Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Preparation of Formulations for Topical Use
2.3. Determination of the Antibacterial Activity of Formulations for Topical Use
2.4. Protocol of Injury, Induction of Infection and Treatment
2.5. Processing of Biopsies and Staining
2.6. Collagen Fiber Content Evaluation
2.7. Stereological Analysis
2.8. Statistical Analysis
3. Results
3.1. Phase 1 of the Experiment
3.1.1. Antibacterial Activity of Formulations for Topical Use
3.1.2. Selection of the Definitive Prototype Based on the F1 and F2 CuNPs Formulations in Uninfected and Infected Lesions
Ulmoplus® with CuNPs, Formulation 1 (U + F1)
Ulmoplus® with CuNPs, Formulation 2 (U + F2)
3.2. Phase II of the Experiment
3.2.1. Experimental Study with Selected Prototype
3.2.2. Ulmoplus ® with F2 CuNPs, without Infection (U + F2NI)
3.2.3. Ulmoplus® with F2 CuNPs, with Infection (U + F2I)
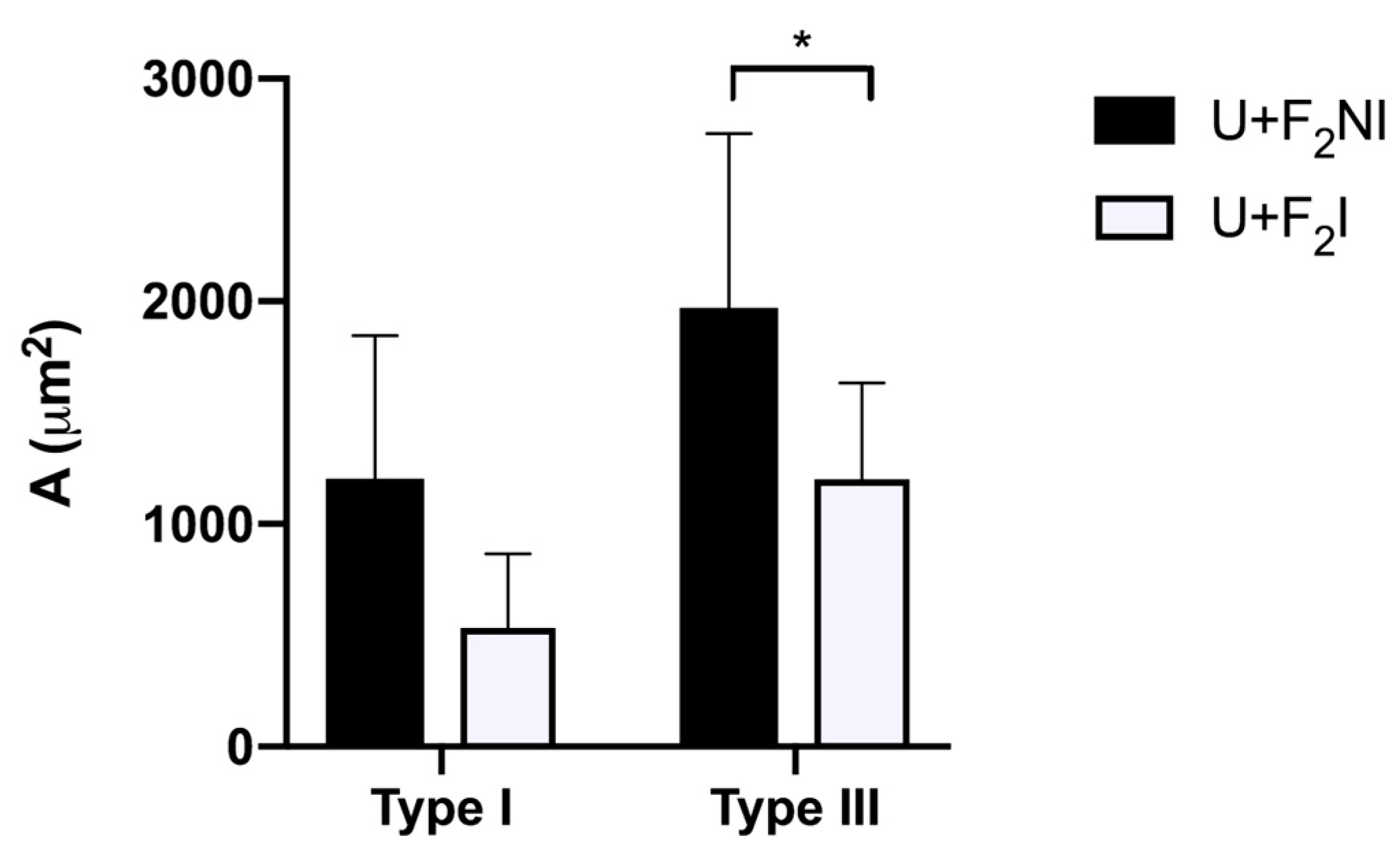
3.2.4. Stereological Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shariati, A.; Moradabadi, A.; Azimi, T.; Ghaznavi-Rad, E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci. Rep. 2020, 10, 1032. [Google Scholar] [CrossRef]
- Dev, S.K.; Choudhury, P.; Srivastava, R.; Sharma, M. Antimicrobial, anti-inflammatory and wound healing activity of polyherbal formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Lohana, P.; Suryaprawira, A.; Woods, E.L.; Dally, J.; Gait-Carr, E.; Alaidaroos, N.Y.A.; Heard, C.M.; Lee, K.Y.; Ruge, F.; Farrier, J.N.; et al. Role of Enzymic Antioxidants in Mediating Oxidative Stress and Contrasting Wound Healing Capabilities in Oral Mucosal/Skin Fibroblasts and Tissues. Antioxidants 2023, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. npj Biofilms Microbiomes 2020, 6, 21. [Google Scholar] [CrossRef]
- Kirker, K.R.; James, G.A. In vitro studies evaluating the effects of biofilms on wound-healing cells: A review. Apmis 2017, 125, 344–352. [Google Scholar] [CrossRef]
- Haesler, E.; Swanson, T.; Ousey, K.; Carville, K. Clinical indicators of wound infection and biofilm: Reaching international consensus. J. Wound Care 2019, 28, s4–s12. [Google Scholar] [CrossRef]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care 2021, 10, 317–327. [Google Scholar] [CrossRef]
