Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monoclonal Antibody Quantification
2.3. Nano Differential Scanning Calorimetry
2.4. Dynamic Light Scattering and Zeta Potential
2.5. Preliminary Studies: Factors Influencing Nano-DSC Results
2.6. Solid Monoclonal Antibody Approaches
2.7. Stability Pilot Study
2.8. Viscosity Studies
3. Results and Discussion
3.1. Preliminary Studies: Factors Influencing Nano-DSC Results
3.2. Solid Monoclonal Antibody Approaches
3.3. Stability Monitoring of Solid Formulations
3.4. Viscosity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jimmy, B.; Jose, J. Patient Medication Adherence: Measures in Daily Practice. Oman Med. J. 2011, 26, 155. [Google Scholar] [CrossRef]
- Kim, J.; Combs, K.; Downs, J.; Tillman, F. Medication Adherence: The Elephant in the Room. US Pharm. 2018, 43, 30–34. [Google Scholar]
- Kleinsinger, F. The Unmet Challenge of Medication Nonadherence. Perm. J. 2018, 22, 18–033. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Therapeutic Proteins. In Therapeutic Proteins: Methods and Protocols; Methods in Molecular Biology; Spinger Science + Business Media LLC: Berlin/Heidelberg, Germany, 2012; Volume 899, pp. 1–26. [Google Scholar] [CrossRef]
- Dewan, S.S. Protein Drugs Market Size, Share & Growth Analysis Report; BCC Publishing: Wellesley, MA, USA, 2021. [Google Scholar]
- Molina, J.T.; Robledillo, J.C.L.; Ruiz, N.C. Potential Benefits of the Self-Administration of Subcutaneous Methotrexate with Autoinjector Devices for Patients: A Review. Drug Healthc. Patient Saf. 2021, 13, 81. [Google Scholar] [CrossRef]
- Vermeire, S.; D’heygere, F.; Nakad, A.; Franchimont, D.; Fontaine, F.; Louis, E.; Van Hootegem, P.; Dewit, O.; Lambrecht, G.; Strubbe, B.; et al. Preference for a Prefilled Syringe or an Auto-Injection Device for Delivering Golimumab in Patients with Moderate-to-Severe Ulcerative Colitis: A Randomized Crossover Study. Patient Prefer. Adherence 2018, 12, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Kivitz, A.; Segurado, O.G. HUMIRA Pen: A Novel Autoinjection Device for Subcutaneous Injection of the Fully Human Monoclonal Antibody Adalimumab. Expert Rev. Med. Devices 2007, 4, 109–116. [Google Scholar] [CrossRef]
