Abstract
Fluoxetine is the recommended first-line antidepressant in many therapeutic guidelines for children and adolescents. However, little is known about the relationships between drug dose and serum level as well as the therapeutic serum reference range in this age group. Within a large naturalistic observational prospective multicenter clinical trial (“TDM-VIGIL”), a transdiagnostic sample of children and adolescents (n = 138; mean age, 15; range, 7–18 years; 24.6% males) was treated with fluoxetine (10–40 mg/day). Analyses of both the last timepoint and all timepoints (n = 292 observations), utilizing (multiple) linear regressions, linear mixed-effect models, and cumulative link (mixed) models, were used to test the associations between dose, serum concentration, outcome, and potential predictors. The receiver operating curve and first to third interquartile methods, respectively, were used to examine concentration cutoff and reference values for responders. A strong positive relationship was found between dose and serum concentration of fluoxetine and its metabolite. Higher body weight was associated with lower serum concentrations, and female sex was associated with lower therapeutic response. The preliminary reference ranges for the active moiety (fluoxetine+norfluoxetine) were 208–328 ng/mL (transdiagnostically) and 201.5–306 ng/mL (depression). Most patients showed marked (45.6%) or minimal (43.5%) improvements and reported no adverse effects (64.9%). This study demonstrated a clear linear dose–serum level relationship for fluoxetine in youth, with the identified reference range being within that established for adults.
1. Introduction
Fluoxetine (FLX) was first characterized in a scientific journal in 1974 as a representative of a new class of antidepressant drugs [1,2]. In 1987, FLX was approved by the US Food and Drug Administration (FDA) as the first selective serotonin (5-hydroxytryptamine) reuptake inhibitor (SSRI) for the treatment of depression in adults [3]. It belongs to the most widely used class of antidepressants worldwide and is the most researched antidepressant in childhood and adolescence [4,5,6,7]. FLX is approved by the US FDA in treatment of minors for major depression (>8 years) and obsessive-compulsive disorder (OCD) (>7 years) and by the European Medicines Agency for treatment of minors aged 8 years or more who are suffering from moderate or severe depression [8,9]. A recent umbrella review shows that FLX is the only psychotropic drug studied that is superior to placebo in terms of primary outcome efficacy as well as response and remission in the treatment of depression in children and adolescents [10]. Hetrick et al. (2021) present a somewhat more reserved conclusion in a Cochrane Review and network meta-analysis, asserting that moderately strong evidence indicates FLX can be considered, along with other specific serotonin reuptake inhibitors (SSRIs), as the first choice for treatment of childhood and adolescent depression [4]. Additionally, evidence supports the use of FLX in children and adolescents, usually in combination with cognitive-behavioral therapy (CBT), for anxiety disorders and OCD [10]. Although the evidence is scarce for the effective use of FLX in treatment of bulimia nervosa and binge eating disorder in adolescents, off-label use is occurring [11,12].
FLX is a highly specific serotonin reuptake inhibitor with no or very low affinity to α- and β-adrenoceptors, histamine, and muscarinic receptors and, hence, fewer adverse effects than tricyclic antidepressants and especially fewer cardiovascular and anticholinergic side effects [13]. Although FLX is almost completely absorbed after oral administration, hepatic first-pass metabolism reduces its bioavailability to less than 90%. This medication has the largest volume of distribution of all SSRIs, but the relative concentration in the brain is lower compared to other SSRIs [14]. FLX also has a high protein binding rate of 94.5% [9].
Norfluoxetine (NORFLX) is the primary active metabolite and occurs after N-demethylation in first-pass hepatic metabolism, mainly by CYP2D6 [5]. Other isoenzymes, like CYP2C9 and CYP2C19, are involved to a lesser extent [5,13,14]. FLX is a potent inhibitor of CYP2D6 and thus inhibits its own metabolism. This can cause nonlinear kinetics with disproportionate increases in serum levels after dose escalation and is responsible for the long half-lives of FLX (4–6 days) and NORFLX (4–15 days) [15]. Additionally, the high binding affinity of FLX and NORFLX to CYP2D6 also causes numerous drug–drug interactions [5]. Under steady-state conditions, the serum levels of NORFLX are normally higher than those of the parent substance FLX [14]. Owing to the similar properties of the parent compound and its metabolite NORFLX, the sum of FLX and NORFLX serum concentrations as the “active moiety” is particularly relevant for therapeutic drug monitoring (TDM) [15].
A study by Meyer et al. (2004) suggests that, for SSRIs, 80% occupancy of serotonin transporters (5-HTT) is necessary for an expected treatment effect in depressive episodes in adults (aged 20–50 years). For FLX, this was achieved on average at a dose of 20 mg in adults [16]. The guideline-recommended and clinically standard dosage for FLX in children and adolescents with depressive disorders aged 8 years and older is a starting dose of 10 mg and a median dose of 20 mg [17,18]. Higher doses, usually up to 60 mg, are prescribed off-label, mainly for the treatment of other disorders such as bulimia nervosa and OCD [19].
About 40–60% of patients treated with FLX do not respond to pharmacologic monotherapy [20,21]. There are many possible influences moderating the effect of treatment with FLX. This is primarily due to pharmacokinetic and pharmacodynamic reasons. Polymorphisms of hepatic enzymes constitute an important class of pharmacokinetic factors that influence serum levels directly. For example, polymorphisms in CYP2D6 lead to different serum concentrations in adults and minors (though CYP2C9 and CYP2C19 polymorphisms seem to have little effect here) [22]. In European/Caucasian populations, 5–10% are poor metabolizers, and about 3% are ultrarapid metabolizers owing to their CYP2D6 genotype [23,24]. This can lead to significant interindividual variability and have an impact on the desired effect as well as possible adverse effects, even toxic effects. Regarding the toxicity of FLX, case reports of intoxications demonstrate relatively low toxicity compared to tricyclic antidepressants. Postmortem studies and case reports indicate values of 1300–7000 ng/mL for FLX serum levels and 400–4000 ng/mL for NORFLX in fatal overdoses [25,26]. For adults, a laboratory alert value of 1000 ng/mL for the active moiety is reported, which is twice the upper limit of the therapeutic reference range [15]. Overdosing may lead to adverse effects such as tachycardia or drowsiness. This can in some cases already occur at approximately 400 ng/mL for the active moiety with significant interindividual variation [27,28,29]. In minors it appears that the dosage per kg body weight is the most relevant factor. Females and adolescents (compared to males and adults, respectively), seem to be at higher risk for adverse effects of antidepressants, including SSRIs [30].