- Lorenzo, M.; Hernández, R.; Soria, M. Chronic wounds treated in an emergency service of primary health care. Enferm. Glob. 2014, 13, 23–31. [Google Scholar]
- Kosaraju, R.; Rennert, R.C.; Maan, Z.N.; Duscher, D.; Barrera, J.; Whittam, A.J.; Januszyk, M.; Rajadas, J.; Rodrigues, M.; Gurtner, G.C. Adipose-derived stem cell-seeded hydrogelsincrease endogenous progenitor cell recruitment and neovascularization in wounds. Tissue Eng. Part A 2016, 22, 295–305. [Google Scholar] [CrossRef]
- Qiang, L.; Sample, A.; Liu, H.; Wu, X.; He, Y.-Y. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 2017, 7, 14110. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Aygin, D. Honey dressing in wound treatment: A systematic review. Complement Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Schencke, C.; Vásquez, B.; Sandoval, C.; del Sol, M. El Rol de la Miel en los Procesos Morfofisiológicos de Reparación de Heridas. Int. J. Morphol. 2016, 34, 385–395. [Google Scholar] [CrossRef]
- Lotfinia, F.; Norouzi, M.R.; Ghasemi-Mobarakeh, L.; Naeimirad, M. Anthocyanin/Honey-Incorporated Alginate Hydrogel as a Bio-Based pH-Responsive/Antibacterial/Antioxidant Wound Dressing. J. Funct. Biomater. 2023, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, M.; Zhang, N.; Wang, G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2019, 34, 123–131. [Google Scholar] [CrossRef]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (Medihoney™) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef]
- Hazrati, M.; Mehrabani, D.; Japoni, A.; Montasery, H.; Azarpira, N.; Hamidian-Shirazi, A.; Tanideh, N. Effect of Honey on Healing of Pseudomonas aeruginosa Infected Burn Wounds in Rat. J. Appl. Anim. Res. 2010, 37, 161–165. [Google Scholar] [CrossRef]
- Schencke, C.; Salvo, J.; Veuthey, C.; Hidalgo, A.; del Sol, M. Cicatrización en quemaduras tipo AB-B utilizando miel de ulmo asociada a vitamina C oral. Int. J. Morphol. 2011, 29, 69–75. [Google Scholar] [CrossRef][Green Version]
- Schencke, C.; Vasconcellos, A.; Salvo, J.; Veuthey, C.; del Sol, M. Efecto Cicatrizante de la Miel de Ulmo (Eucryphia cordifolia) Suplementada con Ácido Ascórbico como Tratamiento en Quemaduras. Int. J. Morphol. 2015, 33, 137–143. [Google Scholar] [CrossRef][Green Version]
- Schencke, C.; Vasconcellos, A.; Sandoval, C.; Torres, P.; Acevedo, F.; del Sol, M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus). Burn. Trauma 2016, 3, 4–25. [Google Scholar] [CrossRef]
