Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment
Abstract
1. Introduction
2. The Main Elements of PDT and Melanoma
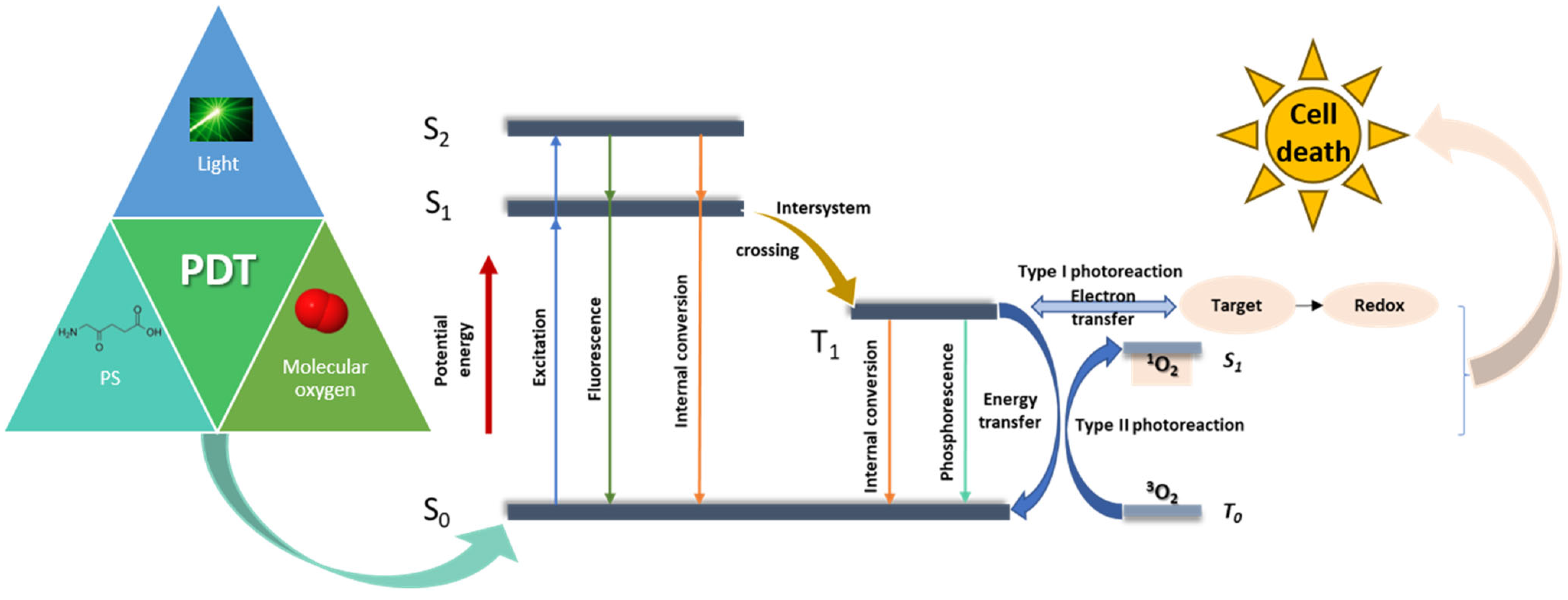
2.1. The Principles of Photodynamic Therapy
2.2. Photosensitizers
| PDT Agents | Activation Wavelength(nm) | Quantum Yield/ Solvent | Reference | |
|---|---|---|---|---|
| Porphyrins | Hematoporphyrins | 350–420, 620–650 | 0.63–0.69/methanol 0.5–0.85/ethanol | [56,57] |
| Protoporphyrins | 405, 500–505, 630–635 | 0.58–0.92/methanol 0.56–0.67/ethanol | [57,58,59] | |
| Metalloporphyrins | 400–435, 500–520,550–560, 590–630 | 0.46–0.59/water 0.36–0.48/chloroform | [60,61] | |
| Pheophorbides | 750–790 | ~1/acetone ~0.5/ micelle solution | [62] | |
| Purpurins | 350–440, 500–550, 600–700 | 0.4–0.82/methanol or MeOD | [63] | |
| Benzoporphyrins | 360–500, 550–600, 670–700 | 0.85/DMF 0.53/DMSO | [64,65] | |
| Chlorins | 380–420, 480–550, 590–660 | 0.43–0.74/pyridine | [66,67] | |
| Phthalocyanines | 350–400, 600–700 | 0.13–0.67/DMSO 0.12–0.62/ water/methanol | [66,68] | |
| Porphycenes | 350–400, 550–600, 630–730 | 0.28–0.36/toluene 0.21–0.28/ D2O/pluronic | [69,70] | |
| Non-porphyrins | Squarines | 550–600, 650–800 | 0.005–0.021/toluene | [71,72] |
| Cyanines | 750–900 | 0.007–0.169/DCM | [73,74] | |
| Xanthenes | 500–570 | 0.75/water | [75] | |
| Phenothiazines | 620–700 | 0.22/water 0.52/DMF | [76] | |
| Curcuminoids | 420–580 | 0.11/toluene/acetonitrile | [77,78] | |
| Boron–dipyrromethene (BODIPY) | 500–580 | ~0.2/toluene | [79,80] |
3. Porphyrins
3.1. Porphyrin Derivatives
| Porphyrin Derivatives | NPs | Parameters | Cell Line | Results | References |
|---|---|---|---|---|---|
| 5-ALA | Au | 628 nm, 220 V, 50 Hz He–Ne laser, 20, 40, 60, and 80 J/cm2 doses | Mel-Rm | The best results were obtained when the 5ALA-gold NP conjugate was irradiated with an optical dose of 60 J/cm2 | [113] |
| 5-ALA | MFLs | 532 nm laser 300 mW/cm2, for 5 min | B16-F10 | The results indicate that at a 5-ALA concentration of 2 mmol/L, the complex after laser irradiation yielded to 20% cell viability. | [116] |
| PpIX (C34H34N4O4) | Poly (D, L lactic-co-glycolic acid) NPs (PLGA NPs) | Wavelength: 630 nm | B16-F10 | PDT effect on melanoma was observed from a low PS concentration in nanocomplex (7.8 µg/mL). | [119] |
| Palladium-meso-tetra (4-carboxyphenyl) porphyrin (PdTCPP) | Layered double hydroxides | 532 nm diode laser, 250 ± 5 mW cm−2 | B16-F10 | LDH-PdTCPP PS induces cytotoxicity against the B16-F10 melanoma cell line. PDT using the LDH-PdTCPP complex reduced tumour growth in mice sevenfold compared to the control group. | [126] |
| Photo protoporphyrin IX dimethyl ester (PppIX-DME). | Polyethylene glycol and polylactic acid (PN-Por) | Halogen light source, 15 s irradiation time in vitro, using light guide and cut-of wavelengths below 600 nm | B16-F10 | The effectiveness of the nanoparticle complex is given by PDT irradiation. The complex of porphyrin derivative and polymeric NPs did not show tumour tissue specificity. | [127] |
| PpIX (C34H34N4O4) | Polysilsesquioxane (PSilQ) NPs | 630 nm, 24.5 mW/cm2, 20 min | A375 | The phototoxicity of the complex was nine times lower than the phototoxicity of porphyrin, thus reducing the side effects of NPs on normal tissue. PpIX-PSilQ NPs showed no cytotoxicity even at equivalent concentrations of PpIX as high as 250 µM. | [120] |
| Verteporfin (Ver) | Silica NPs (SNPs) | Wavelength: 650 nm | A375P and SK-MEL 28 | After irradiation, proliferation of Ver-MSN-treated normal cells and inhibitory effects on tumour cell lines showed a lower metastatic effect in the case of the A375P line. After irradiation, SK-MEL 28 cell proliferation was reduced to half for Ver-MSN nanocomplexes, compared with the free Ver, which inhibits the cell growth by only 30%. | [121] |
| 5,10,15,20-(Tetra-4-sulfonatophenyl) porphyrin tetraammo-nium (TPPS) | γ-Fe2O3 NPs (Iron Oxide NPs) | Wavelength: 405 nm,1 min blue light exposure, 1 mW/cm2 | MelJuso | TPPS photodynamic activity had a significant increase via conjugation with γ-Fe2O3 NPs at a very low irradiation dose (1 mW/cm2 irradiation intensity and 1 min. of exposure) and with cytotoxicity at 1 μg/mL. Antitumour effect of γ-Fe2O3 NPs-TPPS for human melanoma cells subjected to PDT, through the generation of singlet oxygen. A 55% decrease in Mel-Juso cells treated with γ-Fe2O3 NPs-TPPS. | [124] |
| 5,10,15,20-(Tetra-N-methyl-4-pyridyl) porphyrin tetratosylate (TMPyP4) | TiO2 NPs (Titanium Dioxide NPs) | 405 nm LED, 1 mW/cm2, 7.5 min exposure time | MelJuso and CCD-1070Sk | The TMPyP4-TiO2 NPs conjugates enhance the porphyrin efficiency against human melanoma MelJuso cells while being less phototoxic on normal CCD-1070Sk skin fibroblasts, thus having a greater selectivity on cancer cells. | [125] |
| 5-(4-ethynylphenyl)-10,15,20-tris-(4-methylpyridin-4-ium1-yl)porphyrin-zinc(II) triiodide (Zn-EpPor) | Nanotubes formed by nucleoprotein components of the tobacco mosaic virus (TMV) | White light from a Vivitek D950HD projector (~10 mW cm−2 at 430 nm) for 30 min | B16-F10 | The Zn-EpPorTMV complex demonstrated significant anti-melanoma activity with a 40% increase in cellular uptake compared to Free Zn-EpPor and an IC50 value of 0.24 µM after 30 min of irradiation. | [128] |
