Metformin Impedes Oxidation of LDL In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of nLDL
2.2. nLDL Oxidation Using Cu2+ Ions
2.3. Determination of Lipid Hydroperoxides (LPOs)
2.4. Determination of MDA
2.5. Determination of Oxidation-Specific Immune Epitopes
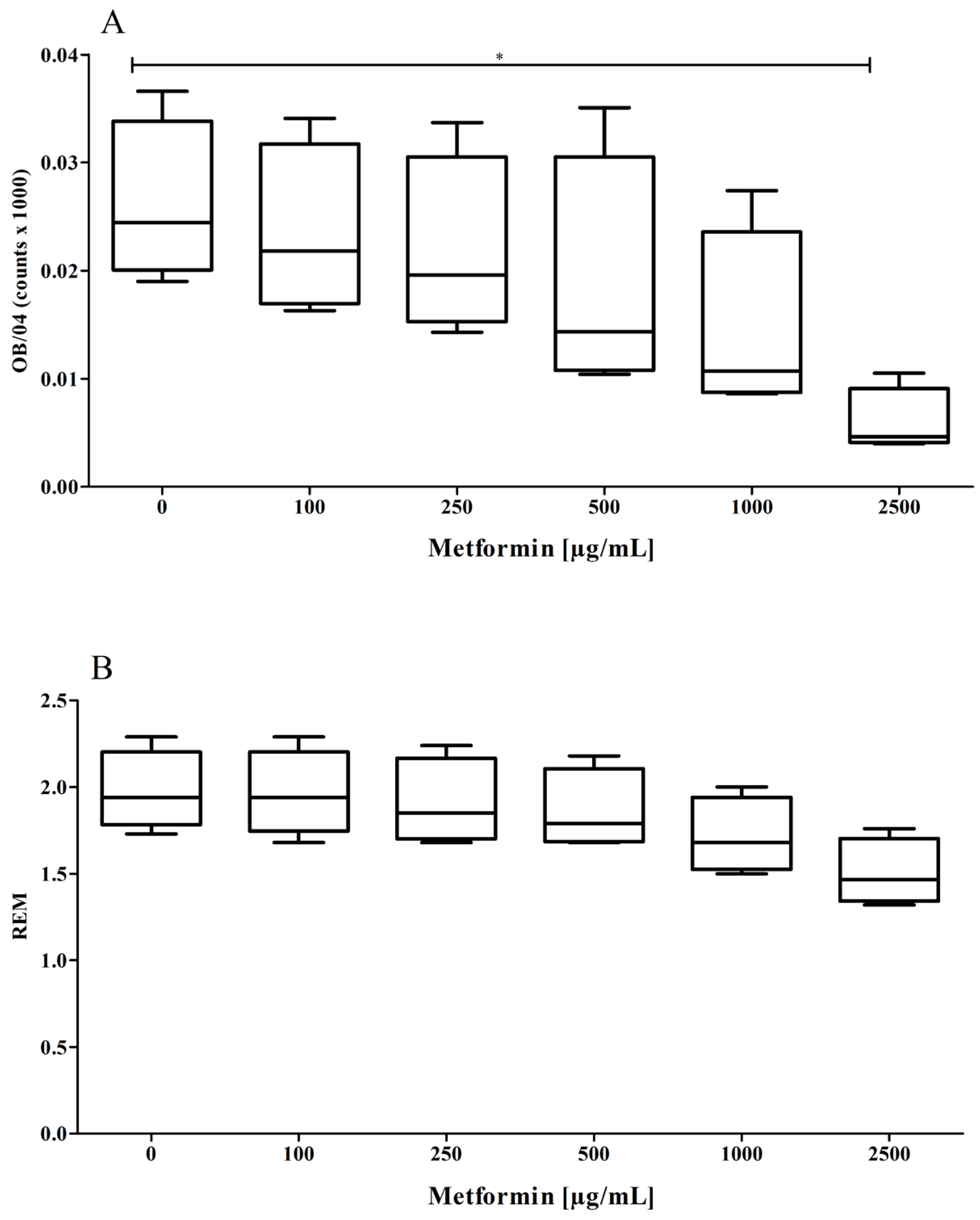
2.6. Determination of REM
2.7. Cell Culture
2.8. Cell Viability (AlamarBlue Assay)
2.9. Quantum Chemical Calculations
2.10. Statistics
3. Results
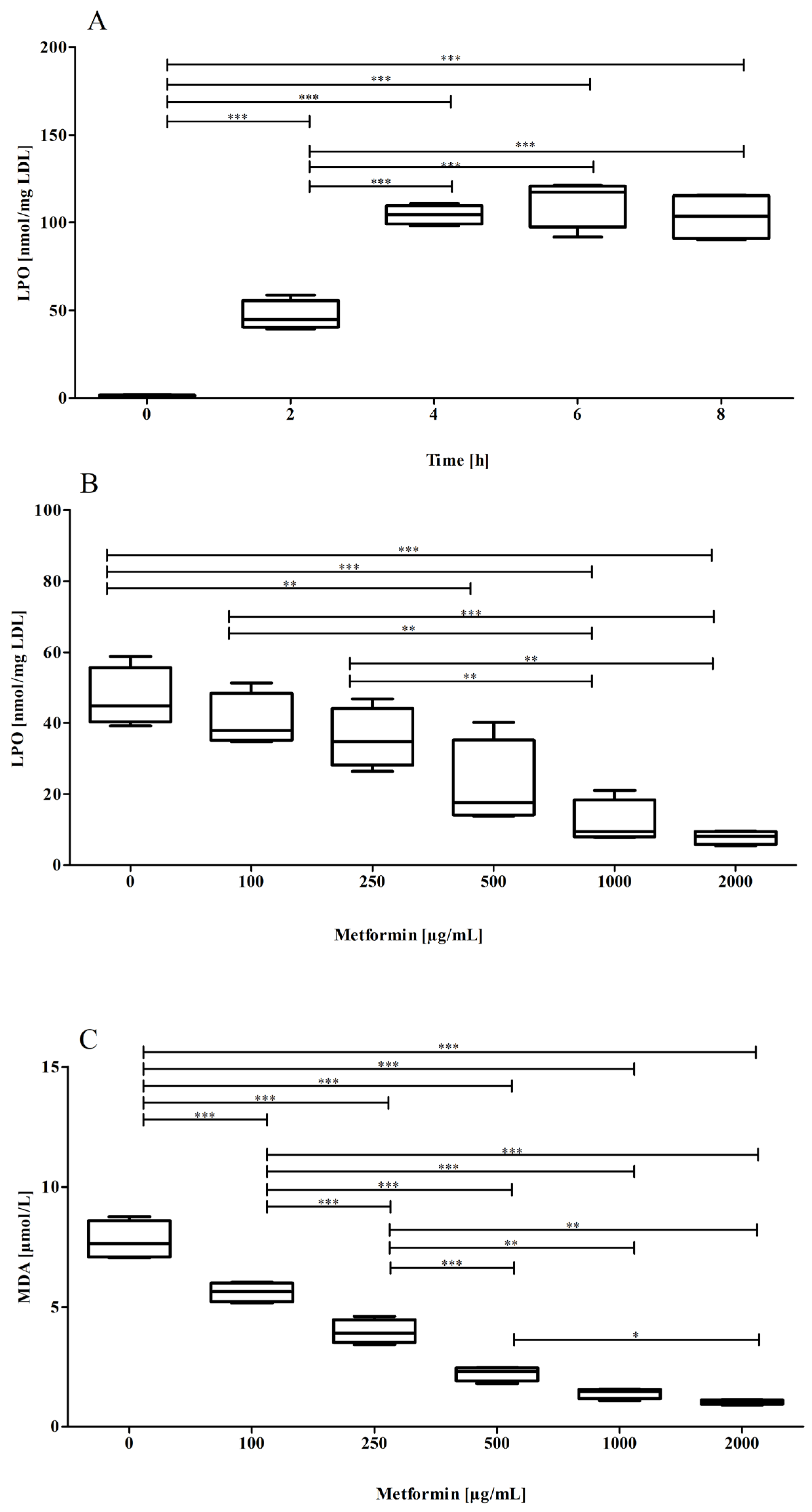
3.1. Effect of Metformin on the Oxidizability of the Lipid Part of the LDL Particle
3.2. Effect of Metformin on the Oxidizability of the Protein Part of the LDL Particle
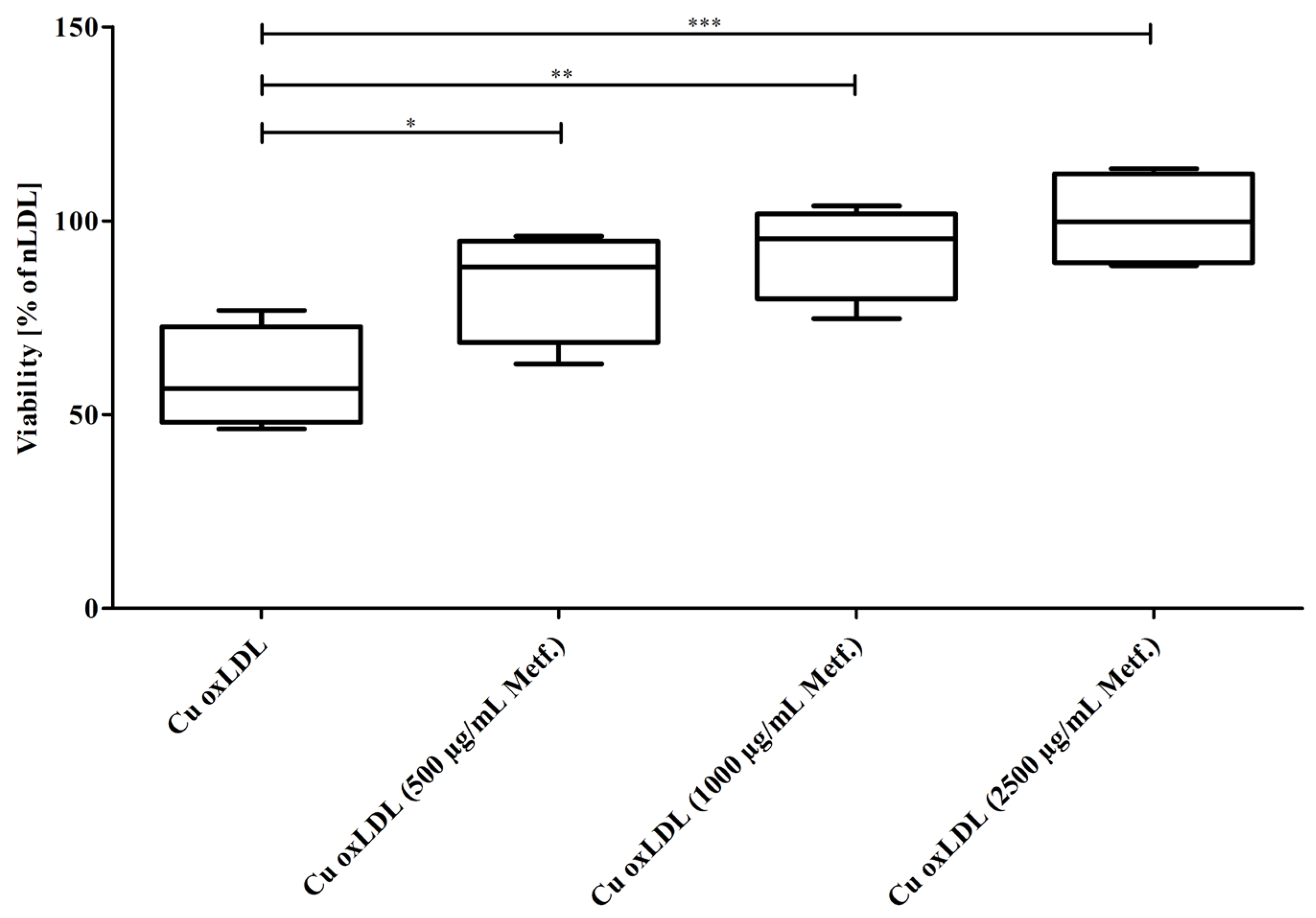
3.3. Cytotoxicity of oxLDL Formed under Increasing Concentrations of Metformin in EA.hy926 Cells
3.4. Reactions of Metformin with Hydroxyl, Hydroperoxyl, or Superoxide Radical Anion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, H.J.; Zhou, Y.H.; Tian, Y.J.; Du, H.Y.; Sun, Y.X.; Zhong, L.Y. Effects of intensive glucose lowering in treatment of type 2 diabetes mellitus on cardiovascular outcomes: A meta-analysis of data from 58,160 patients in 13 randomized controlled trials. Int. J. Cardiol. 2016, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.; Klein, R.; Ooi, T.C.; Rossing, P.; et al. Metformin in adults with type 1 diabetes: Design and methods of REducing with MetfOrmin Vascular Adverse Lesions (REMOVAL): An international multicentre trial. Diabetes Obes. Metab. 2017, 19, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Top, W.M.C.; Kooy, A.; Stehouwer, C.D.A. Metformin: A Narrative Review of Its Potential Benefits for Cardiovascular Disease, Cancer and Dementia. Pharmaceuticals 2022, 15, 312. [Google Scholar] [CrossRef]
- Cusi, K.; Consoli, A.; DeFronzo, R.A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S140–S157. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment. Diabetes Care 2017, 40, S64–S74. [Google Scholar] [CrossRef]
- Aroda, V.R.; Gonzalez-Galvez, G.; Gron, R.; Halladin, N.; Haluzik, M.; Jermendy, G.; Kok, A.; Orsy, P.; Sabbah, M.; Sesti, G.; et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): A multicentre, open-label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 596–605. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodriguez, A. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef]
