1. Introduction
Freeze drying or lyophilization is an essential process for the preservation of pharmaceutical and biological products. It is carried out at temperatures and pressures well below the triple point of water.
Typical mathematical models of freeze drying have been developed for different scales: single particles, aggregates and biological products such as fruits and frozen suspensions in vials or syringes on the small scale, as well as particle beds or complete drying chambers on the large scale. While, on the small scale, the impact of freeze drying conditions on the drying time, temperature of the material and pressure are most often of interest, the large-scale approaches focus on the impact of the spatially and temporally varying process conditions. The latter usually require complex three-dimensional (3D) models that are often implemented in COMSOL [
1], while in the first case, in most situations, 1D models are sufficient due to the anticipation of product homogeneity [
2]. In such models, effective parameters are used and no spatial variation in the pore structure is considered.
In the literature, a wide range of model assumptions can be found, which differ not only in terms of postulated steady-state or transient conditions, but also in terms of the incorporated physical phenomena. The accuracy of the proposed models surely increases with higher complexity, provided that the used parameters are reliably determined, e.g., by experiments. However, the computational efficiency usually suffers from the number of physical mechanisms that are included or increasing size and dimension of the computational domain. Secondly, the accurate prediction of model parameters is challenging in most cases. This can be disadvantageous, especially in cases of model predictive control, e.g., [
3]. The choice of the model is therefore most often a compromise between accuracy and computation time.
Here, we want to provide a brief literature overview in which we focus on the models that have been proposed for the primary freeze drying of frozen bulk solutions, such as suspensions that are frozen as blocks inside vials and syringes. The focus is thereby on the studies of single vials (sample scale), without excluding the work of, e.g., [
4], where the outcomes from simulations of single vials are extrapolated to the drying chamber scale. Secondary drying (occurring before primary drying is finished, i.e., taking place in parallel) is often neglected in these models.
Primary freeze drying is generally constrained by the process conditions, namely pressure and temperature, which dictate the limits of product integrity and therefore restrict drying time and energy efficiency [
5,
6]. Temperature, pressure and drying time are thus the essential parameters that are usually predicted, e.g., for process control, e.g., [
3]. The most critical parameter is thereby clearly the product temperature, which needs to be low enough to avoid material denaturation, melting or product collapse throughout the vial and during the whole drying process [
6]. It depends on the temperature of the shelf and the pressure of the chamber, which both also control the drying rate.
In the 2D dusty gas model presented in [
7], transient heat transfer and coupled mass transfer are solved. In this, the dried region is considered as a porous medium with constant porosity; thermal conductivity, density and heat capacity are averaged for the mixture of solid and empty pores. In the frozen region, these parameters are averaged for water ice and solids. Here, only the top surface is assumed permeable for vapor transfer, whereas all other sides are impermeable. Mass transfer is in most cases computed either as a diffusion problem or as viscous flow, the latter often in presence of an inert gas. For example, [
7,
8] proposed to use the Darcy equation with the permeability of the porous dried region for vapor transfer simulation.
The heat transfer equations, including the transient situation of the warming-up or cooling-down of the material (i.e., the sensible heat), are set up and solved for both dried and frozen regions. In most cases, a moving boundary condition (Stefan problem [
4,
7]) is postulated based on the temporal variation in the sublimation interface, where thermodynamic equilibrium between ice and vapor phase is assumed. The equilibrium vapor pressure is mostly calculated with the Clausius–Clapeyron equation or with the equation from Goff and Gratch (1951) [
9]. The heat supply is usually realized by conduction through the vial bottom, while at the top, surface heat transfer by radiation from the environment can be assumed, e.g., [
10]. Several proposals have been made for the heat exchange with the environment along the side walls, which include radiation and conduction, e.g., [
3]. Heat transfer inside the frozen region is based on conduction through the ice–solid mixture; in the dried region, heat transfer through the interconnected solid walls as well as heat convection are assumed [
11]. At very low chamber pressure (below 10 Pa and in the absence of inert gases), the problem is simplified and heat convection is disregarded, e.g., [
1].
The assumption of homogeneous domains that are assigned with effective, i.e., averaged properties, usually form the basis for further simplification of the problem to 1D, i.e., along the vertical height of the blocks [
11]. This is surely accurate enough, when it can be expected that the sublimation of ice results in the formation of a flat (infinitesimally thin) phase boundary at the top side of the frozen block which moves downwards during the drying process. In other cases, i.e., when either structural heterogeneity or heat transfer in the lateral direction cannot be disregarded, the sublimation front can be convex (curved downwards along the side walls of the vial) [
2], or concave [
12].
Even if the dimension of the domain is reduced to the space coordinate in the vertical direction, the computational effort is still high, making this approach uncomfortable for online process control [
3]. As a consequence, less-complicated model approaches with simplified assumptions for both heat and mass transfer were developed [
4]. Often in these models, a fitting function for the mass transfer, depending on a priori conducted experiments, is used [
3,
4,
13]. Assuming quasi-stationary heat transfer, the partial differential equation (from the transient case with a first order derivative over time and second order derivative over space) is transferred into an ordinary differential equation. This reduces the computational effort significantly without altering the simulation results too much. Alternatively, the analytical solution of the transient heat transfer problem is used [
4]. It was shown in the same study that the simulation results fit well with the experimental results. The overall agreement with other studies, e.g., [
14], is good. The simplified model proposed in [
3] is furthermore used with the model-based determination of mass and heat transfer coefficients with the purpose of determining the limits of primary freeze drying in terms of maximum temperature and chamber pressure [
6]. The in silico determination of model parameters is an alternative option to experimental measurements, which are often elaborate, time consuming and less accurate [
6]. As an important feature, these coefficients can be randomly distributed for the simulations, thus allowing us to predict a wide range of drying behavior in single vials under different conditions, as it can be expected for the vials on the shelf in a drying chamber [
13].
This brief literature overview shows that the mathematical models that describe primary freeze drying of frozen bulk solutions consider the vial contents as a continuum with averaged effective parameters, such as effective porosity, thermal conductivity, heat capacity and transport coefficients. As an example, heat transfer coefficients can incorporate several different effects, including heat transfer by conduction through the solid and ice phase, heat transferred by vapor or heat exchange with the vial and the surroundings. The available approaches are intended to predict the global variation of, e.g., drying time in dependence of the process conditions, i.e., temperature and pressure, which can vary along the position of the vials on the shelf. Thus, they provide data about the inter-vial heterogeneity of freeze drying, which is important for process and apparatus design and process control. However, these models are not suitable for the investigation of intra-vial heterogeneity which is linked to the local, and sometimes even temporal, variation in material properties. The authors of [
15] studied the permeability of the pore structure in dependence of cooling rate based on Darcy’s flow equation. They found that smaller pores and thus smaller permeabilities of the vapor phase are obtained at higher cooling rates, where the nucleation temperature is smaller. A similar finding was recently reported in [
16] where higher nucleation temperatures were associated with larger ice crystals. This shows the need for the development of models for heterogeneous and composite materials [
17].
To the best of our knowledge, this is the first time that a non-isothermal pore network model (PNM) is presented for the mathematical modeling of primary freeze drying. In contrast to the averaging approaches, the PNM resolves the structure of the material on the pore scale. It can therefore consider local effects, such as the variation in pore sizes, which have a direct impact on the local heat and mass transfer conditions. PNMs thus serve for the study of structure–transport correlations, which is already widely established for conventional drying methods [
18]. There, the capillarity of porous media yields strongly structured drying fronts which again affect the drying kinetics. Although capillary transport does not play a role in freeze drying, the heat and mass transfer conditions can alter the sublimation front structure. The use of PNMs can thus be useful to understand and predict the drying kinetics of heterogeneous materials, such as graded or foamed structures, or to study freeze drying at the limits of material collapse. In cases where a length-scale separation is not possible, e.g., in thin porous layers, the use of PNMs may even be mandatory [
19].
With the proposed simulation tool, freeze drying can be studied on a physical base. Temperature, pressure and ice content are locally resolved. Besides this, it yields effective parameters as a function of ice content, which could be used for the improved parameterization of the above-described approaches [
13]. In particular, the local heat and mass transfer coefficients of heterogeneous porous media could be adjusted based on PNM studies. Only a few parameters are involved that need to be a priori defined, and these parameters concern the boundary conditions of heat and mass transfer as well as the material’s morphology. The PNM can be realized with regular lattices, e.g., as proposed in [
20], or reconstructed porous domains can be implemented, e.g., [
21]. The size of the domain and the incorporated physical mechanisms thereby dictate the computational effort.
One major advantage of PNM is its ability to study the impact of structure on heat and mass transfer conditions independently of each other. This makes fundamental physical mechanisms much more accessible.
