Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
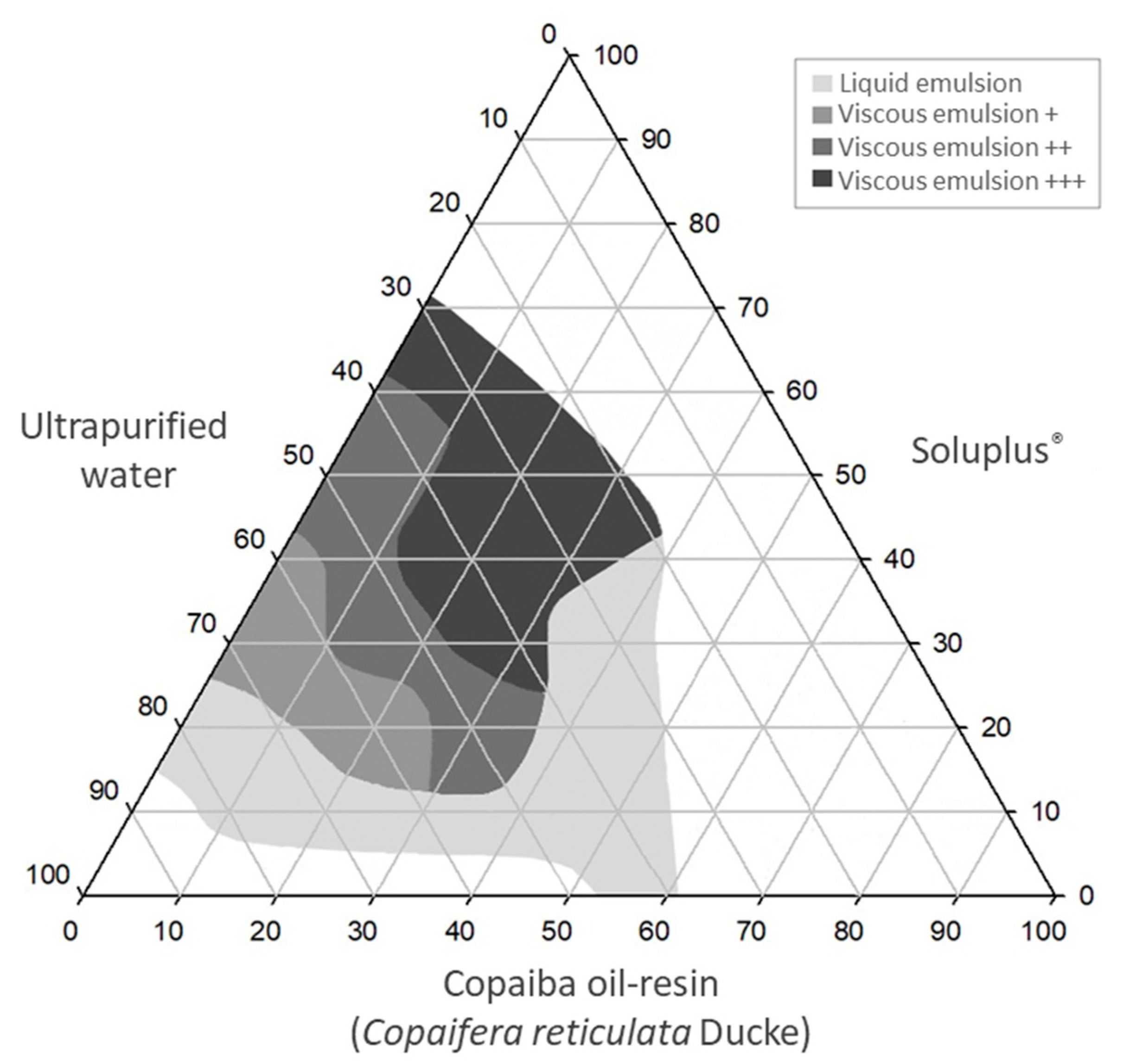
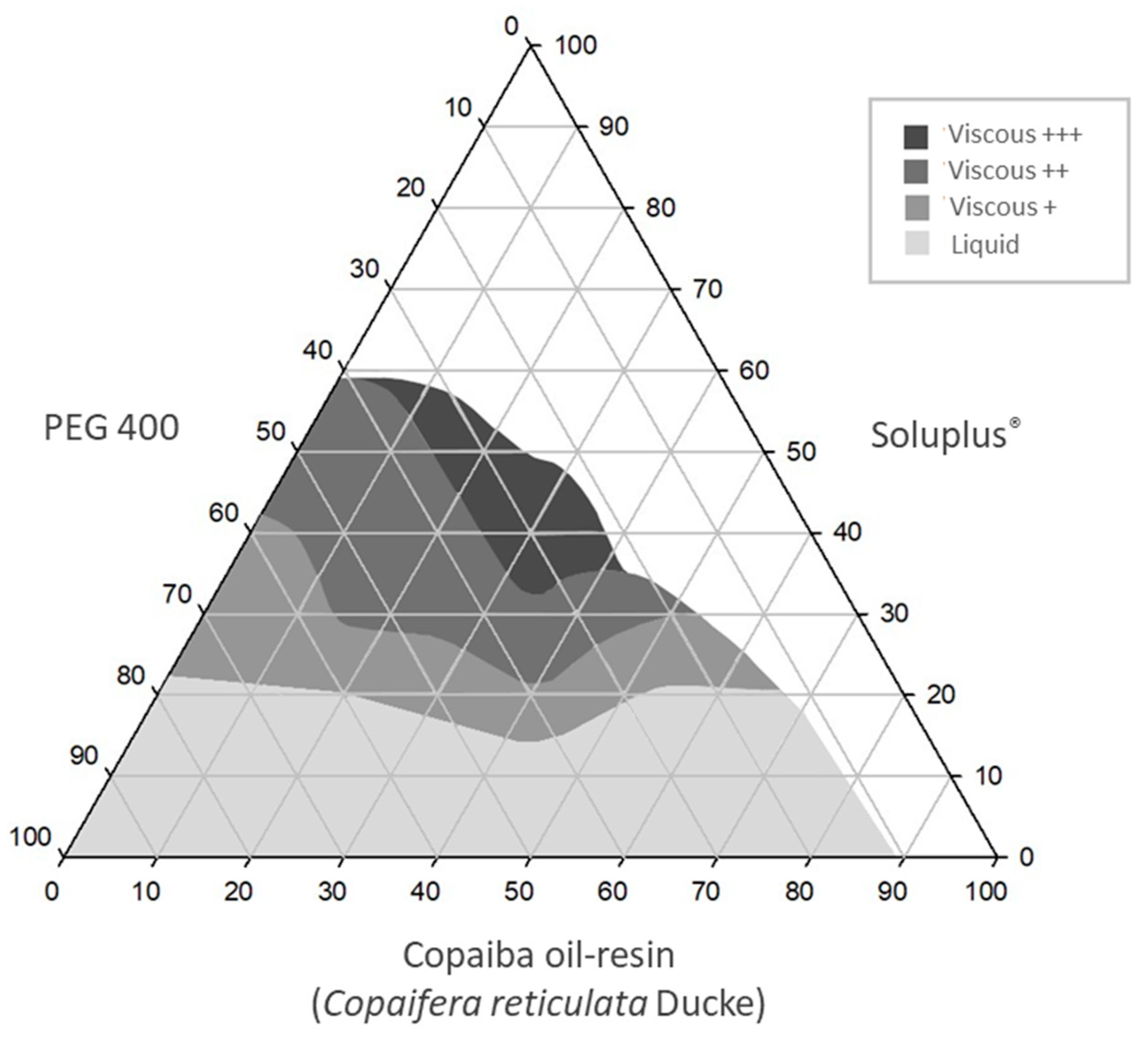
2.2. Construction of Ternary Phase Diagrams
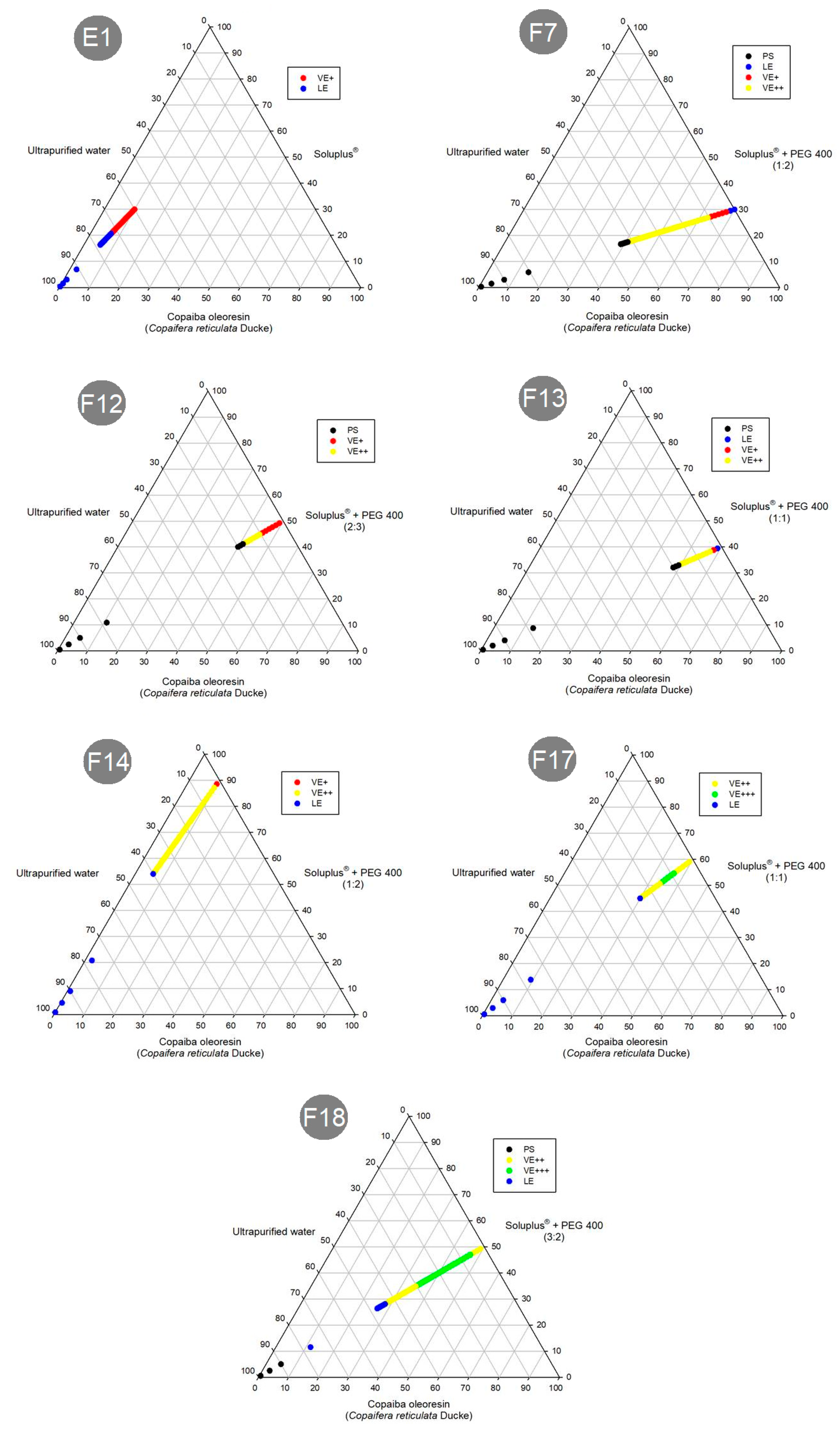
2.3. Preparation of Formulations
2.4. Preliminary Physicochemical Stability Study
2.5. Analysis of Emulsification Properties of Systems
2.5.1. Preparation of Simulated Media
2.5.2. Analysis of Emulsifying Properties
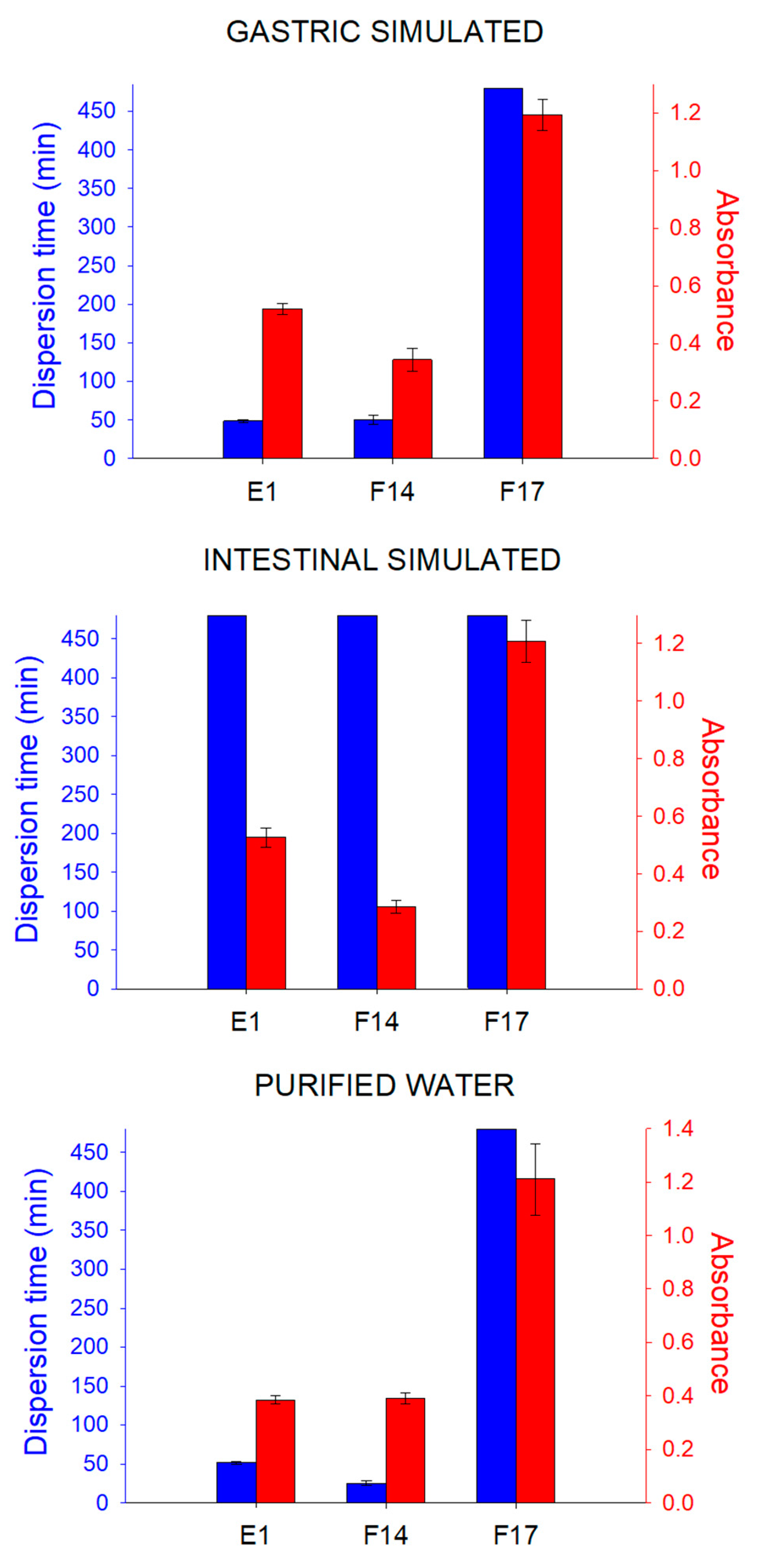
2.5.3. Determination of the Emulsification Time
2.6. Morphological Analysis of Selected Systems
2.6.1. Analysis by Light Microscopy
2.6.2. Analysis by Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.7. Size Analysis and Zeta Potential
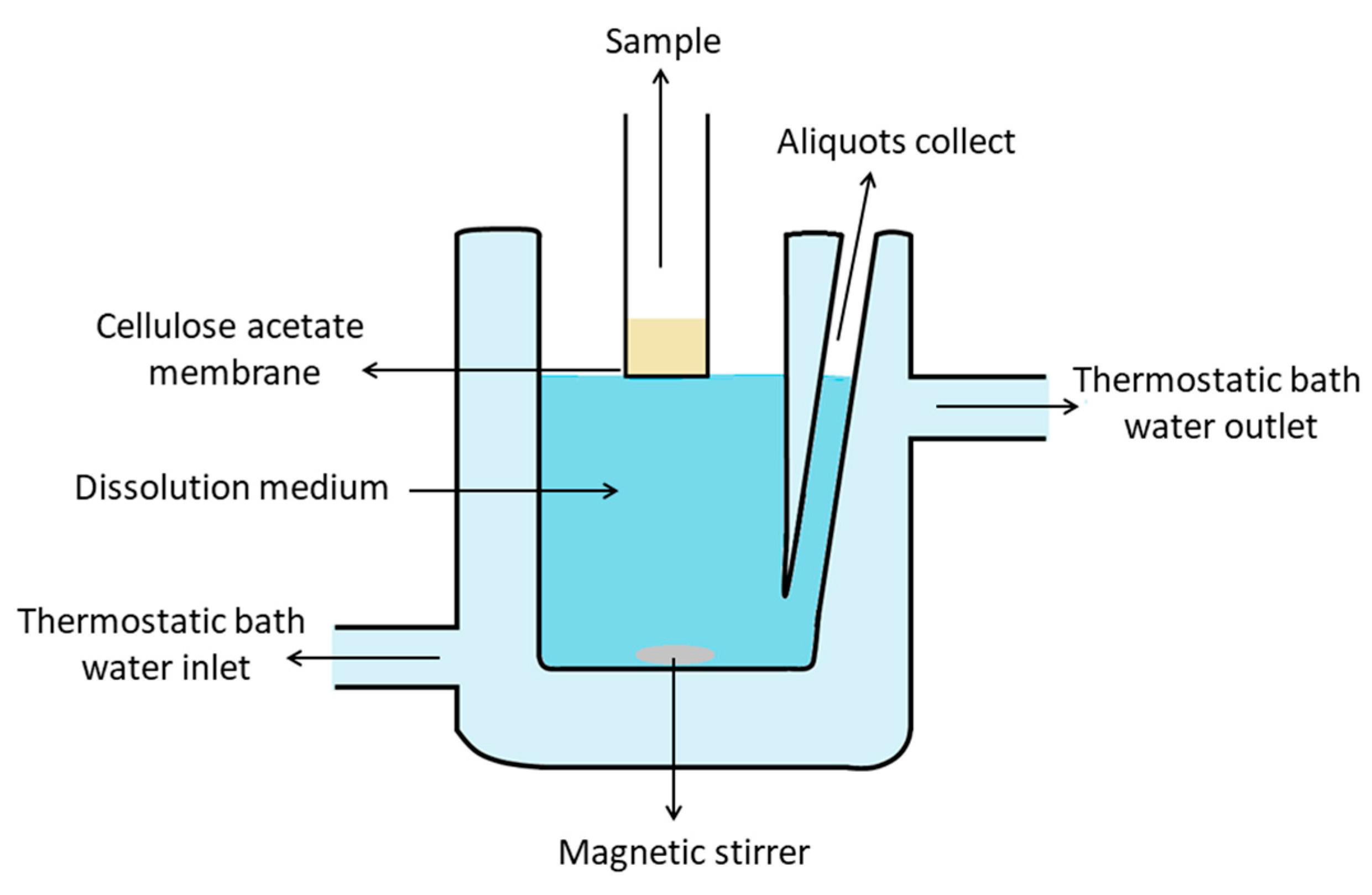
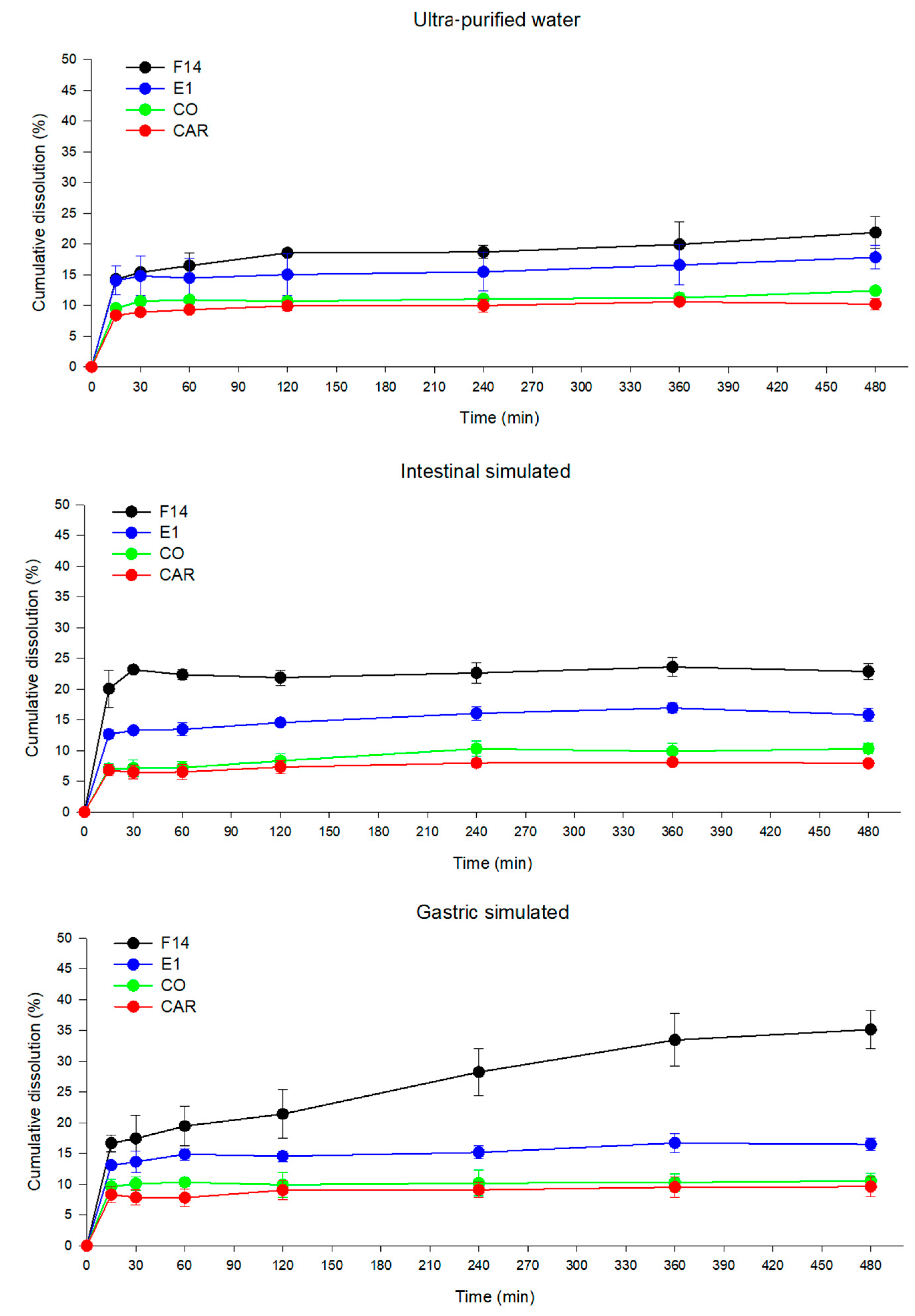
2.8. In Vitro Dissolution Study
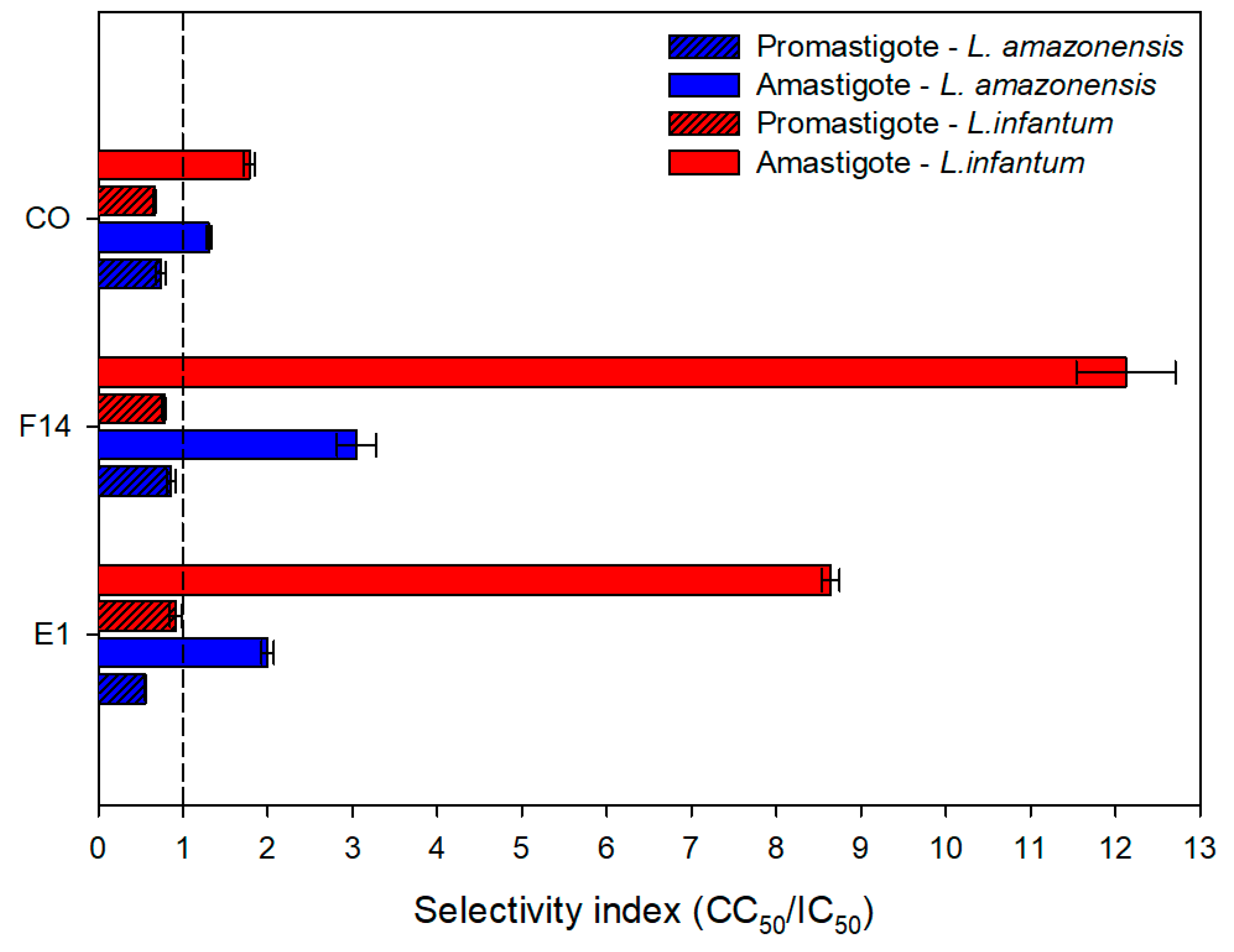
2.9. In Vitro Antiproliferative Activity
2.9.1. Activity against Promastigote Forms
2.9.2. Activity against Amastigote Forms
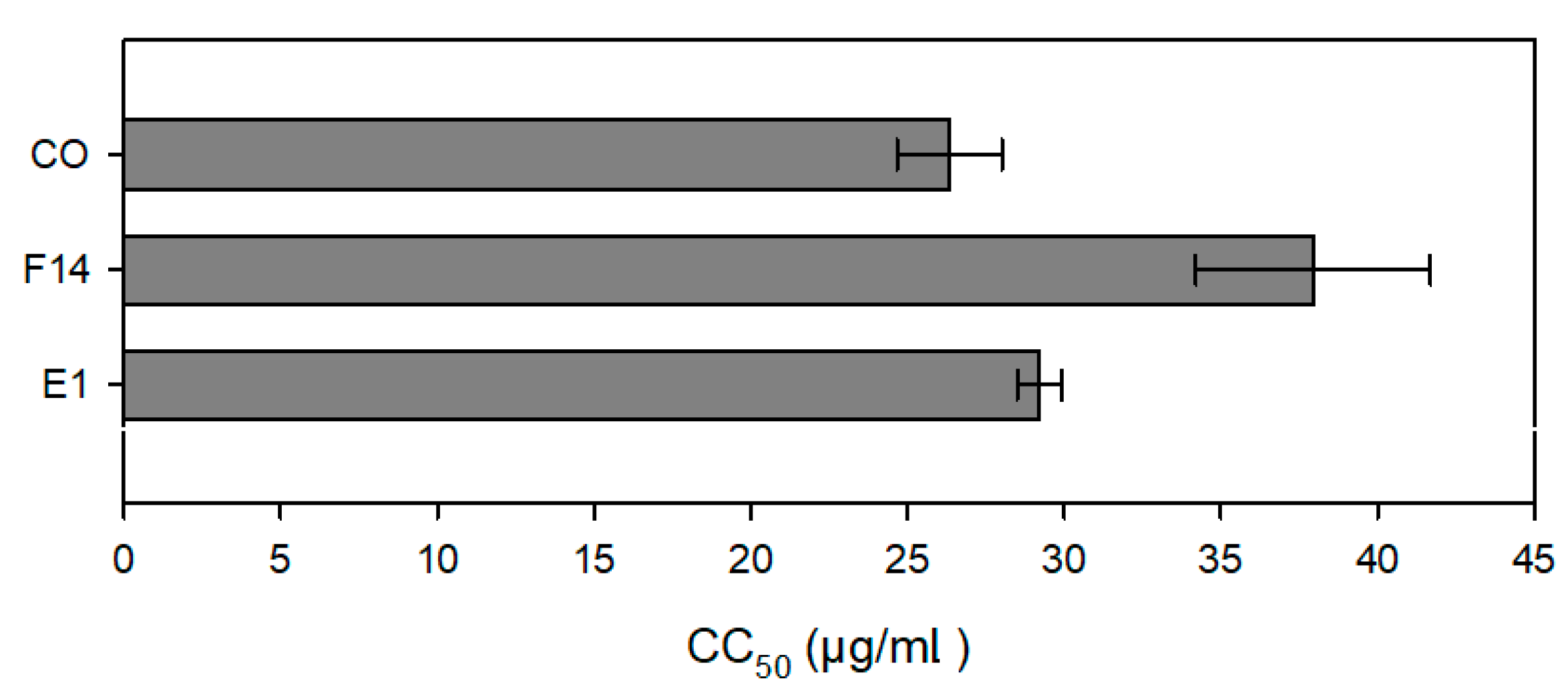
2.10. Cytotoxicity Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Ternary Phase Diagrams
3.2. Preparation and Preliminary Physicochemical Stability Study
3.3. Analysis of Emulsification Properties of Systems
3.4. Morphological Analysis
3.5. Dissolution Study
3.6. Anti-Leishmanial Activity and Cytotoxic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections: A Practical Review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef]
- WHO. Control of the Leishmaniases; WHO: Geneva, Switzerland, 2010.
- Sundar, S.; Chakravarty, J. An Update on Pharmacotherapy for Leishmaniasis. Natl. Inst. Health 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.C.; Rijal, S.; Ostyn, B.; De Vries, P.J.; Singh, R.; Bhattarai, N.; Uranw, S.; Dujardin, J.; Boelaert, M.; Beijnen, J.H.; et al. Failure of Miltefosine in Visceral Leishmaniasis Is Associated With Low Drug Exposure. J. Infect. Dis. 2014, 210, 146–153. [Google Scholar] [CrossRef]