- Schencke, C.; Sandoval, C.; Vásquez, B.; Sol, M.D. Quantitative analysis of dermal scars in deep skin burns treated with Ulmo honey supplemented with ascorbic acid. Int. J. Clin. Exp. Med. 2018, 11, 2422–2429. [Google Scholar]
- Salvo Arias, J.; Schencke Figueroa, C.; Arias Bustamante, A.; Otzen Hernández, T.; del Sol Calderón, M. Validación clínica de enfermería en cicatrización de úlceras venosas con miel nativa chilena suplementada. Rev. Urug. Enferm. 2020, 15, 13. [Google Scholar]
- Schencke, C.; Salvo, J.; Vasconcellos, A.; del Sol, M. Estudio Comparativo de la Cicatrización en Quemaduras con Tratamiento en Base a Miel de Ulmo (Eucryphia cordifolia) y Vitamina C oral versus Hidrogel en Cobayos (Cavia porcellus). Int. J. Morphol. 2013, 31, 839–844. [Google Scholar] [CrossRef][Green Version]
- del Sol Calderon, M.; Schencke Figueroa, C.; Salvo Arias, J.; Hidalgo Sandoval, A.; Ocharan Torre, F. Combined therapy of Ulmo honey (Eucryphia cordifolia) and ascorbic acid to treat venous ulcers. Rev. Lat.-Am. Enferm. 2015, 23, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Torres, P.; Oomah, B.D.; de Alencar, S.M.; Massarioli, A.P.; Martín-Venegas, R.; Albarral-Ávila, V.; Burgos-Díaz, C.; Ferrer, R.; Rubilar, M. Volatile and non-volatile/semi-volatile compounds and in vitro bioactive properties of Chilean Ulmo (Eucryphia cordifolia Cav.) honey. Food Res. Int. 2017, 94, 20–28. [Google Scholar] [CrossRef]
- Schencke, C.; Vásquez, B.; Sandoval, C.; del Sol, M. Ulmoplus® Increases FGF-2 Expression and Promote Burn Wound Healing. Int. J. Morphol. 2021, 39, 1701–1708. [Google Scholar] [CrossRef]
- Melamed, E.; Kiambi, P.; Okoth, D.; Honigber, I.; Tamir, E.; Borkow, G. Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings—Case Series. Medicina 2021, 57, 296. [Google Scholar] [CrossRef]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Barroso, A.; Mestre, H.; Ascenso, A.; Simões, S.; Reis, C. Nanomaterials in wound healing: From material sciences to wound healing applications. Nano Sel. 2020, 1, 443–460. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Michopoulou, A.; Rousselle, P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing. Eur. J. Dermatol. 2015, 25, 33–42. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of Cell Proliferation and Expression of Matrixmetalloproteinase-1 and Interluekin-8 Genes in Dermal Fibroblasts by Copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 12, 21159–21182. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2010, 77, 794–802. [Google Scholar] [CrossRef]