The approach described here can be used to study structure–transport relationships that can be helpful to optimize freeze drying in terms of drying time and energy consumption [
5,
13]. More clearly, it can be studied if heat and/or mass transfer can be improved by a change in geometry, e.g., height or width, as well as by an increase in pore sizes and/or porosity, which might be adjusted according to the freezing conditions or the composition of the mixture.
2. Materials and Methods
The non-isothermal PNM of primary freeze drying is a further development of the model in [
22]. In contrast to the previous model, the computation of mass transfer is only considered for the vapor phase and in the transition regime between molecular (Knudsen) diffusion and viscous flow. The water is contained inside the void space in the frozen state, for which capillary liquid pumping is not computed. Transient heat transfer based on conduction through the solid and ice phases is solved using the Krischer Model [
23]. In addition to that, heat transfer by vapor flow through empty pores, i.e., advection, is implemented in the new model.
2.1. Pore Network Model
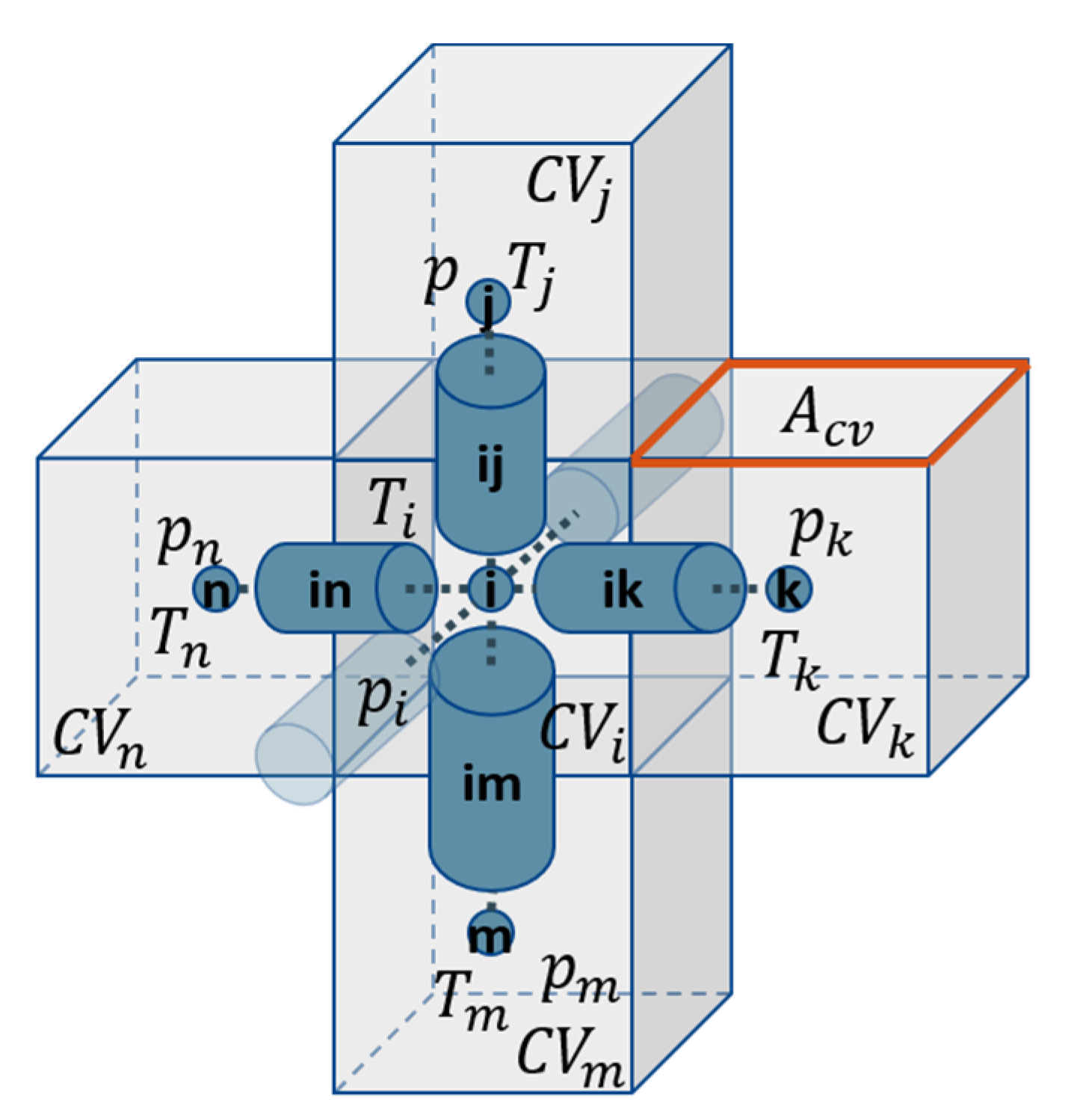
The simulations are conducted using regular 3D square lattices. The network is realized as a bond network, which means that the void space is completely assigned to cylindrical pores that are connected by computational nodes without volume (
Figure 1). While the structural and physical properties, such as length, width, cross-section and mass transfer resistance are allocated to the pores, the computational nodes are used to solve the enthalpy and mass balances and to store the pressure data (mass balances) as well as the temperature data (enthalpy balances).
As shown in
Figure 1, each computational node is surrounded by a control volume (CV). It is cubic in the case of the regular PN studied here. The edge length is constant and identical with the pore length, i.e.,