- Karlsdottir, K.; Gunnarsdottir, A.I.; Grondal, G.; Love, T.J.; Stefansdottir, E.; Davidsdottir, L.G.; Thorleifsdottir, R.H.; Gudbjornsson, B. A Patients’ Perspective Towards the Injection Devices for Humira® and Imraldi® in a Nationwide Switching Program. Front. Med. 2022, 9, 17. [Google Scholar] [CrossRef]
- Melien, R.; Garidel, P.; Hinderberger, D.; Blech, M. Thermodynamic Unfolding and Aggregation Fingerprints of Monoclonal Antibodies Using Thermal Profiling. Pharm. Res. 2020, 37, 3–13. [Google Scholar] [CrossRef]
- Garidel, P.; Eiperle, A.; Blech, M.; Seelig, J. Thermal and Chemical Unfolding of a Monoclonal IgG1 Antibody: Application of the Multistate Zimm-Bragg Theory. Biophys. J. 2020, 118, 1067–1075. [Google Scholar] [CrossRef]
- Mehta, S.B.; Bee, J.S.; Randolph, T.W.; Carpenter, J.F. Partial Unfolding of a Monoclonal Antibody: Role of a Single Domain in Driving Protein Aggregation. Biochemistry 2014, 53, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M. Differential Scanning Calorimetry as a Tool for Protein Folding and Stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, C.; Jiang, B.; Sun, C.C.; Hoag, S.W. Effects of Compaction and Storage Conditions on Stability of Intravenous Immunoglobulin-Implication on Developing Oral Tablets of Biologics. Int. J. Pharm. 2021, 604, 120737. [Google Scholar] [CrossRef]
- Magnenat, L.; Palmese, A.; Fremaux, C.; D’Amici, F.; Terlizzese, M.; Rossi, M.; Chevalet, L. Demonstration of Physicochemical and Functional Similarity between the Proposed Biosimilar Adalimumab MSB11022 and Humira®. mAbs 2017, 9, 127–139. [Google Scholar] [CrossRef]
- Ionescu, R.M.; Vlasak, J.; Price, C.; Kirchmeier, M. Contribution of Variable Domains to the Stability of Humanized IgG1 Monoclonal Antibodies. J. Pharm. Sci. 2008, 97, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R.; Grace, M.; Blumlein, A.; McManus, J.J. Differential Scanning Calorimetry to Quantify Heat-Induced Aggregation in Concentrated Protein Solutions. In Protein Self-Assembly: Methods and Protocols; Methods in Molecular Biology; McManus, J.J., Ed.; Spinger Science + Business Media LLC: Berlin/Heidelberg, Germany, 2019; Volume 2039, pp. 117–129. [Google Scholar]
