Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Que-CD Formulations
2.2.1. Que-CD Lyophilizates
2.2.2. Que-Lyophilizate Solutions for Oral Gavage Administration
2.2.3. Spray-Dried MLMPs
2.2.4. Que-CD-MLMP Blends
2.3. Animal Experiments
2.3.1. Animals and Housing Conditions
2.3.2. Intranasal Administration
2.3.3. In Vivo Study Dosing and Sampling Protocol
2.4. Que Extraction from Biological Samples
2.4.1. Parent-Que Extraction from Serum
2.4.2. Parent-Que Extraction from Rat Brain and Olfactory Bulb
2.4.3. Total Que Quantification in Serum and Brain Samples
2.5. HPLC-PDA Method for Que Quantification in Biological Samples
2.6. Non-Compartmental Analysis
2.7. Relative Drug Targeting Efficiency Percentage and NTB Direct Transport Percentage Indexes
2.8. Statistical Analysis
3. Results
3.1. Administration of Nasal Formulations
3.2. Que HPLC-PDA Assay
3.3. Oral and IN Administration of Que-Me-β-CD Lyophilizate and Its Blend with Mannitol/Lecithin Microparticles
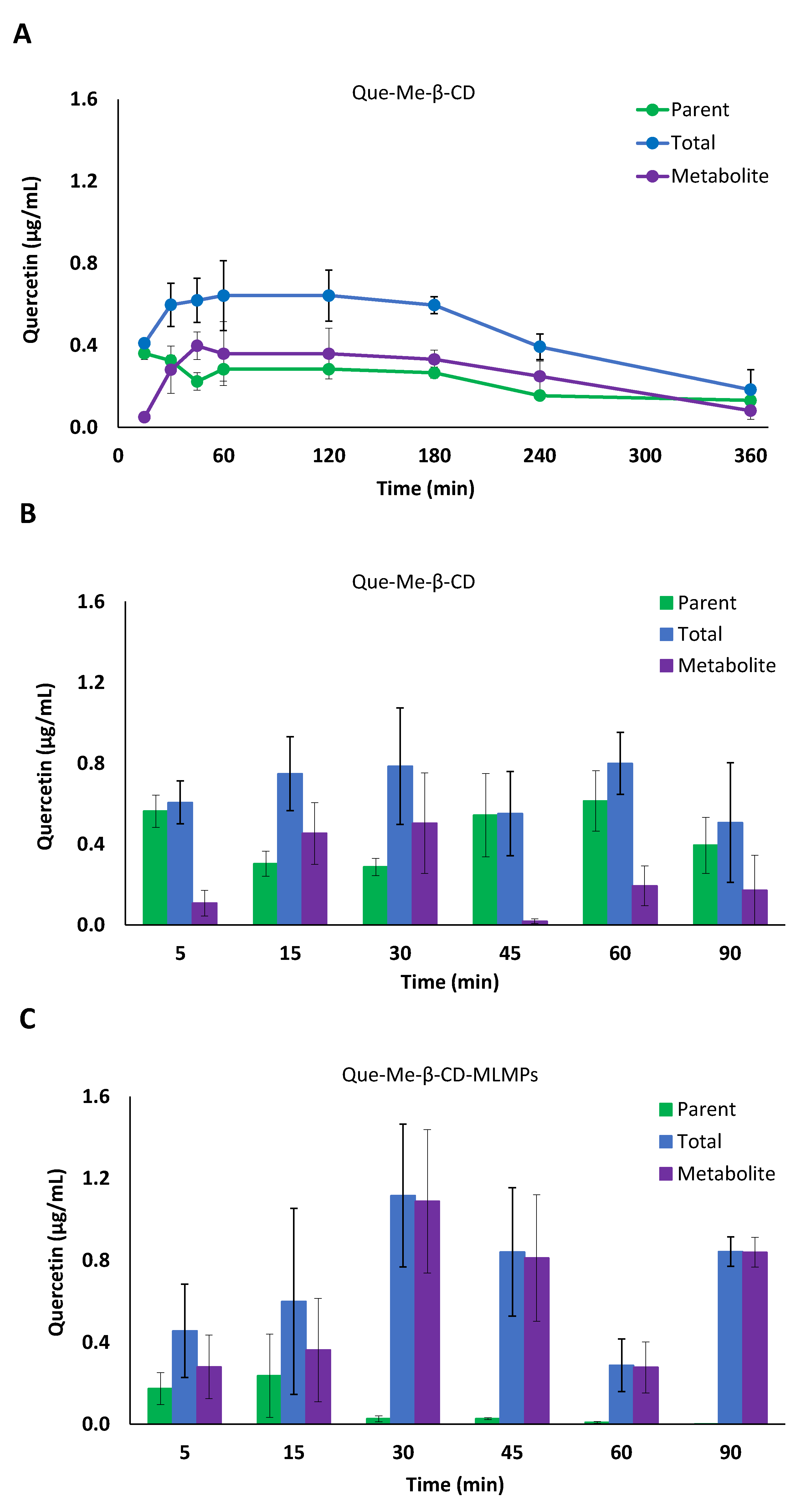
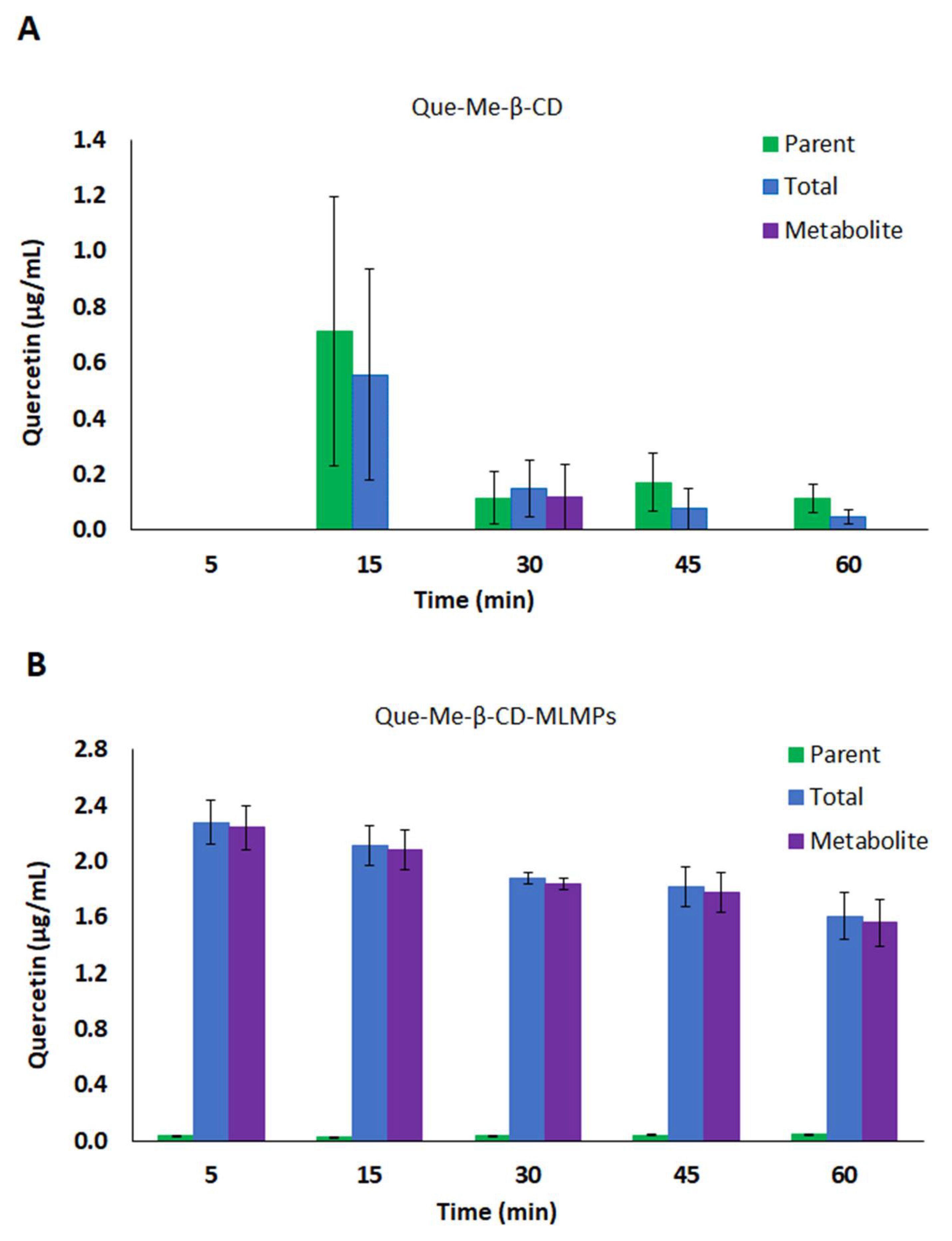
3.3.1. Serum Pharmacokinetic Data