, the volume is
and the contact area between two neighboring CVs is
in this case. The ice phase is equally distributed in partially saturated pores.
The CVs are assigned with thermo-physical properties (thermal conductivity and heat capacity) as well as volume, porosity and density. Thermal conductivity, heat capacity and density depend on local saturation as well as porosity, and are updated during the simulation.
An overview of the geometric parameters of the PNs considered in this work is provided in
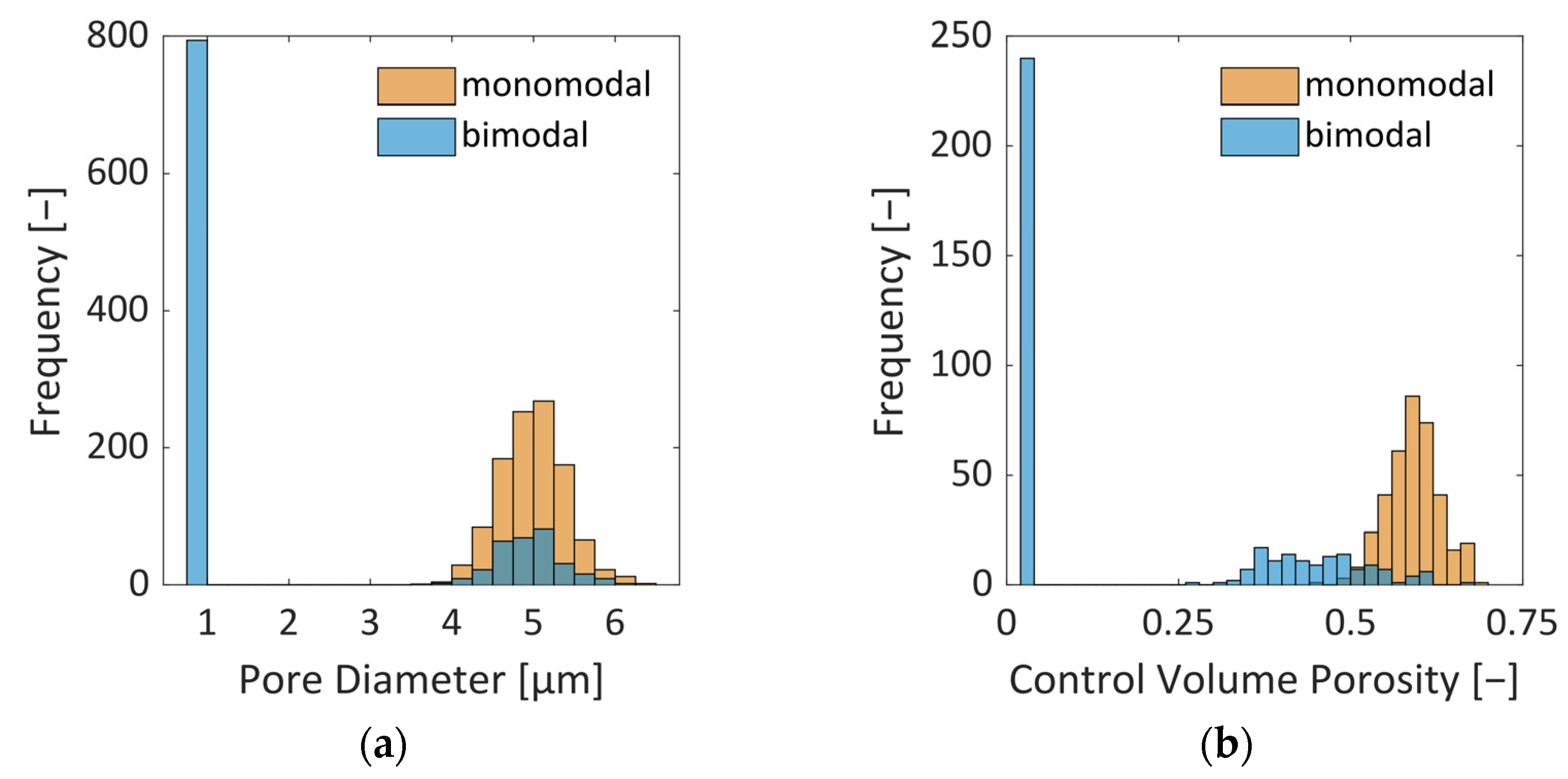
Table 1. The pore sizes are normally distributed (
Figure 2) based on structural analysis carried out in [
21,
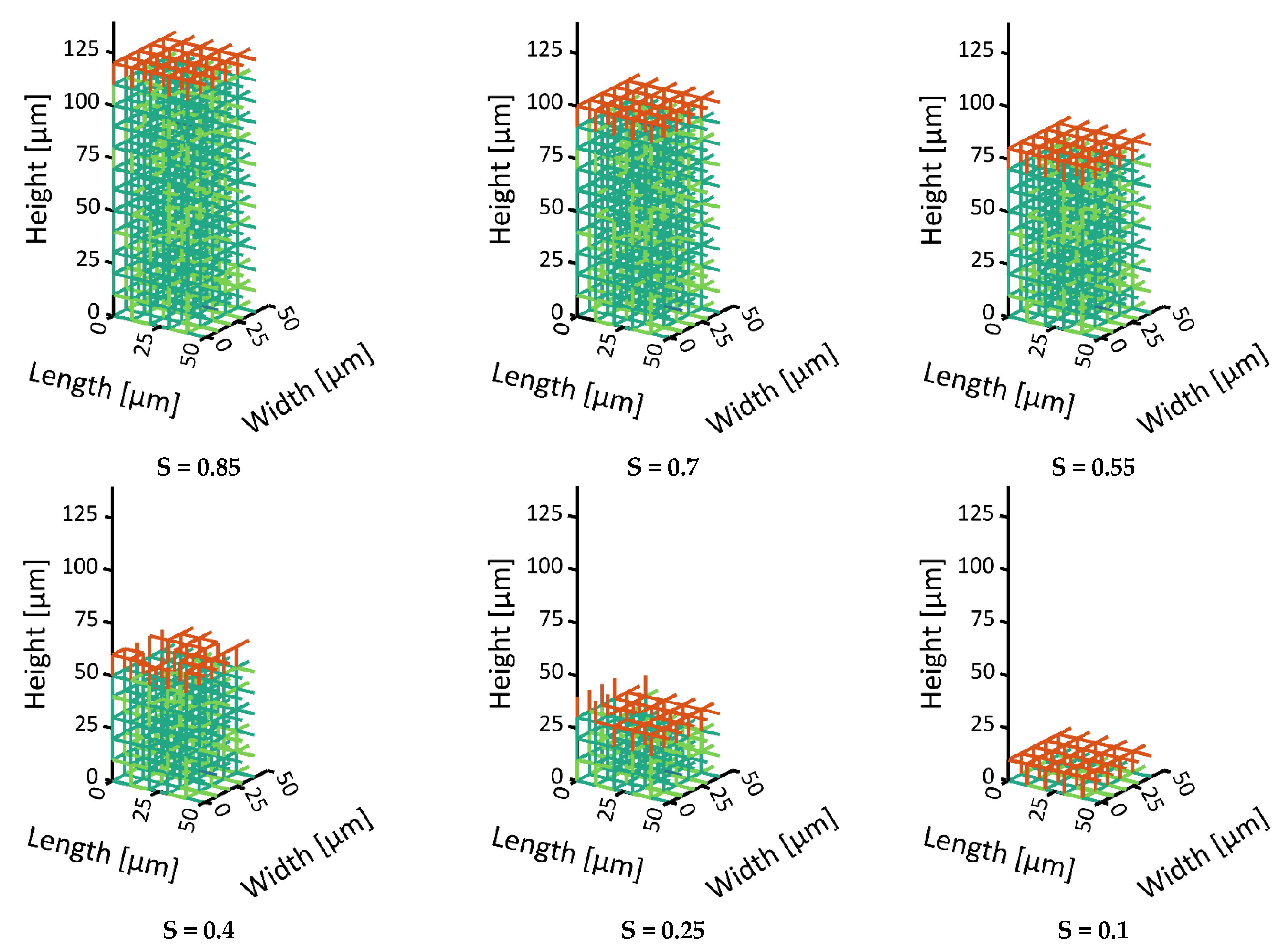
24]. In addition to a PN with monomodal pore size distribution (PSD), representing a homogeneous material, we consider the case of local variation in the PSD here. The second PN is therefore realized with a bimodal PSD. It has larger pores in the center and smaller pores along the sides. The ratio of small to large pores is 2.59. This means that the PN contains a much higher number of smaller pores. This mimics the situation of structural heterogeneity that could be expected from the freezing step [
25]. Here, we assume that the lateral sides could be frozen faster with the result of smaller ice crystals than in the center, where we suppose larger ice crystals and pores exist.
Note that, in the bimodal PN, different CVs occur: (i) CVs in the very center which contain only pores with larger diameters, (ii) CVs at the very periphery which contain only pores with small diameters and (iii) CVs at the boundary of both regions which contain a mixture of larger and smaller pores.
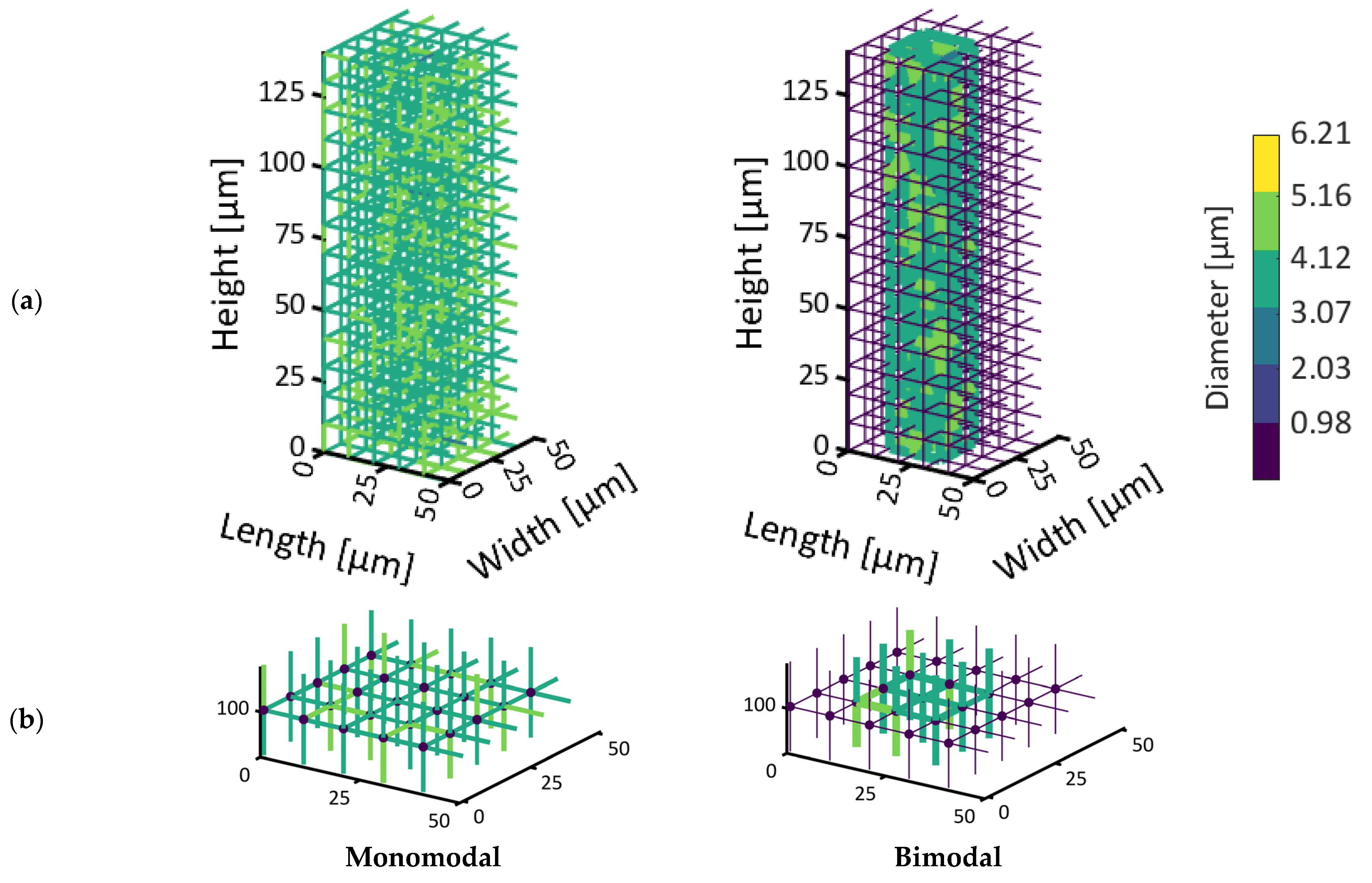
The PNs are shown in
Figure 3. The image in
Figure 3a represents the PN with monomodal PSD, while in
Figure 3b, the PN with bimodal PSD is shown. The color code of the pores refers to their diameter. Brighter colors represent larger pores.
