BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions
Abstract
1. Introduction
2. Overview of Brain-Derived Neurotrophic Factor
3. The Multifaceted Roles of Brain-Derived Neurotrophic Factor
| Function | Description |
|---|---|
| Neuronal Development | Promotes the growth and development of neurons during early brain development, contributing to the formation of neuronal connections and neural circuits [29]. |
| Synaptic Plasticity | Regulates synaptic plasticity. It facilitates the strengthening and formation of new synapses [30]. |
| Learning, Memory, and Mood Regulation | Supports the formation of long-term memories and promotes the consolidation of newly acquired information. BDNF is also implicated in mood regulation, being associated with the pathophysiology of psychiatric disorders, such as major depression disorder [2,32]. |
| Neurogenesis | Promotes the generation of new neurons, replenishing and maintaining a healthy population of neurons [35]. |
| Neuroprotection | Helps to mitigate damage caused by oxidative stress, inflammation, and other harmful processes in the brain [40]. |
4. Exploring BDNF and Its Connection to Mood Disorders
4.1. Oxidative Stress and BDNF: Exploring the Role in Major Depression Disorder
4.2. Exploring the Link between BDNF and HPA Axis Dysregulation: Implications for Major Depression Disorder
4.3. The Intersection of BDNF and Serotonergic Systems in Major Depression Disorder
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lewis, D.A.; Sibille, E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Lu, B. Diverse Functions of Multiple Bdnf Transcripts Driven by Distinct Bdnf Promoters. Biomolecules 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- WHO. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 June 2023).
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Castren, E.; Ieraci, A.; Aprahamian, I.; Arosio, B.; Rosa Guerini, F.; Oude Voshaar, R.C.; Fondazione Don Carlo Gnocchi, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F.; Schatzberg, A.; Murphy, G. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology, and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Kunugi, H.; Hori, H.; Adachi, N.; Numakawa, T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin. Neurosci. 2010, 64, 447–459. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Leschik, J.; Gentile, A.; Cicek, C.; Péron, S.; Tevosian, M.; Beer, A.; Radyushkin, K.; Bludau, A.; Ebner, K.; Neumann, I.; et al. Brain-derived neurotrophic factor expression in serotonergic neurons improves stress resilience and promotes adult hippocampal neurogenesis. Prog. Neurobiol. 2022, 217, 102333. [Google Scholar] [CrossRef]
- Rumajogee, P.; Vergé, D.; Hanoun, N.; Brisorgueil, M.; Hen, R.; Lesch, K.; Hamon, M.; Miquel, M. Adaption of the serotoninergic neuronal phenotype in the absence of 5-HT autoreceptors or the 5-HT transporter: Involvement of BDNF and cAMP. Eur. J. Neurosci. 2004, 19, 937–944. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 1345. [Google Scholar] [CrossRef]
- Yang, B.; Ren, Q.; Zhang, J.C.; Chen, Q.X.; Hashimoto, K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain–liver axis. Transl. Psychiatry 2017, 7, e1128. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Wong-Riley, M.T.T. Transcriptional Regulation of Brain-derived Neurotrophic Factor Coding Exon IX. J. Biol. Chem. 2016, 291, 22583–22593. [Google Scholar] [CrossRef]
