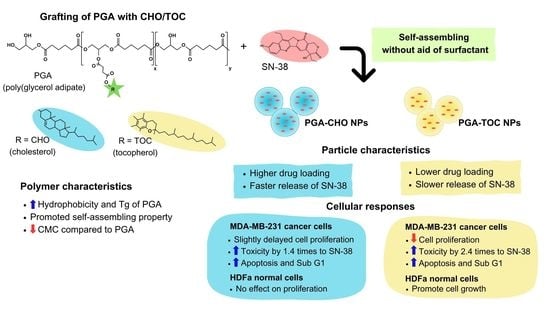
Modification of Poly(Glycerol Adipate) with Tocopherol and Cholesterol Modulating Nanoparticle Self-Assemblies and Cellular Responses of Triple-Negative Breast Cancer Cells to SN-38 Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of CHO- and TOC-Grafted PGA
2.3. Polymer Characterization
2.3.1. Structure Elucidation
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Water Contact Angle
2.4. Nanoparticle Preparation
2.5. Nanoparticle Characterization
2.6. Drug Release Study
2.7. Cell Proliferation Study
2.8. Cell Cycle Analysis
2.9. Cytotoxicity Study
2.10. Apoptosis Assay
2.11. Cellular Uptake Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Polymer Characterization
3.1.1. Structure Elucidation
3.1.2. Thermal Behavior
3.1.3. Water Contact Angle
3.2. Particle Characterization
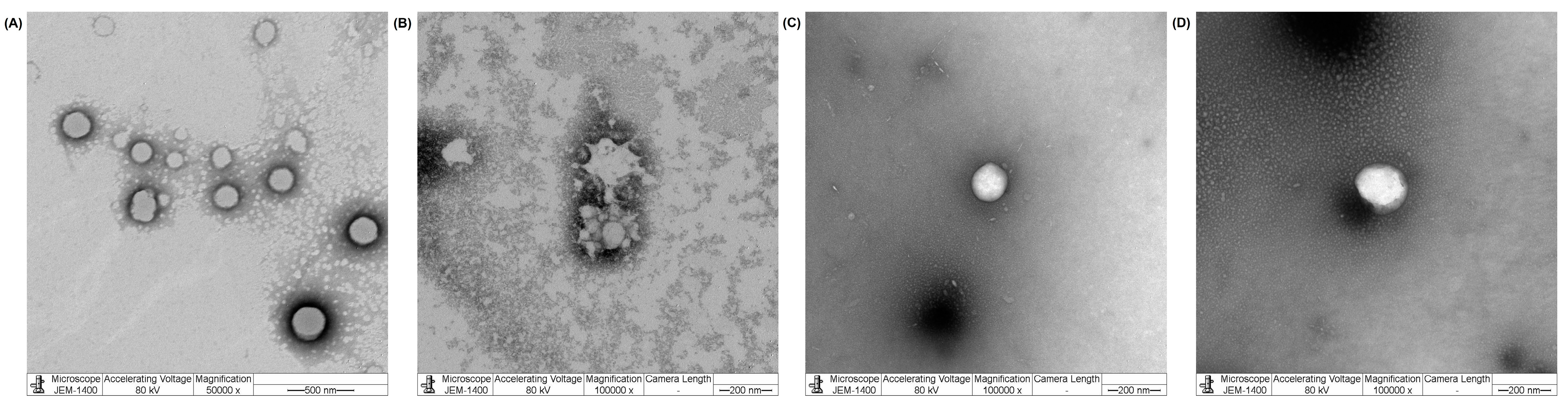
3.2.1. Blank NPs
3.2.2. Drug-Loaded NPs
3.3. Effect on Cellular Responses
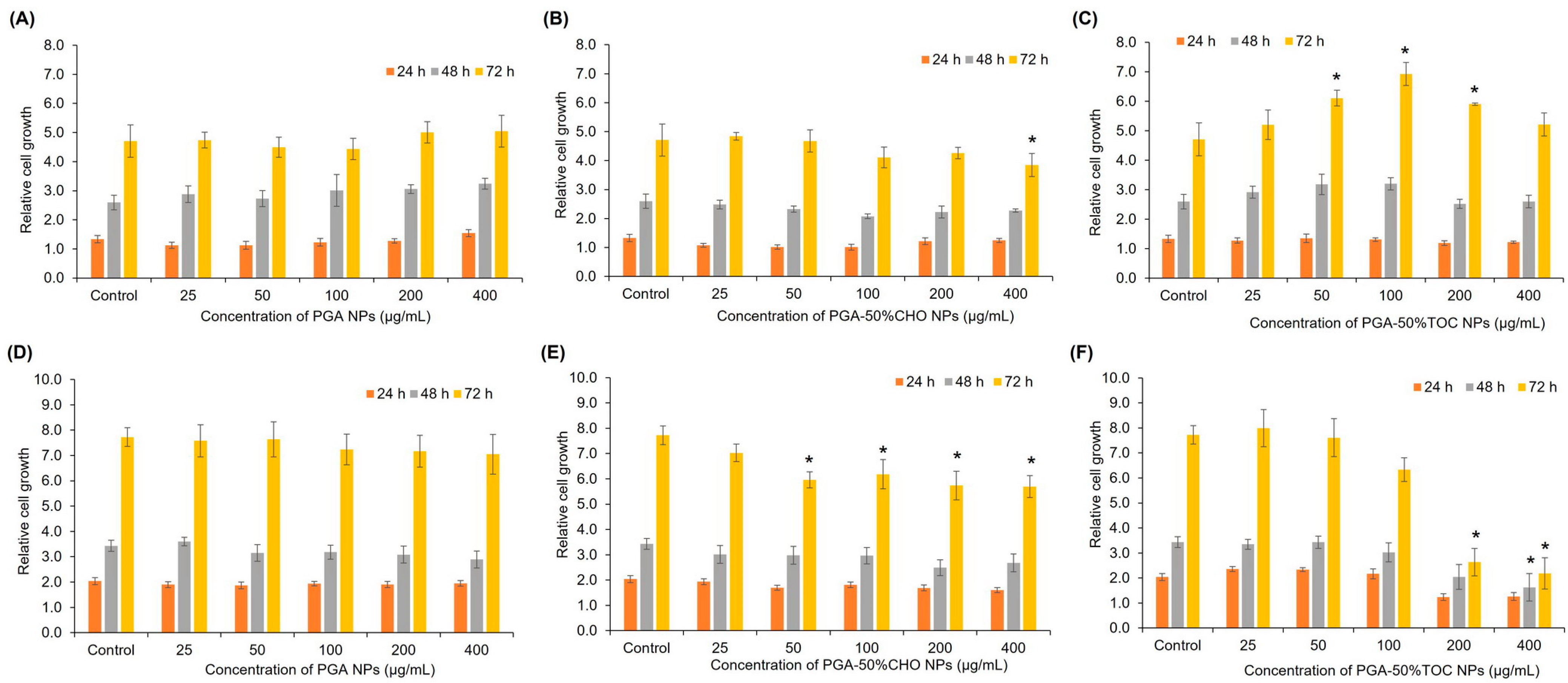
3.3.1. Blank NPs
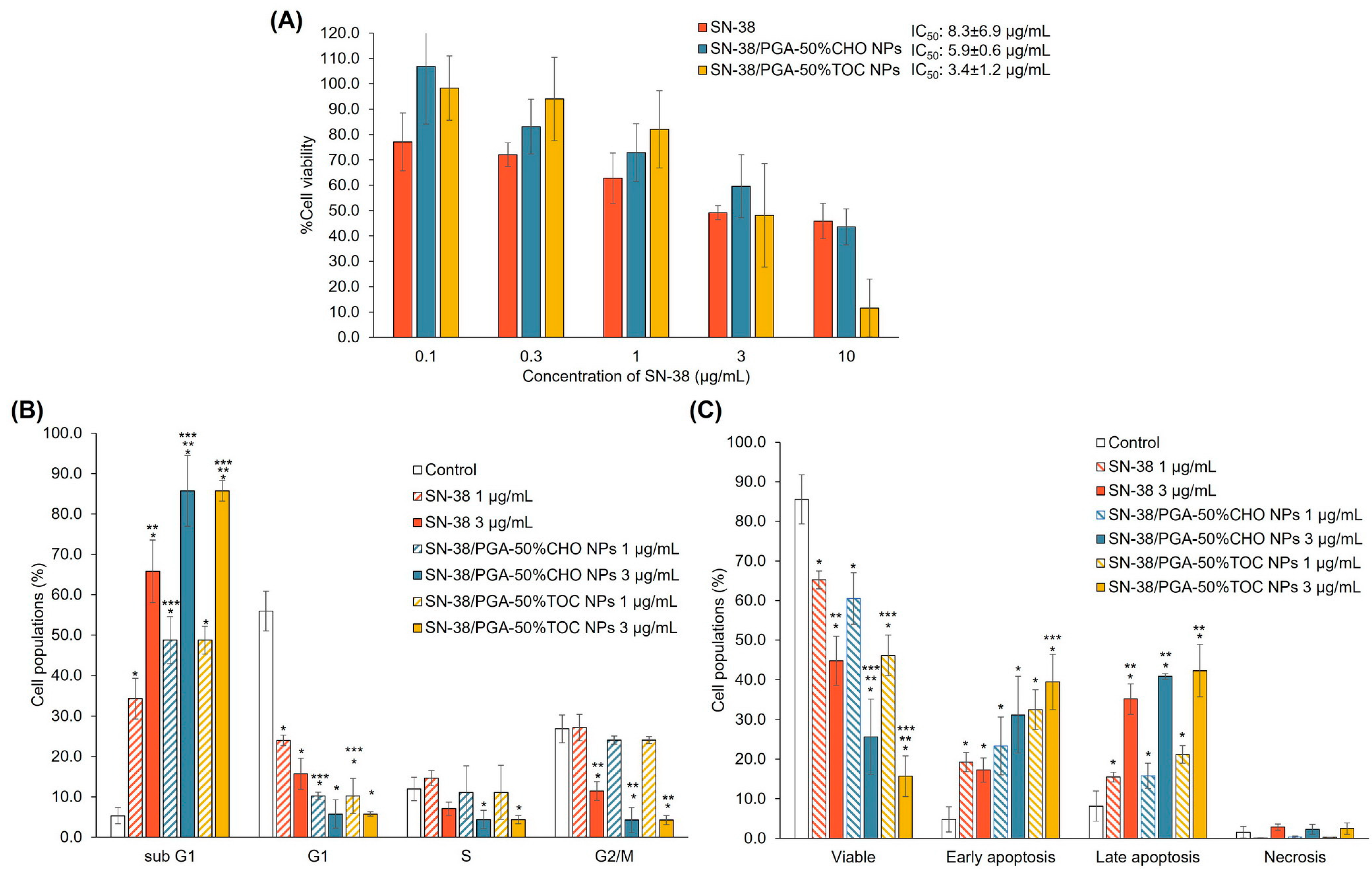
3.3.2. Drug-Loaded NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Ocaña, A.; Castro-Osma, J.A.; Bravo, I.; Alonso-Moreno, C. Polyester Polymeric Nanoparticles as Platforms in the Development of Novel Nanomedicines for Cancer Treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Creasey, R.G.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Meng, W.; Parker, T.L.; Kallinteri, P.; Walker, D.A.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Uptake and metabolism of novel biodegradable poly(glycerol-adipate) nanoparticles in DAOY monolayer. J. Control. Release 2006, 116, 314–321. [Google Scholar] [CrossRef]
- Puri, S.; Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Drug incorporation and release of water soluble drugs from novel functionalised poly(glycerol adipate) nanoparticles. J. Control. Release 2008, 125, 59–67. [Google Scholar] [CrossRef]
- Meng, W.; Kallinteri, P.; Walker, D.A.; Parker, T.L.; Garnett, M.C. Evaluation of poly(glycerol-adipate) nanoparticle uptake in an in vitro 3-D brain tumor co-culture model. Exp. Biol. Med. 2007, 232, 1100–1108. [Google Scholar] [CrossRef]
- Weiss, V.M.; Naolou, T.; Groth, T.; Kressler, J.; Mader, K. In vitro toxicity of stearoyl-poly(glycerol adipate) nanoparticles. J. Appl. Biomater. Funct. Mater. 2012, 10, 163–169. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel Functionalized Biodegradable Polymers for Nanoparticle Drug Delivery Systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef]
- Weiss, V.M.; Naolou, T.; Hause, G.; Kuntsche, J.; Kressler, J.; Mäder, K. Poly(glycerol adipate)-fatty acid esters as versatile nanocarriers: From nanocubes over ellipsoids to nanospheres. J. Control. Release 2012, 158, 156–164. [Google Scholar] [CrossRef]