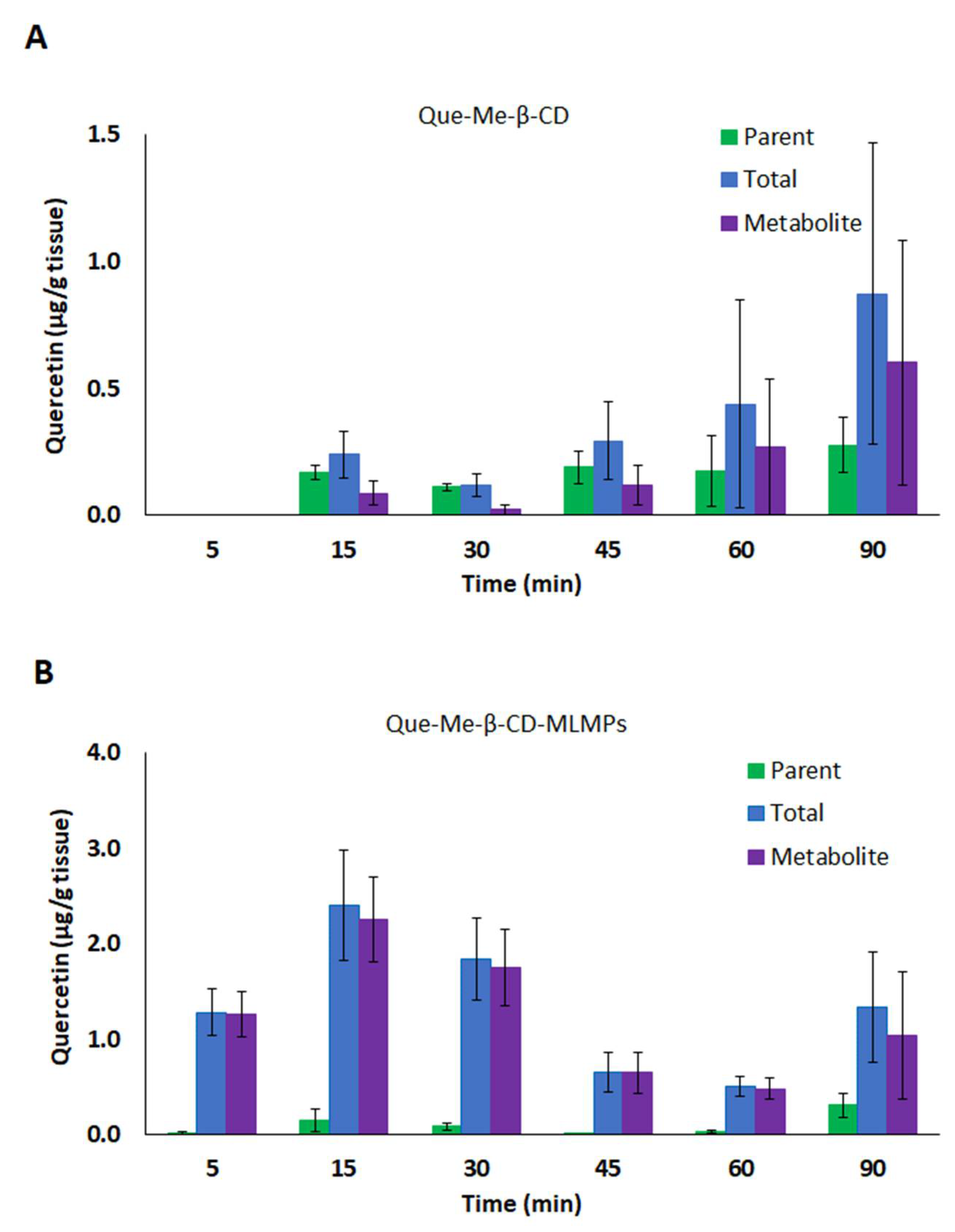
3.3.2. Brain Pharmacokinetic Data
3.4. Oral and IN Administration of Que-HP-β-CD Lyophilizate and Its Blend with Mannitol/Lecithin Microparticles
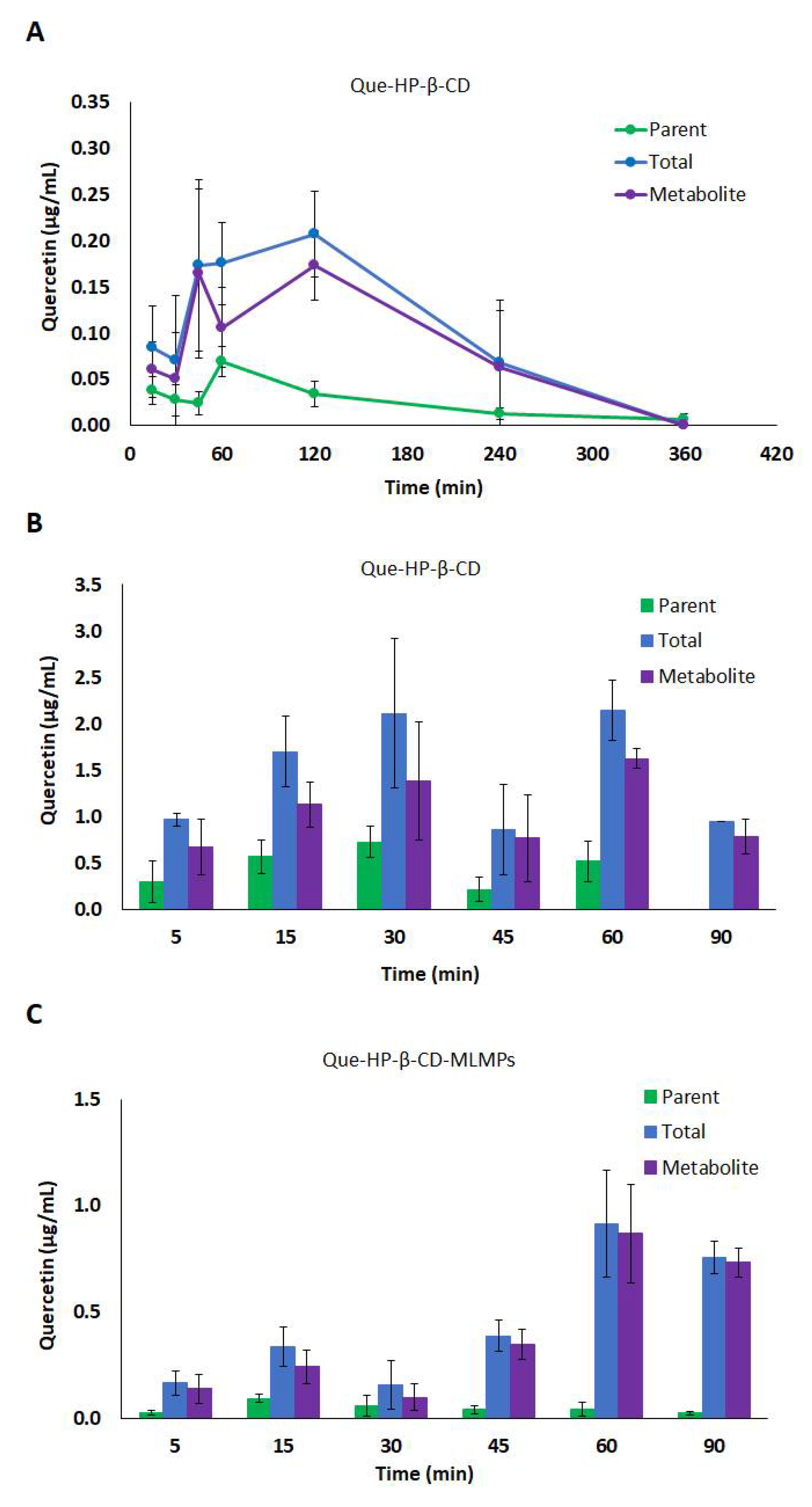
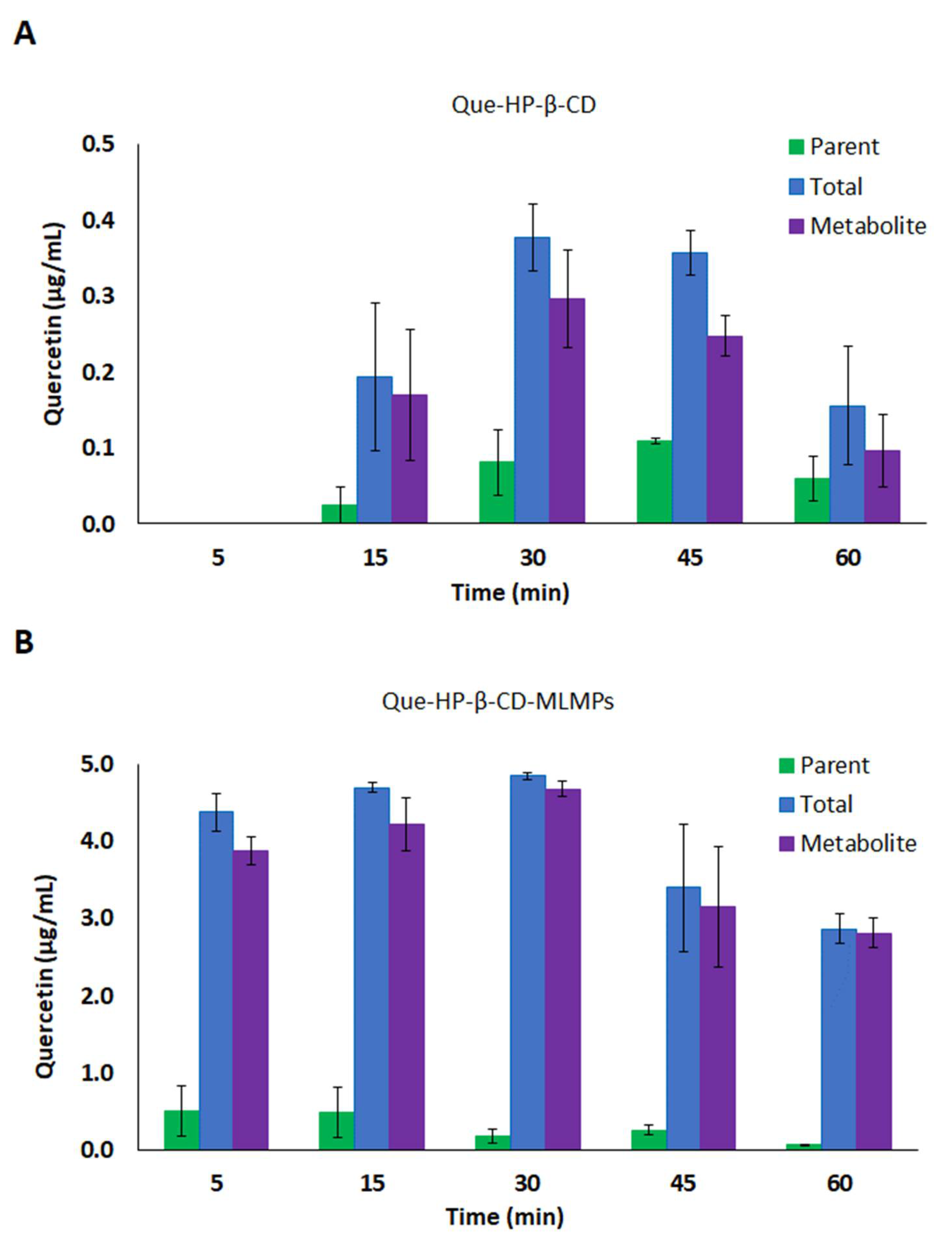
3.4.1. Serum Pharmacokinetic Data
3.4.2. Brain Pharmacokinetic Data
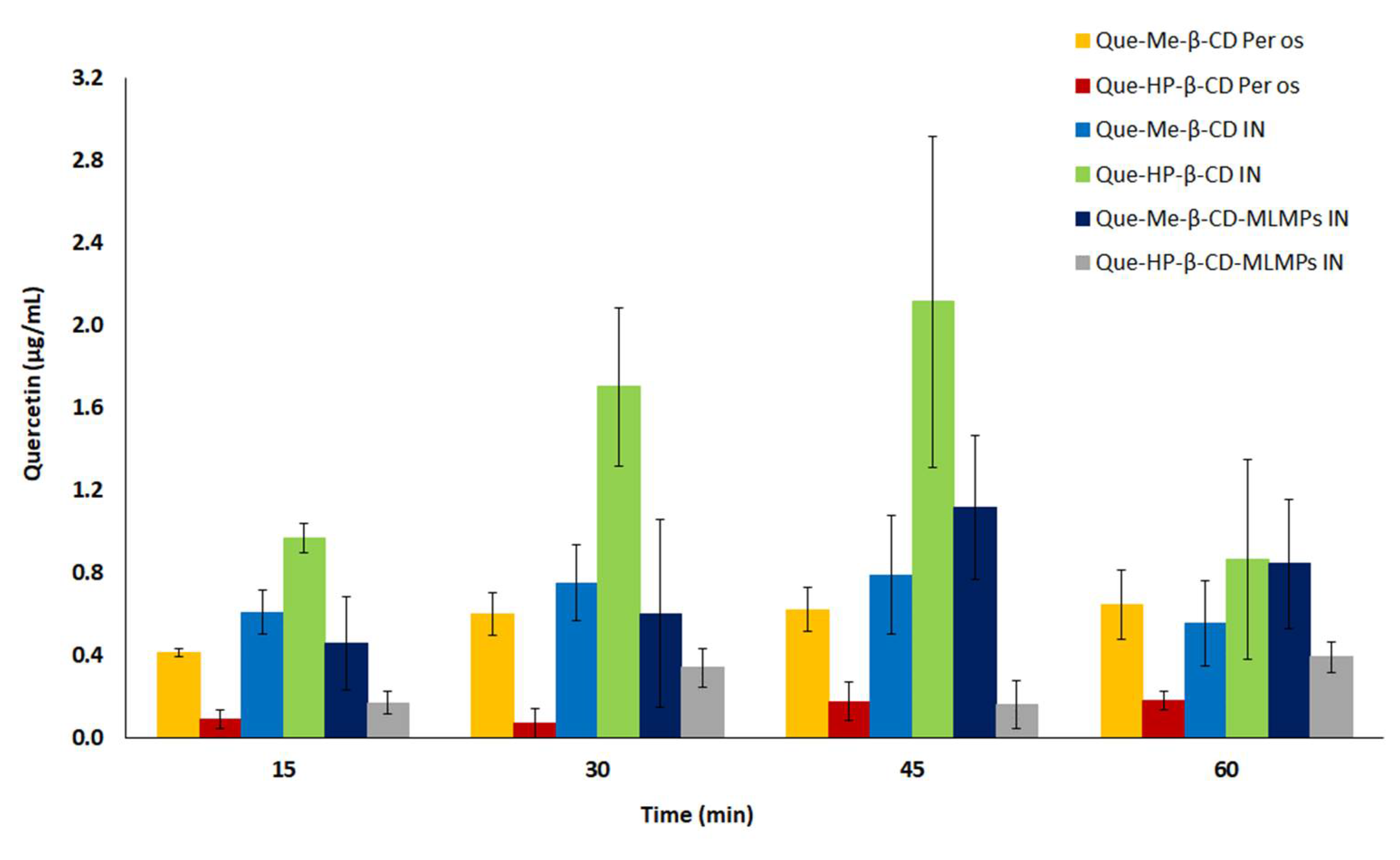
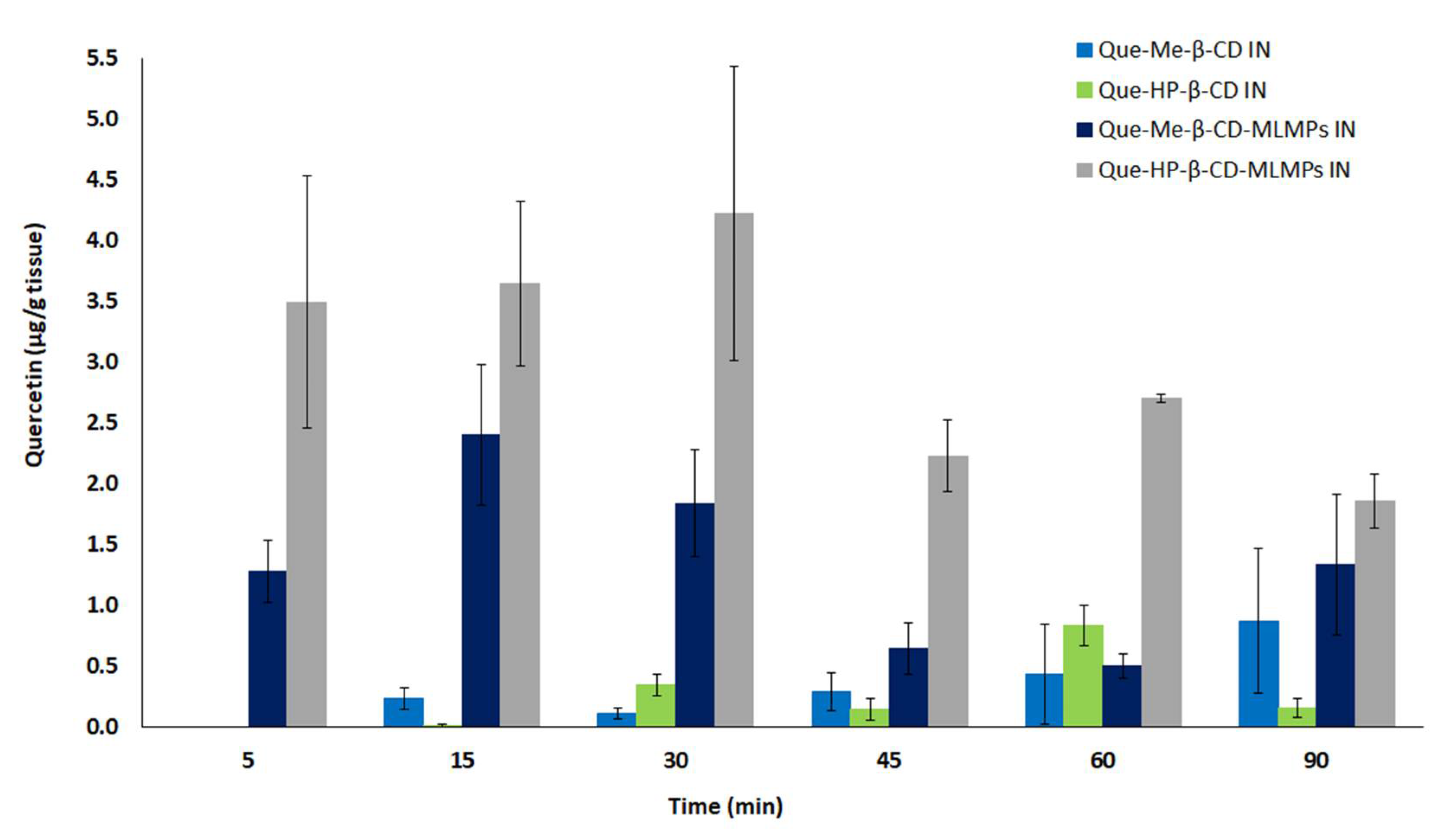
3.5. Comparative Profiles of All the Tested Formulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guaáqueta, A.M.; Munñoz-Manco, J.I. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.; Fernandez, F. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sánchez-López, E.; García, M.L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016, 2016, 8501693. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Echeverry, C.; Arredondo, F. Pretreatment with natural flavones and neuronal cell survival after oxidative stress: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 2111–2115. [Google Scholar] [CrossRef]