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 2011, 14, 1730–1743. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Walger, E.; Marlin, N.; Mortha, G.; Molton, F.; Duboc, C. Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation. Appl. Sci. 2021, 11, 687. [Google Scholar]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Cheloni, G.; Marti, E.; Slaveykova, V.I. Interactive effects of copper oxide nanoparticles and light to green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2016, 170, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Xiong, Q.; Hornburg, D.; Tao, W.; Farokhzad, O.C. Nano–bio interactions in cancer: From therapeutics delivery to early detection. Accounts Chem. Res. 2021, 54, 291–301. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Clasen, S.; Hasan, N.M.; Barry, A.N.; Lutsenko, S. Functional partnership of the copper export machinery and glutathione balance in human cells. J. Biol. Chem. 2012, 287, 26678–26687. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Febré, N.; Silva, V.; Báez, A.; Palza, H.; Delgado, K.; Aburto, I.; Silva, V. Comportamiento antibacteriano de partículas de cobre frente a microorganismos obtenidos de úlceras crónicas infectadas y su relación con la resistencia a antimicrobianos de uso común. Rev. Med. Chile 2016, 144, 1523–1530. [Google Scholar] [CrossRef]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics 2022, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, D.; Lindblad, M.M.; Paidi, M.D.; Hasselholt, S.; Lykkesfeldt, J.; Tveden-Nyborg, P. In vivo vitamin C deficiency in guinea pigs increases ascorbate transporters in liver but not kidney and brain. Nutr. Res. 2014, 34, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal Permeation of Drugs in Various Animal Species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies; National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Aular, Y. Aspectos bioéticos del uso de animales de experimentación en investigación científica: Legislación y comités de bioética animal. Revista Arjé 2018, 12, 94–103. [Google Scholar]