- Out, M.; Kooy, A.; Lehert, P.; Schalkwijk, C.A.; Stehouwer, C.D.A. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomized controlled 4.3year trial. J. Diabetes Complicat. 2018, 32, 171–178. [Google Scholar] [CrossRef]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Bennett, J.; Cheng, Y.J.; Vamos, E.P.; Cross, A.J.; Ezzati, M.; Gregg, E.W. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: An epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021, 9, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Lux, A.; O’Callaghan, F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2018, 85, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.; Boyle, J.G.; Petrie, J.R.; Team, R.S. A new perspective on metformin therapy in type 1 diabetes. Diabetologia 2017, 60, 1594–1600. [Google Scholar] [CrossRef]
- Diabetes, C.; Complications Trial/Epidemiology of Diabetes, I.; Complications Study Research, G. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016, 39, 686–693. [Google Scholar] [CrossRef]
- Nathan, D.M.; Lachin, J.; Cleary, P.; Orchard, T.; Brillon, D.J.; Backlund, J.Y.; O’Leary, D.H.; Genuth, S.; Diabetes, C.; Complications, T.; et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 2003, 348, 2294–2303. [Google Scholar] [CrossRef]
- Wang, C.P.; Lorenzo, C.; Habib, S.L.; Jo, B.; Espinoza, S.E. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 679–686. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Sinawe, H.; Dimri, M. Biochemistry, LDL Cholesterol. In StatPearls; StatPearls Publishing LCC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yandrapalli, S.; Gupta, S.; Andries, G.; Cooper, H.A.; Aronow, W.S. Drug Therapy of Dyslipidemia in the Elderly. Drugs Aging 2019, 36, 321–340. [Google Scholar] [CrossRef]
- Monami, M.; Sesti, G.; Mannucci, E. PCSK9 inhibitor therapy: A systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes. Metab. 2019, 21, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic effects of 4-hydroxynonenal. Free. Radic. Biol. Med. 2017, 111, 127–139. [Google Scholar] [CrossRef]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jurgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free. Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef]
