, 108412. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric nano-encapsulation of curcumin enhances its anti-cancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1α and nuclear p65 (REL A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Golonko, A.; Lewandowska, H.; Świsłocka, R.; Jasińska, U.; Priebe, W.; Lewandowski, W. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur. J. Med. Chem. 2019, 181, 111512. [Google Scholar] [CrossRef]
- Sudhesh Dev, S.; Zainal Abidin, S.A.; Farghadani, R.; Othman, I.; Naidu, R. Receptor tyrosine kinases and their signaling pathways as therapeutic targets of curcumin in cancer. Front. Pharmacol. 2021, 12, 772510. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Lee, M.K.; Kim, J.H. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009, 29, 5039–5044. [Google Scholar]
- Martínez-Castillo, M.; Villegas-Sepúlveda, N.; Meraz-Rios, M.A.; Hernández-Zavala, A.; Berumen, J.; Coleman, M.A.; Orozco, L.; Cordova, E.J. Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells. Oncol. Lett. 2018, 15, 6777–6783. [Google Scholar]
- Peng, Y.; Li, X.; Gu, P.; Cheng, W.; Zhang, R.; Hu, K. Curcumin-loaded zein/pectin nanoparticles: Caco-2 cellular uptake and the effects on cell cycle arrest and apoptosis of human hepatoma cells (HepG2). J. Drug Deliv. Sci. Technol. 2022, 74, 103497. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xie, Z.; Yu, S.; Li, L.; Xiao, H.; Song, Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2021, 203, 111759. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 2021, 252, 117180. [Google Scholar] [CrossRef]
- He, C.; Zhang, L.; Liu, W.; Huang, Y.; Hu, P.; Dai, T.; Xu, J.; Chen, Z. Albumin-based nanoparticles combined with photodynamic therapy enhance the antitumor activity of curcumin derivative C086. Dye. Pigment. 2021, 189, 109258. [Google Scholar] [CrossRef]
- Eslami, S.S.; Jafari, D.; Montazeri, H.; Sadeghizadeh, M.; Tarighi, P. Combination of curcumin and metformin inhibits cell growth and induces apoptosis without affecting the cell cycle in LNCaP prostate cancer cell line. Nutr. Cancer 2021, 73, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment. Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 97, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Gea-Banacloche, J.C. Immunomodulation. In Principles of Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2006; pp. 893–904. [Google Scholar]
- Afolayan, F.I.; Erinwusi, B.; Oyeyemi, O.T. Immunomodulatory activity of curcumin-entrapped poly d, l-lactic-co-glycolic acid nanoparticles in mice. Integr. Med. Res. 2018, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sa, G. Curcumin as an adjuvant to cancer immunotherapy. Front. Oncol. 2021, 11, 675923. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Saeed, B.Q.; Temirgalieva, E.; Yumashev, A.V.; El-Esawi, M.A.; Navashenaq, J.G.; Valizadeh, H.; Sadeghi, A.; Aslani, S.; Yousefi, M. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. 2021, 276, 119437. [Google Scholar] [CrossRef]