- Jacob, P.L.; Ruiz Cantu, L.A.; Pearce, A.K.; He, Y.; Lentz, J.C.; Moore, J.C.; Machado, F.; Rivers, G.; Apebende, E.; Fernandez, M.R.; et al. Poly(glycerol adipate) (PGA) backbone modifications with a library of functional diols: Chemical and physical effects. Polymer 2021, 228, 123912. [Google Scholar] [CrossRef]
- Jacob, P.L.; Brugnoli, B.; Del Giudice, A.; Phan, H.; Chauhan, V.M.; Beckett, L.; Gillis, R.B.; Moloney, C.; Cavanagh, R.J.; Krumins, E.; et al. Poly(diglycerol adipate) variants as enhanced nanocarrier replacements in drug delivery applications. J. Colloid Interface Sci. 2023, 641, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Naolou, T.; Hussain, H.; Baleed, S.; Busse, K.; Lechner, B.-D.; Kressler, J. The behavior of fatty acid modified poly(glycerol adipate) at the air/water interface. Colloids Surf. Physicochem. Eng. Asp. 2015, 468, 22–30. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of acyl modified poly(glycerol-adipate) comb-like polymers and their self-assembly into nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Swainson, S.M.E.; Taresco, V.; Pearce, A.K.; Clapp, L.H.; Ager, B.; McAllister, M.; Bosquillon, C.; Garnett, M.C. Exploring the enzymatic degradation of poly(glycerol adipate). Eur. J. Pharm. Biopharm. 2019, 142, 377–386. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Styliari, I.D.; Argent, R.H.; Swainson, S.M.E.; Booth, J.; Turpin, E.; Laughton, C.A.; Burley, J.C.; Alexander, C.; et al. New N-acyl amino acid-functionalized biodegradable polyesters for pharmaceutical and biomedical applications. RSC Adv. 2016, 6, 109401–109405. [Google Scholar] [CrossRef]
- Sagnelli, D.; Cavanagh, R.; Xu, J.; Swainson, S.M.E.; Blennow, A.; Duncan, J.; Taresco, V.; Howdle, S. Starch/Poly(glycerol-adipate) nanocomposite film as novel biocompatible materials. Coatings 2019, 9, 482. [Google Scholar] [CrossRef]
- Gordhan, D.; Swainson, S.M.E.; Pearce, A.K.; Styliari, I.D.; Lovato, T.; Burley, J.C.; Garnett, M.C.; Taresco, V. Poly(Glycerol Adipate): From a Functionalized Nanocarrier to a Polymeric-Prodrug Matrix to Create Amorphous Solid Dispersions. J. Pharm. Sci. 2020, 109, 1347–1355. [Google Scholar] [CrossRef]
- Wersig, T.; Hacker, M.C.; Kressler, J.; Mader, K. Poly(glycerol adipate)—Indomethacin drug conjugates—Synthesis and in vitro characterization. Int. J. Pharm. 2017, 531, 225–234. [Google Scholar] [CrossRef]
- Suksiriworapong, J.; Taresco, V.; Ivanov, D.P.; Styliari, I.D.; Sakchaisri, K.; Junyaprasert, V.B.; Garnett, M.C. Synthesis and properties of a biodegradable polymer-drug conjugate: Methotrexate-poly(glycerol adipate). Colloids Surf. B Biointerfaces 2018, 167, 115–125. [Google Scholar] [CrossRef]