3.2. Chlorins
| Chlorins | NPs | Parameters | Cell Line | Results | Ref |
|---|---|---|---|---|---|
| Chlorin p6 | Iron oxide | 630 nm 37 J/cm2 fluence | B16-F10 B16-G4F | The highest phototoxic effect was obtained on B16-F10 cell line after irradiation indicated a possible specificity of the nanocomplex. | [131] |
| Chlorin e6 | Superparamagnetic iron oxide | 690 nm laser irradiation 0.5 W/cm2, 30 s | B16-F10 | Chlorin e6 and doxorubicin conjugated with coated polyglycerol NPs have increased absorption in melanoma cells and enhanced photocytotoxicity. | [140] |
| Chlorin e6 | Liposomes | In vitro: 660 nm continuous laser (50 mW/cm2, 5 min). In vivo: 660 nm laser (200 mW/cm2, 10 min) | A375 | PGIL synergistically achieves a high-efficiency PDT effect by enhancing apoptosis, inhibiting invasion, and boosting NK cell-related immune effects in melanoma cells. | [141] |
| Chlorin e6 | Polymer DSPE–PEG2000–biotin | In vitro 2D: ultrasound radiation 1 MHz, 50% duty cycle, 1.5 W/cm2, 2 min and/or LED irradiation 405 nm, 0.5 J/cm2; In vitro 3D: ultrasound radiation 1 MHz, 50% duty cycle, 1.5 W/cm2, 2 min and/or two-photon laser excitation at 730 nm, 15 mW, 5 min; In vivo: ultrasound radiation 1 MHz, 50% duty cycle, 1.5 W/cm2, 2 min 730 nm, 35 mW, 5 min | A375 | In vitro and in vivo tests show that synergistic action of the ultrasonic and light irradiation of the complex lead to enhanced cytotoxic effect and almost fully eradicate the melanoma tumour in the mouse model. | [132] |
| Chlorin e6 | Aluminium-albumin | In vitro: 660 nm laser irradiation, 0.8 W/cm2, 5 min In vivo: 660 nm laser irradiation, 0.8 W/cm2, 5 min, single dose in day 0 | B16-F10 | Al-BSA-Ce6 NPs inhibited growth of the first tumour, significantly prolonged survival, also reduced possibility of recurrence, and inhibited growth of tumour cells in distal and lung metastases by stimulating specific tumour immune response. | [142] |
| Chlorophyll | Pluronic F68 nanocomposite | In vitro: 671-nm laser irradiation, 20 min In vivo: 671 nm laser, 20 min, at 24 h, for 15 days | A375 | The photothermal and PDT effect of encapsulated vegetable chlorophyll into Pluronic F68 polymeric micelle proved high efficiency against melanoma in vitro and in vivo compared with non-encapsulated. | [143] |
| Ferrous chlorophyllin | Liposomes | monochromatic red laser 652 nm, 200 mW/cm2 56.2 J/cm2 | B16-F10 | The cellular uptake of liposomes increased over time (6 to 24 h) via endocytosis, with preferential accumulation in the mitochondria and nucleus; after depigmentation, PDT with liposomes containing Fe-CHL resulted in an LC50 value of 1.77 μM after 48 h incubation, causing cell death through a combination of apoptosis and necrosis. | [41] |
3.3. Phthalocyanines
| Phthalocyanine Derivatives | NPs | Parameters | Cell Line | Results | Ref |
|---|---|---|---|---|---|
| Zinc phthalocyanine (ZnPc) | Magnetic fluid, containing citrate-coated maghemite NPs | Magnetohyperthermia assay: AC magnetic field operating at 1 MHz and 40 Oe amplitude, for 3 min PDT assay: 670 nm, laser diode, 600 mW average power, at 84 mW/cm2 light irradiance, light dose between 0.5 and 2 J/cm2 | B16-F10 | The combined application of laser light and AC magnetic field on B16-F10 cells incubated with the magnetoliposome formulation resulted in a significant reduction in cell viability compared with PDT or magnetic field alone. | [147] |
| ZnPc | Polylactic acid (PLA)/ polyvinyl alcohol (PVA) | 660 nm laser, irradiance of 28 J/cm2 for 2.5 min | MV3 | PLA/PVA-encapsulated dacarbazine and ZnPc substantially augmented cell death in MV3 cells following PDT. | [148] |
| ZnPc | Gold-PEG conjugates | Halogen lamp (620–700) nm, fluence rate of 175 mW/cm2, total fluence of 157 J/cm2, 15 min | B78H1 | Irradiation of the amelanotic melanoma at 3 h following i.v. injection of the ZnPc-AuNPs-PEG conjugates induced a photodynamic destruction of the tumour. In addition, 40% of the mice were completely cured, with no tumour regrowth. | [149] |
| Aluminium chloride phthalocyanine (ClAlPc) | SLN | 670 nm, diode Eagle laser, average power of 0.30 mW and a light radiance of 17 mW/cm2; 0.5, 1.0, and 2.0 J/cm2 light doses | B16-F10 | The best results were obtained when the ClAlPc-SLN complex was used at the highest dosages: 2.0 J/cm2.with a cell viability of 15.06%. | [151] |
| ClAlPc | NLC and SLN | 630 nm LED, total fluence of 25.3 J/cm2 | BF16-F10 | ClAlPc-free exhibited 100% cell viability regardless of LED irradiation, while NLC 40 at concentration of 0.2 μg/mL decreased the cell viability to 0.93%. Without irradiation, NLC 40 caused a significant reduction in cell viability, with 12% at a concentration of 0.2 μg/mL of the drug. | [152] |
| Aluminium phthalocyanine (AlPc) | SLN | 660 nm LED, 10 min, at 10 cm distance, with 25.88 J/cm2 fluence | B16-F10 | After LED exposure, SLN-AlPc demonstrated a decrease in cell viability, demonstrating the potential of PDT for the targeted killing of cancer cells. Higher PDT activity observed with SLN-AlPc-20μM. | [153] |