- Paula, P.C.; Angelica Maria, S.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Chen, J.Y. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fang, Z. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Saito, S. Different profiles of quercetin metabolites in rat plasma: Comparison of two administration methods. Biosci. Biotechnol. Biochem. 2009, 73, 517–523. [Google Scholar] [CrossRef][Green Version]
- Ishisaka, A.; Mukai, R. Specific localization of quercetin-3-O-glucuronide in human brain. Arch. Biochem. Biophys. 2014, 557, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Reyes, D.; Morales, A.I. Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, J.M.; Cho, E.; Jeong, D.; Shinde, V.V.; Kim, H.; Choi, Y.; Jung, S. Enhancement of Solubility and Bioavailability of Quercetin by Inclusion Complexation with the Cavity of Mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Bull. Korean Chem. Soc. 2017, 38, 880–889. [Google Scholar] [CrossRef]
- Uchiyama, H.; Wada, Y.; Takamatsu, M.; Kadota, K.; Tozuka, Y. Improved Solubility of Quercetin by Preparing Amorphous Solid with Transglycosylated Rutin and Isoquercitrin. Environ. Control Biol. 2018, 56, 161–165. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Chen, Y.; Zou, L.; Liu, W.; Cheng, C.; Fu, D.; Liu, C. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Arbain, N.H.; Basri, M.; Salim, N.; Wui, W.T.; Abdul Rahman, M.B. Development and Characterization of Aerosol Nanoemulsion System Encapsulating Low Water Soluble Quercetin for Lung Cancer Treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Shiromani, S.; Patil, M.M.; Nallamuthu, I.; Rajamanickam, R.; Singsit, D.; Anand, T. Shellac/caseinate as a composite nanocarrier for improved bioavailability of quercetin. Food Hydrocoll. Health 2023, 3, 100113. [Google Scholar] [CrossRef]
- Enteshari Najafabadi, R.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Vaz, G.; Clementino, A.; Mitsou, E.; Ferrari, E.; Buttini, F.; Sissa, C.; Xenakis, A.; Sonvico, F.; Dora, C.L. In Vitro Evaluation of Curcumin- and Quercetin-Loaded Nanoemulsions for Intranasal Administration: Effect of Surface Charge and Viscosity. Pharmaceutics 2022, 14, 194. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Zaki, R.M.; Alsaidan, O.A.; Elmowafy, M.; Zafar, A.; Shalaby, K.; Abdelgawad, M.A.; Abo El-Ela, F.I.; Rateb, M.E.; Naguib, I.A.; et al. Intranasal Nanotransferosomal Gel for Quercetin Brain Targeting: I. Optimization, Characterization, Brain Localization, and Cytotoxic Studies. Pharmaceutics 2023, 15, 1805. [Google Scholar] [CrossRef]
- Patil, N.L.; Mahajan, H.S. Quercetin Loaded Nanostructured Lipid Carriers for Nose to Brain Delivery: In Vitro and In Vivo Studies. Am. J. Adv. Drug Deliv. 2018, 6, 9–20. [Google Scholar] [CrossRef]
- Mahmoud, K.Y.; Elhesaisy, N.A.; Rashed, A.R.; Mikhael, E.S.; Fadl, M.I.; Elsadek, M.S.; Mohamed, M.A.; Mostafa, M.A.; Hassan, M.A.; Halema, O.M.; et al. Exploring the potential of intranasally administered naturally occurring quercetin loaded into polymeric nanocapsules as a novel platform for the treatment of anxiety. Sci. Rep. 2023, 13, 510. [Google Scholar] [CrossRef]
- Diamantis, D.A.; Ramesova, S. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1913–1924. [Google Scholar] [CrossRef]
- Manta, K.; Papakyriakopoulou, P. Preparation and biophysical characterization of Quercetin inclusion complexes with β-cyclodextrin derivatives for the preparation of possible nose-to-brain Quercetin delivery systems. Mol. Pharm. 2020, 17, 4241–4255. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Manta, K. Nasal powders of quercetin-β-cyclodextrin derivatives complexes with mannitol/lecithin microparticles for Nose-to-Brain delivery: In vitro and ex vivo evaluation. Int. J. Pharm. 2021, 607, 121016. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Abou-Kaoud, M. Quantitative analysis of drug delivery to the brain via nasal route. J. Control Release 2014, 189, 133–140. [Google Scholar] [CrossRef]
- Tiozzo Fasiolo, L.; Manniello, M.D. Flurbiprofen sodium microparticles and soft pellets for nose-to-brain delivery: Serum and brain levels in rats after nasal insufflation. Int. J. Pharm. 2021, 605, 120827. [Google Scholar] [CrossRef]
- Balducci, A.G.; Ferraro, L. Antidiuretic effect of desmopressin chimera agglomerates by nasal administration in rats. Int. J. Pharm. 2013, 440, 154–160. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 4 June 2023).