- Vestri, A.; Pearce, A.K.; Cavanagh, R.; Styliari, I.D.; Sanders, C.; Couturaud, B.; Schenone, S.; Taresco, V.; Jakobsen, R.R.; Howdle, S.M.; et al. Starch/Poly(glycerol-adipate) nanocomposites: A novel oral drug delivery device. Coatings 2020, 10, 125. [Google Scholar] [CrossRef]
- Taresco, V.; Tulini, I.; Francolini, I.; Piozzi, A. Polyglycerol adipate-grafted polycaprolactone nanoparticles as carriers for the antimicrobial compound usnic acid. Int. J. Mol. Sci. 2022, 23, 14339. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Favretto, M.E.; Onyeagor, N.D.; Khan, G.M.; Douroumis, D.; Casely-Hayford, M.A.; Kallinteri, P. Development of poly(glycerol adipate) nanoparticles loaded with non-steroidal anti-inflammatory drugs. J. Microencapsul. 2012, 29, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tchoryk, A.; Taresco, V.; Argent, R.H.; Ashford, M.; Gellert, P.R.; Stolnik, S.; Grabowska, A.; Garnett, M.C. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjugate Chem. 2019, 30, 1371–1384. [Google Scholar] [CrossRef]
- Meng, W.; Garnett, M.C.; Walker, D.A.; Parker, T.L. Penetration and intracellular uptake of poly(glycerol-adipate) nanoparticles into three-dimensional brain tumour cell culture models. Exp. Biol. Med. (Maywood) 2016, 241, 466–477. [Google Scholar] [CrossRef]
- Animasawun, R.K.; Taresco, V.; Swainson, S.M.E.; Suksiriworapong, J.; Walker, D.A.; Garnett, M.C. Screening and matching polymers with drugs to improve drug incorporation and retention in nanoparticles. Mol. Pharm. 2020, 17, 2083–2098. [Google Scholar] [CrossRef]
- Neuzil, J.; Weber, T.; Gellert, N.; Weber, C. Selective cancer cell killing by α-tocopheryl succinate. Br. J. Cancer 2001, 84, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Papas, A.; Constantinou, A.I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 123, 739–752. [Google Scholar] [CrossRef]
- Zampelas, A.; Magriplis, E. New Insights into Cholesterol Functions: A Friend or an Enemy? Nutrients 2019, 11, 1645. [Google Scholar] [CrossRef]
- Wei, B.; He, M.; Cai, X.; Hou, X.; Wang, Y.; Chen, J.; Lan, M.; Chen, Y.; Lou, K.; Gao, F. Vitamin E succinate-grafted-chitosan/chitosan oligosaccharide mixed micelles loaded with C-DMSA for Hg2+ detection and detoxification in rat liver. Int. J. Nanomed. 2019, 14, 6917–6932. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, S.; Liu, W.; Yuan, Z.; Yin, P.; Gao, F. Vitamin E succinate-grafted-chitosan oligosaccharide/RGD-conjugated TPGS mixed micelles loaded with paclitaxel for U87MG tumor therapy. Mol. Pharm. 2017, 14, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-P.; Lee, K.-J.; Choi, J.-W.; Yun, C.-O.; Nah, J.-W. Targeting delivery of tocopherol and doxorubicin grafted-chitosan polymeric micelles for cancer therapy: In vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2015, 133, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Y.; Chen, W.; Ren, J.; Zhang, L.; Lu, L.; Luo, G.; Huang, H. Preparation, characterization and evaluation of α-tocopherol succinate-modified dextran micelles as potential drug carriers. Materials 2015, 8, 6685–6696. [Google Scholar] [CrossRef]