- Valente, A.J.; Irimpen, A.M.; Siebenlist, U.; Chandrasekar, B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: Inhibition by HDL3 and AMPK activators. Free. Radic. Biol. Med. 2014, 70, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Kager, G.; Dohr, G.; Rabl, H.; Ghassempur, I.; Jurgens, G. Generation, characterization, and histochemical application of monoclonal antibodies selectively recognizing oxidatively modified apoB-containing serum lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, C.; Nusshold, C.; Paar, M.; Ledinski, G.; Tafeit, E.; Koestenberger, M.; Bernhart, E.M.; Sattler, W.; Cvirn, G.; Hallstrom, S. Ethyl pyruvate inhibits oxidation of LDL in vitro and attenuates oxLDL toxicity in EA.hy926 cells. PLoS ONE 2018, 13, e0191477. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen-Laanaya, H.K.; Orloff, M.S. Analysis of cell viability using time-dependent increase in fluorescence intensity. Anal. Biochem. 2012, 429, 32–38. [Google Scholar] [CrossRef]
- el-Saadani, M.; Esterbauer, H.; el-Sayed, M.; Goher, M.; Nassar, A.Y.; Jurgens, G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar] [CrossRef]
- Jurgens, G.; Hoff, H.F.; Chisolm, G.M., III; Esterbauer, H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chem. Phys. Lipids 1987, 45, 315–336. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hoerl, G.; Ledinski, G.; Kager, G.; Thalhammer, M.; Koestenberger, M.; Juergens, G.; Gary, T.; Cvirn, G. Virtually same oxidizability of LDL but higher Lp(a) levels in arterial compared to venous plasma. Chem. Phys. Lipids 2014, 184, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, S.; Cekic Gonenc, O.; Karaca, Y.; Mentese, A.; Demir, S.; Beyhun, E.; Sahin, A.; Gunduz, A.; Yulug, E.; Turedi, S. The effect of ethyl pyruvate and N-acetylcysteine on ischemia-reperfusion injury in an experimental model of ischemic stroke. Am. J. Emerg Med. 2016, 34, 1804–1807. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Emeis, J.J.; Edgell, C.J. Fibrinolytic properties of a human endothelial hybrid cell line (Ea.hy 926). Blood 1988, 71, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Margolis, M.; Schreiner, C.; Edgell, C.J.; Azizkhan, J.; Lazarowski, E.; Juliano, R.L. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: Contributions of induced gene expression, G-proteins, and integrins. J. Cell. Physiol. 1992, 153, 437–449. [Google Scholar] [CrossRef]
- Riesbeck, K.; Billstrom, A.; Tordsson, J.; Brodin, T.; Kristensson, K.; Dohlsten, M. Endothelial cells expressing an inflammatory phenotype are lysed by superantigen-targeted cytotoxic T cells. Clin. Diagn. Lab. Immunol. 1998, 5, 675–682. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.; Khamashta, M.A. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Harrington, J.L.; de Albuquerque Rocha, N.; Patel, K.V.; Verma, S.; McGuire, D.K. Should Metformin Remain First-Line Medical Therapy for Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease? An Alternative Approach. Curr. Diabetes Rep. 2018, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, M.J. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: Roles of lipid hydroperoxides, alpha-tocopherol, thiols, and ceruloplasmin. Arch. Biochem. Biophys. 2001, 394, 117–135. [Google Scholar] [CrossRef]
- De Grey, A.D. HO2•: The forgotten radical. DNA Cell Biol. 2002, 21, 251–257. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Raji, B.; Walrand, S.; Gardes-Albert, M.; Jore, D.; Legrand, A.; Peynet, J.; Vasson, M.P. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003, 52, 586–589. [Google Scholar] [CrossRef]
- Khouri, H.; Collin, F.; Bonnefont-Rousselot, D.; Legrand, A.; Jore, D.; Gardes-Albert, M. Radical-induced oxidation of metformin. Eur. J. Biochem. 2004, 271, 4745–4752. [Google Scholar] [CrossRef]
- Collin, F.; Khoury, H.; Bonnefont-Rousselot, D.; Therond, P.; Legrand, A.; Jore, D.; Gardes-Albert, M. Liquid chromatographic/electrospray ionization mass spectrometric identification of the oxidation end-products of metformin in aqueous solutions. J. Mass Spectrom. 2004, 39, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Chittari, M.V.; Bodmer, C.W.; Zehnder, D.; Ceriello, A.; Thornalley, P.J. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes 2010, 59, 1038–1045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grant, P.J. The genetics of atherothrombotic disorders: A clinician’s view. J. Thromb. Haemost. 2003, 1, 1381–1390. [Google Scholar] [CrossRef]