- Monzote, L. Current Treatment of Leishmaniasis: A Review. Open Antimicrob. Agents J. 2009, 1, 9–19. [Google Scholar]
- Nagle, A.S.; Khare, S.; Kumar, A.B.; Supek, F.; Buchynskyy, A.; Mathison, C.J.N.; Chennamaneni, N.K.; Pendem, N.; Buckner, F.S.; Gelb, M.H.; et al. Recent Developments in Drug Discovery for Leishmaniasis and Human African Trypanosomiasis. Am. Chem. Soc. Publ. 2014, 114, 11305–11347. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.M.d.A.; Fonseca Silva, A.; Brandão, M.; Mesquita Grandi, T.S.; Smânia, E.D.F.A.; Smânia, A.; Zani, C.L. Biological Screening of Brazilian Medicinal Plants. Mem. Inst. Oswaldo Cruz 2000, 95, 367–373. [Google Scholar] [CrossRef]
- Souza Brito, A.R.M.; Souza Brito, A.A. Forty Years of Brazilian Medicinal Plant Research. J. Ethnopharmacol. 1993, 39, 53–67. [Google Scholar] [CrossRef]
- Braga, W.F.; Rezende, C.M.; Antunes, O.A.C.; Pinto, Â.C. Terpenoids from Copaiba Cearensis. Phytochemistry 1998, 49, 263–264. [Google Scholar] [CrossRef]
- Falcão, H.d.S.; Lima, I.O.; dos Santos, V.L.; Dantas, H.d.F.; Diniz, M.d.F.F.M.; Barbosa-Filho, J.M.; Batista, L.M. Review of the Plants with Anti-Inflammatory Activity Studied in Brazil. Brazilian J. Pharmacogn. 2005, 15, 381–391. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Pinto, A.C. O gênero Copaifera L. Quim. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- De Albuquerque, K.C.O.; Da Veiga, A.D.S.S.; Da Silva E Silva, J.V.; Brigido, H.P.C.; Ferreira, E.P.D.R.; Costa, E.V.S.; Marinho, A.M.D.R.; Percário, S.; Dolabela, M.F. Brazilian Amazon Traditional Medicine and the Treatment of Difficult to Heal Leishmaniasis Wounds with Copaifera. Hindawi Publ. Corp. Evid.-Based Complement. Altern. Med. 2017, 2017, 8350320. [Google Scholar]