2.2. Model Assumptions and Parameter Settings
The PNM was developed for the simulation of primary freeze drying. The desorption of bound water, which is usually associated with secondary freeze drying, was not considered. The structural properties of the PN were kept constant, i.e., the void space and solid bridges were not altered, e.g., as a consequence of material shrinkage or collapse.
The void space of the PN is initially completely saturated with frozen water. Sublimation is then initiated at the top side of the PN. The bottom side is made impermeable for mass transfer and periodic boundary conditions are applied at the sides. Heat is supplied by contact of the PNs to a virtual heating shelf at the bottom side, following the set-up in [
26]. A constant temperature boundary condition is applied at this side. Radiative heat transfer is neglected in the simulations. The top side of the PN is virtually connected to the bulk of the freeze drying chamber, which is assigned with constant temperature (
T∞) and pressure (
P∞) (
Figure 4). The process parameters are summarized in
Table 2.
Table 3 summarizes the material properties. In this study, the solid is represented by maltodextrin with the material properties given in
Table 3. As can be seen, the physical properties are assumed to be constant, i.e., independent of temperature and pressure.
2.3. Determination of the Properties of the Control Volumes
The geometry of the control volumes (CVs) is schematically illustrated in
Figure 1. Depending on the local variation in the pore sizes, different values of porosity
εCV (
Figure 2b),
effective density
,
effective heat capacity,
as well as effective thermal conductivity,
are computed. In Equation (4), the thermal conductivity in series is given by
and the thermal conductivity in parallel is given by
The final equation is different to previous works [
22], where the orientation of pores was not considered. The factor
is assumed as
a = 0.5 in this study. The saturation of the CVs is calculated from the sum of the ice volume divided by the overall void space of pores contained inside it:
Additionally, the saturation of single pores is calculated analogously.
The effective parameters thus depend both, on the porosity εCV as well as on the saturation SCV of CVs with water ice.
2.4. Mass Transfer
The transfer of water vapor is computed based on the pressure gradients between computational nodes
i and
j that have at least one empty pore neighbor
ij assuming quasi-steady conditions. The mass transfer resistance is thereby determined by the properties of the empty pores surrounding the nodes:
with
and temperature
computed as the arithmetic mean value of the temperatures stored in the adjacent computational nodes,
Ti and
Tj. The length
Lij is the length of the pore, which can be either empty or partially saturated. Thus, the distance between the ice interface and the surface of the pore is not tracked in the current version of the model. Cylindrical cross sections,
are assumed.
In Equation (8),
Kij is computed from the correlation [
32],
with
and Knudsen number
In Equation (10),
denotes the permeability of the dry cake [
32] and in Equation (11), the mean free path of the vapor in empty pores is computed under usage of the ideal gas constant
from
with the arithmetic mean values again used for temperature and pressure.
Applying the law of conservation of mass to each computational node,
the pressure is available from the set of linear equations, with the number of equations identical to the number of computational nodes,
with the matrix of conductances,
and the vector of boundary conditions,
Equation (14) is solved numerically in MATLAB (R2022a) using the following boundary conditions. At the top side, a constant pressure
P∞ is applied, which represents the pressure in the environment of the freeze drying PN. The connection between the surface pores with pressure
Pi to virtual nodes with
P∞ (
Figure 4) is implemented with a constant mass transfer coefficient
g∞:
The value of g∞ = const. is assumed as g∞ = 1∙10−12 m∙s = const. for both PNs in this study. This value was chosen based on preliminary studies of both networks with the condition to not impede the mass transfer at the top side of the PN.
At the sublimation front, thermodynamic equilibrium is assumed and the pressure is calculated based on [
9]:
In this, T* = 273.16 K and P* = 6.1071 hPa are the temperature and pressure at the triple point of water, respectively. The temperature T is the regarding equilibrium temperature at the sublimation front. This links the mass transfer to the heat transfer problem.
2.5. Sublimation Rate
The local phase change or sublimation rate of each ice-filled pore along the gas–solid phase boundary is computed from the mass balances of the computational nodes that are located at the endings of sublimating pores. Because each pore lays between exactly two nodes, it is possible (especially for horizontal pores), but not necessarily a given, that sublimation occurs concurrently at both endings.
Here, only positive vapor flow rates are regarded to accumulate the sublimation rate. Any negative flow rates that might occur due to the flow of vapor from a warmer towards a colder pore are disregarded and thus the condensation of vapor is not computed in this study.
The sublimation rate of single pores determines the amount of heat consumed inside the CV to which this pore belongs to:
Equation (20) couples the mass transfer balance with the enthalpy balance. The ice phase is equally distributed in partially saturated pores. Thus, in a regular PN, the subliming pore contributes to the energy balance of both adjacent CVs, and the sublimation rate is therefore multiplied by a factor of 1/2 in Equation (20).
2.6. Transient Heat Transfer
The transient heat transfer problem is solved for the temperature field associated with the computational nodes and the according CVs with their distributed properties, as already discussed above. Local thermodynamic equilibrium is assumed in each CV, with TCV = Ti = const.
The transient energy balance of each CV reads as
The term on the LHS denotes the change of enthalpy inside the CV that further results in the change in its temperature. The first term on the RHS denotes heat conduction based on Fourier’s law, the second term the heat transferred by the flow of vapor (thermal convection) and the third term the heat consumed by the sublimation of ice. The numerical solution is computed by using the implicit Euler scheme [
33].
The resulting set of linear equations is solved applying the above given boundary and initial conditions analog to the mass transfer problem in Equation (14). The surface pores at the top side of the PN are treated similarly as in Equation (21). The connection to the environment is given by the following condition:
As the bottom CVs are in direct contact with the heating plate with constant temperature, it is realized in the same way, but anticipating solely heat conduction to the boundary pores:
2.7. Coupling of Heat and Mass Transfer
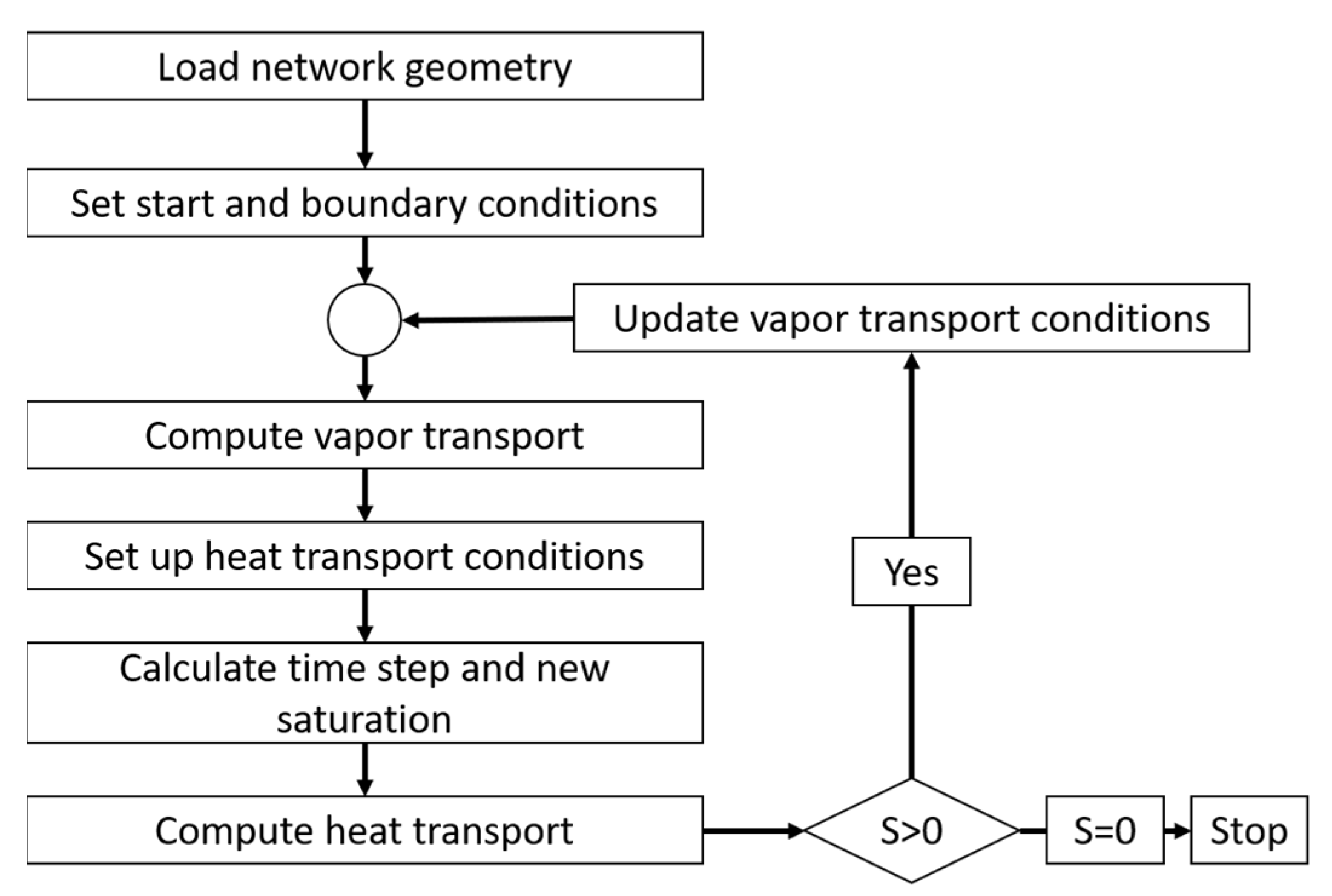
The coupling of heat and mass transfer plays a critical role in the drying algorithm. The heat is consumed for the increase in temperature of the CVs (heating up) as well as for the sublimation of ice along the phase boundary (local cooling). The coupling is considered in the heat and mass balances. A flow chart of the algorithm is provided in
Figure 5. As can be seen, the saturation vapor pressure, the mass transfer conditions and the thermo-physical properties are constantly updated depending on the computed temperature and pressure changes.