- Souillac, P.O. Biophysical Characterization of Insoluble Aggregates of a Multi-Domain Protein: An Insight into the Role of the Various Domains. J. Pharm. Sci. 2005, 94, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Beyschau Andersen, C.; Manno, M.; Rischel, C.; Thórólfsson, M.; Martorana, V. Aggregation of a Multidomain Protein: A Coagulation Mechanism Governs Aggregation of a Model IgG1 Antibody under Weak Thermal Stress. Protein Sci. 2009, 19, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Frey, V.; Corcoran, M.; Zhang-Van Enk, J.; Subramony, J.A. Influence of Arginine Salts on the Thermal Stability and Aggregation Kinetics of Monoclonal Antibody: Dominant Role of Anions. Mol. Pharm. 2016, 13, 3362–3369. [Google Scholar] [CrossRef]
- Hassan, L.A.; Al-Ghobashy, M.A.; Abbas, S.S. Evaluation of the Pattern and Kinetics of Degradation of Adalimumab Using a Stability-Indicating Orthogonal Testing Protocol. Biomed. Chromatogr. 2019, 33, e4676. [Google Scholar] [CrossRef] [PubMed]
- Durowoju, I.B.; Bhandal, K.S.; Hu, J.; Carpick, B.; Kirkitadze, M. Differential Scanning Calorimetry—A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J. Vis. Exp. 2017, 2017, 55262. [Google Scholar] [CrossRef]
- Fukuda, J.; Iwura, T.; Yanagihara, S.; Kano, K. Factors to Govern Soluble and Insoluble Aggregate-Formation in Monoclonal Antibodies. Anal. Sci. 2015, 31, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Truncali, K.; Ritchie, J.; Kroe-Barrett, R.; Singh, S.; Robinson, A.S.; Roberts, C.J. Weak Protein Interactions and PH- and Temperature-Dependent Aggregation of Human Fc1. mAbs 2015, 7, 1072–1083. [Google Scholar] [CrossRef]
- Misra, S.K.; Orlando, R.; Weinberger, S.R.; Sharp, J.S. Compensated Hydroxyl Radical Protein Footprinting Measures Buffer and Excipient Effects on Conformation and Aggregation in an Adalimumab Biosimilar. AAPS J. 2019, 21, 87. [Google Scholar] [CrossRef]