- dos Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; da Veiga Junior, V.F.; Nakamura, C.V. Copaiba Oil: An Alternative to Development of New Drugs against Leishmaniasis. Hindawi Publising Corp. Evid.-Based Complement. Altern. Med. 2012, 2012, 898419. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution an in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.O.; Izumi, E.; Ueda-Nakamura, T.; Dias-Filho, B.P.; da Veiga-Júnior, V.F.; Vataru Nakamura, C. Antileishmanial Activity of Diterpene Acids in Copaiba Oil. Mem. Inst. Oswaldo Cruz 2013, 108, 59–64. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera Demonstrated In Vitro Antiparasitic and Synergic Activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef]
- Mahmood, A.; Bernkop-schnürch, A. SEDDS: A Game Changing Approach for the Oral Administration of Hydrophilic Macromolecular Drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K.M. Aulton’s Pharmaceutics the Design and Manufacture of Medicines, 4th ed.; Aulton, M.E., Taylor, K.M., Eds.; Elsivier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical Characterization and Pharmacokinetics Evaluation of B-Caryophyllene/B-Cyclodextrin Inclusion Complex. Int. J. Pharm. 2013, 450, 304–310. [Google Scholar] [CrossRef]
- Santos, P.S.; Oliveira, T.C.; Júnior, L.M.R.; Figueiras, A.; Nunes, L.C.C. β-Caryophyllene Delivery Systems: Enhancing the Oral Pharmacokinetic and Stability. Curr. Pharm. Des. 2018, 24, 3440–3453. [Google Scholar] [CrossRef]