- O’Neill, R.E.; Cao, X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv. Cancer Res. 2019, 143, 145–194. [Google Scholar]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Role of vital trace elements in nanocurcumin-centered formulation: A novel approach to resuscitate the immune system. Biol. Trace Elem. Res. 2018, 182, 265–277. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef] [PubMed]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Fereidouni, M.; Pirro, M.; Bianconi, V.; Sahebkar, A. Modulation of regulatory T cells by natural products in cancer. Cancer Lett. 2019, 459, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Corbella Bagot, C.; Rappeport, E.; Ba Tis, T.; Park, W. Quantitative modeling and experimental verification of Förster resonant energy transfer in upconversion nanoparticle biosensors. J. Appl. Phys. 2021, 130, 023102. [Google Scholar] [CrossRef]
- Chaurasia, S.; Chaubey, P.; Patel, R.R.; Kumar, N.; Mishra, B. Curcumin-polymeric nanoparticles against colon-26 tumor-bearing mice: Cytotoxicity, pharmacokinetic and anticancer efficacy studies. Drug Dev. Ind. Pharm. 2016, 42, 694–700. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Durgaprasad, S.; Pai, C.G.; Alvres, J.F. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005, 122, 315. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Golombick, T.; Diamond, T.H.; Badmaev, V.; Manoharan, A.; Ramakrishna, R. The Potential Role of Curcumin in Patients with Monoclonal Gammopathy of Undefined Significance—Its Effect on Paraproteinemia and the Urinary N-Telopeptide of Type I Collagen Bone Turnover MarkerThe Role of Curcumin in MGUS. Clin. Cancer Res. 2009, 15, 5917–5922. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Weber, D.M.; Wang, M.; Giralt, S.A.; Thomas, S.K.; Alexanian, R.; Zhou, X.; Patel, P.; Bueso-Ramos, C.E.; Newman, R.A. Curcumin Downregulates NF-kB and Related Genes in Patients with Multiple Myeloma: Results of a Phase I/II Study. Blood 2007, 110, 1177. [Google Scholar] [CrossRef]
- Polasa, K.; Raghuram, T.; Krishna, T.P.; Krishnaswamy, K. Effect of turmeric on urinary mutagens in smokers. Mutagenesis 1992, 7, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Hastak, K.; Lubri, N.; Jakhi, S.; More, C.; John, A.; Ghaisas, S.; Bhide, S. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997, 116, 265–269. [Google Scholar] [CrossRef]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Anwar, S.K.; Elmonaem, S.N.A.; Moussa, E.; Aboulela, A.G.; Essawy, M.M. Curcumin nanoparticles: The topical antimycotic suspension treating oral candidiasis. Odontology 2023, 111, 350–359. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef]
- Patel, M.S.; Mandal, S.D.; Mandal, S.; Faldu, S.; Patel, J. Nasotransmucosal Delivery of Curcumin-Loaded Mucoadhesive Microemulsions for Treating Inflammation-Related CNS Disorders. Turk. J. Pharm. Sci. 2022, 19, 560. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.V.; Pawitan, J.A. Curcumin nanoformulation for pulmonary drug delivery. Res. J. Pharmacogn 2022, 9, 73–81. [Google Scholar]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Oğuz, K.K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C 2017, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A. Affimer tagged cubosomes: Targeting of carcinoembryonic antigen expressing colorectal cancer cells using in vitro and in vivo models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef] [PubMed]