- Papakyriakopoulou, P.; Balafas, E. Nose-to-Brain delivery of donepezil hydrochloride following administration of an HPMC-Me-β-CD-PEG400 nasal film in mice. J. Drug Deliv. Sci. Technol. 2023, 84, 104463. [Google Scholar] [CrossRef]
- Sanghavi, N.; Bhosale, S.D. RP-HPLC method development and validation of Quercetin isolated from the plant Tridax procumbens L. J. Sci. Innov. Res. 2014, 3, 594–597. [Google Scholar] [CrossRef]
- Aranishi, T.; Nagai, Y. Usability of Nasal Glucagon Device: Partially Randomized Caregiver and Third-Party User Experience Trial with Simulated Administration at a Japanese Site. Diabetes Ther. 2020, 11, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Suico, J.G.; Hövelmann, U. Glucagon Administration by Nasal and Intramuscular Routes in Adults With Type 1 Diabetes During Insulin-Induced Hypoglycaemia: A Randomised, Open-Label, Crossover Study. Diabetes Ther. 2020, 11, 1591–1603. [Google Scholar] [CrossRef]
- Neiers, F.; Jarriault, D. The odorant metabolizing enzyme UGT2A1: Immunolocalization and impact of the modulation of its activity on the olfactory response. PLoS ONE 2021, 16, e0249029. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, E.; Esmaeili, A. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, Z.; Cao, X. Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev. Bras. Farmacogn. 2013, 23, 776–782. [Google Scholar] [CrossRef]
- Yang, L.L.; Xiao, N. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci. Rep. 2016, 6, 35460. [Google Scholar] [CrossRef]
- Huebbe, P.; Wagner, A.E. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Buenestado, A. Intranasal drug delivery: An efficient and non-invasive route for systemic administration: Focus on opioids. Pharmacol. Ther. 2012, 134, 366–379. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef]
- Giuliani, A.; Balducci, A.G. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deiv. 2018, 25, 376–387. [Google Scholar] [CrossRef]
- Raffin, R.P.; Colombo, P. Agglomerates containing pantoprazole microparticles: Modulating the drug release. AAPS PharmSciTech 2009, 10, 335–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Luo, Z. Preparation, characterization, and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J.; Bedford, S. Influence of lipophilicity on drug–cyclodextrin interactions: A calorimetric study. Thermochim. Acta 2010, 511, 102–106. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Bhatia-Dey, N.; Heinbockel, T. The Olfactory System as Marker of Neurodegeneration in Aging, Neurological and Neuropsychiatric Disorders. Int. J. Environ. Res. Public Health 2021, 18, 6976. [Google Scholar] [CrossRef] [PubMed]

| Group | Formulation | Mode of Administration | Dose | |
|---|---|---|---|---|
| mg/kg | mg | |||
| a | Que-Me-β-CD lyophilizate | Per os | 2.5 | 0.83 |
| b | Que-HP-β-CD lyophilizate | Per os | 0.45 | 0.145 |
| c | Que-Me-β-CD lyophilizate | Intranasal | 2.7 | 0.88 |
| d | Que-HP-β-CD lyophilizate | Intranasal | 0.45 | 0.147 |
| e | Que-Me-β-CD:MLMPs (75:25) | Intranasal | 1.46 | 0.47 |
| f | Que-HP-β-CD:MLMPs (75:25) | Intranasal | 1 | 0.34 |
| QUE NCA Serum Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic Parameter Value (SE) | |||||||||
| Nasal Administration | Per os Administration | ||||||||
| Pharmacokinetic Parameter | Que-Me-β-CD Lyophilizate | Que-Me-β-CD-MLMPs | Que-Me-β-CD | ||||||
| Parent | Total | Metabolite | Parent | Total | Metabolite | Parent | Total | Metabolite | |
| AUC0−t (min × μg)/mL | 41.2 (5.25) | 59.6 (8.12) | 21.3 (5.42) | 5.4 (2.6) | 59.4 (9.70) | 54.0 (8.33) | 74.6 (4.04) | 153 (13.2) | 81.2 (12.3) |
| Cmax (μg/mL) | 0.69 (0.15) | 0.80 (0.15) | 0.50 (0.25) | 0.23 (0.20) | 1.1 (0.34) | 1.1 (0.35) | 0.36 (0.031) | 0.64 (0.17) | 0.40 (0.067) |
| AUCinf (min × μg)/mL | 89.3 | - | - | 5.42 | - | - | 119 | 193 | 96.8 |
| AUC % Extrapolation | 54 | - | - | <1 | - | - | 37 | 21 | 16 |
| tmax (min) | 5/60 | 60 | 30/90 | 15 | 30 | 30/90 | 15 | 60 | 45 |