The goal in the treatment of patients is achieving the best possible risk–benefit balance. TDM makes a significant contribution to this objective, as it aims to decrease the frequency and duration of psychiatric episodes by improving the probability of the therapeutic effect through verifying serum levels are within the reference range [15]. Measurement of serum concentrations of the parent compound and its relevant metabolites at steady state allows dosage adjustments within a defined reference range for the best efficacy and lowest possible adverse effect risk. In children and adolescents, developmental pharmacokinetic and pharmacodynamic differences in drug metabolism can play a critical role, but TDM studies in minors are limited [31,32].
To date, only three studies have examined the dosage, serum concentration, and efficacy of FLX in children and adolescents [33,34,35]. There are divergent results on the relationship between dosage and serum concentration of NORFLX and the active moiety as well as on the relationship between serum levels and both therapeutic responses and adverse effects. However, one critical commonality of these studies is their relatively small sample sizes (n = 64 to 74).
Therefore, the present naturalistic multicentric study was undertaken to investigate the relationships between dosage, serum concentration, and its predictors in children and adolescents treated with FLX. Additionally, our goal was to identify a preliminary age- and indication-specific therapeutic range of the active moiety, for which we explored serum level associations with clinical effects and adverse effects.
2. Materials and Methods
2.1. Study Design and Population
This study is part of the “TDM-VIGIL” project, a large-scale, prospective, multicenter pharmacovigilance study conducted by the TDM-VIGIL consortium in partnership with child and adolescent psychiatric centers in Germany, Austria, and Switzerland [36]. The project was funded by the German Federal Institute of Drugs and Medical Devices (BfArM) and received approval from the ethics committee at the primary study center, University Hospital Wuerzburg (301/13), as well as from the local ethics committees of participating centers. This study was conducted in accordance with the Declaration of Helsinki and registered in the European Clinical Trials Database (EudraCT, 2013-004881-33). As the subjects were minors, the parents or guardians had to provide consent for their child to participate in the study. From the age of 14 onward, informed assent was also required from the patients themselves.
The study spanned three different routine healthcare settings, including inpatient, outpatient, and day-treatment units. Patients were included across diagnoses if they started treatment with FLX or if an existing treatment was switched to FLX. All dosing steps were recorded along with corresponding dates in the medication protocol. The steady state (after at least 3.3 half-lives in relation to the lower range limit) was confirmed by the treating physicians and documented in the patient’s medical records. The following assessment time points were incorporated into the study design: baseline (before starting FLX), after reaching the target dose and remaining at a steady state, at discharge from the treatment setting (or in outpatients, when the time interval until the next scheduled follow-up appointment was expected to be 6 months), a follow-up two weeks later, and a follow-up six months after the last steady-state assessment. Depending on the clinical course, additional visit times were possible. The actual timeline and number of visits varied among individual patients.
2.2. Patient Assessment
Experienced child and adolescent psychiatrists monitored the treatment course. They diagnosed patients according to the International Classification of Diseases, 10th Revision (ICD-10). Indication for FLX was assessed and recorded. A medical examination for each patient was conducted, which included checking vital signs, electrocardiography, and laboratory blood tests to assess hepatic and renal function.
On the day of blood collection, treatment responses were measured using the Clinical Global Impression (CGI) Scale (Improvement, CGI-I), which clinicians were instructed to rate solely based on the effects of the drug. The scale scores for global improvement were as follows: ‘very much improved’, ‘much improved’, ‘minimally improved’, ‘no change’, ‘minimally worse’, ‘much worse’, ‘very much worse’. To measure adverse reactions to FLX, the Pediatric Adverse Event Rating Scale (PAERS) was employed [37], as it is considered to be a valid and informative assessment tool for pharmacotherapy studies [15]. This instrument was specifically designed to quantify the severity of 56 signs of adverse events occurring in pediatric patients under treatment with a psychotropic drug. The 56 items were rated on a Likert scale with ‘none’, ‘slight’, ‘moderate’, ‘severe’, and ‘extremely severe’ responses. The maximal severity on any of the items was considered in this study. Only adverse effects related to FLX, and not to concurrent psychiatric or somatic medications, were included as relevant for this study. Additionally, indications for the TDM measurement, detailed information on concomitant medications, nicotine use, weight, and height were also recorded. Non-compliance was rated using a pre-defined rating schema (Supplementary S1), which was documented at the time of blood collection/serum concentration measurement.
2.3. Serum Concentration Analysis
TDM analysis was conducted according to the guidelines of the Working Group on Neuropsychopharmacology and Pharmacopsychiatry (AGNP) consensus [15]. Blood samples were collected from cubital veins into 7.5-mL monovettes without any additives or anticoagulants at a steady-state trough level. The samples were immediately analyzed (in the case of samples from Wuerzburg, Germany) or analyzed after being received by the TDM laboratory in Wuerzburg, Germany, shipped by regular mail. The concentrations of FLX and NORFLX were determined using an isocratic reversed-phase high-performance liquid chromatography method with UV-absorbance detection on an Agilent 1200 series system (Agilent Technologies Inc., Santa Clara, CA, USA), as described in detail in the Supplementary S2. Each analytical series included internal quality control samples, and external control samples were analyzed quarterly. The laboratory responsible for the analysis is certified by a quality control program (https://www.instand-ev.de, accessed on 2 July 2023; https://www.ukw.de/psychiatrie/zuweisende-kolleginnen-und-kollegen/tdm-labor/zertifikate/, accessed on 2 July 2023). Analytical grade chemicals and solvents were procured from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
For further information on the study design, see Egberts et al. (2022) [38].
2.4. Data Management and Statistical Analysis
All statistical analyses were performed using R (R Foundation for Statistical Computing) version 4.1.1. The alpha level for all analyses was set to 0.05. To maximize the comparability of the conclusions from the dataset generated in this study, the main analyses were performed with both the last valid timepoint, as in previous relevant studies [33,39], and with all the valid timepoints, as detailed in the next paragraph. Each analysis block was conducted separately for FLX, its active metabolite NORFLX, and the active moiety (FLX+NORFLX), which exhibited anticipated correlations with each other. First, the relationship between the daily dose of FLX (in mg) and serum concentrations (in ng/mL) were examined using linear regressions (the lm function). The model assumptions were verified by means of the semi-automated diagnostic functions in the ‘gvlma’ (Global Validation of Linear Models Assumptions) R package, including evaluation of global statistics, skewness, kurtosis, link function, and heteroscedasticity parameters and inspection of the related plots [40]. As not all the assumptions were met by the original data, a square-root transformation was applied to the outcome variables. In addition to the simple linear regressions, multiple linear regressions were performed with four additional hypothesized predictors: age (years), sex (male/female), body weight (kg), and smoking status (yes/no). The variance inflation factor (VIF) was evaluated as a measure of multicollinearity. Both regressions were compared based upon model fit. Second, proportional odds models using the clm (cumulative link models) function in the ‘ordinal’ R package were fitted to examine the serum concentrations as predictors of clinical effects (measured by CGI-I) and adverse effects (measured by PAERS—maximal severity). As there was only a single ‘very severe’ PAERS response, ‘very severe’ and ‘severe’ responses were pooled together. The analyses were repeated with an extended set of hypothesized predictors. For CGI-I, these were age, sex, psychotropic co-medication (i.e., antipsychotic and antidepressant drug), and diagnostic comorbidity (i.e., a single diagnosis versus multiple diagnoses). For PAERS, the predictors were age, sex, and psychotropic co-medication. The proportional odds assumptions in these statistics were verified with likelihood ratio tests using the nominal_test and scale_test functions, while model convergence was tested with the convergence function.