- Garidel, P.; Kliche, W.; Pisch-Heberle, S.; Thierolf, M. Characterization of Proteins and Related Analytical Techniques. In Protein Pharmaceuticals: Formulation, Analytics and Delivery; Hanns-Christian, M., Ed.; Editio-Cantor-Verlag: Aulendorf, Germany, 2010; ISBN 3871933821. [Google Scholar]
- Monera, O.D.; Kay, C.M.; Hodges, R.S. Protein Denaturation with Guanidine Hydrochloride or Urea Provides a Different Estimate of Stability Depending on the Contributions of Electrostatic Interactions. Protein Sci. 1994, 3, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Ejima, D.; Tsumoto, K.; Obeyama, N.; Tanaka, Y.; Kita, Y.; Timasheff, S.N. Suppression of Protein Interactions by Arginine: A Proposed Mechanism of the Arginine Effects. Biophys. Chem. 2007, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Srimathi, T.; Kumar, T.K.S.; Chi, Y.-H.; Chiu, I.M.; Yu, C. Characterization of the Structure and Dynamics of a Near-Native Equilibrium Intermediate in the Unfolding Pathway of an All Beta-Barrel Protein. J. Biol. Chem. 2002, 277, 47507–47516. [Google Scholar] [CrossRef]
- Mohammad Zadeh, A.H.; Rouholamini Najafabadi, A.; Vatanara, A.; Faghihi, H.; Gilani, K. Effect of Molecular Weight and Ratio of Poly Ethylene Glycols’ Derivatives in Combination with Trehalose on Stability of Freeze-Dried IgG. Drug Dev. Ind. Pharm. 2017, 43, 1945–1951. [Google Scholar] [CrossRef]
- Umerska, A.; Paluch, K.J.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Freeze Drying of Polyelectrolyte Complex Nanoparticles: Effect of Nanoparticle Composition and Cryoprotectant Selection. Int. J. Pharm. 2018, 552, 27–38. [Google Scholar] [CrossRef]
- Mi, Y.; Wood, G. The Application and Mechanisms of Polyethylene Glycol 8000 on Stabilizing Lactate Dehydrogenase during Lyophilization-PubMed. PDA J. Pharm. Sci. Technol. 2004, 58, 192–202. [Google Scholar]
- Puts, C.F.; Berendsen, T.A.; Bruinsma, B.G.; Ozer, S.; Luitje, M.; Usta, O.B.; Yarmush, M.L.; Uygun, K. Polyethylene Glycol Protects Primary Hepatocytes during Supercooling Preservation. Cryobiology 2015, 71, 125–129. [Google Scholar] [CrossRef]
- Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Excipients for Room Temperature Stable Freeze-Dried Monoclonal Antibody Formulations. J. Pharm. Sci. 2020, 109, 807–817. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. The Thermodynamic Mechanism of Protein Stabilization by Trehalose. Biophys. Chem. 1997, 64, 25–43. [Google Scholar] [CrossRef]
- Oddone, I.; Arsiccio, A.; Duru, C.; Malik, K.; Ferguson, J.; Pisano, R.; Matejtschuk, P. Vacuum-Induced Surface Freezing for the Freeze-Drying of the Human Growth Hormone: How Does Nucleation Control Affect Protein Stability? J. Pharm. Sci. 2020, 109, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Anko, M.; Bjelošević, M.; Planinšek, O.; Trstenjak, U.; Logar, M.; Ahlin Grabnar, P.; Brus, B. The Formation and Effect of Mannitol Hemihydrate on the Stability of Monoclonal Antibody in the Lyophilized State. Int. J. Pharm. 2019, 564, 106–116. [Google Scholar] [CrossRef]
- Mehta, M.; Bhardwaj, S.P.; Suryanarayanan, R. Controlling the Physical Form of Mannitol in Freeze-Dried Systems. Eur. J. Pharm. Biopharm. 2013, 85, 207–213. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.J. How Sugars Protect Proteins in the Solid State and during Drying (Review): Mechanisms of Stabilization in Relation to Stress Conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Sudrik, C.M.; Cloutier, T.; Mody, N.; Sathish, H.A.; Trout, B.L. Understanding the Role of Preferential Exclusion of Sugars and Polyols from Native State IgG1 Monoclonal Antibodies and Its Effect on Aggregation and Reversible Self-Association. Pharm. Res. 2019, 36, 109. [Google Scholar] [CrossRef]
- Wang, S.S.; Yan, Y.; Ho, K. US FDA-Approved Therapeutic Antibodies with High-Concentration Formulation: Summaries and Perspectives. Antib. Ther. 2021, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Milton, N.; Groleau, E.G.; Mishra, D.S.; Vansickle, R.E. Existence of a Mannitol Hydrate during Freeze-drying and Practical Implications. J. Pharm. Sci. 1999, 88, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Krishnamurthy, R.; Suryanarayanan, R. Influence of Processing Conditions on the Physical State of Mannitol-Implications in Freeze-Drying. Pharm. Res. 2007, 24, 370–376. [Google Scholar] [CrossRef]