| Zn(II)-phthalocyanine disulphide (C11Pc) | Gold | 600–700 nm wavelength range form quartz-halogen lamp; at a fluence-rate of 175 mW/cm2 for a total fluence of 157 J/cm2 | B78H1 transplanted in C57 mice | PDT studies show that tumour growth is slowed following light activation of C11Pc conjugated to gold NP. | [150] |
4. Non-Porphyrin Photosensitizers
| Non-Porphyrin PS | NPs | Parameters | Cell Line | Results | Ref |
|---|---|---|---|---|---|
| ICG | HA-PAMAM-COOH | 808 nm, 1.5 W/cm2, 10 min | A375 | Almost total tumour ablation due to synergistic effect of PDT, PTT, and chemotherapy after treatment with ICG- and TMZ-loaded HA-PAMAM-COOH exposed to NIR radiation. | [159] |
| ICG | AuBPs | 785 nm, 190 mW, 15 min | B16-F10 | More than 90% of the melanoma cells were killed due to the combined PDT/PTT effect after treatment with hybrid nanosystem AuBPs@PLA@ICG@FA. | [161] |
| ICG | MnO2@BSA | 808 nm, 1 W/cm2, 5 min | B16-F10 | A 65–75% kill rate of the melanoma cells, according to used MnO2-ICG@BSA concentrations, due to PDT/PTT effect. | [163] |
| ICG | Chitosan-coated liposomes | 775 nm, 250 mW, 2.5 min | B16-F10 | Cytotoxicity was enhanced by chitosan concentration. At 0.1% chitosan concentration, ICG-loaded liposomes’ cytotoxicity was increased by irradiation (approximately 70% cell viability). | [164] |
| IR820 | CAT-PLGA-HA | 808 nm, power density of 4 W/cm2, 5 min | MV3, M14, and A375 | The novel drug delivery nanoplatform could be used to alleviate hypoxia in the tumour microenvironment and improve the efficacy of PDT, providing a foundation for further research into novel melanoma treatment techniques. | [170] |
| RB | NH2-MSNs | 540 nm, 5 min | SK-MEL-28 | Reduced cell proliferation after irradiation. | [171] |
| RB + NaYF4:Er3+,Yb3+ | PLGA | 520–560 nm, 10 J/cm2, 5 min | MeWo and Me-45 | Significant reduction in cell viability for both melanoma cell lines (>90% for MeWo). | [173] |
| RB + NaYF4:Er3+,Yb3+ | PLGA | 980 nm, 6.2 W/cm2, 5 min | MeWo | Restructuration and destabilization of cytoskeletons F-actin fibres. | [173] |
| RB conjugated with methoxypolyethylene glycol | Self-assembly hydrophilic NP | λ > 500 nm, 27 J/cm2, 30 min | HepG2, MCF-7 and B16-F10 | Boosted cellular uptake, enhanced intracellular ROS generation, improved synergistic anticancer efficacy. | [174] |
| MB | HGNSs-PEG | 670 nm, 14.9 mW/cm2, 9 min | DFW and MCF-7 | A 2% cell survival rate for DFW and a 3% cell survival rate for MCF-7, at a HGNSs-PEG-MB concentration of 20µM. | [175] |
| MB + veliparib | PLGA | 660 nm, 102 J/cm2 | B16-F10-Nex2 | Cell viability decreased by 36% for 1 µM concentration of MB and 8.3 µM concentration of veliparib. | [176] |
| Quinizarin | Poly-(methyl methacrylate) | blue LED 450 (±20 nm), fluences ranging from 1.0 to 25 J.cm−2 | B16-F10 | PDT assays demonstrated significantly increased cell death with higher QZ-PP-NP concentration and LED light fluence, mainly inducing apoptotic cell death. | [177] |
| I2-BDP | UiO-66 | visible light irradiation at a power density of 80 mW cm−2 for 10 min | B16-F10 | The IC50 for the light-activated UiO-PDT on B16-F10 cell line was obtained at a concentration of 0.70, μg mL−1. | [178] |
| I2BDP | PCN-222 | 405 nm, 4 mW/cm2, 3 h | B16-F10 | Irradiation of PCN–I2BDP(Nano) with light induces high cytotoxic activity. | [179] |
| Hyp | F127-FA/ F127-SN/ F127-BT | 550−625 nm, 35 mW/cm2, 46.8 J/cm2 light dose, 40 min | B16-F10 | CC50 of 0.24 ± 0.02 µmol/L for Hyp—F127-BT | [180] |
| Curcumin | Silica | blue LED 465 nm; 34 mW/cm2 | A375 | Curcumin–Si nanocomplex in PDT displays low toxicity on normal cells, but toxic effect on A375 cells at 50 µg/mL, inducing apoptosis, inhibiting cancer cell proliferation, and enhancing intracellular ROS generation. | [181] |
| ISQ | BSA-AuNC@AuNR | 808 nm, power density of 1 W/cm2, 4 min | A375 | The combined effect of the targeted photo- and chemotherapies produced an appreciable toxicity to the melanoma cells. | [182] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; De Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495. [Google Scholar] [CrossRef] [PubMed]
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 25 May 2023).
- Zeng, L.; Gowda, B.H.J.; Ahmed, M.G.; Abourehab, M.A.S.; Chen, Z.-S.; Zhang, C.; Li, J.; Kesharwani, P. Advancements in Nanoparticle-Based Treatment Approaches for Skin Cancer Therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular Genetics, Metastasis, Targeted Therapies, Immunotherapies, and Therapeutic Resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.L.S.A.; Novoloaca, A.; Cahais, V.; Awada, Z.; Cuenin, C.; Spitz, N.; Carvalho, A.L.; Evangelista, A.F.; Crovador, C.S.; Reis, R.M.; et al. Cutaneous and Acral Melanoma Cross-OMICs Reveals Prognostic Cancer Drivers Associated with Pathobiology and Ultraviolet Exposure. Nat. Commun. 2022, 13, 4115. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Neagu, M.; Caruntu, C.; Constantin, C.; Boda, D.; Zurac, S.; Spandidos, D.A.; Tsatsakis, A.M. Chemically Induced Skin Carcinogenesis: Updates in Experimental Models (Review). Oncol. Rep. 2016, 35, 2516–2528. [Google Scholar] [CrossRef]
- Risks and Causes of Skin Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/skin-cancer/risks-causes (accessed on 25 May 2023).