- Mauro, M.; de Grandis, R.A.; Campos, M.L.; Bauermeister, A.; Peccinini, R.G.; Pavan, F.R.; Lopes, N.P.; De Moraes, N.V. Acid Diterpenes from Copaiba oleoresin (Copaifera langsdorffii): Chemical and Plasma Stability and Intestinal Permeability Using Caco-2 Cells. J. Ethnopharmacol. 2019, 235, 183–189. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, B.; Yang, X.-W.; Xu, W.; Zhang, P.; Zou, L.; Zhang, L.-X. Intestinal Permeability of Sesquiterpenes in the Caco-2 Cell Monolayer Model. Planta Med 2010, 76, 319–324. [Google Scholar] [CrossRef]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral Lipid-Based Drug Delivery Systems—An Overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef]
- Rani, S.; Rana, R.; Saraogi, G.K.; Kumar, V.; Gupta, U. Self-Emulsifying Oral Lipid Drug Delivery Systems: Advances and Challenges. AAPS PharmSciTech 2019, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Kohli, S. Lipids and Surfactants: The inside Story of Lipid-Based Drug Delivery Systems. Crit. Rev. Ther. Drug Carrier Syst. 2018, 35, 99–155. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.C.; Bruschi, M.L. Self-Emulsifying Systems for Delivery of Bioactive Compounds from Natural Origin. AAPS PharmSciTech 2022, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Cespi, M.; Casettari, L.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. Rheological Characterization of Polyvinyl Caprolactam-Polyvinyl Acetate-Polyethylene Glycol Graft Copolymer (Soluplus®) Water Dispersions. Colloid Polym. Sci. 2014, 292, 235–241. [Google Scholar] [CrossRef]
- BASF Technical Information Soluplus®. Tech. Inf. 2019. Available online: https://pharma.basf.com/technicalinformation (accessed on 6 August 2023).
- Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 3rd ed.; The MacMillan Press, London, UK, 2003.