In the analyses of all datapoints, the lmer (linear mixed-effect model) function from the ‘lme4’ package was used to investigate the associations with daily dose of FLX and serum concentrations using square-root-transformed FLX, NORFLX, and FLX+NORFLX concentrations (separately) as outcome variables, dose as a fixed predictor, and subject ID as a random intercept. The clmm (cumulative link mixed models) function from the ‘ordinal’ package was used to test the impact of metabolite level on clinical and adverse effects with CGI-I and PAEARS (separately) as outcome variables, FLX, NORFLX, and FLX+NORFLX concentrations (separately) as fixed predictors, and subject ID as a random intercept. The model with multiple predictors included the variables, as in the single timepoint analyses, as well as the data collection time (i.e., days from the baseline timepoint).
To determine a possible concentration threshold of efficacy, receiver operating curve (ROC) analyses were performed based on a binary logistic regression, and the optimal cutoff was calculated by finding an optimal trade-off between sensitivity and specificity. The “good responders” and “poor/non-responders” groups were constructed by pooling the CGI-I responses of ‘very much improved’ and ‘much improved’ as “good responders” and ‘minimally improved’, ‘no change’, ‘minimally worse’, ‘much worse’, and ‘very much worse’ as “poor/non-responders”. Finally, we determined an effective concentration level range (i.e., reported for good clinical responders to FLX) as the mean ± one standard deviation and the 25th–75th interquartile range, which approximate the reference therapeutic range according to previous recommendations [41]. The last valid datapoints were included in these analyses as deemed most appropriate. Transdiagnostic responders and responders with depression (F32.X and F33.X) were considered separately.
The quality control criteria for data exclusion were the interval between blood collection and laboratory analysis exceeding 72 h, uncertain patient medication compliance noted by a clinician, and blood not being collected during steady-state conditions. In addition, anorexia nervosa patients with a body mass index (BMI) lower than the 10th percentile were also excluded.
3. Results
3.1. Descriptive Statistics
Study population characteristics expressed as means with standard deviations or counts with corresponding percentages are listed in Table 1. After excluding patients with data that did not meet the quality control criteria and/or missing data (missing dose, blood, or CGI/PAERS information), 138 patients were included in this study (mean age, 15 years; age range, 7–18; 34 males [24.6%]). The mean daily FLX dosage was 19.93 ± 5.30 mg (range, 10–40 mg), and the related mean steady-state serum concentration for FLX was 123.66 ± 65.17 ng/mL (range, 17–396 ng/mL), for NORFLX was 144.74 ± 65.93 ng/mL (range, 21–339, ng/mL), and for FLX+NORFLX was 264.21 ± 110.85 ng/mL (range, 62–673 ng/mL). Most patients responded with marked improvement (38.4% ‘much improved’ and 7.2% ‘very much improved’), followed by minimal improvement (43.5%). The majority reported no adverse effects (64.9%), followed by moderate (19.4%) and then slight (13.4%) adverse effects.

Table 1.
Demographic and clinical characteristics of the study sample.
The number of timepoints in the analyses including more than one observation ranged from two to four. There were between 292 and 281 observations in each of the analyses involving dosage and serum concentrations and between 216 and 205 observations in each of the analyses involving serum concentrations and clinical/adverse effects.
3.2. Dose and Metabolite Concentrations (Last Datapoints)
In the simple linear regressions (FLX, F1,136 = 16.84, p < 0.001, adj. R2 = 0.104; NORFLX, F1,132 = 19.07, p < 0.001, adj. R2 = 0.120; FLX+NORFLX, F1,136 = 22.34, p < 0.001, adj. R2 = 0.135) there was a positive association between dosage and serum concentrations (FLX, β = 0.18, CI 0.09–0.26, p < 0.001; NORFLX, β = 0.19, CI 0.10–0.27, p < 0.001; FLX+NORFLX, β = 0.24, CI 0.14–0.34, p < 0.001). Scatter plots showing these effects for the untransformed outcome variables are presented in Figure 1. In the multiple linear regressions, two predictors, dose and body weight, were significant (Table 2A). All VIF values were lower than 2, indicating there were no multicollinearity issues. All models with multiple predictors explained significantly more variance than the simple models (FLX, adj. R2M2−M1 = 0.153, SS = 188.0, F4,132 = 8.003, p < 0.001; NORFLX, adj. R2M2−M1 = 0.092, SS = 116.8, F4,128 = 4.857, p = 0.001; FLX+NORFLX, adj. R2M2−M1 = 0.156, SS = 272.46, F4,131 = 8.679, p < 0.001).
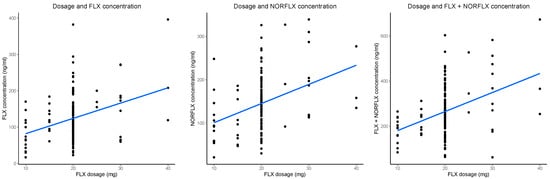
Figure 1.
Significant linear associations between fluoxetine (FLX) daily dose (x-axis) and steady-state serum concentrations of FLX, norfluoxetine (NORFLX), and the active moiety (FLX+NORFLX) (y-axis; from left to right). The blue diagonal line is the best-fit line. The black dots represent data points.

Table 2.
Analyses with serum concentrations and multiple predictors.
3.3. Dose and Metabolite Concentrations (All Datapoints)
Inclusion of all datapoints confirmed the findings from the last datapoint analysis showing a strong positive effect of dose on concentration (FLX, β = 0.31, CI 0.25–0.36, p < 0.001, marginal R2 = 0.285, conditional R2 = 0.562; NORFLX, β = 0.25, CI 0.20–0.30, p < 0.001, marginal R2 = 0.216, conditional R2 = 0.679; FLX+NORFLX, β = 0.38, CI 0.31–0.44, p < 0.001, marginal R2 = 0.311, conditional R2 = 0.548). In all three models with multiple predictors, the effects of dose and body weight were significant (Table 2B). In addition, there was a significant effect of time (positive direction) in the model including FLX and a significant effect of sex (maleness being a negative predictor) in the model including NORFLX.