- Salvo, J.; Schencke, C.; Pavez, M.; Veuthey, C.; del Sol, M. Thermal injury protocol to study healing, with and without infection, in Guinea pig (Cavia porcellus) model. Int. J. Morphol. 2023, 41, 1053–1057. [Google Scholar]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Institut Municipal d’Investigació Mèdica. Calculadora de Grandària Mostral GRANMO. Version 7.12. Barcelona, Spain. Available online: https://www.imim.es/ofertadeserveis/software-public/granmo/ (accessed on 28 May 2022).
- Althubaiti, A. Sample size determination: A practical guide for health researchers. J. Gen. Fam. Med. 2022, 24, 72–78. [Google Scholar] [CrossRef]
- Pérez-Olvera, O.; Arellano, B.S.; Rodríguez, M.H.A. Overview of Stereological Methods and their Applications for Cell Biology. Patol. Rev. Latinoam. 2012, 50, 63–71. [Google Scholar]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 21, 47. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar]
- Vries, C.R.d.; Sweere, J.M.; Ishak, H.; Sunkari, V.; Bach, M.S.; Liu, D.; Manasherob, R.; Bollyky, P.L. A Delayed Inoculation Model of Chronic Pseudomonas aeruginosa Wound Infection. J. Vis. Exp. 2020, 156, e60599. [Google Scholar]
- López De Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Fallah Huseini, H.; Abdolghaffari, A.H.; Ahwazi, M.; Jasemi, E.; Yaghoobi, M.; Ziaee, M. Topical Application of Teucrium polium Can Improve Wound Healing in Diabetic Rats. Int. J. Low. Extrem. Wounds 2020, 19, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mandarim-de-Lacerda, C.A.; del Sol, M. Tips for Studies with Quantitative Morphology (Morphometry and Stereology). Int. J. Morphol. 2017, 35, 1482–1494. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 795281. [Google Scholar] [CrossRef]
- Hayes, G.; Wright, N.; Gardner, S.L.; Telzrow, C.L.; Wommack, A.J.; Vigueira, P.A. Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett. Appl. Microbiol. 2018, 66, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Safi, S.Z.; Singh, I.; Bin Emran, T. Wound dressings: Recent updates. Int. J. Surg. 2022, 104, 106793. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of honey in advanced wound care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef] [PubMed]