- Jena, S.K.; Samal, S.K.; Kaur, S.; Chand, M.; Sangamwar, A.T. Potential of amphiphilic graft copolymer α-tocopherol succinate-g-carboxymethyl chitosan in modulating the permeability and anticancer efficacy of tamoxifen. Eur. J. Pharm. Sci. 2017, 101, 149–159. [Google Scholar] [CrossRef]
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles of amphiphilic graft copolymer of α-tocopherol succinate-g-carboxymethyl chitosan for tamoxifen delivery: Synthesis, characterization and in vivo pharmacokinetic study. Carbohydr. Polym. 2016, 151, 1162–1174. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Wu, M.; Fan, L.; He, C.; Wan, J.B.; Li, P.; Chen, M.; Li, H. Vitamin E succinate-conjugated F68 micelles for mitoxantrone delivery in enhancing anticancer activity. Int. J. Nanomed. 2016, 11, 3167–3178. [Google Scholar] [CrossRef]
- Battogtokh, G.; Kang, J.H.; Ko, Y.T. Long-circulating self-assembled cholesteryl albumin nanoparticles enhance tumor accumulation of hydrophobic anticancer drug. Eur. J. Pharm. Biopharm. 2015, 96, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Yang, W.; Li, X.; Liu, L.; Zhou, Z.; Wang, Y.; Li, R.; Zhang, Q. Preparation and characterization of self-assembled nanoparticles of 6-O-cholesterol-modified chitosan for drug delivery. Carbohydr. Polym. 2011, 84, 1244–1251. [Google Scholar] [CrossRef]
- Tao, X.; Xie, Y.; Zhang, Q.; Qiu, X.; Yuan, L.; Wen, Y.; Li, M.; Yang, X.; Tao, T.; Xie, M.; et al. Cholesterol-modified amino-pullulan nanoparticles as a drug carrier: Comparative study of cholesterol-modified carboxyethyl Pullulan and Pullulan nanoparticles. Nanomaterials 2016, 6, 165. [Google Scholar] [CrossRef]
- Yinsong, W.; Lingrong, L.; Jian, W.; Zhang, Q. Preparation and characterization of self-aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan conjugates. Carbohydr. Polym. 2007, 69, 597–606. [Google Scholar] [CrossRef]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zhang, Q.; Liang, D.; Huang, Y. Interaction between human serum albumin and cholesterol-grafted polyglutamate as the potential carriers of protein drugs. Acta Pharm. Sin. B 2019, 9, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. The efficacy of cholesterol-based carriers in drug delivery. Molecules 2020, 25, 4330. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, Q.; Tan, S.; Zhuang, X.; Bao, Y.; Wu, T.; Zhang, Z. D-α-tocopherol polyethylene glycol succinate-based derivative nanoparticles as a novel carrier for paclitaxel delivery. Int. J. Nanomed. 2015, 10, 5219–5235. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef]