- Krstić, M.; Ražić, S.; Đekić, L.; Dobričić, V.; Momčilović, M.; Vasiljević, D.; Ibrić, S. Application of a mixture experimental design in the optimization of the formulation of solid self-emulsifying drug delivery systems containing carbamazepine. Latin Amer. J. Pharm 2015, 34, 885–894. [Google Scholar]
- ANVISA. Cosmetic Product Stability Guide; Quality Cosmetics Series; ANVISA, Ed.; ANVISA: Brasilia, Brazil, 2004; Volume 1.
- ANVISA Brazilian Pharmacopoeia. Brazilian Pharmacopoeia; ANVISA, Ed.; ANVISA: Brasilia, Brazil, 2019; Volume 1.
- Friedl, J.D.; Steinbring, C.; Zaichik, S.; Le, N.N.; Bernkop-Schurch, A. Cellular Uptake of Self-Emulsifying Drug-Delivery Systems: Polyethylene Glycol versus Polyglycerol Surface. Nanomedicine 2020, 15, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Meshulam, T.; Levitz, S.M.; Diamond, R.D. A Simplified New Assay for Assessment of Fungal Cell Damage with the Tetrazolium Dye, (2,3)-Bis-(2-Methoxy-4-Nitro-5-Sulphenyl)-(2H)-Tetrazolium-5-Carboxanil Ide (XTT). J. Infect. Dis. 1995, 172, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Ferrua, B.; Lang, T.; Maddugoda, M.P.; Munro, P.; Pomares, C.; Lemichez, E.; Marty, P. Luciferase-Expressing Leishmania Infantum Allows the Monitoring of Amastigote Population Size, in Vivo, Ex Vivo and In Vitro. PLoS Negl. Trop. Dis. 2011, 5, e1323. [Google Scholar] [CrossRef] [PubMed]
- Mossmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Dano, M.E.L.; dos Santos, R.S.; Villa Nova, M.; Nakamura, C.V.; Caetano, W.; Bruschi, M.L. HPLC Analysis of β-Caryophyllene in Copaiba Oleoresin: Development, Validation and Applicability. Res. Soc. Dev. 2022, 11, e129111032525. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaiferamultijuga Hayne-A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Bardají, D.K.; da Silva, J.J.; Bianchi, T.C.; de Souza Eugênio, D.; de Oliveira, P.F.; Leandro, L.F.; Martins, C.H. Copaifera reticulata oleo-resin: Chemical characterization and antibacterial properties againstoral pathogens. Anaerobe. 2016, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Borges, V.R.; Ribeiro, A.F.; de Souza Anselmo, C.; Cabral, L.M.C.; de Souza, V.P. Development of a high performance liquid chromatography method for quantification of isomers b-caryophyllene and a-humulene in copaiba oleoresin using the Box-Behnken design. J. Chromatogr. B. 2013, 940, 34–41. [Google Scholar]
- Da Silva, J.A.; Damasceno, B.P.G.d.L.; Borba, V.F.d.C.; Do Egito, E.S.T.; De Santana, D.P. Uso de Diagramas de Fase Pseudoternários Como Ferramenta de Obtenção de Nanoemulsões Transdérmicas. Rev. Bras. Farm 2009, 90, 245–249. [Google Scholar]
- Sinko, P.J. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 5th ed.; Artmed: Guelph, ON, Canada, 2008. [Google Scholar]
- Syed, H.K.; Peh, K.K. Identification of Phases of Various Oil, Surfactant/Co-Surfactants and Water System by Ternary Phase Diagram. Acta Pol. Pharm.-Drug Res. 2014, 71, 301–309. [Google Scholar]
- Ujhelyi, Z.; Vecsernyés, M.; Fehér, P.; Kósa, D.; Arany, P.; Nemes, D.; Sinka, D.; Vasvári, G.; Fenyvesi, F.; Váradi, J.; et al. Physico-Chemical Characterization of Self-Emulsifying Drug Delivery Systems. Drug Discov. Today Technol. 2018, 27, 81–86. [Google Scholar] [CrossRef]
- Singh, A.; Singh, V.; Juyal, D.; Rawat, G. Self Emulsifying Systems: A Review. Asian J. Pharm. 2015, 9, 13–18. [Google Scholar] [CrossRef]
- Gursoy, R.N.; Benita, S. Self-Emulsifying Drug Delivery Systems (SEDDS) for Improved Oral Delivery of Lipophilic Drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Daltin, D. Surfactants: Chemistry, Properties and Applications; Blucher: Vildbjerg, Denmark, 2011. [Google Scholar]