- Liao, X.; Krishnamurthy, R.; Suryanarayanan, R. Influence of the Active Pharmaceutical Ingredient Concentration on the Physical State of Mannitol-Implications in Freeze-Drying. Pharm Res 2005, 22, 1978–1985. [Google Scholar] [CrossRef]
- Cao, W.; Xie, Y.; Krishnan, S.; Lin, H.; Ricci, M. Influence of Process Conditions on the Crystallization and Transition of Metastable Mannitol Forms in Protein Formulations during Lyophilization. Pharm. Res. 2013, 30, 131–139. [Google Scholar] [CrossRef]
- Shields, R.L.; Namenuk, A.K.; Hong, K.; Meng, Y.G.; Rae, J.; Briggs, J.; Xie, D.; Lai, J.; Stadlen, A.; Li, B.; et al. High Resolution Mapping of the Binding Site on Human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and Design of IgG1 Variants with Improved Binding to the FcγR. J. Biol. Chem. 2001, 276, 6591–6604. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular Mechanisms of Action of Anti-TNF-α Agents–Comparison among Therapeutic TNF-α Antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef]
- Roche, A.; Gentiluomo, L.; Sibanda, N.; Roessner, D.; Friess, W.; Trainoff, S.P.; Curtis, R. Towards an Improved Prediction of Concentrated Antibody Solution Viscosity Using the Huggins Coefficient. J. Colloid Interface Sci. 2022, 607, 1813–1824. [Google Scholar] [CrossRef]
- Woldeyes, M.A.; Josephson, L.L.; Leiske, D.L.; Galush, W.J.; Roberts, C.J.; Furst, E.M. Viscosities and Protein Interactions of Bispecific Antibodies and Their Monospecific Mixtures. Mol. Pharm. 2018, 15, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Kuhn, A.B.; Schäfer, L.V.; Karow-Zwick, A.R.; Blech, M. High-Concentration Protein Formulations: How High Is High? Eur. J. Pharm. Biopharm. 2017, 119, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.A.; Sologuren, R.R.; Narwal, R. Do Clustering Monoclonal Antibody Solutions Really Have a Concentration Dependence of Viscosity? Biophys. J. 2013, 104, 913–923. [Google Scholar] [CrossRef][Green Version]
- Laue, T. Proximity Energies: A Framework for Understanding Concentrated Solutions. J. Mol. Recognit. 2012, 25, 165–173. [Google Scholar] [CrossRef]