3.4. Metabolite Concentrations and Clinical/Adverse Effects (Last Datapoints)
The simple ordinal regressions with CGI-I responses as outcome variables revealed no statistically significant effect for any of the three serum concentration parameters (all p > 0.184). Similarly, there were no significant results for PAERS as a measure of adverse reactions (all p > 0.481). Among the covariates, there was a significant effect of sex (being male was a positive predictor of clinical efficacy) in all three models with CGI-I (Table 3A,B).

Table 3.
Analyses with serum concentrations and clinical/adverse effects.
3.5. Metabolite Concentrations and Clinical/Adverse Effects (All Datapoints)
No significant effects were observed in the simple linear models involving CGI-I (all p > 0.169) or PAERS (all p > 0.469). In all three models including CGI-I with multiple predictors, there was a significant positive effect of maleness (Table 3C). No statistically significant effect of predictors were found in the models including PAERS (Table 3D).
To additionally explore the role of diagnosis, we entered five diagnostic categories as a predictor (i.e., affective disorders, anxiety disorders, obsessive-compulsive disorders, eating disorders, other disorders) in each of the above models. All the described effects remained significant with ‘other disorders’ being the only additionally significant diagnosis with reference to the affective disorder group (FLX: OR = 11.84, CI = 3.12–44.94, p < 0.001; NORFLX: OR = 10.57, CI = 2.97–37.63 p < 0.001; FLX+NORFLX: OR = 11.78, CI = 3.10–44.72, p < 0.001) in the model with PAERS with all datapoints. The same model with PAERS with the last point did not converge.
3.6. ROC Analysis and Effective Interquartile Ranges
The ROC analysis indicated unsatisfactory discrimination between poor (n = 74) and good responders (n = 64) transdiagnostically (FLX, area under the curve [AUC] = 0.491 [95% CI, 0.392–0.59]; NORFLX, AUC = 0.437 [95% CI, 0.338–0.535]; FLX+NORFLX, AUC = 0.427 [95% CI, 0.33–0.524]). Similar results were found between poor (n = 47) and good responders (n = 44) with a main diagnosis of depression (FLX, AUC = 0.496 [95% CI, 0.376–0.617]; NORFLX, AUC = 0.469 [95% CI, 0.347–0.591]; FLX+NORFLX, AUC = 0.451 [95% CI, 0.332–0.571]). The mean ± 1 standard deviation and 25th–75th interquartile concentration ranges for FLX, NORFLX, and FLX+NORFLX are listed in Table 4.

Table 4.
Serum concentrations for responders to fluoxetine.
4. Discussion
The aim of this study was to examine the relationships between dosage, serum concentration, and its predictors in children and adolescents treated with FLX. We also explored serum level associations with clinical/adverse effects and determined a tentative age-/indication-specific therapeutic range of the active moiety. The results indicated a significant association between the FLX dose and the serum levels of FLX, NORFLX, and the active moiety, which was observed for the last timepoint and was even more pronounced when considering all serum level datapoints collected. Body weight was significantly and inversely associated with the serum concentrations in both models (holding other predictors constant). In terms of efficacy, there was no significant relationship between serum levels of FLX, NORFLX, or the active moiety and therapeutic effect as assessed by CGI-I. Among the variables considered, females showed lower odds of clinical effect (holding other predictors constant). In terms of adverse effects measured by PAERS, there was no significant association with serum levels, and no other tested variable was found to be a significant predictor.
The observed associations between daily FLX dose and serum concentrations of FLX, NORFLX, and the active moiety together with the impact of body weight are in accordance with a study by Blázquez et al. (2014) [34] in adolescents and with a study by Lundmark et al. (2001) in adults [42]. Koelch et al. (2012) observed a positive correlation between the dose of FLX per kg of body weight and serum levels of FLX but not for NORFLX and the active moiety [33]. Other study results in adults revealed a similar but age-dependent heterogeneous pattern [43]. A possible explanation for these inconsistencies could be phenoconversion. Specifically, drug-induced altered enzyme activity modifies the genetically determined metabolism. The inhibition of CYP2D6 enzyme activity by FLX impairs its own metabolism and may lead to an increased FLX-to-NORFLX ratio [43,44,45]. In about 20–30% of patients taking an enzyme inhibitor of CYP2D6, such as FLX, this leads to phenoconversion to poor metabolizer status [46]. Influencing factors appear to include genetic vulnerability, dosage, and length of drug exposure [44]. While dosage and duration of exposure were considered in most statistical analyses of the mentioned studies, no conclusion can be drawn regarding genetic vulnerability.
Koelch et al. observed a lower serum level for the active moiety and NORFLX in smokers [31]. This was not replicated in our data, but it is worth noting that the proportion of smokers was considerably lower in our study (5.7% regular smokers versus 20% in the study by Koelch and colleagues), likely obscuring such a possible effect. Notably, a study of adults including a large proportion of smokers (24%) also found no difference regarding the active moiety or NORFLX serum levels [42]. Blázquez et al. did not include smoking in their analyses [32]. Nicotine is not a known general inducer or inhibitor of CYP2D6 and thus is not expected to affect serum concentrations of FLX through known mechanisms [47]. Only in the brain does nicotine appear to induce CYP2D6 expression [48].
Consistent with other TDM studies on FLX conducted in this age group, we did not find any age effect. Many factors determining pharmacokinetics mature within the first two years of life [49]. Interindividual genetic variation in CYP2D6 activity also seems to have more influence on its activity than developmental aspects in late childhood and adolescence [50]. This may explain why no effect of age was found in the present study population of individuals aged 7–18 years, which comprised predominantly adolescents.
In our data, males tended to show lower levels of NORFLX, which may be related to the higher average CYP2D6 activity in females [51]. Koelch et al. found no evidence that sex exerts a moderating influence on serum concentration of FLX and its metabolite NORFLX [31]. However, Blázquez et al. in minors and Amsterdam et al. in adults found higher FLX, NORFLX, and the active moiety concentrations in females [34,52]. There is convincing evidence of pharmacokinetic differences between the sexes, for example, related to the volume of distribution and drug clearance. This often leads to higher serum concentrations in females [51,53]. The heterogeneous findings are probably owing to the numerous influencing factors, such as genetic polymorphism in CYP2D6 and interindividual pharmacokinetic differences, which were not controlled in these studies.
The reference range calculated in our study for responders with depression was 201.5–306 ng/mL, which is within the reference range for adults (120–500 ng/mL) [15]. When all diagnostic groups were considered, the range was slightly higher (208–328 ng/mL).