- Pathak, H.; Borchert, A.; Garaali, S.; Burkert, A.; Frieling, H. BDNF exon IV promoter methylation and antidepressant action: A complex interplay. Clin. Epigenetics 2022, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Xu, M.; Gao, Z.-H.; Wang, Y.-Q.; Yue, Z. Alterations of Serum Levels of BDNF-Related miRNAs in Patients with Depression. PLoS ONE 2013, 8, e63648. [Google Scholar] [CrossRef] [PubMed]
- Woo, N.H.; Teng, H.K.; Siao, C.-J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H. Brain-Derived Neurotrophic Factor Signaling in the Pathophysiology of Alzheimer’s Disease: Beneficial Effects of Flavonoids for Neuroprotection. Int. J. Mol. Sci. 2021, 22, 5719. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- García-Suárez, P.C.; Rentería, I.; Plaisance, E.P.; Moncada-Jiménez, J.; Jiménez-Maldonado, A. The effects of interval training on peripheral brain derived neurotrophic factor (BDNF) in young adults: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 8937. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacol. Rev. 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Yao, H.; Imamura, Y.; Hashimoto, M.; Monji, A. Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: The Sefuri study. Sci. Rep. 2020, 10, 16442. [Google Scholar] [CrossRef]
- Jewett, B.E.; Thapa, B. Physiology, NMDA receptor. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Horch, H.W.; Katz, L.C. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat. Neurosci. 2002, 5, 1177–1184. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Cui, R.; Li, B.; Luo, W.; Yu, S.; Liu, H.; Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; et al. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Yao, M.; Zhang, W.; Yang, B.; Wang, G. Panax notoginseng Saponins Stimulates Neurogenesis and Neurological Restoration After Microsphere-Induced Cerebral Embolism in Rats Partially via mTOR Signaling. Front. Pharmacol. 2022, 13, 2213. [Google Scholar] [CrossRef]
- Chen, S.-D.; Wu, C.-L.; Hwang, W.-C.; Yang, D.-I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Paduchová, Z.; Katrenčíková, B.; Vaváková, M.; Laubertová, L.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z.; Trebatická, J. The Effect of Omega-3 Fatty Acids on Thromboxane, Brain-Derived Neurotrophic Factor, Homocysteine, and Vitamin D in Depressive Children and Adolescents: Randomized Controlled Trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Kaltenboeck, A.; Harmer, C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018, 2, 2398212818799269. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; De Gomes, M.G.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; Souza, L.C. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef]
- Broux, B.; Pabelick, C.; Bai Xu, S.; Jin, Y.; Hua Sun, L.; Yang, W.; Ji Cui, R. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front. Neurol. 2019, 1, 515. [Google Scholar] [CrossRef]