- Griffith, C.F. Skin Cancer in Immunosuppressed Patients. JAAPA 2022, 35, 19. [Google Scholar] [CrossRef]
- Wang, L.L.; Lin, S.K.; Stull, C.M.; Shin, T.M.; Higgins, H.W.; Giordano, C.N.; McMurray, S.L.; Etzkorn, J.R.; Miller, C.J.; Walker, J.L. Cutaneous Oncology in the Immunosuppressed. Dermatol. Clin. 2023, 41, 141–162. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Hou, K.; Wu, Y.; Chen, H.; Ning, Z. Melanoma and Nanotechnology-Based Treatment. Front. Oncol. 2022, 12, 858185. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and Treatment of Melanoma. European Consensus-Based Interdisciplinary Guideline—Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Wada-Ohno, M.; Ito, T.; Furue, M. Adjuvant Therapy for Melanoma. Curr. Treat. Options Oncol. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef] [PubMed]
- Skudalski, L.; Waldman, R.; Kerr, P.E.; Grant-Kels, J.M. Melanoma: An Update on Systemic Therapies. J. Am. Acad. Dermatol. 2022, 86, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Costa, L.D.; Guieu, S.; Biazzotto, J.C.; da Neves, M.G.P.M.S.; Faustino, M.A.F.; da Silva, R.S.; Tomé, A.C. Photodynamic Treatment of Melanoma Cells Using Aza-Dipyrromethenes as Photosensitizers. Photochem. Photobiol. Sci. 2020, 19, 885–891. [Google Scholar] [CrossRef]
- Jung, E.; Shim, I.; An, J.; Ji, M.S.; Jangili, P.; Chi, S.-G.; Kim, J.S. Phenylthiourea-Conjugated BODIPY as an Efficient Photosensitizer for Tyrosinase-Positive Melanoma-Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 2120–2127. [Google Scholar] [CrossRef]
- Baldea, I.; Olteanu, D.E.; Bolfa, P.; Ion, R.M.; Decea, N.; Cenariu, M.; Banciu, M.; Sesarman, A.V.; Filip, A.G. Efficiency of Photodynamic Therapy on WM35 Melanoma with Synthetic Porphyrins: Role of Chemical Structure, Intracellular Targeting and Antioxidant Defense. J. Photochem. Photobiol. B Biol. 2015, 151, 142–152. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular Photosensitizers Rejuvenate Photodynamic Therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors1. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. How to Treat Melanoma? The Current Status of Innovative Nanotechnological Strategies and the Role of Minimally Invasive Approaches like PTT and PDT. Pharmaceutics 2022, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Han, T.; Xia, H.; Dong, L.; Chen, L.; Lei, L. Advances in Photodynamic Therapy Based on Nanotechnology and Its Application in Skin Cancer. Front. Oncol. 2022, 12, 836397. [Google Scholar] [CrossRef] [PubMed]
- Adnane, F.; El-Zayat, E.; Fahmy, H.M. The Combinational Application of Photodynamic Therapy and Nanotechnology in Skin Cancer Treatment: A Review. Tissue Cell 2022, 77, 101856. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Liu, W.-T.; Wang, H.-T.; Yeh, Y.-H.; Wong, T.-W. An Update on Recent Advances of Photodynamic Therapy for Primary Cutaneous Lymphomas. Pharmaceutics 2023, 15, 1328. [Google Scholar] [CrossRef]
- Chauhan, A.; Gretz, N. Role of Visible Light on Skin Melanocytes: A Systematic Review. Photochem. Photobiol. 2021, 97, 911–915. [Google Scholar] [CrossRef]
- Al Khatib, M.; Harir, M.; Costa, J.; Baratto, M.; Schiavo, I.; Trabalzini, L.; Pollini, S.; Rossolini, G.; Basosi, R.; Pogni, R. Spectroscopic Characterization of Natural Melanin from a Streptomyces Cyaneofuscatus Strain and Comparison with Melanin Enzymatically Synthesized by Tyrosinase and Laccase. Molecules 2018, 23, 1916. [Google Scholar] [CrossRef]
- Madkhali, N.; Alqahtani, H.R.; Al-Terary, S.; Laref, A.; Hassib, A. Control of Optical Absorption and Fluorescence Spectroscopies of Natural Melanin at Different Solution Concentrations. Opt. Quant. Electron. 2019, 51, 227. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin Broadband Absorption Develops from Aggregation-Modulated Chromophore Interactions under Structural and Redox Control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef]
- Sadiq, I.; Kollias, N.; Baqer, A. Spectroscopic Observations on Human Pigmentation. Photodermatol. Photoimmunol. Photomed. 2019, 35, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Tan, L.-C.; Dong, L.-W.; Zhang, W.-Q.; Shen, X.-X.; Lu, X.; Zheng, H.; Lu, Y.-G. Susceptibility and Resistance Mechanisms During Photodynamic Therapy of Melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Freitas, L.F.; Hamblin, M.R.; Anzengruber, F.; Perussi, J.R.; Ribeiro, A.O.; Martins, V.C.A.; Plepis, A.M.G. Zinc Phthalocyanines Attached to Gold Nanorods for Simultaneous Hyperthermic and Photodynamic Therapies against Melanoma in Vitro. J. Photochem. Photobiol. B Biol. 2017, 173, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.E.; Lamberti, M.J.; Rivarola, V.A.; Rumie Vittar, N.B. Developing Strategies to Predict Photodynamic Therapy Outcome: The Role of Melanoma Microenvironment. Tumour Biol. 2015, 36, 9127–9136. [Google Scholar] [CrossRef]
- Meira, W.V.; Heinrich, T.A.; Cadena, S.M.S.C.; Martinez, G.R. Melanogenesis Inhibits Respiration in B16-F10 Melanoma Cells Whereas Enhances Mitochondrial Cell Content. Exp. Cell Res. 2017, 350, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wu, Z.; Zheng, R.; Yin, N.; Han, F.; Zhao, Z.; Dai, M.; Han, D.; Wang, W.; Niu, L. Inhibition Mechanism of Melanin Formation Based on Antioxidant Scavenging of Reactive Oxygen Species. Analyst 2022, 147, 2703–2711. [Google Scholar] [CrossRef]
- Gomaa, I.; Sebak, A.; Afifi, N.; Abdel-Kader, M. Liposomal Delivery of Ferrous Chlorophyllin: A Novel Third Generation Photosensitizer for in Vitro PDT of Melanoma. Photodiagn. Photodyn. Ther. 2017, 18, 162–170. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Liu, B.; Tan, Y.N. Emerging Strategies in Enhancing Singlet Oxygen Generation of Nano-Photosensitizers Toward Advanced Phototherapy. Nano-Micro Lett. 2022, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation Therapy. II. Cure of Animal Tumors with Hematoporphyrin and Light. J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Carrillo-Carrión, C.; Carmen Castillejos, M.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic Therapy: Photosensitizers and Nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Boscencu, R.; Radulea, N.; Manda, G.; Machado, I.F.; Socoteanu, R.P.; Lupuliasa, D.; Burloiu, A.M.; Mihai, D.P.; Ferreira, L.F.V. Porphyrin Macrocycles: General Properties and Theranostic Potential. Molecules 2023, 28, 1149. [Google Scholar] [CrossRef]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and its Applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Linares, I.A.P.; Martinelli, L.P.; Moritz, M.N.O.; Selistre-de-Araujo, H.S.; De Oliveira, K.T.; Rodrigues Perussi, J. Cytotoxicity of Structurally-Modified Chlorins Aimed for Photodynamic Therapy Applications. J. Photochem. Photobiol. A Chem. 2022, 425, 113647. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological Properties of Phthalocyanine Photosensitizers Photosens, Holosens and Phthalosens: A Comparative in Vitro Analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; Van Nostrum, C.F.; De La Escosura, A.; Torres, T. Amphiphilic Phthalocyanines in Polymeric Micelles: A Supramolecular Approach toward Efficient Third-Generation Photosensitizers. J. Mater. Chem. B 2020, 8, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Nejat, A.; Kalhori, K.A.M. Antimicrobial Photodynamic Therapy with Nanoparticles Versus Conventional Photosensitizer in Oral Diseases. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 237–259. ISBN 978-0-323-46152-8. [Google Scholar]