In line with other comparable studies, there was no association of therapeutic response with serum levels of FLX, NORFLX, or the active moiety in our study. The lack of an association between FLX treatment plasma levels and therapeutic response has been repeatedly observed both in adults and children and adolescents [33,34,52]. A recent meta-analysis also suggests that no such association exists for other SSRIs. In addition, for adults, high doses of FLX (60 mg/day) were shown to be less effective than 20 mg/day [54]. A key exception is reported by Sakolsky et al. (2011), who observed a non-significant trend in adolescents (12–18 years) for a higher likelihood of response to FLX when patients had serum concentrations above the geometric mean compared with those who were below it, as well as a significantly higher likelihood of response when the dose was increased, thus raising serum concentrations from below the geometric mean to above it [35]. Small samples, a lack of systematic and detailed symptom assessments, and several other confounders may explain the failure to detect an association between dose or serum level and response in the vast majority of studies.
First, genetic variants may influence the efficacy of SSRIs in treating major depression and other psychiatric disorders, as exemplified by a polymorphism of the SCL6A4 gene that encodes the sodium-dependent serotonin transporter (5-hydroythyptamine transporter). Although study results are heterogeneous, there is evidence that different polymorphisms are associated with response to FLX and other SSRIs or treatment failure [55,56]. Studies of anxiety disorders and obsessive-compulsive disorders in addition to depression have also shown that other genetic variants in genes encoding both enzymes involved in serotonin biosynthesis (tryptophan hydroxylase, TPH2) and serotonin receptors (5-hydroxytryptamine-receptor, HTR1B) also have an impact on clinical outcomes in children and adolescents [57]. For the ABCB1 gene, which encodes P-glycoprotein, a protein that transports harmful substances out of the cell to protect it, there is also evidence that polymorphisms influence the therapeutic effect of FLX in children and adolescents [22,58].
Second, in study populations with depressive disorders in particular, placebo responders, verum responders, and non-responders tend to each comprise a third of all subjects. This results in a low signal-to-noise ratio and blurs the true concentration–response association [41]. A meta-analysis showed that to detect the effect of SSRIs administered in childhood and adolescence, the number of subjects needed to treat in randomized controlled trials is nine on average and higher in younger patients [59].
Third, as argued by Meyer et al. (2004) based on positron emission tomography imaging studies, observed saturation dynamics of inhibition of the serotonin transporter suggest a nonlinear concentration–response relationship rather than a linear relationship [16]. However, this is not supported by our results. Our ROC analysis showed no evidence of a FLX concentration threshold differentiating good responders from poor responders. Nevertheless, 80% saturation is already achieved at a dose of 20 mg, which was usually the targeted dose in our study.
Notably, our results also indicated higher odds of males (versus females) benefiting from FLX in terms of clinical improvement, which contrasts with the available literature [60,61]. Although there are several studies that suggest males are more likely to benefit from tricyclic antidepressants than females, for SSRIs, either no difference or even a better response in females, especially among those of reproductive age, was found [61,62,63]. Therefore, our finding that being male is a significant predictor of clinical response to SSRIs is not easily interpretable. One consideration was that the significant proportion of female patients with anorexia (n = 13) could potentially may have had an influence here, even though we excluded underweight female patients. However, the exploratory analysis with diagnostic groups as predictors in the models did not yield a significant result. Pharmacokinetic differences have been suggested as a mechanism causing sex differences in efficacy in general [61]. However, we observed a trend-level effect for a smaller increase in serum levels in males compared to females only for NORFLX, but this would not explain the observed superior effectiveness.
Overall, there is no conclusive prior data analysis demonstrating a difference between sexes in response to SSRIs. Presumably, numerous possible confounders play some role in our results (e.g., disease severity, adherence, and pharmacokinetic as well as pharmacodynamic sex differences) [61].
Like Koelch et al. and Blázquez et al., we could not find an association between serum levels and adverse effects [33,34]. In general, there is compelling evidence that FLX administered for the treatment of depression is well tolerated compared with other antidepressants in childhood and adolescence [7,10]. In our study, the majority of participants reported no adverse effects (64.9%).
The strengths of this study include its extensive analytic strategy maximizing the use of data as well as its large sample size compared to similar prior studies. Participants were recruited from everyday clinical practice through a multicenter approach, making the patient sample representative of real-world clinical care. The study adhered to quality criteria for TDM studies, including using serum analyses and collecting blood samples under steady-state conditions to establish a therapeutic reference range. This was calculated for all diagnoses and separately for the approved indication of depression. In addition, conservative exclusion criteria were applied to avoid data bias, e.g., exclusion of underweight patients.
The results of this study should be considered through the lens of its limitations. First, this is a naturalistic study conducted under some uncontrolled conditions, with a flexible dosing strategy and the inclusion of patients with concomitant psychotropic and somatic medications. While psychotropic co-medication was taken into account for efficacy assessment, influences on the therapeutic response that depend on the target symptomatology cannot be excluded. Second, we did not consider co-medication for the dose-serum concentration correlations. An effect here would have been expected only from drugs known to interact with CYP2D6, and the group of patients with corresponding co-medication was too small (n = 2) to influence the findings substantially. Third, participants were included for whom prior treatment with another psychotropic drug was switched to FLX owing to a lack of response. This resulted in a heterogeneous group with varying degrees of clinical severity and response status, which complicated the identification of a possible relationship between medication and therapeutic response, which may have additionally been hampered by the use of a broad transdiagnostic instrument like the CGI-I. Fourth, in this naturalistic observational study, patients from all diagnostic groups were included. Although the identified reference range for depression did not significantly differ from the transdiagnostic range, due to the typically distinct neurobiological etiology of each disorder, only diagnosis-specific statements can be made. Fifth, enantiomers were not differentiated in the study, despite the considerable interindividual variation revealed by previous research [64]. Sixth, due to clinical practicability, the prompt detection of potential poor metabolizers, and the capture of serum levels for early treatment effects within the first two weeks, we opted for the earliest possible time point as steady state [65]. It is important to note that this choice could potentially result in reference ranges that are rather underestimated. Seventh, statistical modeling of multi-level data from a naturalistic study, which prioritizes the clinical objectives and leaves some factors less controllable or even unmeasured, can be a challenging task. In general, the goodness-of-fit indices for our more complex models were relatively low. Future studies should explore additional explanatory variables and employ different data collection strategies. Finally, there was no rigorous control of medication adherence, although practitioner assessments were collected for each patient to mitigate this issue.