- Mohammadi, S.; Beh-Pajooh, A.; Ahmadimanesh, M.; Amini, M.; Ghazi-Khansari, M.; Moallem, S.A.; Hosseini, R.; Nourian, Y.H.; Ghahremani, M.H. Evaluation of DNA methylation in BDNF, SLC6A4, NR3C1 and FKBP5 before and after treatment with selective serotonin-reuptake inhibitor in major depressive disorder. Epigenomics 2022, 14, 1269–1280. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.; Namgung, E.; Lee, D.-W.; Kim, G.H.; Kim, M.; Kim, N.; Kim, T.D.; Kim, S.; Lyoo, I.K. The BDNF Val66Met polymorphism affects the vulnerability of the brain structural network. Front. Hum. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef]
- Pathak, P.; Mehra, A.; Ram, S.; Pal, A.; Grover, S. Association of serum BDNF level and Val66Met polymorphism with response to treatment in patients of major depressive disease: A step towards personalized therapy. Behav. Brain Res. 2022, 430, 113931. [Google Scholar] [CrossRef]
- Jahromy, M.H.; Baghchesara, B.; Javanshir, S. Effects of Allopurinol as a xanthine oxidase inhibitor on depressive-like behavior of rats and changes in serum BDNF level. IBRO Neurosci. Rep. 2022, 13, 373–377. [Google Scholar] [CrossRef]
- Wu, S.; Ning, K.; Wang, Y.; Zhang, L.; Liu, J. Up-regulation of BDNF/TrkB signaling by δ opioid receptor agonist SNC80 modulates depressive-like behaviors in chronic restraint-stressed mice. Eur. J. Pharmacol. 2023, 942, 175532. [Google Scholar] [CrossRef]
- Ryu, D.; Jee, H.-J.; Kim, S.-Y.; Hwang, S.-H.; Pil, G.-B.; Jung, Y.-S. Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients 2022, 14, 3314. [Google Scholar] [CrossRef]
- Cai, T.; Zheng, S.; Shi, X.; Yuan, L.; Hu, H.; Zhou, B.; Xiao, S.; Wang, F. Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front. Cell. Infect. Microbiol. 2022, 1101. [Google Scholar] [CrossRef] [PubMed]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; Macdonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB Neurotrophin Receptor Is Induced by Antidepressant Drugs and Is Required for Antidepressant-Induced Behavioral Effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Homberg, J.R.; Molteni, R.; Calabrese, F.; Riva, M.A. The serotonin–BDNF duo: Developmental implications for the vulnerability to psychopathology. Neurosci. Biobehav. Rev. 2014, 43, 35–47. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 6888. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, M.; Xie, M.; Yang, Y.; Xue, F.; Li, W.; Zhang, M.; Li, Z.; Li, X.; Jia, N.; et al. Protective role of antioxidant supplementation for depression and anxiety: A meta-analysis of randomized clinical trials. J. Affect. Disord. 2023, 323, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Du, X.; Zhou, J.; Yuan, L.; Ren, H.; Yang, X.; Zhang, G.; Chen, X.; Cuellar-Barboza, A.B.; et al. Circulating Brain-Derived Neurotrophic Factor, Antioxidant Enzymes Activities, and Mitochondrial DNA in Bipolar Disorder: An Exploratory Report. Psychiatry 2020, 11, 514658. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, K.; Tripathi, A.K.; Bhatia, M.S.; Gupta, L.K. Effect of Mirtazapine Treatment on Serum Levels of Brain-Derived Neurotrophic Factor and Tumor Necrosis Factor-α in Patients of Major Depressive Disorder with Severe Depression. Pharmacology 2016, 97, 184–188. [Google Scholar] [CrossRef]
- Correia, A.S.; Fraga, S.; Teixeira, J.P.; Vale, N. Cell Model of Depression: Reduction of Cell Stress with Mirtazapine. Int. J. Mol. Sci. 2022, 23, 4942. [Google Scholar] [CrossRef]