- McLoone, P.; Tabys, D.; Fyfe, L. Honey Combination Therapies for Skin and Wound Infections: A Systematic Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2020, 13, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Gunton, J.E.; Girgis, C.M.; Lau, T.; Vicaretti, M.; Begg, L.; Flood, V. Vitamin C improves healing of foot ulcers: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2021, 126, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A.; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Vissers, M.C.; Pullar, J.M. Re-opening old wounds—Vitamin C and wound healing deserve a re-examination. Am. J. Clin. Nutr. 2016, 115, 1–2. [Google Scholar] [CrossRef]
- Gemalmaz, H.C.; Sarıyılmaz, K.; Ozkunt, O.; Gurgen, S.G.; Silay, S. Role of a combination dietary supplement containing mucopolysaccharides, vitamin C, and collagen on tendon healing in rats. Acta Orthop. Traumatol. Turc. 2018, 52, 452–458. [Google Scholar] [CrossRef]
- Gref, R.; Deloménie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaële, D.; Zouhiri, F.; Couvreur, P. Vitamin C–squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mat. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional Copper-Containing Mesoporous Glass Nanoparticles as Antibacterial and Proangiogenic Agents for Chronic Wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Konerding, M.A.; Ziebart, T.; Wolloscheck, T.; Wellmann, A.; Ackermann, M. Impact of single-dose application of TGF-β, copper peptide, stanozolol and ascorbic acid in hydrogel on midline laparatomy wound healing in a diabetic mouse model. Int. J. Mol. Med. 2012, 30, 271–276. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, F.; Li, Z.; Lin, S.; Chen, L. Hydrogel cross-linked with dynamic covalent bonding and micellization for pro- moting burn wound healing. ACS Appl. Mater. Interfaces 2018, 10, 25194–25202. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kang, H.W. Engineering pharmaceutical nanocarriers for photodynamic therapy on wound healing: Review. Mater. Sci. Eng. C 2019, 105, 110110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zi, L.; Cen, Y.; You, C.; Tian, M. Copper sulfide nanoparticles-incorporated hyaluronic acid injectable hydrogel with enhanced angiogenesis to promote wound healing. Front. Bioeng. Biotechnol. 2020, 8, 417. [Google Scholar] [CrossRef]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazăr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar] [PubMed]