- Gontijo, C.M.F.; Melo, M.N. Leishmaniose Visceral No Brasil: Quadro Atual, Desafios e Perspectivas. Rev. Bras. Epidemiol. 2004, 7, 338–349. [Google Scholar] [CrossRef]
- Wu, H.; Wang, K.; Wang, H.; Chen, F.; Huang, W.; Chen, Y.; Chen, J.; Tao, J.; Wen, X.; Xiong, S. Novel Self-Assembled Tacrolimus Nanoparticles Cross-Linking Thermosensitive Hydrogels for Local Rheumatoid Arthritis Therapy. Colloids Surf. B Biointerfaces 2017, 149, 97–104. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not ? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- WHO. W.H.O. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis (accessed on 15 November 2021).
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Effect of Brazilian Copaiba Oils on Leishmania amazonensis. J. Ethnopharmacol. 2008, 120, 204–208. [Google Scholar] [CrossRef]
- Gontijo, B.; De Carvalho, M.d.L.R. Leishmaniose Tegumentar Americana. Rev. Soc. Bras. Med. Trop. 2003, 36, 71–80. [Google Scholar] [CrossRef]
- Bates, P.A. Transmission of Leishmania Metacyclic Promastigotes by Phlebotomine Sand Flies. Int. J. Parasitol. 2007, 37, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology; Artmed: Guelph, ON, Canada, 2012. [Google Scholar]




| Formulations | Mean Diameter (nm) | Polydispersity Index | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | |
| E1 | 716.07 ± 52.94 | 81.27 ± 4.19 | 0.50 ± 0.01 | 0.31 ± 0.01 | −5.85 ± 0.20 | −14.68 ± 0.12 |
| F14 | 76.50 ± 0.30 | 122.63 ± 2.05 | 0.38 ± 0.01 | 0.23 ± 0.02 | −12.82 ± 0.79 | −18.05 ± 2.52 |
| Sample | CC50 (µg/mL) | L. amazonensis | L. infantum | ||
|---|---|---|---|---|---|
| Promastigote | Amastigote | Promastigote | Amastigote | ||
| IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | ||