- Alfirevic, A.; Pirmohamed, M. Chapter 10—Pharmacogenetics and Pharmacogenomics. In Medical and Health Genomics; Kumar, D., Antonarakis, S., Eds.; Academic Press: Oxford, UK, 2016; pp. 121–137. [Google Scholar] [CrossRef]
- Damrongrak, K.; Kloysawat, K.; Bunsupa, S.; Sakchasri, K.; Wongrakpanich, A.; Taresco, V.; Cuzzucoli Crucitti, V.; Garnett, M.C.; Suksiriworapong, J. Delivery of acetogenin-enriched Annona muricata Linn leaf extract by folic acid-conjugated and triphenylphosphonium-conjugated poly(glycerol adipate) nanoparticles to enhance toxicity against ovarian cancer cells. Int. J. Pharm. 2022, 618, 121636. [Google Scholar] [CrossRef]
- Ali, M.A.; Noguchi, S.; Iwao, Y.; Oka, T.; Itai, S. Preparation and Characterization of SN-38-Encapsulated Phytantriol Cubosomes Containing α-Monoglyceride Additives. Chem. Pharm. Bull. 2016, 64, 577–584. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef]
- Mingkwan, T.; Suksiriworapong, J.; Chantasart, D. Pluronic® P123/TPGS and Pluronic® F127/TPGS mixed micelles for the entrapment of itraconazole. Chiang Mai J. Sci. 2015, 42, 946–956. [Google Scholar]
- Thakur, R.; Sivakumar, B.; Savva, M. Thermodynamic studies and loading of 7-ethyl-10-hydroxycamptothecin into mesoporous silica particles MCM-41 in strongly acidic solutions. J. Phys. Chem. B 2010, 114, 5903–5911. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Nazari, M.; Safaeijavan, R.; Vaziri Yazdi, A.; Moniri, E. Investigation of the adsorption and release kinetics of the anticancer drug, methotrexate, from chitosan nanocapsules modified by caffeic acid and oleic acid. Inorg. Chem. Commun. 2023, 153, 110769. [Google Scholar] [CrossRef]
- Azadi, S.; Ashrafi, H.; Azadi, A. Mathematical Modeling of Drug Release from Swellable Polymeric Nanoparticles. J. Appl. Pharm. Sci. 2017, 7, 125–133. [Google Scholar]
- Mendyk, A.; Jachowicz, R. Unified methodology of neural analysis in decision support systems built for pharmaceutical technology. Expert Syst. Appl. 2007, 32, 1124–1131. [Google Scholar] [CrossRef]
- Sant, V.P.; Nagarsenker, M.S. Synthesis of monomethoxypolyethyleneglycol—Cholesteryl ester and effect of its incorporation in liposomes. AAPS PharmSciTech 2011, 12, 1056–1063. [Google Scholar] [CrossRef]
- Palem, C.R.; Dudhipala, N.R.; Battu, S.K.; Repka, M.A.; Rao Yamsani, M. Development, optimization and in vivo characterization of domperidone-controlled release hot-melt-extruded films for buccal delivery. Drug Dev. Ind. Pharm. 2016, 42, 473–484. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 5997, Cholesterol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cholesterol (accessed on 1 March 2023).
- PubChem Compound Summary for CID 14985, Vitamin E. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14985 (accessed on 1 March 2023).