It is important to note that the time-constants of the kinetics of heat transfer are very different compared to the mass transfer kinetics in the regarded situation of primary freeze drying. The update of the temperature field can therefore not be coupled to the emptying of a pore, but needs to be realized more frequently. For the stability of the transient heat transfer equations it is necessary to consider the minimum time step
, calculated from the following equations:
and
All symbols and indices used in this study are summarized in
Table 4 below.
4. Conclusions
In this paper, a non-isothermal pore network (PN) model of primary freeze drying with quasi-steady vapor transport and transient heat transfer was presented. To the best of our knowledge, this is the first PN model of freeze drying. It was realized with constant temperature boundary conditions at the top and bottom sides and periodic boundary conditions along the vertical interfaces. The advantage of the PN model is the representation of the continuous void space by discrete pores, which allows one to set-up and solve the mass and enthalpy balances in a discrete way with relatively low computational effort. This makes it possible to study the impact of the porous structure on transport conditions, as well as the evolution and progress of the sublimation front.
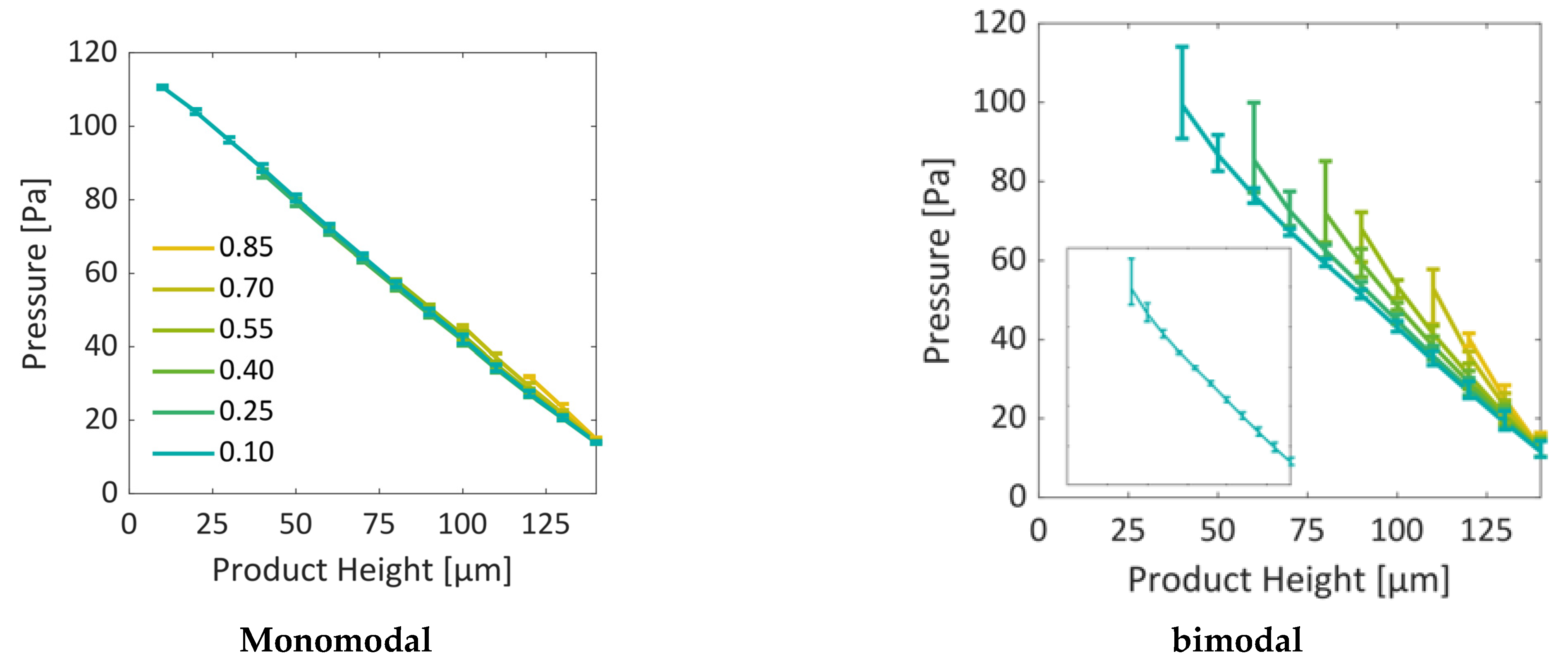
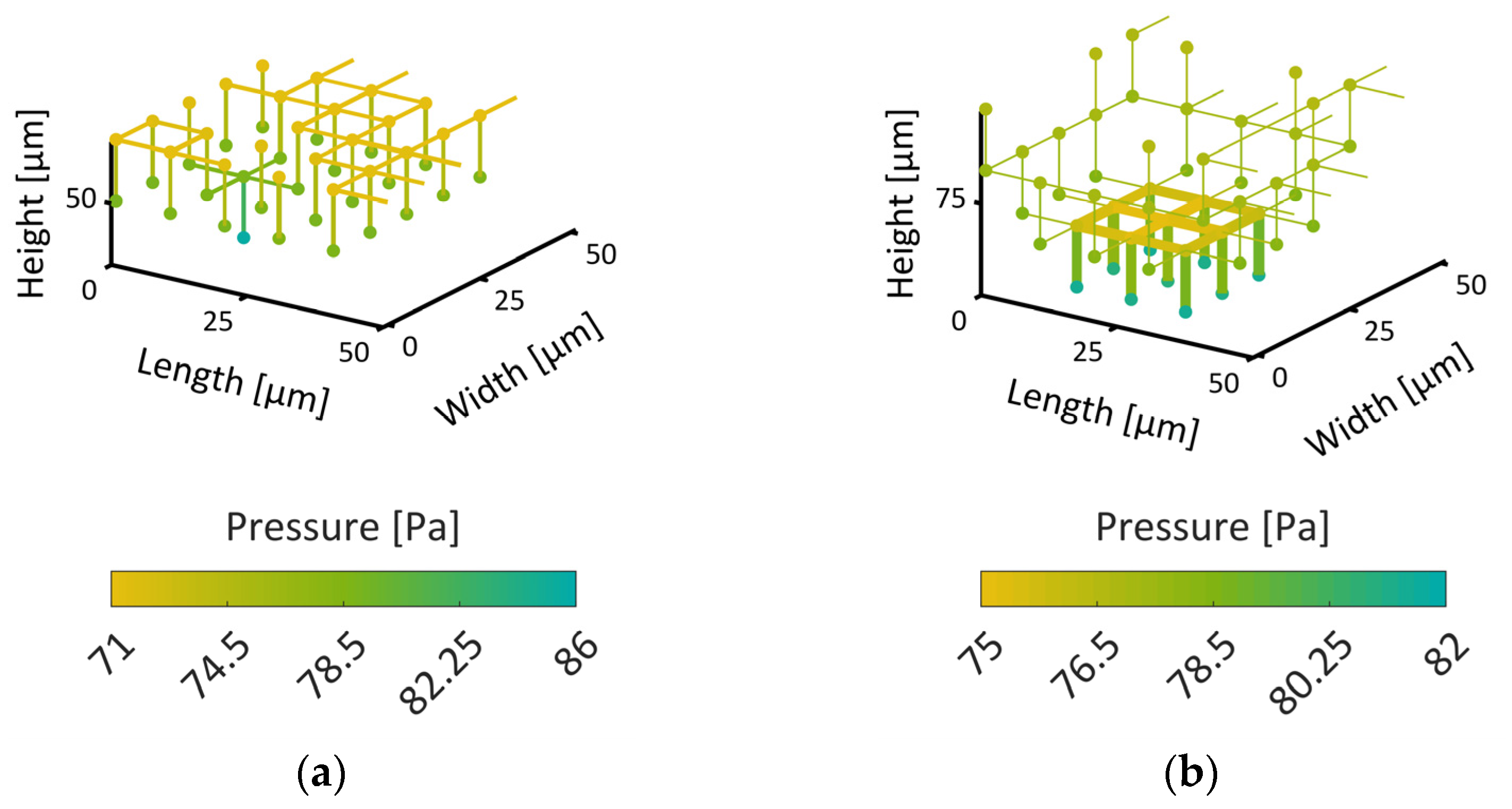
Simulation results were therefore presented for two significantly different situations. In the first case, a homogeneous PN with monomodal PSD was considered, and in the second case, a PN with local variation in pore structure was studied. In the latter case, pores along the vertical sides of the PN were smaller by a factor of 5 than the pores in the center. The first scenario revealed a roughly planar sublimation front, in good agreement with literature references, e.g., [
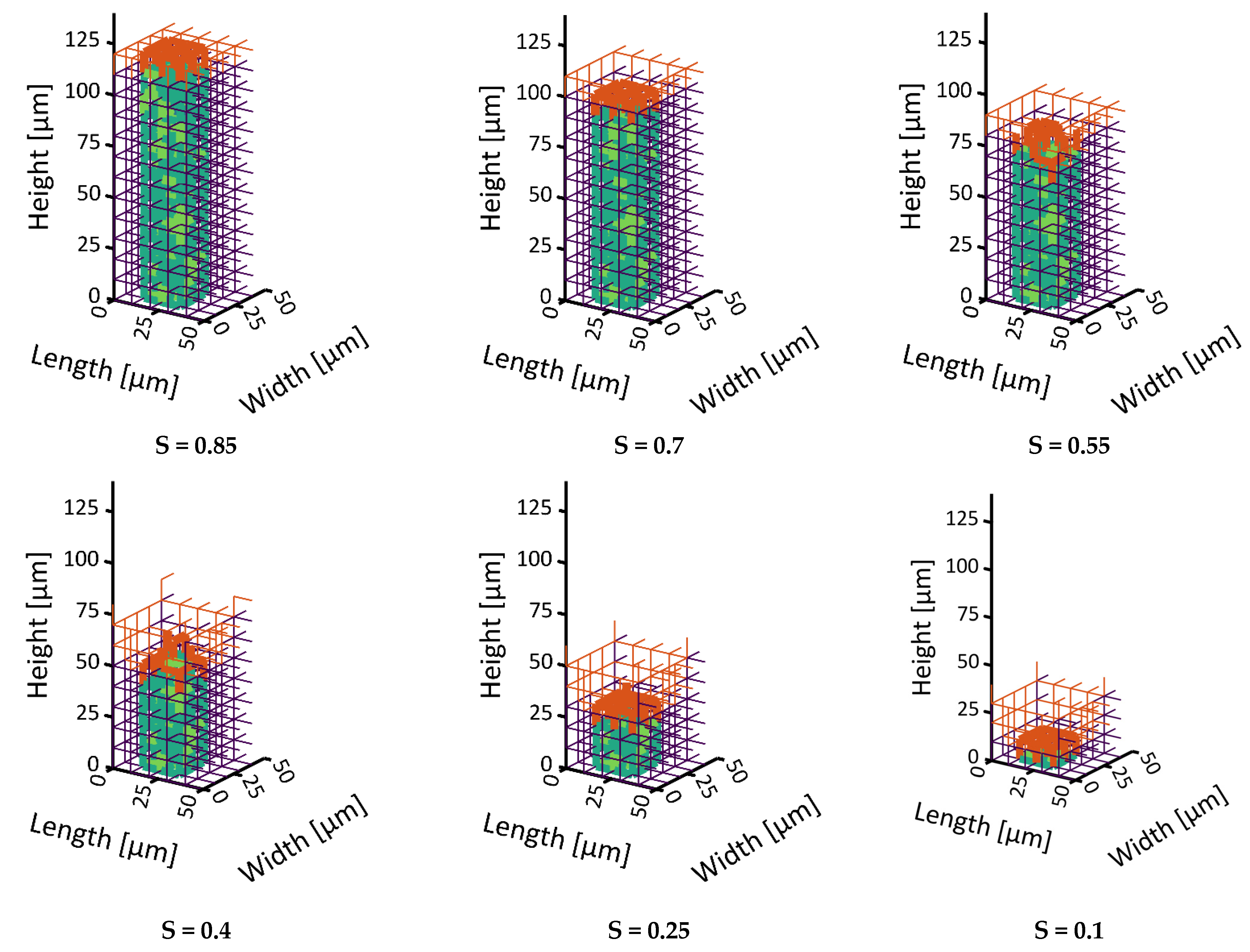
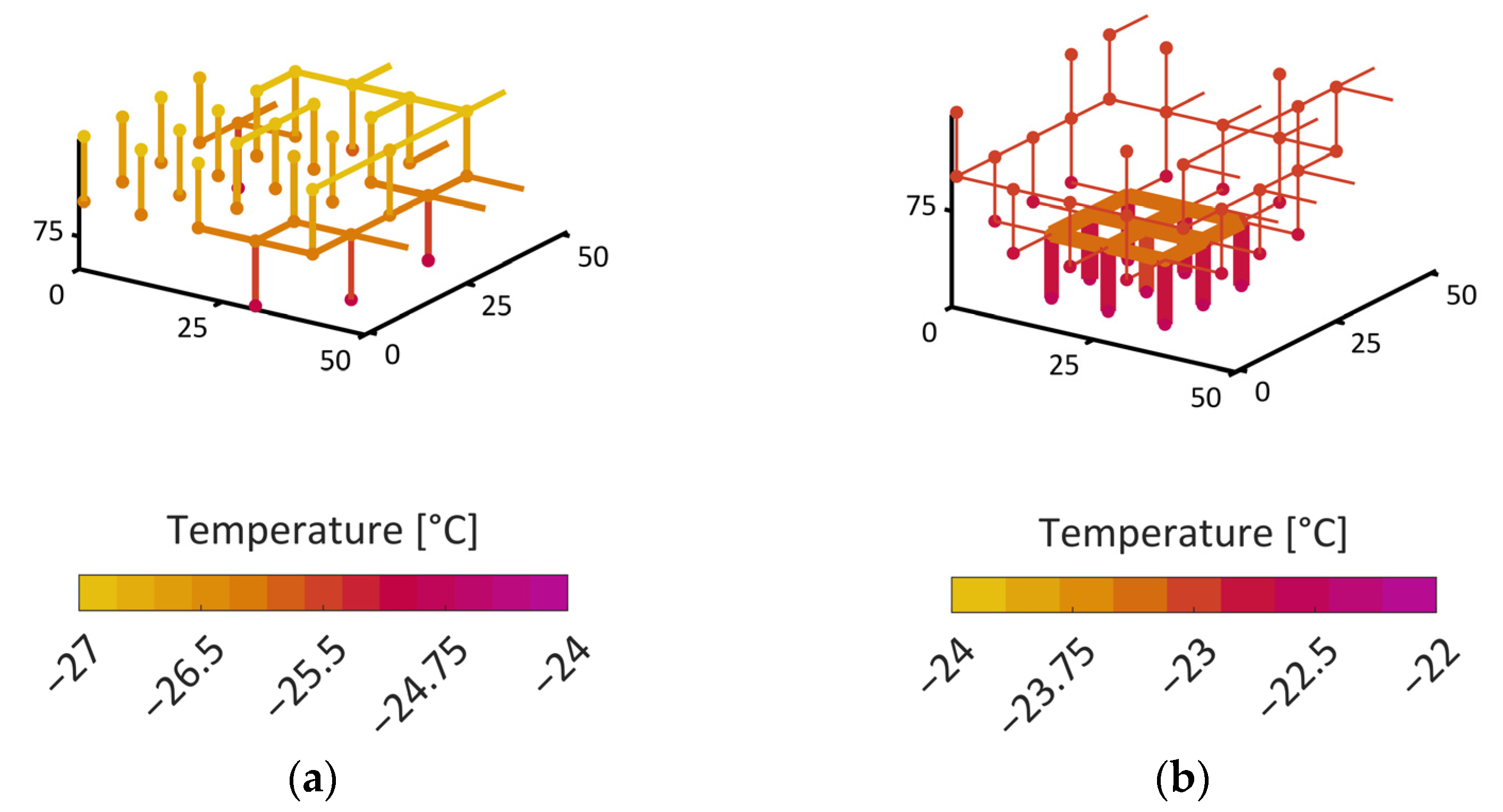
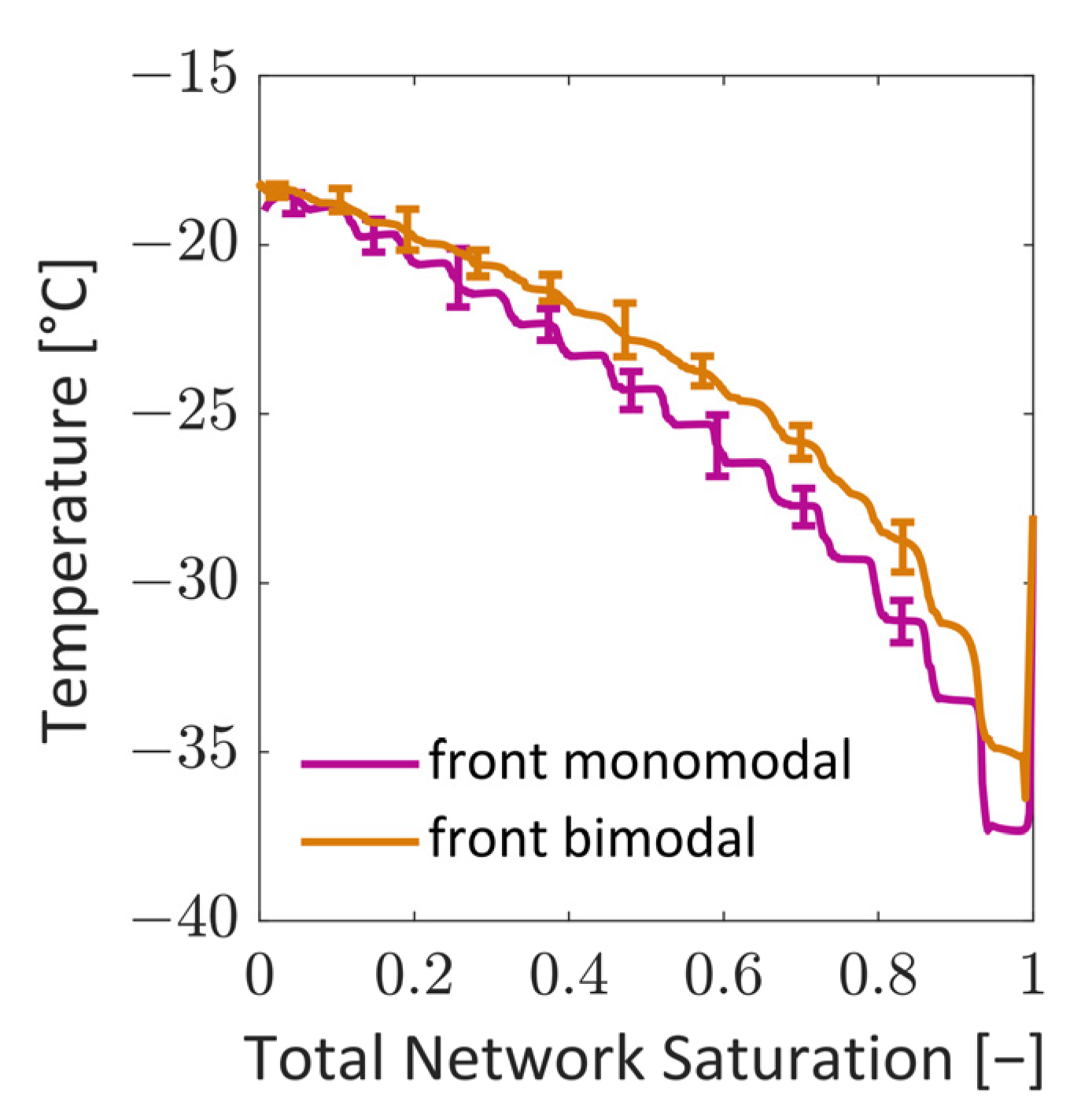
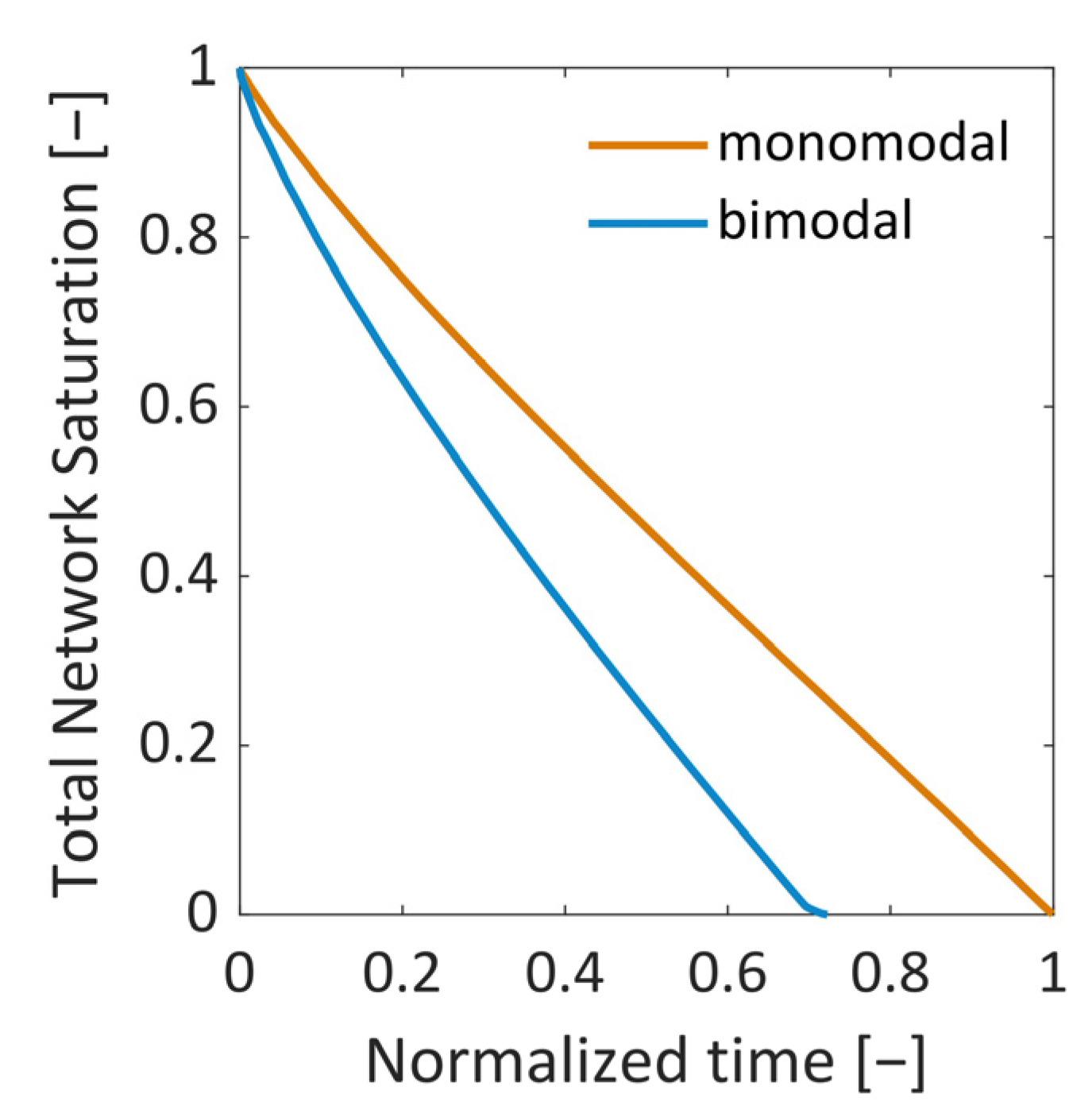
34]. The second situation instead showed the formation of distinct structures of the sublimation front, similar to what has already been observed in experiments. At first, the smaller pores dried faster and yielded a sublimation front which was slightly curved downwards at the sides, i.e., it had a convex shape. This was explained by the high initial mass transfer rates, which were achieved by the high vapor pressure gradients between the PN and the surroundings, as well as the high vapor transport coefficients at the top side of the PN, and the low ice content in the small pores. Once the first row of pores was dried and higher mass transfer resistances occurred inside these pores, the progress of the sublimation front slowed down in this region of the PN. In contrast, the faster drying of the larger pores in the center finally resulted in a concave shape of the sublimation front. The distance between the LAP and the MAP continuously increased until the first pore in the bottom layer dried out. The maximum width of the sublimation front was limited by the small PN height in this study. It is expected that it depends on the spatial variation in the PSD, which could prospectively be studied in larger PNs, where the extension of the sublimation front width would not be limited by the small height of the PN.