5. Conclusions
This study reinforces recent evidence of a linear relationship between FLX dosing and serum concentrations of FLX, NORFLX, and the active moiety in children and adolescents. It also elucidates some other clinical variables, which may be of relevance in this age group. Given the inverse association between weight and serum concentrations, in further studies over- or underweight status should be considered as an influencing factor that may require dose adjustment. The lack of significant associations between serum concentration and clinical/adverse effects, as shown in numerous naturalistic studies, may be owing to uncontrolled confounders and a low signal-to-noise ratio, as well as complex interactions and genetic polymorphisms that influence pharmacodynamics. More sophisticated study designs are needed to isolate possible differential contributions to clinical response to antidepressants in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15092202/s1, Supplement S1: Rating scheme adherence. Supplement S2: Methods TDM laboratory FLX and NORFLX.
Author Contributions
Conceptualization and methodology, M.G., K.M.E., M.R., P.H. and U.M.; performing und evaluating the laboratory measurements, S.U., R.T., S.F. and K.M.E.; data analysis, L.S. and M.F.; resources, S.W. and M.R.; data curation, H.R.; writing—original draft preparation, M.F. and L.S.; writing—review and editing, M.F., L.S. and K.M.E.; visualization, L.S.; supervision, K.M.E.; project administration, M.R. and K.M.E.; funding acquisition, K.M.E., R.T., P.L.P. and M.R. Recruiting of patients, W.B., C.U.C., C.F., T.H., E.T., S.K., L.W., M.B., H.I., A.K., K.R., T.R., J.M.F., S.-Y.R.-D., C.R., T.B., M.K. (Michael Kaess), M.K. (Michael Kölch), P.L.P., G.S.-K., C.W., S.W. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a research grant from the German Federal Institute for Drugs and Medical Devices (BfArM, reference number, V-15322/68605/2013-2018). The patient registry of the ‘Competence Network on Therapeutic Drug Monitoring in Child and Adolescent Psychiatry’ was additionally supported by the German Federal Ministry of Education and Research (BMBF-FKZ: 001EZ0937) and the ‘Verein zur Durchführung Neurowissenschaftlicher Tagungen e.V.
Institutional Review Board Statement
This study received approval from the ethics committee at the primary study center, University Hospital Wuerzburg (301/13), as well as from the local ethics committees of participating centers. This study was conducted in accordance with the Declaration of Helsinki and registered in the European Clinical Trials Database (EudraCT, 2013-004881-33).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. As the study involved minors, the parents or legal guardians had to agree to participate in the study. From the age of 14, informed assent was also required from the patients themselves.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because these are sensitive health patient data.
Acknowledgments
Gisela Antony, CIO Marburg GmbH. Jürgen Deckert and the staff of the TDM laboratory, Center of Mental Health, University Hospital Wuerzburg.
Conflicts of Interest
K.E., R.T., M.R., M.G. and P.P. received grant research support from BfArM. MR currently receives a research grant from Kids-Safe, Innovation Committee of the German Federal Joint Committee (G-BA grant number 01NVF16021). P.P. receives grant research support from the German Federal Ministry of Education and Research (BMBF) and was involved in clinical trials from Servier and Lundbeck; he received an advisor honorarium from Boehringer Ingelheim and speaker’s honoraria from Shire, Infectopharm, and Gerot Lannach. C.C. has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Sage, Supernus, Tolmar, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, PsiloSterics, and Quantic. T.B. received personal fees from serving as an advisor or consultant to eyelevel, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche, and Takeda; received conference attendance support, conference support, or speaking fees from Janssen, Medice, and Takeda; and received royalties from Hogrefe, Kohlhammer, CIP-Medien, and Oxford University Press. J.M.F. received research funding in the last five years from European Union, BMG (Federal Ministry of Health), BMBF (Federal Ministry of Education and Research), BMFSFJ (Federal Ministry of Family, Senior Citizens, Women and Youth), DFG (German Research Foundation), G-BA Innovation Fund, State Ministries of Baden-Württemberg and Saarland, State Foundation Baden-Württemberg, Porticus Foundation, and Evangelical-Lutheran Church in Württemberg. He also received travel grants, honoraria, sponsorship for conferences, and medical educational purposes from APK, Adenauer- and Ebertstiftung, Deutschlandfunk, DFG, DJI, DKSB, Infectopharm, med update, UNICEF, professional associations, universities and federal, and state ministries and was a consultant for APK, federal and state ministries industry-sponsored lecture series but has no shareholdings and no participation in pharmaceutical companies. Every grant and every honorarium was declared to the law office of the University Hospital Ulm. M.K (Michael Kölch). receives royalties from publishing companies as an author of books. He served as PI or CI in clinical trials for Lundbeck, Pascoe, and Janssen-Cilag. He served as scientific advisor for Janssen. The present work is unrelated to the above grants and relationships. S.W. has received in the last 5 years royalties from Thieme, Hogrefe, Kohlhammer, Springer, and Beltz. Her work was supported in the last 5 years by the Swiss National Science Foundation, different EU FP7s programs, Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, BfArM, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann, Vontobel, Unicentia, Erika Schwarz Fonds, and Gesundheitsforderung Schweiz. M.F. received royalties from Kohlhammer and Elsevier. The other authors. (L.S., E.T., L.S., C.W., A.K., K.R., U.M., S.U., S.K., M.B., L.W., P.H., W.B., C.F., T.H., H.I., M.K. (Michael Kaess), T.R., C.R., G.S.-K. and S.F.) declare no conflict of interest.
References
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.W.; Perry, K.W.; Molloy, B.B. Effect of an uptake inhibitor on serotonin metabolism in rat brain: Studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140). Life Sci. 1974, 15, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, S.E.; McKenzie, J.E.; Bailey, A.P.; Sharma, V.; Moller, C.I.; Badcock, P.B.; Cox, G.R.; Merry, S.N.; Meader, N. New generation antidepressants for depression in children and adolescents: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, CD013674. [Google Scholar]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the mechanism of fluoxetine-mediated CYP2D6 inhibition. Pharmaceutics 2021, 13, 148. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Eaton, L. European Agency Approves Use of Fluoxetine for Children and Teens. Available online: https://www-bmj-com.emedien.ub.uni-muenchen.de/content/332/7555/1407.2.short (accessed on 24 October 2022).
- Food and Drug Administration. Fluoxetine-Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf (accessed on 3 April 2023).