- Wang, T.; Gu, Q.; Zhao, J.; Mei, J.; Shao, M.; Pan, Y.; Zhang, J.; Wu, H.; Zhang, Z.; Liu, F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6636–6645. [Google Scholar]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Gan, S.H.; Khalil, M.I. Honey: A potential therapeutic agent for managing diabetic wounds. Evid. Based Complement. Alternat. Med. 2014, 2014, 169130. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The neglected role of copper ions in wound healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl starch-based hydrogels decorated with CuO nanoparticles: Antioxidant and antimicrobial properties and accelerated wound healing in vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Toro, M.; Jacob, R.; Navarrete, P.; Troncoso, M.; Figueroa, G.; Reyes-Jara, A. Antimicrobial effect of copper surfaces on bacteria isolated from poultry meat. Braz. J. Microbiol. 2018, 49 (Suppl. S1), 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
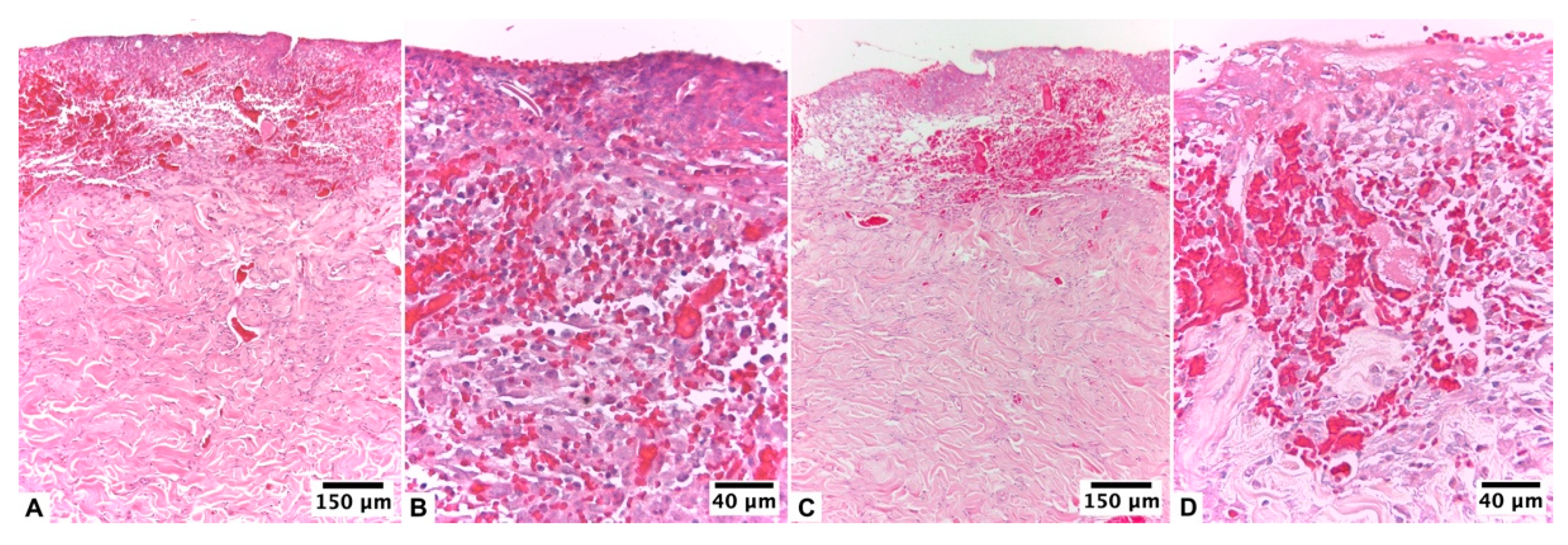



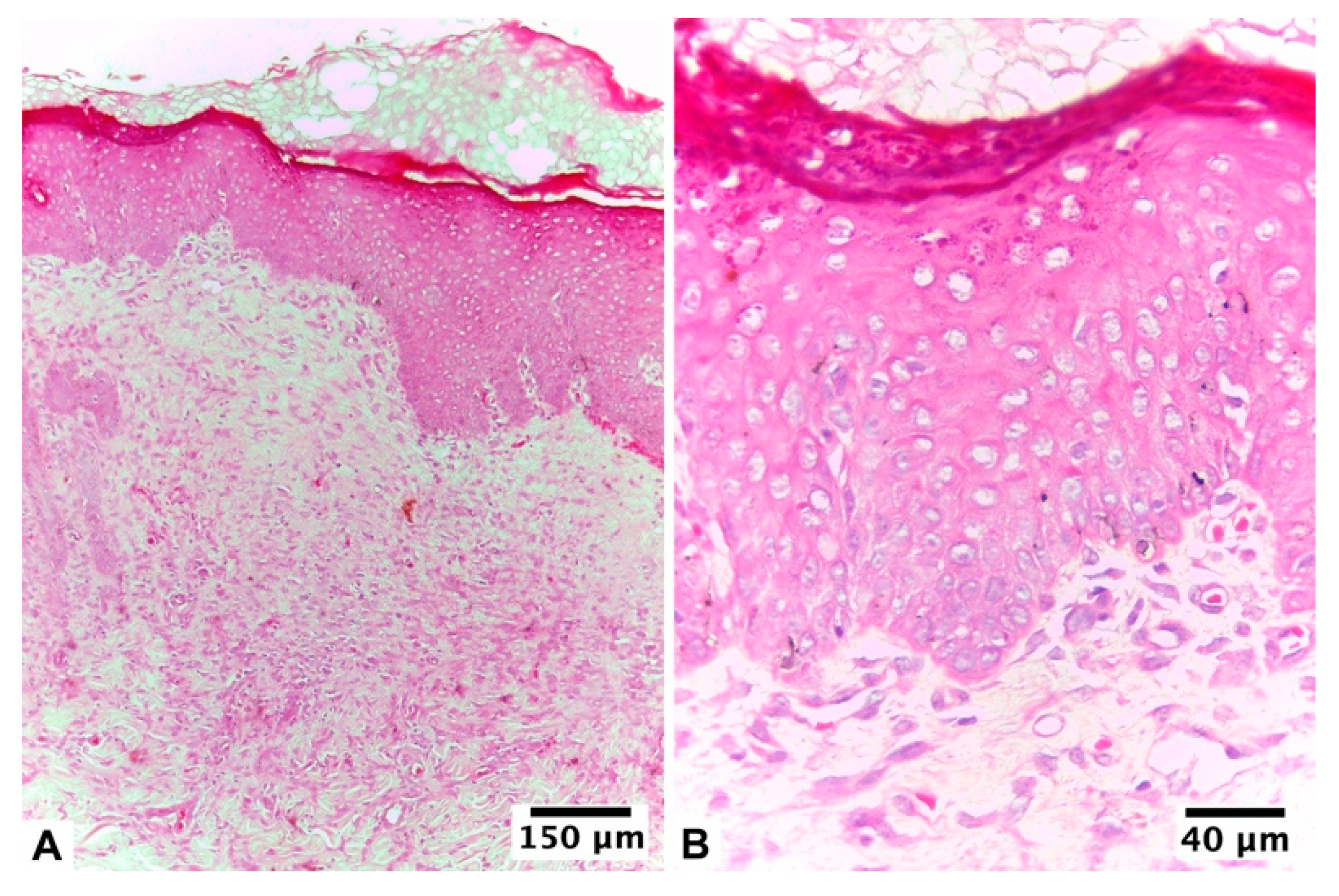
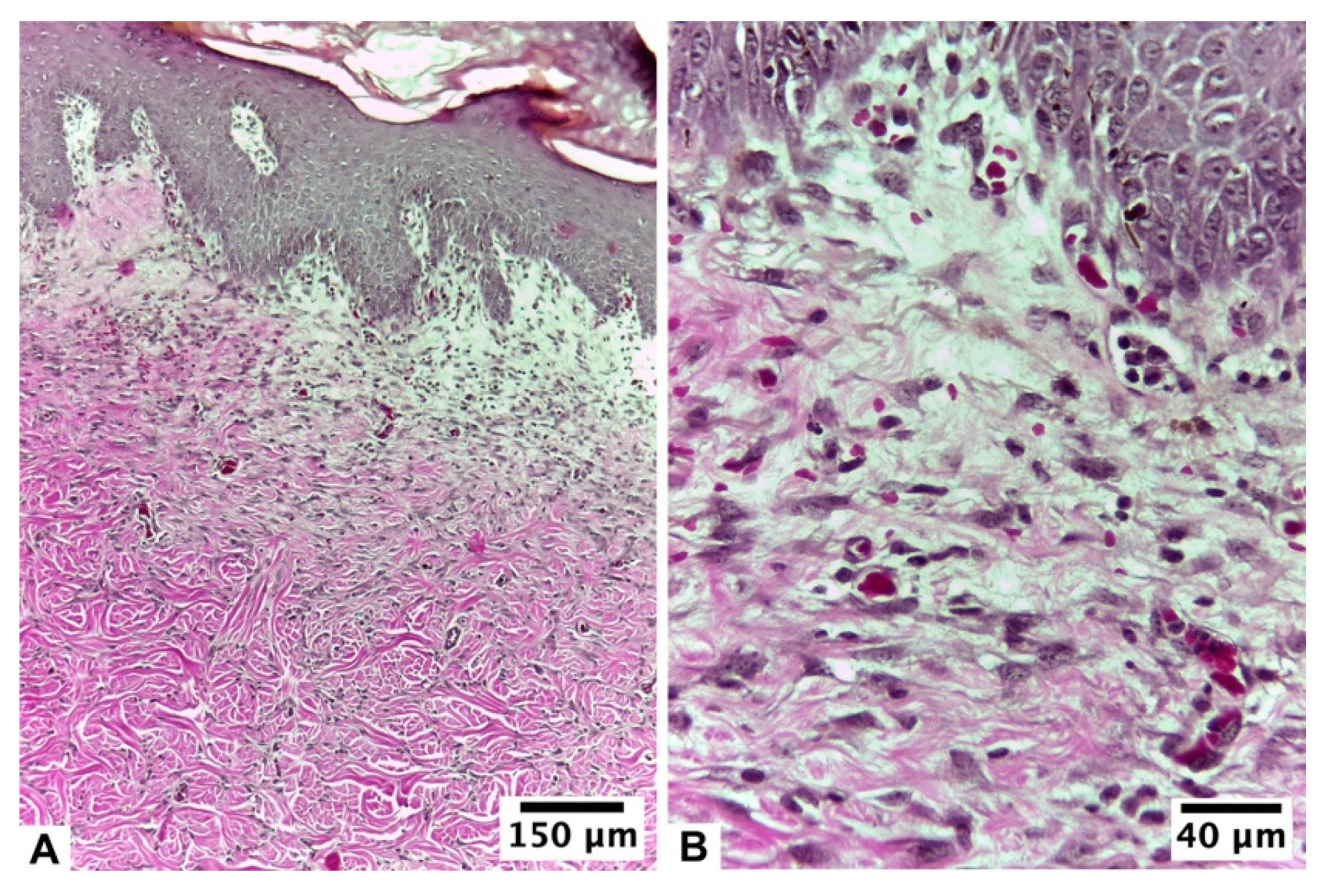
| Score | Re-Epithelization | Granulation | Inflammatory Cells | Angiogenesis |
|---|---|---|---|---|
| 0 | Absence of epithelial proliferation in ≥70% of the tissue | Immature and inflammatory tissue in ≥70% of the tissue | 13–15 inflammatory cells per histological field | Absence of angiogenesis, presence of congestion, hemorrhage, and edema |
| 1 | Poor epidermal organization in ≥60% of the tissue | Sparse immature and inflammatory tissue in ≥60% of the tissue | 10–13 inflammatory cells per histological field | 1–2 vessels per site, edema, hemorrhage, and congestion |
| 2 | Incomplete epidermal organization in ≥40% of the tissue | Moderate remodeling in ≥40% of the tissue | 7–10 inflammatory cells per histological field | 3–4 vessels per site, moderate edema, and congestion |
| 3 | Moderate epithelial proliferation in ≥60% of the tissue | Layer of coarse granulation and well-formed collagen matrix in ≥60% of the tissue | 4–7 inflammatory cells per histological field | 5–6 vessels per site, slight edema, and congestion |