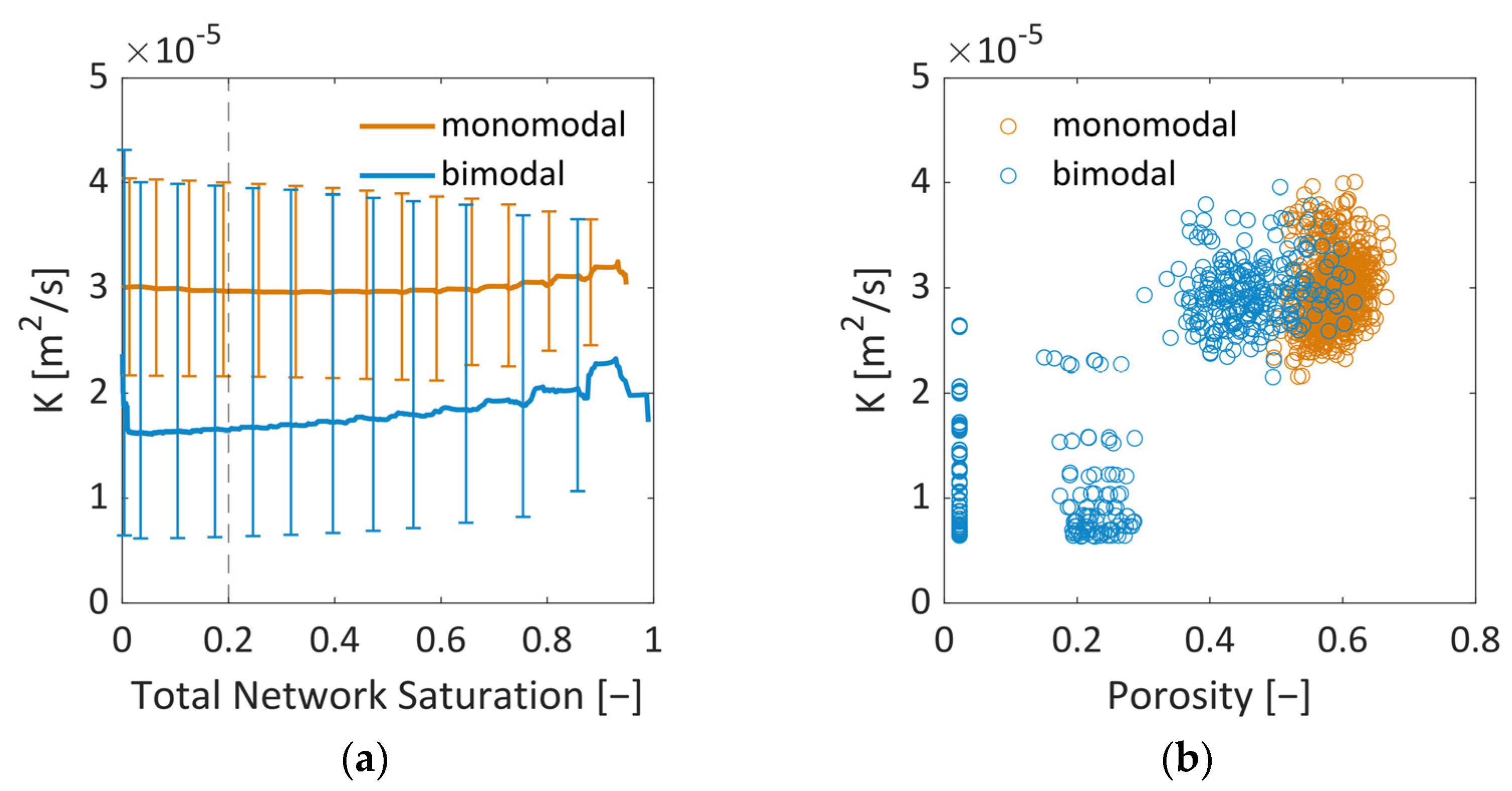
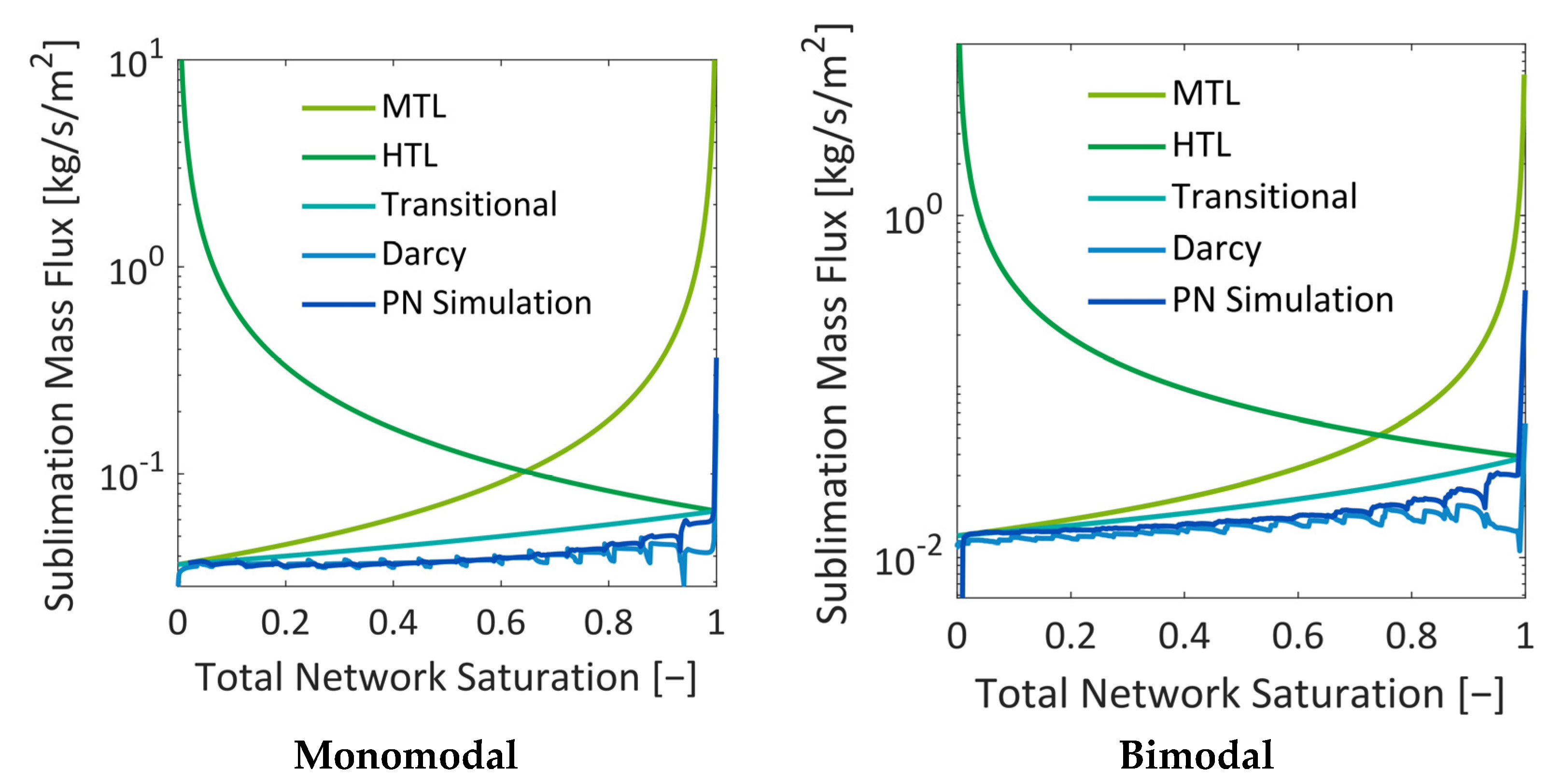
In summary, the influence of the pore structure on the shape of the sublimation front could be nicely demonstrated in this study, although the mass transfer was found to be in the Darcy regime. It could be shown that the increase in mass transfer resistances due to the presence of smaller pores can reduce the overall drying rates in the case of the bimodal PN. This was a major effect in this study. The variation in the thermal properties did indeed change the PN temperatures slightly in the studied cases, but this seemed to not impact strongly on the drying behavior.
The comparison of the sublimation rates with references from the literature showed an overall good agreement, especially for the monomodal PN. However, it also revealed that the pore-scale structure variations and the associated spread of transport parameters (heat and mass) results in stronger deviations. These deviations are expected to become even stronger when the structure is more distributed, e.g., in PNs reconstructed from real porous structures [
21]. Consequently, after the successful demonstration of the new mathematical approach on regular PN lattices in this paper, the algorithm shall be adopted for the simulation of reconstructed porous materials [
21] in the next step.