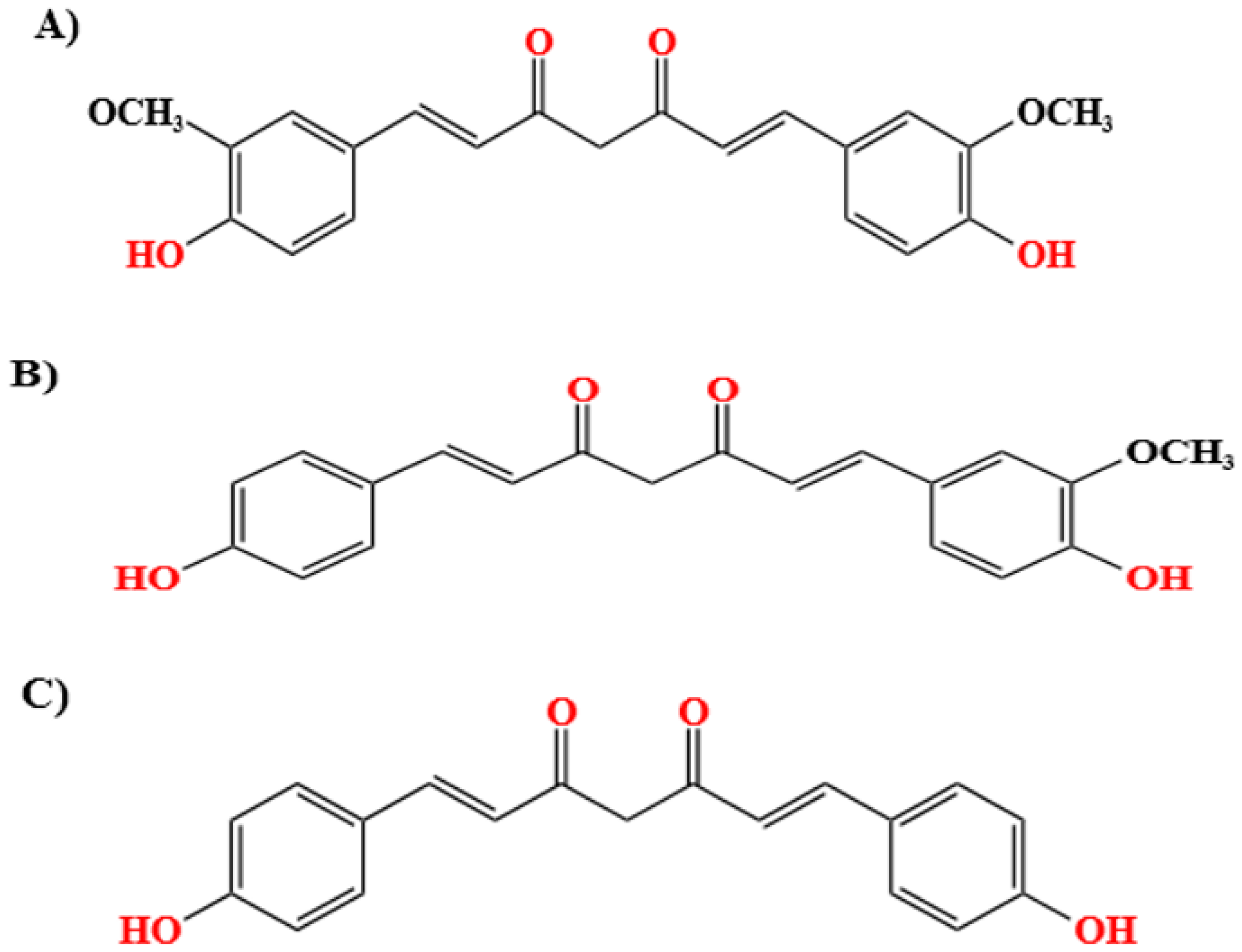


| Indigenous Practice | Curative Effect | Reference |
|---|---|---|
| A poultice of turmeric is applied to the perineum | The healing of any lacerations in the birth canal after childbirth | [3] |
| Powdered turmeric is taken with boiled milk | Cure cough and related respiratory ailments | [3] |
| Roasted turmeric | Given as an anti-dysenteric for children | [4] |
| Turmeric paste applied on bruises | Cures bruises and relieves pain | [5] |
| Turmeric decoction | Relieves
| [6] |
| Poultice of turmeric | Applied on
| [7] |
| ClinicalTrials.gov Identifier | Cancer Type | Phase of Study | Primary Purpose of the Study |
|---|---|---|---|
| NCT03769766 | Prostate cancer | Phase 3 | Treatment |
| NCT03980509 | Breast cancer | Phase 1 | Treatment |
| NCT01042938 | Breast cancer | Phase 2 | Treatment |
| NCT02439385 | Colorectal cancer | Phase 2 | Treatment |
| NCT03211104 | Prostate cancer | - | Treatment |
| NCT02724202 | Metastatic colon cancer | Early phase 1 | Treatment |
| NCT01160302 | Head and neck cancer | Early phase 1 | Basic science |
| NCT03847623 | Breast cancer | - | Other |
| NCT03072992 | Advanced breast cancer Metastatic breast cancer | Phase 2 | Treatment |
| NCT01859858 | Advanced colorectal cancer | Phase 1 | Basic Science |
| NCT01490996 | Colonic cancer metastasis | Phase 1,2 | Treatment |
| NCT01917890 | Prostate cancer radiation therapy | - | Supportive care |
| NCT00192842 | Pancreatic cancer | Phase 2 | Treatment |
| NCT03865992 | Breast cancer | - | Supportive Care |
| NCT01740323 | Breast cancer | Phase 2 | Treatment |
| NCT02321293 | Lung cancer | Phase 1 | Interventional |
| NCT04294836 | Cervical cancer, stage IIB | Phase 2 | Treatment |
| NCT00094445 | Pancreatic neoplasms, Adenocarcinoma | Phase 2 | Treatment |
| NCT01333917 | Colorectal cancer | Phase 1 | Interventional |
| NCT01294072 | Colon cancer | Phase 1 | Basic Science |
| NCT00027495 | Colorectal cancer | Phase 1 | Interventional |
| NCT02554344 | Cervical intraepithelial neoplasia | Early phase 1 | Treatment |
| NCT00295035 | Colon neoplasm | Phase 3 | Treatment |
| NCT02724618 | Prostate cancer | Phase 2 | Supportive Care |
| NCT02064673 | Prostate cancer | Phase 3 | Treatment |
| NCT04731844 | Prostate cancer | Phase 2 | Treatment |
| NCT03290417 | Prostate cancer | - | Treatment |
| NCT02598726 | Bladder spasm, malignant neoplasm | Phase 1 | Treatment |
| NCT02095717 | Prostate cancer | Phase 2 | Intervention |
| NCT01975363 | Atypical ductal breast hyperplasia | NA | Intervention |
| NCT01201694 | Advanced cancers | Phase 1 | Treatment |
| NCT02556632 | Breast carcinoma | Phase 2 | Supportive Care |
| NCT03192059 | Cervical, endometrial and uterine cancers | Phase 2 | Treatment |
| NCT02017353 | Endometrial carcinoma | Phase 2 | Treatment |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Experimental studies | In vivo studies |
| Publication in English | Other language publications |
| Full text | Publications with only abstracts |