| t1/2 (min) | 85 | - | - | 13 | - | - | 235 | 152 | 134 |
| kel (1/min) | 0.008 | - | - | 0.054 | - | - | 0.003 | 0.005 | 0.005 |
| QUE NCA Brain Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic Parameter Value (SE) | |||||||||
| Nasal Administration | Per os Administration | ||||||||
| Pharmacokinetic Parameter | Que-Me-β-CD Lyophilizate | Que-Me-β-CD-MLMPs | Que-Me-β-CD | ||||||
| Parent | Total | Metabolite | Parent | Total | Metabolite | Parent | Total | Metabolite | |
| AUC0−t (min × μg)/g | 14.7 (3.66) | 32.0 (13.1) | 18.3 (9.51) | 8.6 (2.5) | 108 (14.6) | 99.8 (14.6) | - | - | - |
| Cmax (μg/g) | 0.27 (0.10) | 0.84 (0.59) | 0.60 (0.48) | 0.31 (0.13) | 2.4 (0.57) | 2.3 (0.45) | - | - | - |
| AUCinf (min × μg)/g | - | - | - | - | - | - | - | - | - |
| AUC % Extrapolation | - | - | - | - | - | - | - | - | - |
| tmax (min) | 90 | 90 | 90 | 90 | 15 | 15 | - | - | - |
| t1/2 (min) | - | - | - | - | - | - | - | - | - |
| kel (1/min) | - | - | - | - | - | - | - | - | - |
| QUE NCA Serum Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic Parameter Value (SE) | |||||||||
| Nasal Administration | Per os Administration | ||||||||
| Pharmacokinetic Parameter | Que-HP-β-CD Lyophilizate | Que-HP-β-CD-MLMPs | Que-HP-β-CD Lyophilizate | ||||||
| Parent | Total | Metabolite | Parent | Total | Metabolite | Parent | Total | Metabolite | |
| AUC0−t (min × μg)/mL | 37.6 (6.53) | 136 (14.8) | 99.8 (10.8) | 4.2 (0.012) | 45.5 (6.27) | 41.3 (5.67) | 8.9 (1.7) | 34.2 (6.36) | 27.5 (5.50) |
| Cmax (μg/mL) | 0.73 (0.20) | 2.2 (0.37) | 1.6 (0.19) | 0.094 (0.018) | 0.91 (0.25) | 0.87 (0.25) | 0.07 (0.02) | 0.21 (0.47) | 0.17 (0.037) |
| AUCinf (min × μg)/mL | 46.6 | - | - | 6.1 | - | - | 9.8 | 45.7 | 43.9 |
| AUC % Extrapolation | 19 | - | - | 30 | - | - | 9 | 25 | 37 |
| tmax (min) | 30/60 | 30/60 | 30/60 | 15 | 60 | 60 | 60 | 120 | 120 |
| t1/2 (min) | 37 | - | - | 52 | - | - | 99 | 118 | 178 |
| kel (1/min) | 0.019 | - | - | 0.013 | - | - | 0.007 | 0.006 | 0.004 |
| QUE NCA Brain Pharmacokinetics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic Parameter Value (SE) | |||||||||
| Nasal Administration | Per os Administration | ||||||||
| Pharmacokinetic Parameter | Que-HP-β-CD Lyophilizate | Que-HP-β-CD-MLMPs | Que-HP-β-CD Lyophilizate | ||||||
| Parent | Total | Metabolite | Parent | Total | Metabolite | Parent | Total | Metabolite | |
| AUC0−t (min × μg)/g | 6.0 (1.0) | 28.9 (5.14) | 23.9 (4.54) | 1.0 (0.4) | 257 (22.4) | 256 (22.2) | - | - | - |
| Cmax (μg/g) | 0.15 (0.026) | 0.84 (0.20) | 0.68 (0.18) | 0.039 (0.031) | 4.2 (1.2) | 4.2 (1.2) | - | - | - |
| AUCinf (min × μg)/g | - | - | - | 1.1 | 421.6 | 421.2 | - | - | - |
| AUC % Extrapolation | - | - | - | 3 | 39 | 39 | - | - | - |
| tmax (min) | 60 | 60 | 60 | 15 | 30 | 30 | - | - | - |
| t1/2 (min) | - | - | - | 17 | 61 | 62 | - | - | - |
| kel (1/min) | - | - | - | 0.041 | 0.011 | 0.011 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manta, K.; Papakyriakopoulou, P.; Nikolidaki, A.; Balafas, E.; Kostomitsopoulos, N.; Banella, S.; Colombo, G.; Valsami, G. Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics 2023, 15, 2036. https://doi.org/10.3390/pharmaceutics15082036
Manta K, Papakyriakopoulou P, Nikolidaki A, Balafas E, Kostomitsopoulos N, Banella S, Colombo G, Valsami G. Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics. 2023; 15(8):2036. https://doi.org/10.3390/pharmaceutics15082036
Chicago/Turabian StyleManta, Konstantina, Paraskevi Papakyriakopoulou, Anna Nikolidaki, Evangelos Balafas, Nikolaos Kostomitsopoulos, Sabrina Banella, Gaia Colombo, and Georgia Valsami. 2023. "Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles" Pharmaceutics 15, no. 8: 2036. https://doi.org/10.3390/pharmaceutics15082036
APA StyleManta, K., Papakyriakopoulou, P., Nikolidaki, A., Balafas, E., Kostomitsopoulos, N., Banella, S., Colombo, G., & Valsami, G. (2023). Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics, 15(8), 2036. https://doi.org/10.3390/pharmaceutics15082036