- Mathai, S.; Smith, T.A.; Ghiggino, K.P. Singlet Oxygen Quantum Yields of Potential Porphyrin-Based Photosensitisers for Photodynamic Therapy. Photochem. Photobiol. Sci. 2007, 6, 995. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hara, K.; Honda, N.; Okazaki, S.; Hazama, H.; Awazu, K. Determination and Analysis of Singlet Oxygen Quantum Yields of Talaporfin Sodium, Protoporphyrin IX, and Lipidated Protoporphyrin IX Using near-Infrared Luminescence Spectroscopy. Lasers Med. Sci. 2020, 35, 1289–1297. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Monteiro, C.J.P.; Pereira, M.M.; Azenha, M.E.; Burrows, H.D.; Serpa, C.; Arnaut, L.G.; Tapia, M.J.; Sarakha, M.; Wong-Wah-Chung, P.; Navaratnam, S. A Comparative Study of Water Soluble 5,10,15,20-Tetrakis(2,6-Dichloro-3-Sulfophenyl)Porphyrin and Its Metal Complexes as Efficient Sensitizers for Photodegradation of Phenols. Photochem. Photobiol. Sci. 2005, 4, 617–624. [Google Scholar] [CrossRef]
- Deda, D.K.; Pavani, C.; Caritá, E.; Baptista, M.S.; Toma, H.E.; Araki, K. Correlation of Photodynamic Activity and Singlet Oxygen Quantum Yields in Two Series of Hydrophobic Monocationic Porphyrins. J. Porphyr. Phthalocyanines 2012, 16, 55–63. [Google Scholar] [CrossRef]
- Vakrat-Haglili, Y.; Weiner, L.; Brumfeld, V.; Brandis, A.; Salomon, Y.; Mcllroy, B.; Wilson, B.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; et al. The Microenvironment Effect on the Generation of Reactive Oxygen Species by Pd−Bacteriopheophorbide. J. Am. Chem. Soc. 2005, 127, 6487–6497. [Google Scholar] [CrossRef]
- Macpherson, A.N.; Kessel, D.; Morgan, A.R.; Munro, I.; Truscott, T.G. A Photophysical Study of Some Purpurins. Faraday Trans. 1990, 86, 3081. [Google Scholar] [CrossRef]
- Staicu, A.; Smarandache, A.; Pascu, A.; Pascu, M.L. Photophysics of Covalently Functionalized Single Wall Carbon Nanotubes with Verteporfin. Appl. Surf. Sci. 2017, 417, 170–174. [Google Scholar] [CrossRef]
- Clement, S.; Sobhan, M.; Deng, W.; Camilleri, E.; Goldys, E.M. Nanoparticle-Mediated Singlet Oxygen Generation from Photosensitizers. J. Photochem. Photobiol. A Chem. 2017, 332, 66–71. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and Photochemical Properties of Potential Porphyrin and Chlorin Photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Ömeroğlu, İ.; Akçay, H.T.; Durmuş, M.; Kantekin, H. Synthesis, Characterization, Photophysical and Photochemical Properties of Peripherally Tetra Benzodioxane Substituted Metal-Free Phthalocyanine and its Zinc(II) and Magnesium(II) Derivatives. J. Mol. Struct. 2021, 1223, 128992. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Müller, M.; Mártire, D.O.; Pörting, S.; Bertolotti, S.G.; Chakravorti, S.; Koç-Weier, G.; Knipp, B.; Schaffner, K. Photophysical Properties of Porphycene Derivatives (18 π Porphyrinoids). J. Photochem. Photobiol. B Biol. 1997, 40, 191–198. [Google Scholar] [CrossRef]
- Masiera, N.; Ostapko, J.; Gorski, A.; Bojarska, A.; Gawryszewska, I.; Sadowy, E.; Hryniewicz, W.; Waluk, J. Photoeradication of Bacteria with Porphycenes: Substituent Effects on the Efficiency. Eur. J. Med. Chem. 2020, 200, 112472. [Google Scholar] [CrossRef]
- Salice, P.; Arnbjerg, J.; Pedersen, B.W.; Toftegaard, R.; Beverina, L.; Pagani, G.A.; Ogilby, P.R. Photophysics of Squaraine Dyes: Role of Charge-Transfer in Singlet Oxygen Production and Removal. J. Phys. Chem. A 2010, 114, 2518–2525. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine Dyes in PDT: From Basic Design to in Vivo Demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef]
- Zhao, X.; Yao, Q.; Long, S.; Chi, W.; Yang, Y.; Tan, D.; Liu, X.; Huang, H.; Sun, W.; Du, J.; et al. An Approach to Developing Cyanines with Simultaneous Intersystem Crossing Enhancement and Excited-State Lifetime Elongation for Photodynamic Antitumor Metastasis. J. Am. Chem. Soc. 2021, 143, 12345–12354. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- DeRosa, M. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Shahinyan, G.A.; Amirbekyan, A.Y.; Markarian, S.A. Photophysical Properties of Methylene Blue in Water and in Aqueous Solutions of Dimethylsulfoxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 170–175. [Google Scholar] [CrossRef]
- Sofyan, N.; Situmorang, F.W.; Ridhova, A.; Yuwono, A.H.; Udhiarto, A. Visible Light Absorption and Photosensitizing Characteristics of Natural Yellow 3 Extracted from Curcuma Longa L. for Dye-Sensitized Solar Cell. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012073. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and Photochemical Properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, K.; Ayouchia, H.B.E.; Anane, H.; Mihaljević, B.; Basarić, N.; Rohand, T. Spectroscopic and Photophysical Properties of Mono- and Dithiosubstituted BODIPY Dyes. J. Photochem. Photobiol. A Chem. 2020, 388, 112206. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, G.Q.; Zhu, J. Methylated Unsymmetric BODIPY Compounds: Synthesis, High Fluorescence Quantum Yield and Long Fluorescence Time. J. Fluoresc. 2019, 29, 407–416. [Google Scholar] [CrossRef]
- Sevieri, M.; Silva, F.; Bonizzi, A.; Sitia, L.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Indocyanine Green Nanoparticles: Are They Compelling for Cancer Treatment? Front. Chem. 2020, 8, 535. [Google Scholar] [CrossRef]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-Based Rose Bengal: A Broad-Spectrum Biomedical Tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, J.; Li, Z.; Chen, H.; Gao, Y. Recent Progress in Sono-Photodynamic Cancer Therapy: From Developed New Sensitizers to Nanotechnology-Based Efficacy-Enhancing Strategies. Acta Pharm. Sin. B 2021, 11, 2197–2219. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.P.; de Souza, T.F.; Cerchiaro, G.; Pinhal, M.A.; Ribeiro, A.O.; Girão, M.J. Hypericin in Photobiological Assays: An Overview. Photodiagn. Photodyn. Ther. 2021, 35, 102343. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Abrahamse, H.; Kruger, C.A.; Kadanyo, S.; Mishra, A. Nanoparticles for Advanced Photodynamic Therapy of Cancer. Photomed. Laser Surg. 2017, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hao, X.; Liang, X.; Zhang, Q.; Zhang, C.; Zhou, G.; Shen, S.; Jia, G.; Zhang, J. Inorganic Nanomaterials as Carriers for Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1–27. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Wang, H.; Tran, T.T.; Duong, K.T.; Nguyen, T.; Le, U.M. Options of Therapeutics and Novel Delivery Systems of Drugs for the Treatment of Melanoma. Mol. Pharm. 2022, 19, 4487–4505. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Pellosi, D.S.; De Jesus, P.D.C.C.; Tedesco, A.C. Spotlight on the Delivery of Photosensitizers: Different Approaches for Photodynamic-Based Therapies. Expert Opin. Drug Deliv. 2017, 14, 1395–1406. [Google Scholar] [CrossRef]
- Gamaleia, N.F.; Shton, I.O. Gold Mining for PDT: Great Expectations from Tiny Nanoparticles. Photodiagn. Photodyn. Ther. 2015, 12, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Singh, S.K.; Kapoor, B.; Dureja, H.; Chellappan, D.K.; Anand, K.; Dua, K.; Khursheed, R.; et al. Nanocarriers for Treatment of Dermatological Diseases: Principle, Perspective and Practices. Eur. J. Pharmacol. 2021, 890, 173691. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-Targeted Nanocarriers for Therapeutic Delivery to Cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, N.; Xu, J.; Zhu, X.; Ling, G.; Zhang, P. Novel Strategies in Melanoma Treatment Using Silver Nanoparticles. Cancer Lett. 2023, 561, 216148. [Google Scholar] [CrossRef]