- Lieberknecht, V.; Engel, D.; Rodrigues, A.L.S.; Gabilan, N.H. Neuroprotective effects of mirtazapine and imipramine and their effect in pro- and anti-apoptotic gene expression in human neuroblastoma cells. Pharmacol. Rep. 2020, 72, 563–570. [Google Scholar] [CrossRef]
- Dionisie, V.; Ciobanu, A.M.; Toma, V.A.; Manea, M.C.; Baldea, I.; Olteanu, D.; Sevastre-Berghian, A.; Clichici, S.; Manea, M.; Riga, S.; et al. Escitalopram Targets Oxidative Stress, Caspase-3, BDNF and MeCP2 in the Hippocampus and Frontal Cortex of a Rat Model of Depression Induced by Chronic Unpredictable Mild Stress. Int. J. Mol. Sci. 2021, 22, 7483. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, Z.; Lei, Y.; et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef]
- Hacioglu, G.; Senturk, A.; Ince, I.; Alver, A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran. J. Basic Med. Sci. 2016, 19, 388. [Google Scholar] [PubMed]
- Birmann, P.T.; Casaril, A.M.; Zugno, G.P.; Acosta, G.G.; Severo Sabedra Sousa, F.; Collares, T.; Seixas, F.K.; Jacob, R.G.; Brüning, C.A.; Savegnago, L.; et al. Flower essential oil of Tagetes minuta mitigates oxidative stress and restores BDNF-Akt/ERK2 signaling attenuating inflammation- and stress-induced depressive-like behavior in mice. Brain Res. 2022, 1784, 147845. [Google Scholar] [CrossRef]
- Wu, Q.; Lin, M.; Wu, P.; Zhao, C.; Yang, S.; Yu, H.; Xian, W.; Song, J. TPPU Downregulates Oxidative Stress Damage and Induces BDNF Expression in PC-12 Cells. Comput. Math. Methods Med. 2022, 2022, 7083022. [Google Scholar] [CrossRef] [PubMed]
- Amiry, G.Y.; Haidary, M.; Azhdari-Zarmehri, H.; Beheshti, F.; Ahmadi-Soleimani, S.M. Omega-3 fatty acids prevent nicotine withdrawal-induced exacerbation of anxiety and depression by affecting oxidative stress balance, inflammatory response, BDNF and serotonin metabolism in rats. Eur. J. Pharmacol. 2023, 947, 175634. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Makvandi, A.; Khalili, M.; Roghani, M.; Amiri Moghaddam, S. Hesperetin ameliorates electroconvulsive therapy-induced memory impairment through regulation of hippocampal BDNF and oxidative stress in a rat model of depression. J. Chem. Neuroanat. 2021, 117, 102001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Li, H.; Fang, J. Red Raspberry Extract Decreases Depression-Like Behavior in Rats by Modulating Neuroinflammation and Oxidative Stress. Biomed Res. Int. 2022, 2022, 9943598. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef]
- Srinivasan, S.; Shariff, M.; Bartlett, S.E.; Lawrence, A.; Neuroscience Institutes, F.; Leandro Vendruscolo, A. The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 2013, 4, 68. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Wong, M.L.; Lewis, M.; Licinio, J. Translational research in endocrinology and neuroimmunology applied to depression. In Biomedical Chemistry: Current Trends and Developments; De Gruyter: Berlin, Germany, 2015; pp. 119–131. [Google Scholar] [CrossRef]
- Sapolsky, R.; Krey, L.; McEwen, B. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Naert, G.; Zussy, C.; Tran Van Ba, C.; Chevallier, N.; Tang, Y.-P.; Maurice, T.; Givalois, L. Involvement of Endogenous Brain-Derived Neurotrophic Factor in Hypothalamic-Pituitary-Adrenal Axis Activity. J. Neuroendocrinol. 2015, 27, 850–860. [Google Scholar] [CrossRef]