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Yang, L.; Dong, Y.; Zhang, Y.; Yan, G.; Tang, R. pH-sensitive micelles self-assembled from star-shaped TPGS copolymers with ortho ester linkages for enhanced MDR reversal and chemotherapy. Asian J. Pharm. Sci. 2021, 16, 363–373. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Lappe, S.; Mulac, D.; Langer, K. Polymeric nanoparticles—Influence of the glass transition temperature on drug release. Int. J. Pharm. 2017, 517, 338–347. [Google Scholar] [CrossRef]
- Matsumoto, J.; Nakada, Y.; Sakurai, K.; Nakamura, T.; Takahashi, Y. Preparation of nanoparticles consisted of poly(L-lactide)-poly(ethylene glycol)-poly(L-lactide) and their evaluation in vitro. Int. J. Pharm. 1999, 185, 93–101. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly(lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Makpol, S.; Zainuddin, A.; Rahim, N.A.; Yusof, Y.A.M.; Ngah, W.Z.W. Alpha-tocopherol modulates hydrogen peroxide-induced DNA damage and telomere shortening of human skin fibroblasts derived from differently aged individuals. Planta Medica 2010, 76, 869–875. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, I.; Kim, W.K.; Choi, J.Y.; Kim, D.Y.; Moon, S.G.; Min, H.K.; Song, M.K.; Sung, J.H. Mitochondria-targeted vitamin E protects skin from UVB-irradiation. Biomol. Ther. 2016, 24, 305–311. [Google Scholar] [CrossRef]
- Butt, H.; Mehmood, A.; Ali, M.; Tasneem, S.; Anjum, M.S.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Protective role of vitamin E preconditioning of human dermal fibroblasts against thermal stress in vitro. Life Sci. 2017, 184, 1–9. [Google Scholar] [CrossRef]
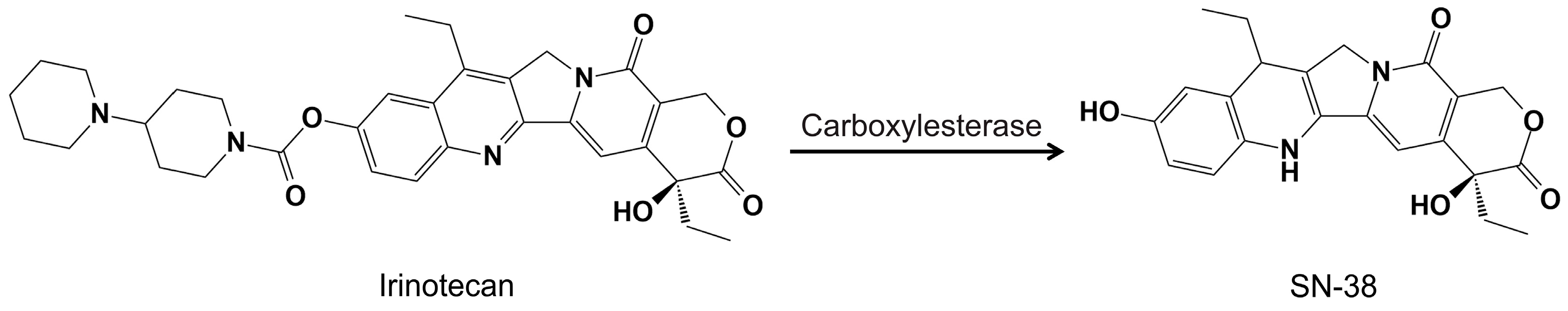
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an important player in cancer chemotherapy: A comprehensive overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef]