- Malindi, Z.; Barth, S.; Abrahamse, H. The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines 2022, 10, 2158. [Google Scholar] [CrossRef]
- Marzi, M.; Osanloo, M.; Vakil, M.K.; Mansoori, Y.; Ghasemian, A.; Dehghan, A.; Zarenezhad, E. Applications of Metallic Nanoparticles in the Skin Cancer Treatment. BioMed Res. Int. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Wang, P.; Tang, H.; Zhang, P. Plasmonic Nanoparticle-Based Hybrid Photosensitizers with Broadened Excitation Profile for Photodynamic Therapy of Cancer Cells. Sci. Rep. 2016, 6, 34981. [Google Scholar] [CrossRef]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold Nanoshells-Mediated Bimodal Photodynamic and Photothermal Cancer Treatment Using Ultra-Low Doses of near Infra-Red Light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Singh, S.K.; Mazumder, S.; Vincy, A.; Hiremath, N.; Kumar, R.; Banerjee, I.; Vankayala, R. Review of Photoresponsive Plasmonic Nanoparticles That Produce Reactive Chemical Species for Photodynamic Therapy of Cancer and Bacterial Infections. ACS Appl. Nano Mater. 2023, 6, 1508–1521. [Google Scholar] [CrossRef]
- Nkune, N.W.; Abrahamse, H. Nanoparticle-Based Drug Delivery Systems for Photodynamic Therapy of Metastatic Melanoma: A Review. Int. J. Mol. Sci. 2021, 22, 12549. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Botteon, C.E.A.; Ribeiro, P.A.; Marcato, P.D.; Raposo, M. Nanoparticle Systems for Cancer Phototherapy: An Overview. Nanomaterials 2021, 11, 3132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Lin, K.; Yang, L. Progress in Nanocarriers Codelivery System to Enhance the Anticancer Effect of Photodynamic Therapy. Pharmaceutics 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma—Where Do We Stand? Curr. Med. Chem. 2019, 25, 5540–5563. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Karocki, A.; Elas, M.; Pawlak, A.; Stochel, G.; Urbanska, K. Verteporfin, Photofrin II, and Merocyanine 540 as PDT Photosensitizers against Melanoma Cells. Biochem. Biophys. Res. Commun. 2006, 349, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Chiang, L.Y.; Lakshmanan, S.; Huang, Y.-Y.; Garcia-Diaz, M.; Karimi, M.; de Souza Rastelli, A.N.; Chandran, R. Nanotechnology for Photodynamic Therapy: A Perspective from the Laboratory of Dr. Michael R. Hamblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School. Nanotechnol. Rev. 2015, 4, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Lou, L.; Zhou, S.; Tan, S.; Xiang, M.; Wang, W.; Yuan, C.; Gao, L.; Xiao, Q. Amplifying the Efficacy of ALA-Based Prodrugs for Photodynamic Therapy Using Nanotechnology. Front. Pharmacol. 2023, 14, 1137707. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sazgarnia, A.; Rajabi, O.; Soudmand, S.; Esmaily, H.; Sadeghi, H.R. An in Vitro Study on the Photosensitivity of 5-Aminolevulinic Acid Conjugated Gold Nanoparticles. Photodiagn. Photodyn. Ther. 2013, 10, 382–388. [Google Scholar] [CrossRef]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted Delivery of 5-Aminolevulinic Acid by Multifunctional Hollow Mesoporous Silica Nanoparticles for Photodynamic Skin Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Harmatys, K.M.; Musso, A.J.; Clear, K.J.; Smith, B.D. Small Molecule Additive Enhances Cell Uptake of 5-Aminolevulinic Acid and Conversion to Protoporphyrin IX. Photochem. Photobiol. Sci. 2016, 15, 1408–1416. [Google Scholar] [CrossRef]
- Li, A.; Liang, C.; Xu, L.; Wang, Y.; Liu, W.; Zhang, K.; Liu, J.; Shi, J. Boosting 5-ALA-Based Photodynamic Therapy by a Liposomal Nanomedicine through Intracellular Iron Ion Regulation. Acta Pharm. Sin. B 2021, 11, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, J.; Wray, A.A. Deferoxamine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557654/ (accessed on 22 May 2023).
- Li, Y.; Ruan, S.; Guo, J.; He, Z.; Xia, Q.; Wu, T.; Wang, Z.; Li, Z.; Hu, H.; Jing, Q.; et al. B16F10 Cell Membrane-Based Nanovesicles for Melanoma Therapy Are Superior to Hyaluronic Acid-Modified Nanocarriers. Mol. Pharm. 2022, 19, 2840–2853. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.B.; da Silva, C.L.; Davanzo, N.N.; da Silva Souza, R.; Correa, R.J.; Tedesco, A.C.; Riemma Pierre, M.B. Protoporphyrin IX (PpIX) Loaded PLGA Nanoparticles for Topical Photodynamic Therapy of Melanoma Cells. Photodiagn. Photodyn. Ther. 2021, 35, 102317. [Google Scholar] [CrossRef]
- Vadarevu, H.; Juneja, R.; Lyles, Z.; Vivero-Escoto, J.L. Light-Activated Protoporphyrin IX-Based Polysilsesquioxane Nanoparticles Induce Ferroptosis in Melanoma Cells. Nanomaterials 2021, 11, 2324. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Estevão, B.M.; Gianotti, E.; Marchese, L.; Renò, F. Verteporfin Based Silica Nanoparticle for in Vitro Selective Inhibition of Human Highly Invasive Melanoma Cell Proliferation. J. Photochem. Photobiol. B Biol. 2017, 167, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Ramakrishnan, S.K.; Triner, D.; Centofanti, B.; Maitra, D.; Győrffy, B.; Sebolt-Leopold, J.S.; Dame, M.K.; Varani, J.; Brenner, D.E.; et al. Tumor-Selective Proteotoxicity of Verteporfin Inhibits Colon Cancer Progression Independently of YAP1. Sci. Signal 2015, 8, ra98. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Nistorescu, S.; Udrea, A.-M.; Badea, M.A.; Lungu, I.; Boni, M.; Tozar, T.; Dumitrache, F.; Maraloiu, V.-A.; Popescu, R.G.; Fleaca, C.; et al. Low Blue Dose Photodynamic Therapy with Porphyrin-Iron Oxide Nanoparticles Complexes: In Vitro Study on Human Melanoma Cells. Pharmaceutics 2021, 13, 2130. [Google Scholar] [CrossRef]
- Balas, M.; Nistorescu, S.; Badea, M.A.; Dinischiotu, A.; Boni, M.; Dinache, A.; Smarandache, A.; Udrea, A.-M.; Prepelita, P.; Staicu, A. Photodynamic Activity of TMPyP4/TiO2 Complex under Blue Light in Human Melanoma Cells: Potential for Cancer-Selective Therapy. Pharmaceutics 2023, 15, 1194. [Google Scholar] [CrossRef]
- Chen, Z.-A.; Kuthati, Y.; Kankala, R.K.; Chang, Y.-C.; Liu, C.-L.; Weng, C.-F.; Mou, C.-Y.; Lee, C.-H. Encapsulation of Palladium Porphyrin Photosensitizer in Layered Metal Oxide Nanoparticles for Photodynamic Therapy against Skin Melanoma. Sci. Technol. Adv. Mater. 2015, 16, 054205. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Shiraishi, T.; Araki, T.; Watanabe, T.; Ono, T.; Higaki, K. Efficient Anti-Tumor Effect of Photodynamic Treatment with Polymeric Nanoparticles Composed of Polyethylene Glycol and Polylactic Acid Block Copolymer Encapsulating Hydrophobic Porphyrin Derivative. Eur. J. Pharm. Sci. 2016, 82, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Carpenter, B.L.; Wen, A.M.; Ghiladi, R.A.; Steinmetz, N.F. High Aspect Ratio Nanotubes Formed by Tobacco Mosaic Virus for Delivery of Photodynamic Agents Targeting Melanoma. ACS Biomater. Sci. Eng. 2016, 2, 838–844. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, X.; Dai, Z. Porphyrin-Loaded Nanoparticles for Cancer Theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef] [PubMed]
- Chlorin (CHEBI:36303). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:36303 (accessed on 11 April 2023).