- Pietzsch, J.; Lattke, P.; Julius, U. Oxidation of apolipoprotein B-100 in circulating LDL is related to LDL residence time. In vivo insights from stable-isotope studies. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E63–E67. [Google Scholar] [CrossRef]
- Petrucci, G.; Rizzi, A.; Hatem, D.; Tosti, G.; Rocca, B.; Pitocco, D. Role of Oxidative Stress in the Pathogenesis of Atherothrombotic Diseases. Antioxidants 2022, 11, 1408. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.; Dai, H.; Deng, Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res. Clin. Pract. 2020, 160, 108001. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Wang, G.; Xu, Y. The Effects of Exenatide and Metformin on Endothelial Function in Newly Diagnosed Type 2 Diabetes Mellitus Patients: A Case-Control Study. Diabetes Ther. 2018, 9, 1295–1305. [Google Scholar] [CrossRef]
- Formoso, G.; De Filippis, E.A.; Michetti, N.; Di Fulvio, P.; Pandolfi, A.; Bucciarelli, T.; Ciabattoni, G.; Nicolucci, A.; Davi, G.; Consoli, A. Decreased in vivo oxidative stress and decreased platelet activation following metformin treatment in newly diagnosed type 2 diabetic subjects. Diabetes Metab. Res. Rev. 2008, 24, 231–237. [Google Scholar] [CrossRef]
- Abdelrahman, S.; Alghrably, M.; Campagna, M.; Hauser, C.A.E.; Jaremko, M.; Lachowicz, J.I. Metal Complex Formation and Anticancer Activity of Cu(I) and Cu(II) Complexes with Metformin. Molecules 2021, 26, 4730. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Lin, H.C.; Tsai, K.L. Metformin regulates oxLDL-facilitated endothelial dysfunction by modulation of SIRT1 through repressing LOX-1-modulated oxidative signaling. Oncotarget 2016, 7, 10773–10787. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Gundewar, S.; Jha, S.; Greer, J.J.; Bestermann, W.H.; Tian, R.; Lefer, D.J. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 2008, 57, 696–705. [Google Scholar] [CrossRef]