- Barfield, E.T.; Gourley, S.L. Prefrontal cortical trkB, glucocorticoids, and their interactions in stress and developmental contexts. Neurosci. Biobehav. Rev. 2018, 95, 535–558. [Google Scholar] [CrossRef]
- Hennings, J.M.; Kohli, M.A.; Uhr, M.; Holsboer, F.; Ising, M.; Lucae, S. Polymorphisms in the BDNF and BDNFOS genes are associated with hypothalamus-pituitary axis regulation in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109686. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, F.; Zhai, M.; He, M.; Hu, Y.; Feng, L.; Li, Y.; Yang, J.; Wu, C. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics 2023, 13, 1059–1075. [Google Scholar] [CrossRef]
- Mori, M.; Shizunaga, H.; Harada, H.; Tajiri, Y.; Murata, Y.; Terada, K.; Ohe, K.; Enjoji, M. Oxytocin treatment improves dexamethasone-induced depression-like symptoms associated with enhancement of hippocampal CREB-BDNFsignaling in female mice. Neuropsychopharmacol. Rep. 2022, 42, 356–361. [Google Scholar] [CrossRef]
- He, Z.; Yu, H.; Wu, H.; Su, L.; Shi, K.; Zhao, Y.; Zong, Y.; Chen, W.; Du, R. Antidepressant effects of total alkaloids of Fibraurea recisa on improving corticosterone-induced apoptosis of HT-22 cells and chronic unpredictable mild stress-induced depressive-like behaviour in mice. Pharm. Biol. 2022, 60, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.O.; Olubodun-Obadun, T.G.; Bakre, O.A.; Ojo, E.S.; Adeyemi, O.O. Kolaviron ameliorates chronic unpredictable mild stress-induced anxiety and depression: Involvement of the HPA axis, antioxidant defense system, cholinergic, and BDNF signaling. Drug Metab. Pers. Ther. 2022, 37, 277–287. [Google Scholar] [CrossRef]
- Lin, L.; Herselman, M.F.; Zhou, X.-F.; Bobrovskaya, L. Effects of corticosterone on BDNF expression and mood behaviours in mice. Physiol. Behav. 2022, 247, 113721. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes de Assis, G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. BDNF Impact on Biological Markers of Depression-Role of Physical Exercise and Training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Martinowich, K.; Lu, B. Interaction between BDNF and Serotonin: Role in Mood Disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Baudat, M.; Kort, A.R.; Hove, D.L.A.; Joosten, E.A. Early-life exposure to selective serotonin reuptake inhibitors: Long-term effects on pain and affective comorbidities. Eur. J. Neurosci. 2022, 55, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, M.; Vaidya, V.A. 5-HT 2A receptors and BDNF regulation: Implications for psychopathology. In 5-HT2A Receptors in the Central Nervous System; Spring: Berlin/Heidelberg, Germany, 2018; pp. 395–438. [Google Scholar] [CrossRef]
- Ilchibaeva, T.; Tsybko, A.; Zeug, A.; Müller, F.E.; Guseva, D.; Bischoff, S.; Ponimaskin, E.; Naumenko, V. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells 2022, 11, 2384. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Fogaça, M.V.; Liu, R.-J.; Duman, C.H.; Li, X.-Y.; Chaki, S.; Duman, R.S. Medial PFC AMPA receptor and BDNF signaling are required for the rapid and sustained antidepressant-like effects of 5-HT1A receptor stimulation. Neuropsychopharmacology 2020, 45, 1725–1734. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Central nervous system effects of 5-HT7 receptors: A potential target for neurodegenerative diseases. Mol. Med. 2022, 28, 70. [Google Scholar] [CrossRef]