- Lee, B.; Min, J.A.; Nashed, A.; Lee, S.-O.; Yoo, J.C.; Chi, S.-W.; Yi, G.-S. A novel mechanism of irinotecan targeting MDM2 and Bcl-xL. Biochem. Biophys. Res. Commun. 2019, 514, 518–523. [Google Scholar] [CrossRef]
- Takeba, Y.; Kumai, T.; Matsumoto, N.; Nakaya, S.; Tsuzuki, Y.; Yanagida, Y.; Kobayashi, S. Irinotecan activates p53 with its active metabolite, resulting in human hepatocellular carcinoma apoptosis. J. Pharmacol. Sci. 2007, 104, 232–242. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Kim, S.; LoRusso, P.; Li, J. Pharmacometabolomics reveals irinotecan mechanism of action in cancer patients. J. Clin. Pharmacol. 2019, 59, 20–34. [Google Scholar] [CrossRef]
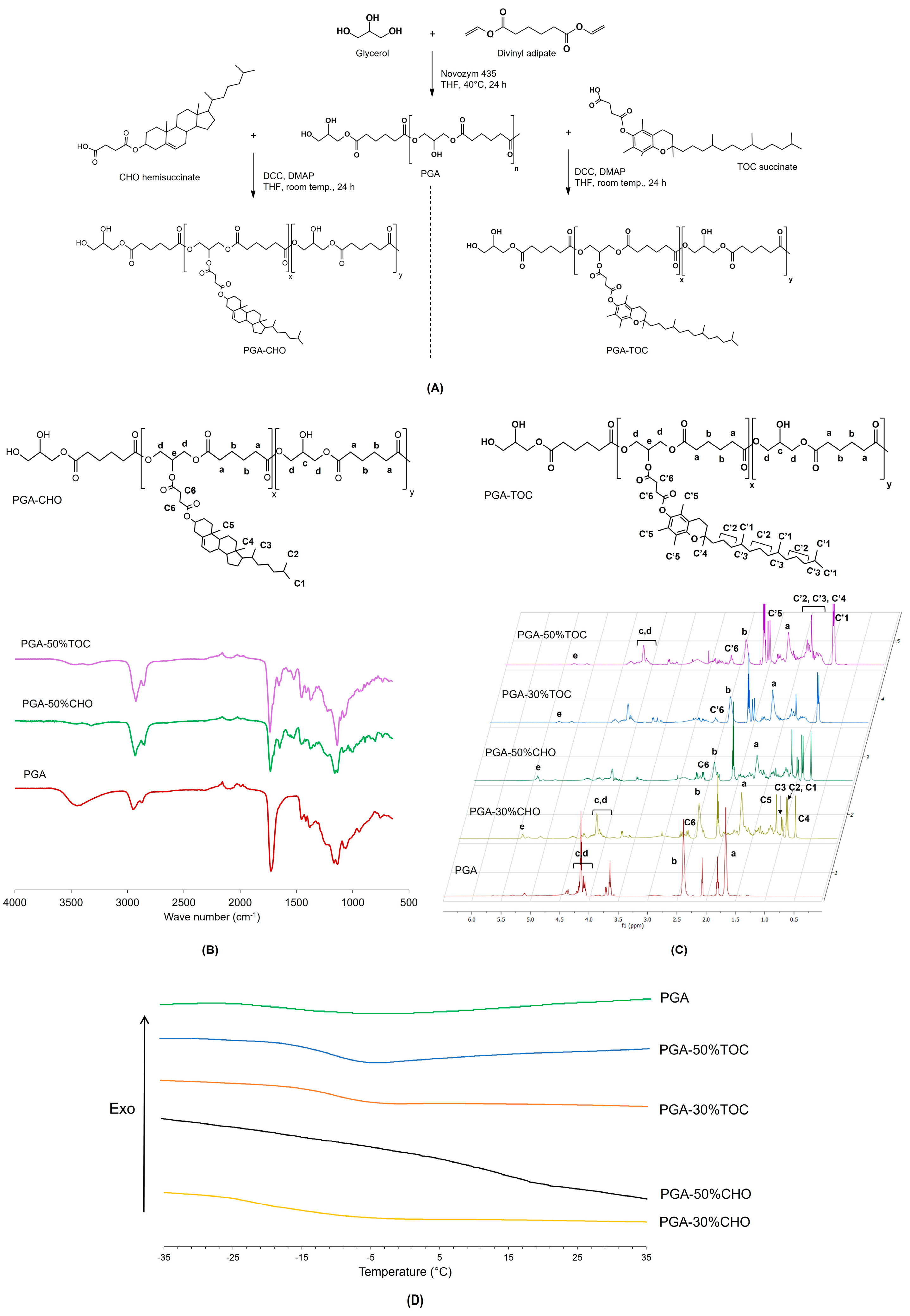
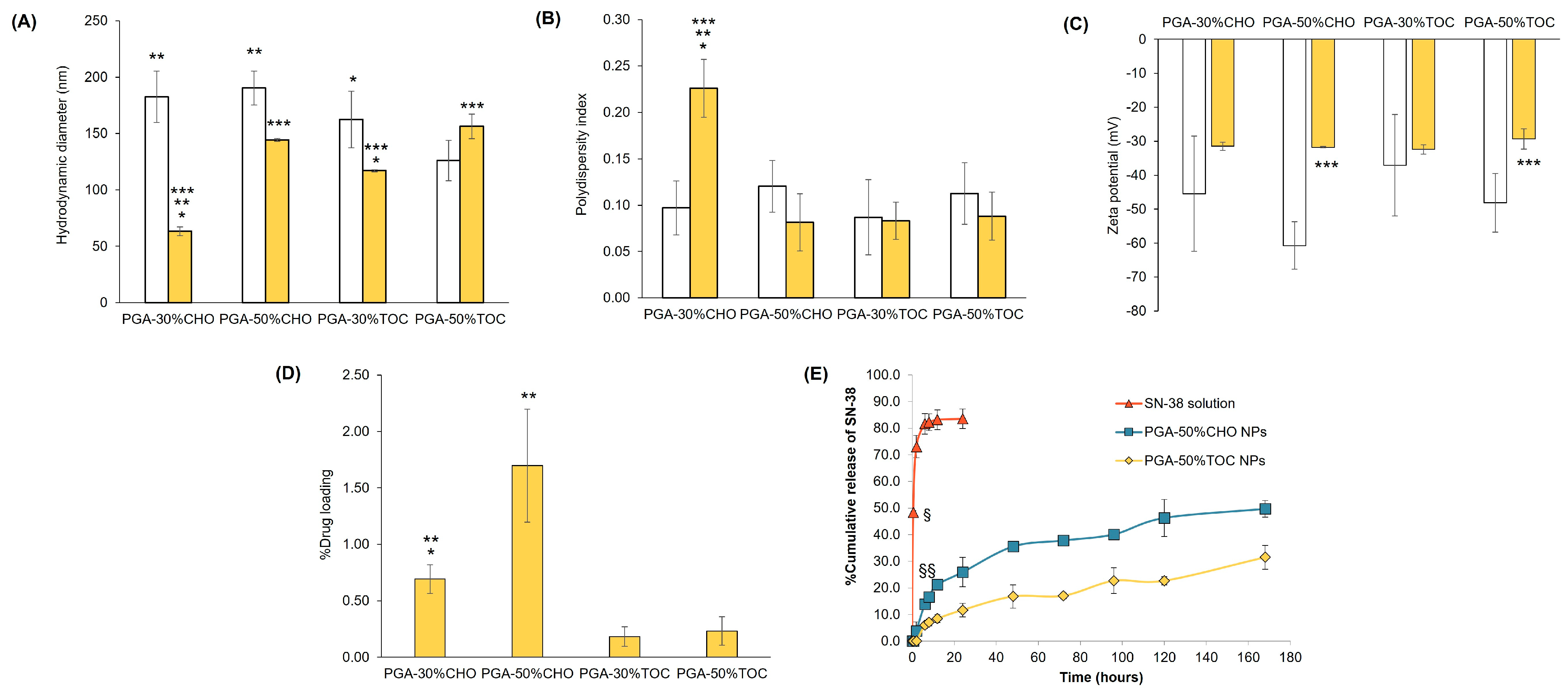


| Polymer | % Mole Grafting | Mn (×103 g/mol) [Mw/Mn] | Tg (°C) | WCA (°) | CMC (×10−3 g/L) |
|---|---|---|---|---|---|
| PGA | - | 11.6 [1.4] | −33 | 55 ± 2 | 170 c |
| PGA-30%CHO | 33.2 a | 9.30 [3.2] | −17 | 96 ± 1 | 4.41 |
| PGA-50%CHO | 50.8 a | 10.5 [2.9] | 8 | 104 ± 1 | 1.79 |
| PGA-30%TOC | 26.4 b | 9.70 [2.6] | −10 | 88 ± 1 | 4.52 |
| PGA-50%TOC | 50.4 b | 9.90 [3.4] | −11 | 113 ± 1 | 1.99 |
| Model [Equation] a | SN-38-Loaded PGA-50%CHO NPs | SN-38-Loaded PGA-50%TOC NPs | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Adjusted R2 | AIC b | Slope | R2 | Adjusted R2 | AIC b | Slope | |
| Zero order [F = kt] | 0.895 | 0.895 | −34.33 | k = 0.0021 | 0.963 | 0.963 | −50.84 | k = 0.0015 |
| First order [ln(1 − F) = −kt] | 0.791 | 0.739 | −26.18 | k = 0.0071 | 0.870 | 0.838 | −37.46 | k = 0.0096 |
| Higuchi’s [F = kt1/2] | 0.723 | 0.723 | −25.62 | k = 0.0492 | 0.970 | 0.970 | −52.50 | k = 0.0233 |
| Korsmeyer–Peppas [ln F = ln K + n ln t] | 0.985 | 0.983 | −50.80 | K = 0.0793 n = 0.3665 | 0.985 | 0.969 | −52.18 | K = 0.0255 n = 0.4746 |