- de Jager, J.; Kooy, A.; Schalkwijk, C.; van der Kolk, J.; Lehert, P.; Bets, D.; Wulffele, M.G.; Donker, A.J.; Stehouwer, C.D. Long-term effects of metformin on endothelial function in type 2 diabetes: A randomized controlled trial. J. Intern. Med. 2014, 275, 59–70. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Torres, G.; Wu, S.; Ouyang, C.; Xie, Z.; Zou, M.H. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes 2017, 66, 193–205. [Google Scholar] [CrossRef]
- Duan, Q.; Song, P.; Ding, Y.; Zou, M.H. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br. J. Pharmacol. 2017, 174, 2140–2151. [Google Scholar] [CrossRef]
- Gutierrez-Repiso, C.; Rodriguez-Pacheco, F.; Garcia-Arnes, J.; Valdes, S.; Gonzalo, M.; Soriguer, F.; Moreno-Ruiz, F.J.; Rodriguez-Canete, A.; Gallego-Perales, J.L.; Alcain-Martinez, G.; et al. The expression of genes involved in jejunal lipogenesis and lipoprotein synthesis is altered in morbidly obese subjects with insulin resistance. Lab. Investig. 2015, 95, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, N.; Wang, Y.; Cheng, J.; Xu, Z.; Wang, S. Metformin protects against oxidized low density lipoprotein-induced macrophage apoptosis and inhibits lipid uptake. Exp. Ther. Med. 2018, 15, 2485–2491. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Welsh, P.; Petrie, J.R. Metformin, lipids and atherosclerosis prevention. Curr. Opin. Lipidol. 2018, 29, 346–353. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Kang, L.; Li, J.; Yang, L.; Zhang, J.; Liu, J.; Zhu, M.; Zhang, Q.; Shen, Y.; et al. Metformin attenuates myocardial ischemia-reperfusion injury via up-regulation of antioxidant enzymes. PLoS ONE 2017, 12, e0182777. [Google Scholar] [CrossRef]
- Rey-Renones, C.; Baena-Diez, J.M.; Aguilar-Palacio, I.; Miquel, C.; Grau, M. Type 2 Diabetes Mellitus and Cancer: Epidemiology, Physiopathology and Prevention. Biomedicines 2021, 9, 1429. [Google Scholar] [CrossRef]
- Zhou, J.B.; Tang, X.; Han, M.; Yang, J.; Simo, R. Impact of antidiabetic agents on dementia risk: A Bayesian network meta-analysis. Metabolism 2020, 109, 154265. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Makkar, S.; Crawford, J.D.; Kochan, N.A.; Wen, W.; Draper, B.; Trollor, J.N.; Brodaty, H.; Sachdev, P.S. Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care 2020, 43, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Chang, Y.L.; Gau, S.Y.; Tsai, T.H.; Lee, C.Y. Dose-Response Association of Metformin with Parkinson’s Disease Odds in Type 2 Diabetes Mellitus. Pharmaceutics 2022, 14, 946. [Google Scholar] [CrossRef] [PubMed]