| 4 | Complete epidermal remodeling in ≥80% of the tissue | Complete tissue organization in ≥80% of the tissue | 1–4 inflammatory cells per histological field | More than seven vessels per site arranged vertically toward the epithelial surface |
| Test Organisms | MIC (v/v) | ||
|---|---|---|---|
| Ulmoplus® | U + F1 | U + F2 | |
| Staphylococcus aureus ATCC 25923 | 6.25 | 6.25 | 6.25 |
| Pseudomonas aeruginosa ATCC 27853 | 12.5 | 12.5 | 12.5 |
| Escherichia coli ATCC 25922 | 12.5 | 25 | 25 |
| Parameters | Score | Group Frequency (%) | |
|---|---|---|---|
| U + F2NI Group | U + F2I Group | ||
| Re-epithelization | 0 | 0 | 0 |
| 1 | 0 | 0 | |
| 2 | 0 | 0 | |
| 3 | 80 | 0 | |
| 4 | 20 | 100 | |
| Granulation | 0 | 0 | 0 |
| 1 | 0 | 0 | |
| 2 | 60 | 0 | |
| 3 | 40 | 0 | |
| 4 | 0 | 100 | |
| Inflammatory cells | 0 | 0 | 0 |
| 1 | 0 | 0 | |
| 2 | 0 | 0 | |
| 3 | 60 | 20 | |
| 4 | 40 | 80 | |
| Angiogenesis | 0 | 0 | 0 |
| 1 | 0 | 0 | |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| 4 | 100 | 100 | |
| Variable | Mean ± SD | ||
|---|---|---|---|
| U + F2NI | U + F2I | p Value | |
| NA Fibroblasts (mm−2) | 2127.613 ± 484.164 | 2250.825 ± 433.992 | 0.035 |
| VV Fibroblasts (%) | 12.800 ± 4.164 | 15.238 ± 3.861 | <0.001 |
| SV Fibroblasts (mm−1) | 78.324 ± 25.966 | 91.505 ± 23.791 | <0.001 |
| NA PMN (mm−2) | 206.821 ± 225.434 | 226.623 ± 181.709 | 0.445 |
| VV PMN (%) | 1.238 ± 1.645 | 1.143 ± 1.560 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvo, J.; Sandoval, C.; Schencke, C.; Acevedo, F.; del Sol, M. Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds. Pharmaceutics 2023, 15, 2187. https://doi.org/10.3390/pharmaceutics15092187
Salvo J, Sandoval C, Schencke C, Acevedo F, del Sol M. Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds. Pharmaceutics. 2023; 15(9):2187. https://doi.org/10.3390/pharmaceutics15092187
Chicago/Turabian StyleSalvo, Jessica, Cristian Sandoval, Carolina Schencke, Francisca Acevedo, and Mariano del Sol. 2023. "Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds" Pharmaceutics 15, no. 9: 2187. https://doi.org/10.3390/pharmaceutics15092187
APA StyleSalvo, J., Sandoval, C., Schencke, C., Acevedo, F., & del Sol, M. (2023). Healing Effect of a Nano-Functionalized Medical-Grade Honey for the Treatment of Infected Wounds. Pharmaceutics, 15(9), 2187. https://doi.org/10.3390/pharmaceutics15092187