| CO | 26.34 ± 1.68 | 35.89 ± 4.98 | 20.20 ± 1.70 | 39.43 ± 1.81 | 14.80 ± 1.48 |
| E1 | 292.06 ± 7.10 (29.21 ± 0.71) | 525.77 ± 19.25 (52.58 ± 1.93) | 146.50 ± 9.06 (14.65 ± 0.91) | 320.47 ± 32.02 (32.05 ± 3.20) | 33.81 ± 1.24 (3.38 ± 0.12) |
| BE1 ** | >2000 | >2000 | >1000 | >2000 | >1000 |
| F14 | 379.38 ± 37.39 (37.94 ± 3.74) | 441.24 ± 17.51 (44.12 ± 1.75) | 124.34 ± 2.77 (12.43 ± 0.28) | 488.13 ± 37.72 (48.81 ± 3.72) | 31.24 ± 1.58 (3.12 ± 0.16) |
| BF14 ** | >1000 | >2000 | >1000 | >2000 | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.C.; Balbinot, R.B.; Villa Nova, M.; Gonçalves, R.S.; Bidóia, D.L.; Caetano, W.; Nakamura, C.V.; Bruschi, M.L. Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment. Pharmaceutics 2023, 15, 2127. https://doi.org/10.3390/pharmaceutics15082127
de Oliveira MC, Balbinot RB, Villa Nova M, Gonçalves RS, Bidóia DL, Caetano W, Nakamura CV, Bruschi ML. Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment. Pharmaceutics. 2023; 15(8):2127. https://doi.org/10.3390/pharmaceutics15082127
Chicago/Turabian Stylede Oliveira, Mariana Carla, Rodolfo Bento Balbinot, Mônica Villa Nova, Renato Sonchini Gonçalves, Danielle Lazarin Bidóia, Wilker Caetano, Celso Vataru Nakamura, and Marcos Luciano Bruschi. 2023. "Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment" Pharmaceutics 15, no. 8: 2127. https://doi.org/10.3390/pharmaceutics15082127
APA Stylede Oliveira, M. C., Balbinot, R. B., Villa Nova, M., Gonçalves, R. S., Bidóia, D. L., Caetano, W., Nakamura, C. V., & Bruschi, M. L. (2023). Development of Environmentally Responsive Self-Emulsifying System Containing Copaiba Oil-Resin for Leishmaniasis Oral Treatment. Pharmaceutics, 15(8), 2127. https://doi.org/10.3390/pharmaceutics15082127