- Correll, C.U.; Cortese, S.; Croatto, G.; Monaco, F.; Krinitski, D.; Arrondo, G.; Ostinelli, E.G.; Zangani, C.; Fornaro, M.; Estradé, A. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: An umbrella review. World Psychiatry 2021, 20, 244–275. [Google Scholar] [CrossRef]
- Hagan, K.E.; Walsh, B.T. State of the art: The therapeutic approaches to Bulimia Nervosa. Clin. Ther. 2021, 43, 40–49. [Google Scholar] [CrossRef]
- Duan, H.; Zhu, L.; Li, M.; Zhang, X.; Zhang, B.; Fang, S. Comparative efficacy and acceptability of selective serotonin reuptake inhibitor antidepressants for binge eating disorder: A network meta-analysis. Front. Pharmacol. 2022, 13, 949823. [Google Scholar] [CrossRef]
- Cheer, S.M.; Goa, K.L. Fluoxetine: A review of its therapeutic potential in the treatment of depression associated with physical illness. Drugs 2001, 61, 81–110. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Härtter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [PubMed]
- Meyer, J.H.; Wilson, A.A.; Sagrati, S.; Hussey, D.; Carella, A.; Potter, W.Z.; Ginovart, N.; Spencer, E.P.; Cheok, A.; Houle, S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C] DASB positron emission tomography study. Am. J. Psychiatry 2004, 161, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Luxton, R.; Kyriakopoulos, M. Depression in children and young people: Identification and management NICE guidelines. Arch. Dis. Child.-Educ. Pract. 2022, 107, 36–38. [Google Scholar] [CrossRef]
- Dolle, K.; Schulte-Körne, G. The treatment of depressive disorders in children and adolescents. Dtsch. Ärzteblatt Int. 2013, 110, 854. [Google Scholar] [CrossRef][Green Version]
- Jannini, T.B.; Lorenzo, G.D.; Bianciardi, E.; Niolu, C.; Toscano, M.; Ciocca, G.; Jannini, E.A.; Siracusano, A. Off-label uses of selective serotonin reuptake inhibitors (SSRIs). Curr. Neuropharmacol. 2022, 20, 693–712. [Google Scholar] [CrossRef]
- March, J.S.; Vitiello, B. Clinical messages from the treatment for adolescents with depression study (TADS). Am. J. Psychiatry 2009, 166, 1118–1123. [Google Scholar] [CrossRef]
- Hetrick, S.E.; McKenzie, J.E.; Merry, S.N. The use of SSRIs in children and adolescents. Curr. Opin. Psychiatry 2010, 23, 53–57. [Google Scholar] [CrossRef]
- Gassó, P.; Rodríguez, N.; Mas, S.; Pagerols, M.; Blázquez, A.; Plana, M.; Torra, M.; Lázaro, L.; Lafuente, A. Effect of CYP2D6, CYP2C9 and ABCB1 genotypes on fluoxetine plasma concentrations and clinical improvement in children and adolescent patients. Pharmacogenomics J. 2014, 14, 457–462. [Google Scholar] [CrossRef]
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Klein, T.; Leeder, J.S. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 2017, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.D. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Barbey, J.T.; Roose, S.P. SSRI safety in overdose. J. Clin. Psychiatry 1998, 59, 42. [Google Scholar] [PubMed]
- Reis, M.; Aamo, T.; Ahlner, J.; Druid, H. Reference concentrations of antidepressants. A compilation of postmortem and therapeutic levels. J. Anal. Toxicol. 2007, 31, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Brent, J.; Kulig, K.; Heiligenstein, J.; Birkett, M.; Group, A.S. Fluoxetine versus tricyclic antidepressants: A prospective multicenter study of antidepressant drug overdoses. J. Emerg. Med. 1997, 15, 439–445. [Google Scholar] [CrossRef]
- Borys, D.J.; Setzer, S.C.; Ling, L.J.; Reisdorf, J.J.; Day, L.C.; Krenzelok, E.P. The effects of fluoxetine in the overdose patient. J. Toxicol. Clin. Toxicol. 1990, 28, 331–340. [Google Scholar] [CrossRef]
- Borys, D.J.; Setzer, S.C.; Ling, L.J.; Reisdorf, J.J.; Day, L.C.; Krenzelok, E.P. Acute fluoxetine overdose: A report of 234 cases. Am. J. Emerg. Med. 1992, 10, 115–120. [Google Scholar] [CrossRef]
- Barthez, S.; Revet, A.; Chouchana, L.; Jonville-Bera, A.-P.; Pizzoglio, V.; Raynaud, J.-P.; Chebane, L.; Lapeyre-Mestre, M.; Montastruc, F. Adverse drug reactions in infants, children and adolescents exposed to antidepressants: A French pharmacovigilance study. Eur. J. Clin. Pharmacol. 2020, 76, 1591–1599. [Google Scholar] [CrossRef]
- Gerlach, M.; Egberts, K.; Dang, S.-Y.; Plener, P.; Taurines, R.; Mehler-Wex, C.; Romanos, M. Therapeutic drug monitoring as a measure of proactive pharmacovigilance in child and adolescent psychiatry. Expert Opin. Drug Saf. 2016, 15, 1477–1482. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Saracino, M.A.; Raggi, M.A. Selective serotonin reuptake inhibitors (SSRIs): Therapeutic drug monitoring and pharmacological interactions. Curr. Med. Chem. 2012, 19, 1846–1863. [Google Scholar] [CrossRef]
- Koelch, M.; Pfalzer, A.-K.; Kliegl, K.; Rothenhöfer, S.; Ludolph, A.; Fegert, J.M.; Burger, R.; Mehler-Wex, C.; Stingl, J.; Taurines, R. Therapeutic drug monitoring of children and adolescents treated with fluoxetine. Pharmacopsychiatry 2012, 45, 72–76. [Google Scholar] [CrossRef]
- Blázquez, A.; Mas, S.; Plana, M.T.; Gassó, P.; Méndez, I.; Torra, M.; Arnaiz, J.A.; Lafuente, A.; Lázaro, L. Plasma fluoxetine concentrations and clinical improvement in an adolescent sample diagnosed with major depressive disorder, obsessive-compulsive disorder, or generalized anxiety disorder. J. Clin. Psychopharmacol. 2014, 34, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sakolsky, D.J.; Perel, J.M.; Emslie, G.J.; Clarke, G.N.; Wagner, K.D.; Vitiello, B.; Keller, M.B.; Birmaher, B.; Asarnow, J.R.; Ryan, N.D. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J. Clin. Psychopharmacol. 2011, 31, 92. [Google Scholar] [CrossRef] [PubMed]
- Egberts, K.; Plener, P.; Malzhan, U.; Taurines, R.; Reuter-Dang, S.; Gerlach, M. Sicherheit von psychopharmaka bei kindern und jugendlichen in der klinischen praxis–Erkenntnisse einer prospektiven studie. Bull. Zur Arzneimittelsicherheit 2020, 3, 4–10. [Google Scholar]