| Hixson–Crowell [1 − (1 − F)1/3 = −kt] | 0.829 | 0.818 | −29.41 | k = 0.0016 | 0.911 | 0.915 | −43.27 | k = 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suksiriworapong, J.; Achayawat, C.; Juangrattanakamjorn, P.; Taresco, V.; Crucitti, V.C.; Sakchaisri, K.; Bunsupa, S. Modification of Poly(Glycerol Adipate) with Tocopherol and Cholesterol Modulating Nanoparticle Self-Assemblies and Cellular Responses of Triple-Negative Breast Cancer Cells to SN-38 Delivery. Pharmaceutics 2023, 15, 2100. https://doi.org/10.3390/pharmaceutics15082100
Suksiriworapong J, Achayawat C, Juangrattanakamjorn P, Taresco V, Crucitti VC, Sakchaisri K, Bunsupa S. Modification of Poly(Glycerol Adipate) with Tocopherol and Cholesterol Modulating Nanoparticle Self-Assemblies and Cellular Responses of Triple-Negative Breast Cancer Cells to SN-38 Delivery. Pharmaceutics. 2023; 15(8):2100. https://doi.org/10.3390/pharmaceutics15082100
Chicago/Turabian StyleSuksiriworapong, Jiraphong, Chittin Achayawat, Phutthikom Juangrattanakamjorn, Vincenzo Taresco, Valentina Cuzzucoli Crucitti, Krisada Sakchaisri, and Somnuk Bunsupa. 2023. "Modification of Poly(Glycerol Adipate) with Tocopherol and Cholesterol Modulating Nanoparticle Self-Assemblies and Cellular Responses of Triple-Negative Breast Cancer Cells to SN-38 Delivery" Pharmaceutics 15, no. 8: 2100. https://doi.org/10.3390/pharmaceutics15082100
APA StyleSuksiriworapong, J., Achayawat, C., Juangrattanakamjorn, P., Taresco, V., Crucitti, V. C., Sakchaisri, K., & Bunsupa, S. (2023). Modification of Poly(Glycerol Adipate) with Tocopherol and Cholesterol Modulating Nanoparticle Self-Assemblies and Cellular Responses of Triple-Negative Breast Cancer Cells to SN-38 Delivery. Pharmaceutics, 15(8), 2100. https://doi.org/10.3390/pharmaceutics15082100