| Effect of curcumin/nanocurcumin on cancer cells | Effect of curcumin/nanocurcumin on non-cancerous cells |
| Detailed data on the mode of therapy | Experiments that need extra data from the author |
| In vitro and clinical studies | |
| Functionalized NPs |
| Treatment | Type of Cancer | Phase of Study | Number of Patients | Outcome | Ref. |
|---|---|---|---|---|---|
| Curcumin, docetaxel | Metastatic breast cancer | Phase I | 14 | The feasibility, safety, and tolerability of the combination of curcumin and docetaxel therapy were established. Phase II trial in advanced and metastatic breast cancer patients started. | [120] |
| Curcumin capsules | Colorectal cancer | Phase I | 12 | Levels of MiG decreased | [121] |
| Curcumin combined with quercetin | Familial adenomatous polyposis developing into adenocarcinoma | Phase I | 5 | Polyps decreased after 6 months of combinational therapy. No appreciable toxicity was observed | [122] |
| Curcumin with piperine | Tropical pancreatitis | Phase I | 20 | Reduction in the erythrocyte MDA levels with a significant increase in GSH levels | [123] |
| Curcumin | Pancreatic cancer | Phase II | 25 | Down-regulation of NF–κB, COX-2, and pSTAT3 | [124] |
| Curcumin in combination with gemcitabine | Pancreatic cancer | Phase I/II | 21 | Curcumin dose (8 g/day) was observed as above the maximum tolerated dose when taken with gemcitabine with a modest efficacy | [125] |
| Docetaxel and curcumin | Advanced and metastatic breast cancer | Phase I | 14 | The maximum tolerable and recommended doses of curcumin were determined in combination with a a standard dose of docetaxel | [120] |
| Curcumin | Multiple myeloma | Phase I | 26 | Decreased levels of urinary N-telopeptide of type I collagen Bone turnover Marker | [126] |
| Phase I/II | - | Significant down-regulation of the constitutive activation of NF–κB, STAT3, and COX-2 expression | [127] | ||
| Turmeric | Lung cancer | Phase I | 16 | Anti-mutagenic effect | [128] |
| Turmeric oil and turmeric oleoresin | submucous fibrosis | Groups 1–15 Groups 2–22 Groups 3–21 | The potential of turmeric extract against micronuclei formation | [129] | |
| Curcumin | Urinary bladder cancer, arsenic-associated Bowen disease of the skin, uterine cervical intraepithelial neoplasm (CIN), oral leucoplakia, and intestinal metaplasia of the stomach | Phase I | 25 | Chemopreventive potential of curcumin against cancerous lesions. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundekkad, D.; Cho, W.C. Applications of Curcumin and Its Nanoforms in the Treatment of Cancer. Pharmaceutics 2023, 15, 2223. https://doi.org/10.3390/pharmaceutics15092223
Mundekkad D, Cho WC. Applications of Curcumin and Its Nanoforms in the Treatment of Cancer. Pharmaceutics. 2023; 15(9):2223. https://doi.org/10.3390/pharmaceutics15092223
Chicago/Turabian StyleMundekkad, Deepa, and William C. Cho. 2023. "Applications of Curcumin and Its Nanoforms in the Treatment of Cancer" Pharmaceutics 15, no. 9: 2223. https://doi.org/10.3390/pharmaceutics15092223
APA StyleMundekkad, D., & Cho, W. C. (2023). Applications of Curcumin and Its Nanoforms in the Treatment of Cancer. Pharmaceutics, 15(9), 2223. https://doi.org/10.3390/pharmaceutics15092223