- March, J.; Karayal, O.; Chrisman, A. CAPTN: The pediatric adverse event rating scale. In Proceedings of the The Scientific Proceedings of the 2007 Annual Meeting of the American Academy of Child and Adolescent Psychiatry, Boston, MA, USA, 23–28 October 2007; pp. 23–28. [Google Scholar]
- Egberts, K.M.; Gerlach, M.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P.; Unterecker, S.; Scherf-Clavel, M.; Rock, H.; Antony, G. Serious adverse drug reactions in children and adolescents treated on-and off-label with antidepressants and antipsychotics in clinical practice. Pharmacopsychiatry 2022, 55, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tini, E.; Smigielski, L.; Romanos, M.; Wewetzer, C.; Karwautz, A.; Reitzle, K.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P. Therapeutic drug monitoring of sertraline in children and adolescents: A naturalistic study with insights into the clinical response and treatment of obsessive-compulsive disorder. Compr. Psychiatry 2022, 115, 152301. [Google Scholar] [CrossRef]
- Peña, E.A.; Slate, E.H. Global validation of linear model assumptions. J. Am. Stat. Assoc. 2006, 101, 341–354. [Google Scholar] [CrossRef]
- Hiemke, C. Concentration-Effect Relationships of Psychoactive Drugs and the Problem to Calculate Therapeutic Reference Ranges. Ther. Drug Monit. 2019, 41, 174–179. [Google Scholar] [CrossRef]
- Lundmark, J.; Reis, M.; Bengtsson, F. Serum concentrations of fluoxetine in the clinical treatment setting. Ther. Drug Monit. 2001, 23, 139–147. [Google Scholar] [CrossRef]
- Reis, M.; Aamo, T.; Spigset, O.; Ahlner, J. Serum concentrations of antidepressant drugs in a naturalistic setting: Compilation based on a large therapeutic drug monitoring database. Ther. Drug Monit. 2009, 31, 42–56. [Google Scholar] [CrossRef]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.F.; Gaedigk, A.; Ereshefsky, L.; Alfaro, C.L.; Simpson, J. CYP2D6 inhibition by selective serotonin reuptake inhibitors: Analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Cicali, E.J.; Wiisanen, K. The importance of phenoconversion when using the CYP2D6 genotype in clinical practice. Pharmacogenomics 2022, 23, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Sychev, D.A.; Ashraf, G.M.; Svistunov, A.A.; Maksimov, M.L.; Tarasov, V.V.; Chubarev, V.N.; Otdelenov, V.A.; Denisenko, N.j.P.; Barreto, G.E.; Aliev, G. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des. Dev. Ther. 2018, 12, 1147–1156. [Google Scholar] [CrossRef]
- Miksys, S.; Tyndale, R. Nicotine induces brain CYP enzymes: Relevance to Parkinson’s disease. Park. Dis. Relat. Disord. 2006, 70, 177–180. [Google Scholar]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef]
- Leeder, J.S.; Gaedigk, A.; Wright, K.J.; Staggs, V.S.; Soden, S.E.; Lin, Y.S.; Pearce, R.E. A longitudinal study of cytochrome P450 2D6 (CYP2D6) activity during adolescence. Clin. Transl. Sci. 2022, 15, 2514–2527. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Amsterdam, J.; Fawcett, J.; Quitkin, F.; Reimherr, F.; Rosenbaum, J.; Michelson, D.; Hornig-Rohan, M.; Beasley, C. Fluoxetine and norfluoxetine plasma concentrations in major depression: A multicenter study. Am. J. Psychiatry 1997, 154, 963–969. [Google Scholar]
- Unterecker, S.; Riederer, P.; Proft, F.; Maloney, J.; Deckert, J.; Pfuhlmann, B. Effects of gender and age on serum concentrations of antidepressants under naturalistic conditions. J. Neural Transm. 2013, 120, 1237–1246. [Google Scholar] [CrossRef]
- Braun, C.; Adams, A.; Rink, L.; Bschor, T.; Kuhr, K.; Baethge, C. In search of a dose–response relationship in SSRIs—A systematic review, meta-analysis, and network meta-analysis. Acta Psychiatr. Scand. 2020, 142, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.; Mas, S.; Plana, M.T.; Lafuente, A.; Lázaro, L. Fluoxetine pharmacogenetics in child and adult populations. Eur. Child Adolesc. Psychiatry 2012, 21, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Siqueira, J.; Silva, C.; Ferreira, E.; Silva, I. 5HTTLPR genetic variant and major depressive disorder: A review. Genes 2020, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Blázquez, A.; Rodríguez, N.; Boloc, D.; Lafuente, A.; Arnaiz, J.A.; Lázaro, L.; Gassó, P. Pharmacogenetic study focused on fluoxetine pharmacodynamics in children and adolescent patients: Impact of the serotonin pathway. Pharmacogenetics Genom. 2016, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Alves, G.; Fortuna, A.; Llerena, A.; Falcão, A. Pharmacogenetics and therapeutic drug monitoring of fluoxetine in a real-world setting: A PK/PD analysis of the influence of (non-) genetic factors. Exp. Clin. Psychopharmacol. 2020, 28, 589. [Google Scholar] [CrossRef]
- Tsapakis, E.M.; Soldani, F.; Tondo, L.; Baldessarini, R.J. Efficacy of antidepressants in juvenile depression: Meta-analysis. Br. J. Psychiatry 2008, 193, 10–17. [Google Scholar] [CrossRef]
- Romanescu, M.; Buda, V.; Lombrea, A.; Andor, M.; Ledeti, I.; Suciu, M.; Danciu, C.; Dehelean, C.A.; Dehelean, L. Sex-related differences in pharmacological response to CNS drugs: A narrative review. J. Pers. Med. 2022, 12, 907. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2022, 18, 447–457. [Google Scholar] [CrossRef]
- Altemus, M.; Sarvaiya, N.; Epperson, C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef]
- Keers, R.; Aitchison, K.J. Gender differences in antidepressant drug response. Int. Rev. Psychiatry 2010, 22, 485–500. [Google Scholar] [CrossRef]
- Jannuzzi, G.; Gatti, G.; Magni, P.; Spina, E.; Pacifici, R.; Zuccaro, P.; Torta, R.; Guarneri, L.; Perucca, E. Plasma concentrations of the enantiomers of fluoxetine and norfluoxetine: Sources of variability and preliminary observations on relations with clinical response. Ther. Drug Monit. 2002, 24, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, T.; Sakurai, H.; Takeuchi, H.; Suzuki, T.; Mimura, M.; Uchida, H. Predictors of response to pharmacotherapy in children and adolescents with psychiatric disorders: A combined post hoc analysis of four clinical trial data. Neuropsychopharmacol. Rep. 2022, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).