- Mbakidi, J.P.; Drogat, N.; Granet, R.; Ouk, T.-S.; Ratinaud, M.-H.; Rivière, E.; Verdier, M.; Sol, V. Hydrophilic Chlorin-Conjugated Magnetic Nanoparticles—Potential Anticancer Agent for the Treatment of Melanoma by PDT. Bioorg. Med. Chem. Lett. 2013, 23, 2486–2490. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, T.; Shen, J.; Karges, J.; Jin, C.; Zhao, Y.; Ji, L.; Chao, H. A Mitochondria-Localized Iridium(III)–Chlorin E6 Conjugate for Synergistic Sonodynamic and Two-Photon Photodynamic Therapy against Melanoma. Inorg. Chem. Front. 2022, 9, 3034–3046. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles Loading Porphyrin Sensitizers in Improvement of Photodynamic Therapy for Ovarian Cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102156. [Google Scholar] [CrossRef]
- Gaio, E.; Conte, C.; Esposito, D.; Reddi, E.; Quaglia, F.; Moret, F. CD44 Targeting Mediated by Polymeric Nanoparticles and Combination of Chlorine TPCS2a-PDT and Docetaxel-Chemotherapy for Efficient Killing of Breast Differentiated and Stem Cancer Cells In Vitro. Cancers 2020, 12, 278. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic Nanoparticle Based Active Targeting for Photodynamic Therapy Treatment of Breast Cancer Cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef] [PubMed]
- Vignion-Dewalle, A.-S.; Baert, G.; Thecua, E.; Vicentini, C.; Mortier, L.; Mordon, S. Photodynamic Therapy for Actinic Keratosis: Is the European Consensus Protocol for Daylight PDT Superior to Conventional Protocol for Aktilite CL 128 PDT? J. Photochem. Photobiol. B Biol. 2017, 174, 70–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Kulichenko, A.; Farrakhova, D.S.; Yakovlev, D.V.; Maklygina, Y.S.; Shiryaev, A.A.; Loschenov, V.B. Fluorescence Diagnostics and Photodynamic Therapy of Squamous Cell Carcinoma of the Lateral Surface of the Tongue Using the Photosensitizer Chlorin E6 by Spectroscopic Video Fluorescence Methods. J. Phys. Conf. Ser. 2021, 2058, 012021. [Google Scholar] [CrossRef]
- Li, T.-F.; Xu, H.-Z.; Xu, Y.-H.; Yu, T.-T.; Tang, J.-M.; Li, K.; Wang, C.; Peng, X.-C.; Li, Q.-R.; Sang, X.-Y.; et al. Efficient Delivery of Chlorin E6 by Polyglycerol-Coated Iron Oxide Nanoparticles with Conjugated Doxorubicin for Enhanced Photodynamic Therapy of Melanoma. Mol. Pharm. 2021, 18, 3601–3615. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Xin, J.; Rahmanzadeh, R.; Wang, J.; Yao, C.; Zhang, Z. Chlorin-Based Photoactivable Galectin-3-Inhibitor Nanoliposome for Enhanced Photodynamic Therapy and NK Cell-Related Immunity in Melanoma. ACS Appl. Mater. Interfaces 2019, 11, 41829–41841. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Chen, W.; Bai, S.; Zheng, T.; He, C.; Guo, Z.; Jiang, M.; Du, G.; Sun, X. Albumin-Biomineralized Nanoparticles to Synergize Phototherapy and Immunotherapy against Melanoma. J. Control. Release 2020, 322, 300–311. [Google Scholar] [CrossRef]
- Chu, M.; Li, H.; Wu, Q.; Wo, F.; Shi, D. Pluronic-Encapsulated Natural Chlorophyll Nanocomposites for in Vivo Cancer Imaging and Photothermal/Photodynamic Therapies. Biomaterials 2014, 35, 8357–8373. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Wright, J.D. Phthalocyanines. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 6987–6991. ISBN 978-0-08-043152-9. [Google Scholar]
- Staicu, A.; Pascu, A.; Nuta, A.; Sorescu, A.; Ratitoiu, V.; Pascu, M.L. Studies About Phthalocyanine Photosensitizers to be Used in Photodynamic Therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. [Google Scholar]
- Bolfarini, G.C.; Siqueira-Moura, M.P.; Demets, G.J.F.; Morais, P.C.; Tedesco, A.C. In Vitro Evaluation of Combined Hyperthermia and Photodynamic Effects Using Magnetoliposomes Loaded with Cucurbituril Zinc Phthalocyanine Complex on Melanoma. J. Photochem. Photobiol. B Biol. 2012, 115, 1–4. [Google Scholar] [CrossRef]
- Do Reis, S.R.R.; Helal-Neto, E.; Da Silva De Barros, A.O.; Pinto, S.R.; Portilho, F.L.; De Oliveira Siqueira, L.B.; Alencar, L.M.R.; Dahoumane, S.A.; Alexis, F.; Ricci-Junior, E.; et al. Dual Encapsulated Dacarbazine and Zinc Phthalocyanine Polymeric Nanoparticle for Photodynamic Therapy of Melanoma. Pharm. Res. 2021, 38, 335–346. [Google Scholar] [CrossRef]
- Camerin, M.; Moreno, M.; Marín, M.J.; Schofield, C.L.; Chambrier, I.; Cook, M.J.; Coppellotti, O.; Jori, G.; Russell, D.A. Delivery of a Hydrophobic Phthalocyanine Photosensitizer Using PEGylated Gold Nanoparticle Conjugates for the in Vivo Photodynamic Therapy of Amelanotic Melanoma. Photochem. Photobiol. Sci. 2016, 15, 618–625. [Google Scholar] [CrossRef]
- Camerin, M.; Magaraggia, M.; Soncin, M.; Jori, G.; Moreno, M.; Chambrier, I.; Cook, M.J.; Russell, D.A. The in Vivo Efficacy of Phthalocyanine–Nanoparticle Conjugates for the Photodynamic Therapy of Amelanotic Melanoma. Eur. J. Cancer 2010, 46, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Goto, P.L.; Siqueira-Moura, M.P.; Tedesco, A.C. Application of Aluminum Chloride Phthalocyanine-Loaded Solid Lipid Nanoparticles for Photodynamic Inactivation of Melanoma Cells. Int. J. Pharm. 2017, 518, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.D.P.; Dipieri, L.V.; Rossetti, F.C.; Marchetti, J.M.; Bentley, M.V.L.B.; Nunes, R.D.S.; Sarmento, V.H.V.; Valerio, M.E.G.; Rodrigues Júnior, J.J.; Montalvão, M.M.; et al. Skin Permeation, Biocompatibility and Antitumor Effect of Chloroaluminum Phthalocyanine Associated to Oleic Acid in Lipid Nanoparticles. Photodiagn. Photodyn. Ther. 2018, 24, 262–273. [Google Scholar] [CrossRef]
- Mello, V.C.; Araújo, V.H.S.; De Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; Da Silva Costa, N.R.; De Souza, I.F.; Da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Priefer, R. Non-Porphyrin Dyes Used as Photosensitizers in Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Xing, E.; Du, Y.; Su, Y.; Feng, Y.; Meng, S. Halogenated Cyanine Dyes for Synergistic Photodynamic and Photothermal Therapy. Dye. Pigment. 2021, 190, 109327. [Google Scholar] [CrossRef]
- Staurenghi, G.; Bottoni, F.; Giani, A. Chapter 2—Clinical Applications of Diagnostic Indocyanine Green Angiography. In Retina, 5th ed.; Ryan, S.J., Sadda, S.R., Hinton, D.R., Schachat, A.P., Sadda, S.R., Wilkinson, C.P., Wiedemann, P., Schachat, A.P., Eds.; W.B. Saunders: London, UK, 2013; pp. 51–81. ISBN 978-1-4557-0737-9. [Google Scholar]
- Olubiyi, O.I.; Lu, F.-K.; Calligaris, D.; Jolesz, F.A.; Agar, N.Y. Chapter 17—Advances in Molecular Imaging for Surgery. In Image-Guided Neurosurgery; Golby, A.J., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 407–439. ISBN 978-0-12-800870-6. [Google Scholar]