| Radical | Position of H-Abstraction | Gibbs’ Free Energy (kcal/mol) | |
|---|---|---|---|
| Water | Benzene | ||
| 1 | −25.25 | −23.95 | |
| 2 | −25.19 | −20.78 | |
| Hydroxyl radical | 3 | −21.89 | −19.05 |
| 4 | −20.37 | −18.43 | |
| 5 | −20.51 | −18.34 | |
| 6 | −27.35 | −26.60 | |
| 7 | −28.55 | −28.07 | |
| 1 | 24.01 | 31.22 | |
| 2 | 24.07 | 34.38 | |
| Superoxide radical anion | 3 | 27.37 | 36.11 |
| 4 | 28.89 | 36.73 | |
| 5 | 28.74 | 36.83 | |
| 6 | 21.91 | 28.57 | |
| 7 | 20.70 | 27.10 | |
| 1 | 8.24 | 9.53 | |
| 2 | 8.30 | 12.70 | |
| Hydroperoxyl radical | 3 | 11.60 | 14.42 |
| 4 | 13.12 | 15.04 | |
| 5 | 12.98 | 15.14 | |
| 6 | 6.14 | 6.88 | |
| 7 | 4.94 | 5.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossmann, C.; Ranz, C.; Kager, G.; Ledinski, G.; Koestenberger, M.; Wonisch, W.; Wagner, T.; Schwaminger, S.P.; Di Geronimo, B.; Hrzenjak, A.; et al. Metformin Impedes Oxidation of LDL In Vitro. Pharmaceutics 2023, 15, 2111. https://doi.org/10.3390/pharmaceutics15082111
Rossmann C, Ranz C, Kager G, Ledinski G, Koestenberger M, Wonisch W, Wagner T, Schwaminger SP, Di Geronimo B, Hrzenjak A, et al. Metformin Impedes Oxidation of LDL In Vitro. Pharmaceutics. 2023; 15(8):2111. https://doi.org/10.3390/pharmaceutics15082111
Chicago/Turabian StyleRossmann, Christine, Cornelia Ranz, Gerd Kager, Gerhard Ledinski, Martin Koestenberger, Willibald Wonisch, Thomas Wagner, Sebastian P. Schwaminger, Bruno Di Geronimo, Andelko Hrzenjak, and et al. 2023. "Metformin Impedes Oxidation of LDL In Vitro" Pharmaceutics 15, no. 8: 2111. https://doi.org/10.3390/pharmaceutics15082111
APA StyleRossmann, C., Ranz, C., Kager, G., Ledinski, G., Koestenberger, M., Wonisch, W., Wagner, T., Schwaminger, S. P., Di Geronimo, B., Hrzenjak, A., Hallstöm, S., Reibnegger, G., Cvirn, G., & Paar, M. (2023). Metformin Impedes Oxidation of LDL In Vitro. Pharmaceutics, 15(8), 2111. https://doi.org/10.3390/pharmaceutics15082111