- Xu, A.Y.; Castellanos, M.M.; Mattison, K.; Krueger, S.; Curtis, J.E. Studying Excipient Modulated Physical Stability and Viscosity of Monoclonal Antibody Formulations Using Small-Angle Scattering. Mol. Pharm. 2019, 16, 4319–4338. [Google Scholar] [CrossRef]




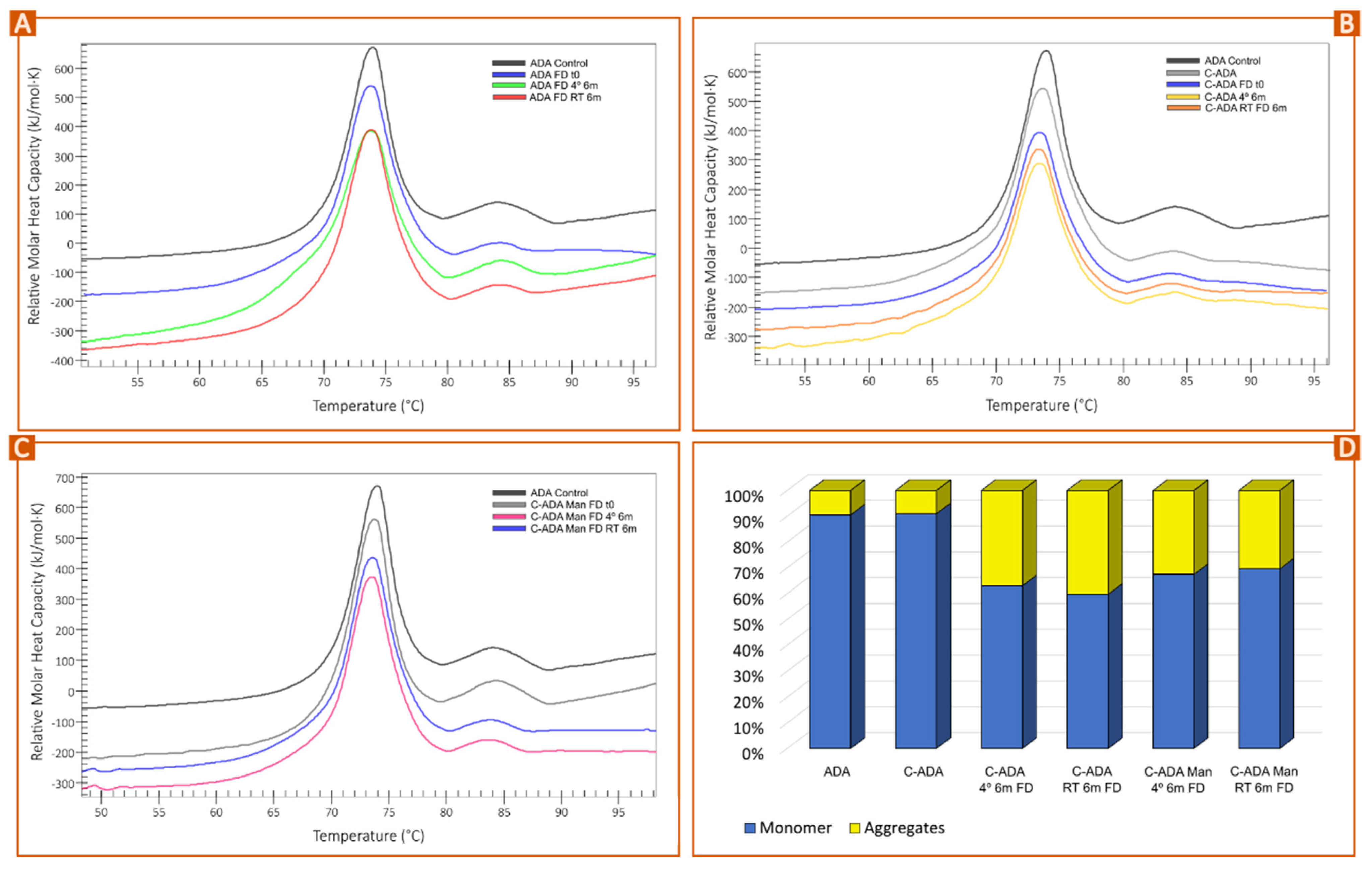
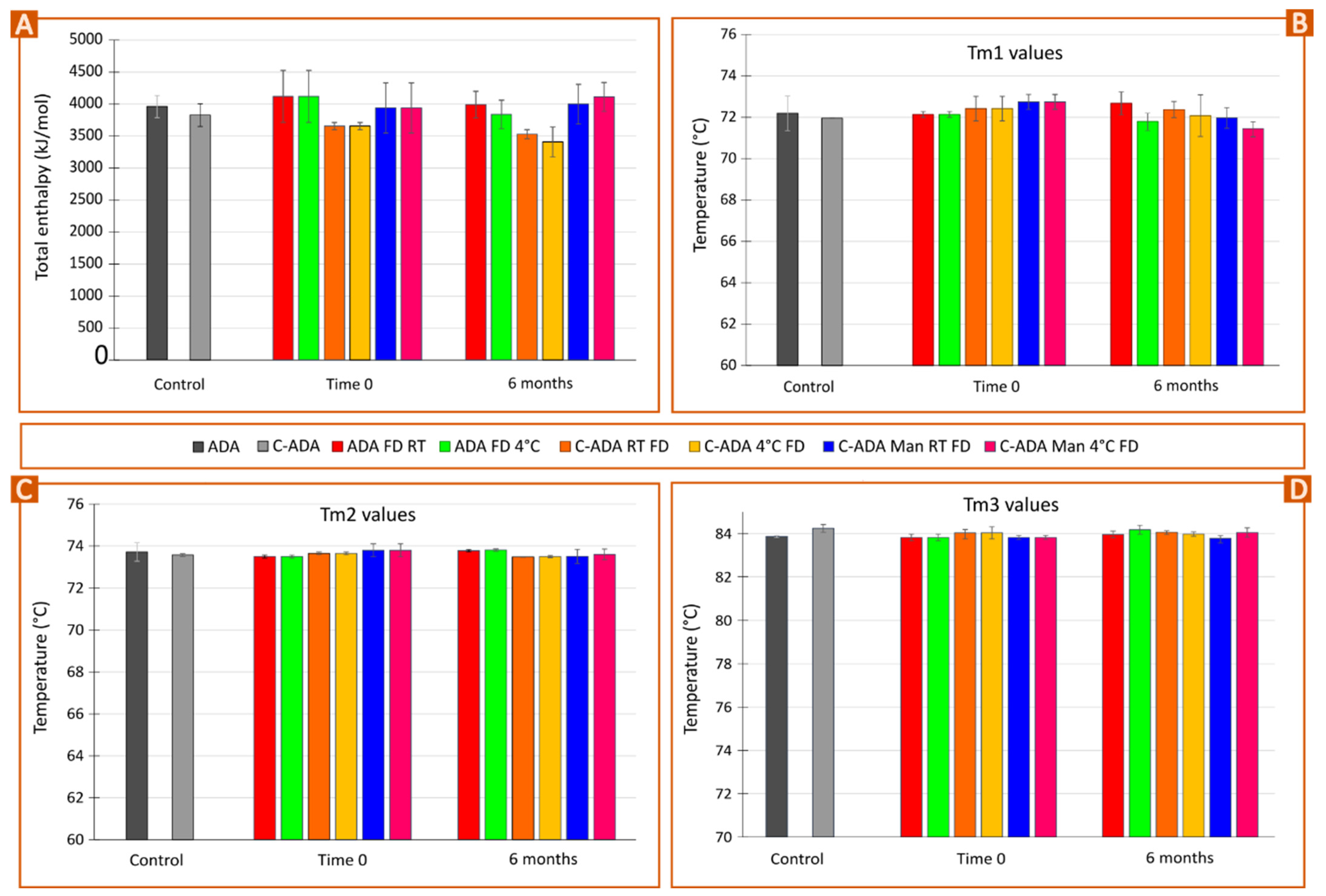
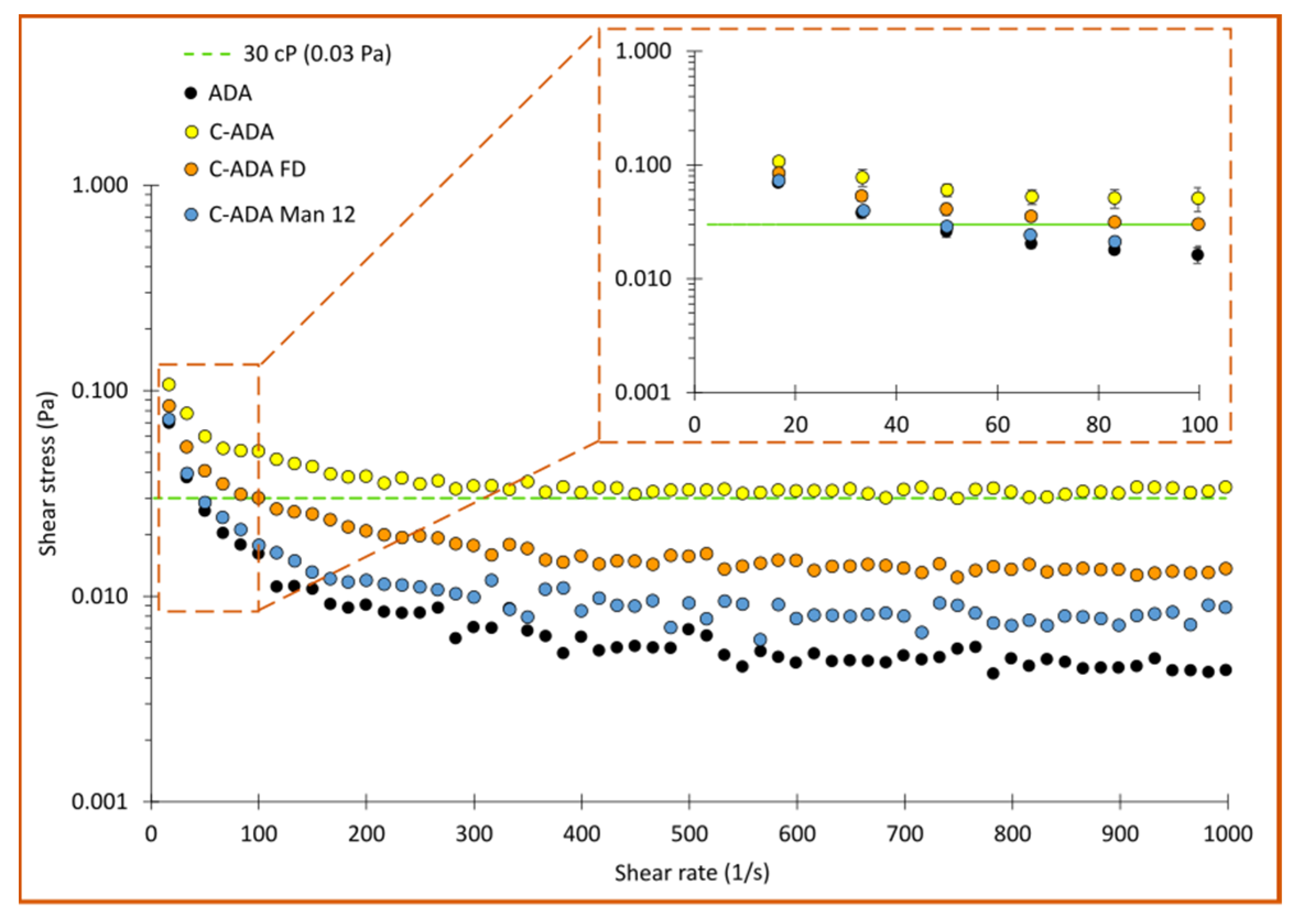
| Sample | Total ΔH (kJ/mol) | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) |
|---|---|---|---|---|
| ADA | 3959.78 | 72.78 | 74.02 | 83.83 |
| C-ADA | 3934.38 | 71.95 | 73.52 | 84.12 |
| C-ADA FD | 3515.73 | 71.97 | 73.61 | 84.37 |
| C-ADA Man 24 FD | 4091.45 | 68.80 | 73.67 | 83.77 |
| C-ADA Man 12 FD | 4130.36 | 72.60 | 73.68 | 83.72 |
| C-ADA Tre 12 | 3885.89 | 68.50 | 73.80 | 83.59 |
| C-ADA Dex 34 FD | 3424.06 | 72.64 | 73.78 | 83.84 |
| C-ADA Dex 18 FD | 4097.09 | 68.45 | 73.54 | 83.80 |
| C-ADA PEG 6000 0.1 FD | 3247.79 | 72.98 | 73.78 | 83.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Villen, F.; Gallego, I.; Sainz-Ramos, M.; Ordoyo-Pascual, J.; Ruiz-Alonso, S.; Saenz-del-Burgo, L.; O’Mahony, C.; Pedraz, J.L. Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study. Pharmaceutics 2023, 15, 2049. https://doi.org/10.3390/pharmaceutics15082049
Garcia-Villen F, Gallego I, Sainz-Ramos M, Ordoyo-Pascual J, Ruiz-Alonso S, Saenz-del-Burgo L, O’Mahony C, Pedraz JL. Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study. Pharmaceutics. 2023; 15(8):2049. https://doi.org/10.3390/pharmaceutics15082049
Chicago/Turabian StyleGarcia-Villen, Fatima, Idoia Gallego, Myriam Sainz-Ramos, Jorge Ordoyo-Pascual, Sandra Ruiz-Alonso, Laura Saenz-del-Burgo, Conor O’Mahony, and Jose Luis Pedraz. 2023. "Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study" Pharmaceutics 15, no. 8: 2049. https://doi.org/10.3390/pharmaceutics15082049
APA StyleGarcia-Villen, F., Gallego, I., Sainz-Ramos, M., Ordoyo-Pascual, J., Ruiz-Alonso, S., Saenz-del-Burgo, L., O’Mahony, C., & Pedraz, J. L. (2023). Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study. Pharmaceutics, 15(8), 2049. https://doi.org/10.3390/pharmaceutics15082049