- Hu, H.; Chen, J.; Yang, H.; Huang, X.; Wu, H.; Wu, Y.; Li, F.; Yi, Y.; Xiao, C.; Li, Y.; et al. Potentiating Photodynamic Therapy of ICG-Loaded Nanoparticles by Depleting GSH with PEITC. Nanoscale 2019, 11, 6384–6393. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, H.; Hou, X.; Wang, L.; Li, Y.; Pang, Y.; Chen, C.; Jiang, G.; Liu, Y. Enhanced Anti-Tumor Efficacy of Temozolomide-Loaded Carboxylated Poly(Amido-Amine) Combined with Photothermal/Photodynamic Therapy for Melanoma Treatment. Cancer Lett. 2018, 423, 16–26. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Termsarasab, U.; Ko, S.-H.; Shim, J.-S.; Chong, S.; Chung, S.-J.; Shim, C.-K.; Cho, H.-J.; Kim, D.-D. Hyaluronic Acid Derivative-Based Self-Assembled Nanoparticles for the Treatment of Melanoma. Pharm. Res. 2012, 29, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-Loaded Gold Nano-Bipyramids with NIR Activatable Dual PTT-PDT Therapeutic Potential in Melanoma Cells. Colloids Surf. B Biointerfaces 2020, 194, 111213. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite Nano Bioceramics Optimized 3D Printed Poly Lactic Acid Scaffold for Bone Tissue Engineering Application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Wen, L.; Hyoju, R.; Wang, P.; Shi, L.; Li, C.; Li, M.; Wang, X. Hydrogen-Peroxide-Responsive Protein Biomimetic Nanoparticles for Photothermal-Photodynamic Combination Therapy of Melanoma. Lasers Surg. Med. 2021, 53, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Lim, S.-J.; Lee, M.-K. Chitosan-Coated Liposomes to Stabilize and Enhance Transdermal Delivery of Indocyanine Green for Photodynamic Therapy of Melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kankala, R.; Wang, S.-B.; Chen, A.-Z. Leveraging Engineering of Indocyanine Green-Encapsulated Polymeric Nanocomposites for Biomedical Applications. Nanomaterials 2018, 8, 360. [Google Scholar] [CrossRef]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Sekar, M.; Karupiah, S.; Porwal, O.; Sahoo, A.; et al. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef]
- Alavi, S.; Haeri, A.; Dadashzadeh, S. Utilization of Chitosan-Caged Liposomes to Push the Boundaries of Therapeutic Delivery. Carbohydr. Polym. 2017, 157, 991–1012. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Liao, J.; Wei, X.; Ran, B.; Peng, J.; Qu, Y.; Qian, Z. Polymer Hybrid Magnetic Nanocapsules Encapsulating IR820 and PTX for External Magnetic Field-Guided Tumor Targeting and Multifunctional Theranostics. Nanoscale 2017, 9, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, E.; Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Rizzi, M.; Renò, F.; Marchese, L. An Efficient Rose Bengal Based Nanoplatform for Photodynamic Therapy. Chem. Eur. J. 2014, 20, 10921–10925. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Mesoporous Silica Nanoparticles: A Promising Multifunctional Drug Delivery System. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. ISBN 978-0-323-46144-3. [Google Scholar]
- Bazylińska, U.; Wawrzyńczyk, D.; Szewczyk, A.; Kulbacka, J. Engineering and Biological Assessment of Double Core Nanoplatform for Co-Delivery of Hybrid Fluorophores to Human Melanoma. J. Inorg. Biochem. 2020, 208, 111088. [Google Scholar] [CrossRef]
- Chen, H.-J.; Zhou, X.-B.; Wang, A.-L.; Zheng, B.-Y.; Yeh, C.-K.; Huang, J.-D. Synthesis and Biological Characterization of Novel Rose Bengal Derivatives with Improved Amphiphilicity for Sono-Photodynamic Therapy. Eur. J. Med. Chem. 2018, 145, 86–95. [Google Scholar] [CrossRef]
- Mohseni, H.; Imanparast, A.; Salarabadi, S.S.; Sazgarnia, A. In Vitro Evaluation of the Intensifying Photodynamic Effect Due to the Presence of Plasmonic Hollow Gold Nanoshells Loaded with Methylene Blue on Breast and Melanoma Cancer Cells. Photodiagn. Photodyn. Ther. 2022, 40, 103065. [Google Scholar] [CrossRef]
- Magalhães, J.A.; Arruda, D.C.; Baptista, M.S.; Tada, D.B. Co-Encapsulation of Methylene Blue and PARP-Inhibitor into Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Enhanced PDT of Cancer. Nanomaterials 2021, 11, 1514. [Google Scholar] [CrossRef]
- Gobo, G.G.; Piva, H.L.; Tedesco, A.C.; Primo, F.L. Novel Quinizarin Spray-Dried Nanoparticles for Treating Melanoma with Photodynamic Therapy. Mater. Today Commun. 2022, 33, 104882. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Li, Z.; Xie, Z. BODIPY-Containing Nanoscale Metal–Organic Frameworks for Photodynamic Therapy. Chem. Commun. 2016, 52, 5402–5405. [Google Scholar] [CrossRef]
- Oh, J.S.; You, Y.; Park, K.C.; Gupta, G.; Kang, D.-K.; Lee, C.Y. Toward an Efficient Photosensitizer for Photodynamic Therapy: Incorporating BODIPY into Porphyrinic Nanoscale MOFs through the Solvent-Assisted Ligand Incorporation. Dye. Pigment. 2019, 170, 107576. [Google Scholar] [CrossRef]
- De Morais, F.A.P.; De Oliveira, A.C.V.; Balbinot, R.B.; Lazarin-Bidóia, D.; Ueda-Nakamura, T.; De Oliveira Silva, S.; Da Silva Souza Campanholi, K.; Da Silva Junior, R.C.; Gonçalves, R.S.; Caetano, W.; et al. Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma. Polymers 2022, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Ghazaeian, M.; Khorsandi, K.; Hosseinzadeh, R.; Naderi, A.; Abrahamse, H. Curcumin–Silica Nanocomplex Preparation, Hemoglobin and DNA Interaction and Photocytotoxicity against Melanoma Cancer Cells. J. Biomol. Struct. Dyn. 2021, 39, 6606–6616. [Google Scholar] [CrossRef] [PubMed]
- Sujai, P.T.; Joseph, M.M.; Karunakaran, V.; Saranya, G.; Adukkadan, R.N.; Shamjith, S.; Thomas, R.; Nair, J.B.; Swathi, R.S.; Maiti, K.K. Biogenic Cluster-Encased Gold Nanorods as a Targeted Three-in-One Theranostic Nanoenvelope for SERS-Guided Photochemotherapy against Metastatic Melanoma. ACS Appl. Bio Mater. 2019, 2, 588–600. [Google Scholar] [CrossRef]
- Benstead, M.; Mehl, G.H.; Boyle, R.W. 4,4′-Difluoro-4-Bora-3a,4a-Diaza-s-Indacenes (BODIPYs) as Components of Novel Light Active Materials. Tetrahedron 2011, 67, 3573–3601. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Chen, Z.; Dong, X.; Zhao, W.; Shi, Y.; Zhu, Q. Discovery of an Amino Acid-Modified Near-Infrared Aza-BODIPY Photosensitizer as an Immune Initiator for Potent Photodynamic Therapy in Melanoma. J. Med. Chem. 2022, 65, 3616–3631. [Google Scholar] [CrossRef]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grunberger, W. Hypericin—The Facts About a Controversial Agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, A.M.; Smarandache, A.; Dinache, A.; Mares, C.; Nistorescu, S.; Avram, S.; Staicu, A. Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment. Pharmaceutics 2023, 15, 2124. https://doi.org/10.3390/pharmaceutics15082124
Udrea AM, Smarandache A, Dinache A, Mares C, Nistorescu S, Avram S, Staicu A. Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment. Pharmaceutics. 2023; 15(8):2124. https://doi.org/10.3390/pharmaceutics15082124
Chicago/Turabian StyleUdrea, Ana Maria, Adriana Smarandache, Andra Dinache, Catalina Mares, Simona Nistorescu, Speranta Avram, and Angela Staicu. 2023. "Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment" Pharmaceutics 15, no. 8: 2124. https://doi.org/10.3390/pharmaceutics15082124
APA StyleUdrea, A. M., Smarandache, A., Dinache, A., Mares, C., Nistorescu, S., Avram, S., & Staicu, A. (2023). Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment. Pharmaceutics, 15(8), 2124. https://doi.org/10.3390/pharmaceutics15082124