- Benmansour, S.; Deltheil, T.; Piotrowski, J.; Nicolas, L.; Reperant, C.; Gardier, A.M.; Frazer, A.; David, D.J. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur. J. Pharmacol. 2008, 587, 90–98. [Google Scholar] [CrossRef]
- Yu, H.; Lv, D.; Shen, M.; Zhang, Y.; Zhou, D.; Chen, Z.; Wang, C. BDNF mediates the protective effects of scopolamine in reserpine-induced depression-like behaviors via up-regulation of 5-HTT and TPH1. Psychiatry Res. 2019, 271, 328–334. [Google Scholar] [CrossRef]
- Wang, D.; Wu, J.; Zhu, P.; Xie, H.; Lu, L.; Bai, W.; Pan, W.; Shi, R.; Ye, J.; Xia, B.; et al. Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: The potential involvement of gut-brain axis. Food Res. Int. 2022, 157, 111289. [Google Scholar] [CrossRef]
- Bazzari, A.H.; Bazzari, F.H. BDNF Therapeutic Mechanisms in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 8417. [Google Scholar] [CrossRef]
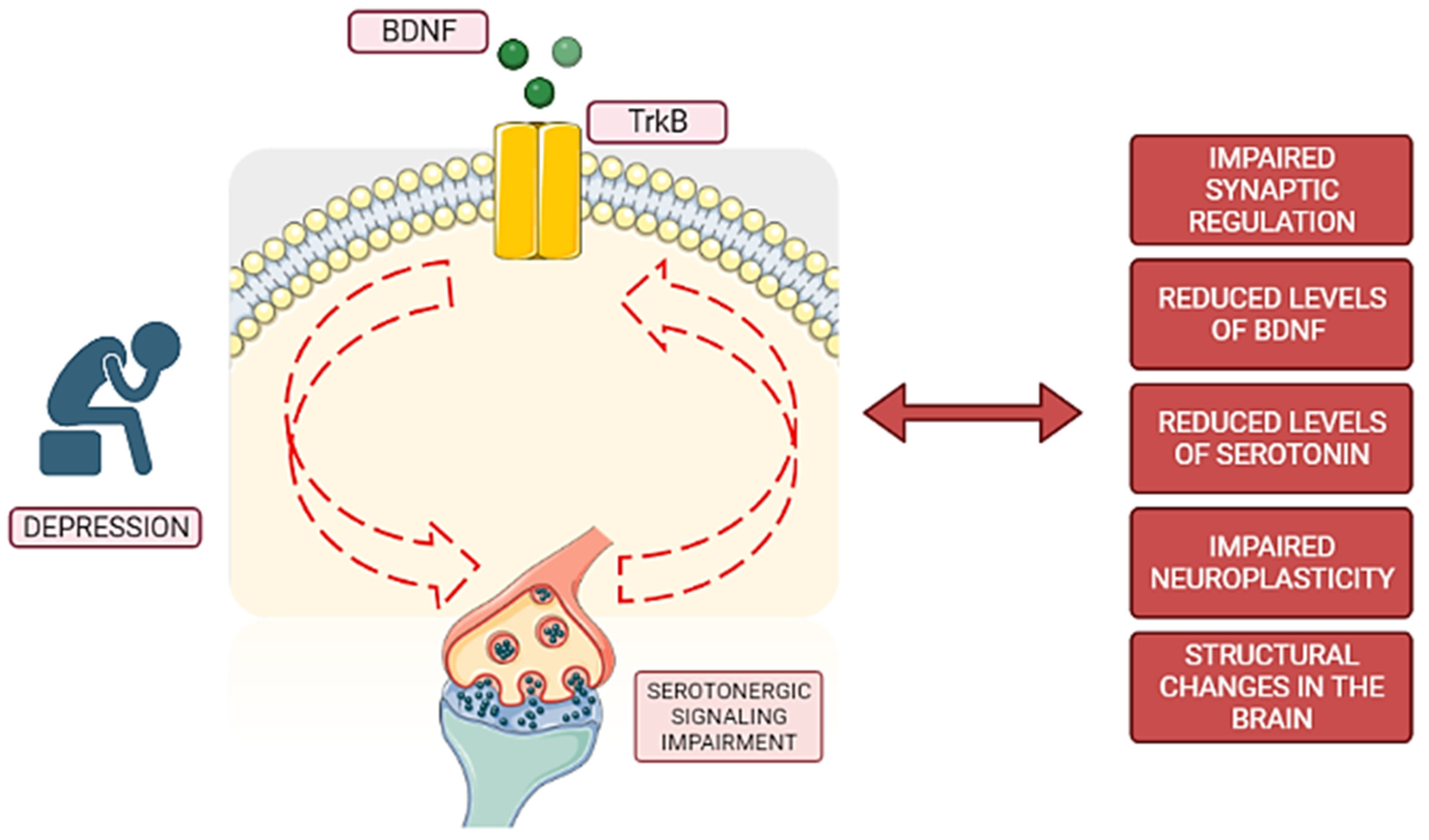
| Aspect | BDNF and Major Depressive Disorder Connection |
|---|---|
| Levels of BDNF | Reduced BDNF levels have been observed in individuals with major depressive disorder [45]. |
| Changes in structure and function | Deficiencies or imbalances in BDNF levels may contribute to the development of depression by promoting structural and functioning changes [7], such as reduced dendritic complexity [37]. |
| Serotonin influence | BDNF is influenced by serotonin, and serotonin activation can stimulate BDNF synthesis and release. Serotonin receptors can also modulate BDNF expression, influencing neuronal function and, consequently, mood regulation [59]. |
| Neuroplasticity | BDNF is involved in neuroplasticity, which is crucial for synaptic connections and structural changes in the brain related to depressive disorder [37]. |
| Antidepressant effects | Different antidepressants can enhance BDNF gene expression, contributing to their therapeutic effects [7]. |
| Oxidative stress | Oxidative stress can lower BDNF production and damage its signaling pathways. The connection between oxidative stress and BDNF levels plays a significant role in the development and progression of depression [11]. |
| Hypothalamic–pituitary–adrenal (HPA) axis dysregulation | Stress-induced HPA axis hyperactivity and the resulting increase in glucocorticoid levels diminish BDNF expression, playing an important role in the development of depression [10]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Cardoso, A.; Vale, N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics 2023, 15, 2081. https://doi.org/10.3390/pharmaceutics15082081
Correia AS, Cardoso A, Vale N. BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics. 2023; 15(8):2081. https://doi.org/10.3390/pharmaceutics15082081
Chicago/Turabian StyleCorreia, Ana Salomé, Armando Cardoso, and Nuno Vale. 2023. "BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions" Pharmaceutics 15, no. 8: 2081. https://doi.org/10.3390/pharmaceutics15082081
APA StyleCorreia, A. S., Cardoso, A., & Vale, N. (2023). BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics, 15(8), 2081. https://doi.org/10.3390/pharmaceutics15082081







