High-Performance Hybrid Phototheranostics for NIR-IIb Fluorescence Imaging and NIR-II-Excitable Photothermal Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
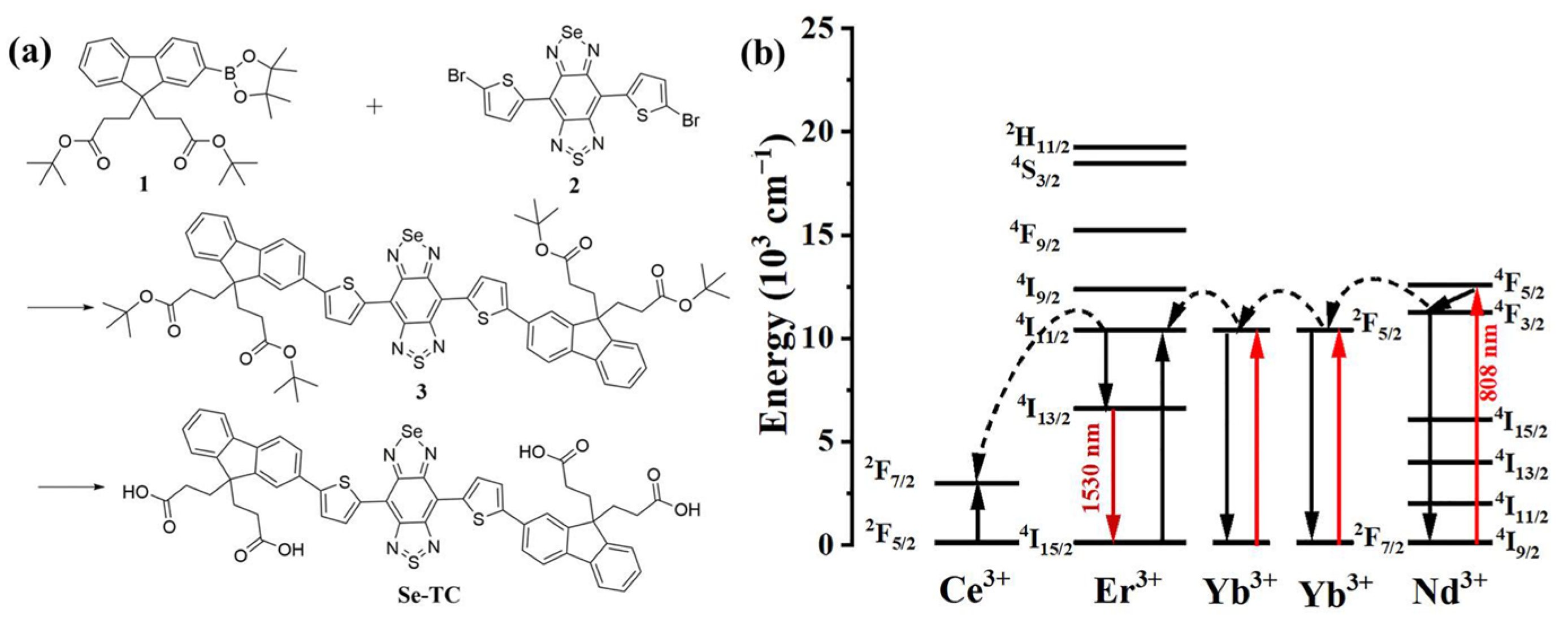
2.2. Synthesis of Se-TC
2.3. Synthesis of RENP
2.4. Characterization
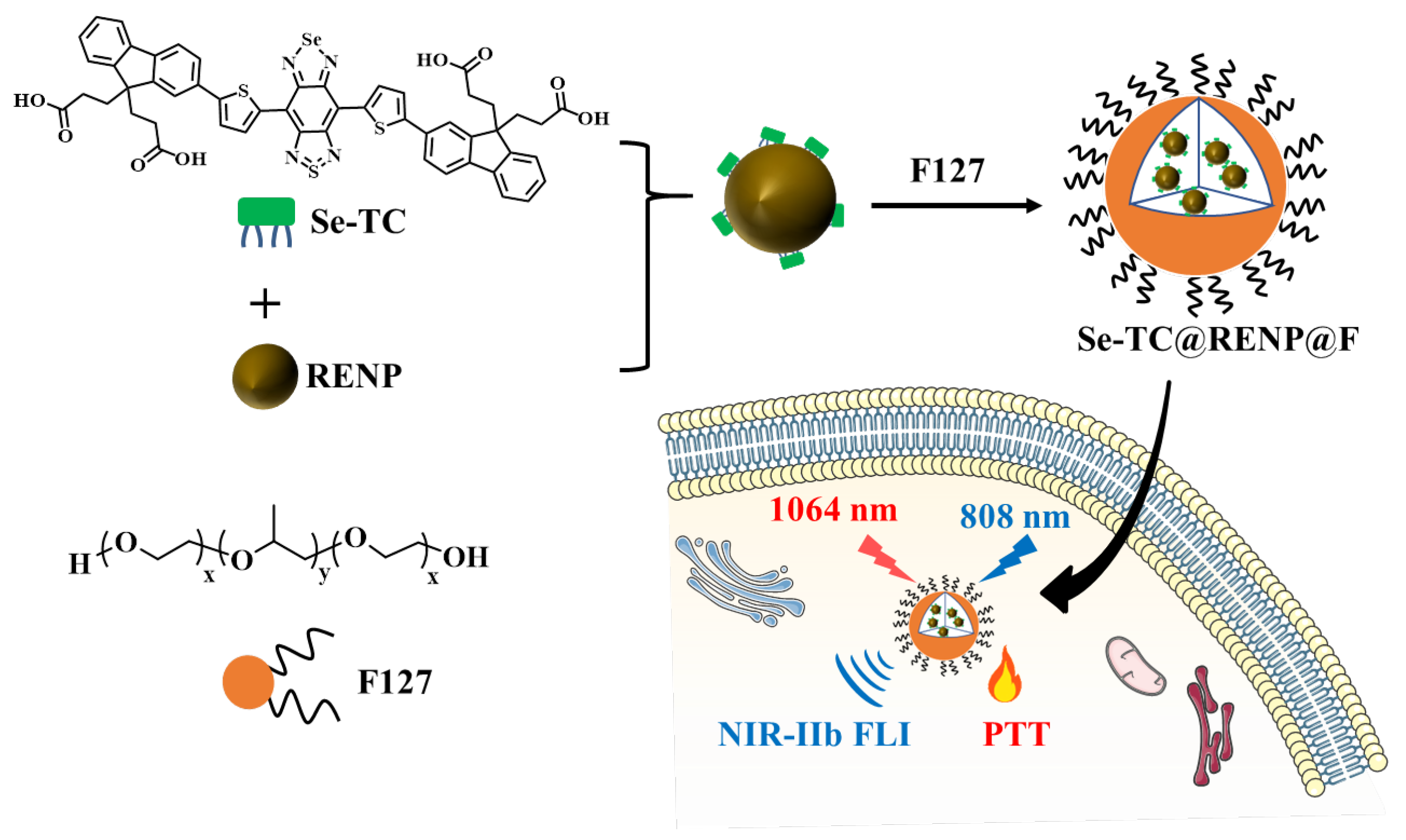
2.5. Preparation of Se-TC@RENP@F
2.6. Photothermal Performance of Se-TC@RENP@F
2.7. Measurement of Extinction Coefficient
2.8. MTT Assay
2.9. Live and Dead Cell Assay
2.10. In Vivo NIR-IIb FLI
3. Results and Discussion
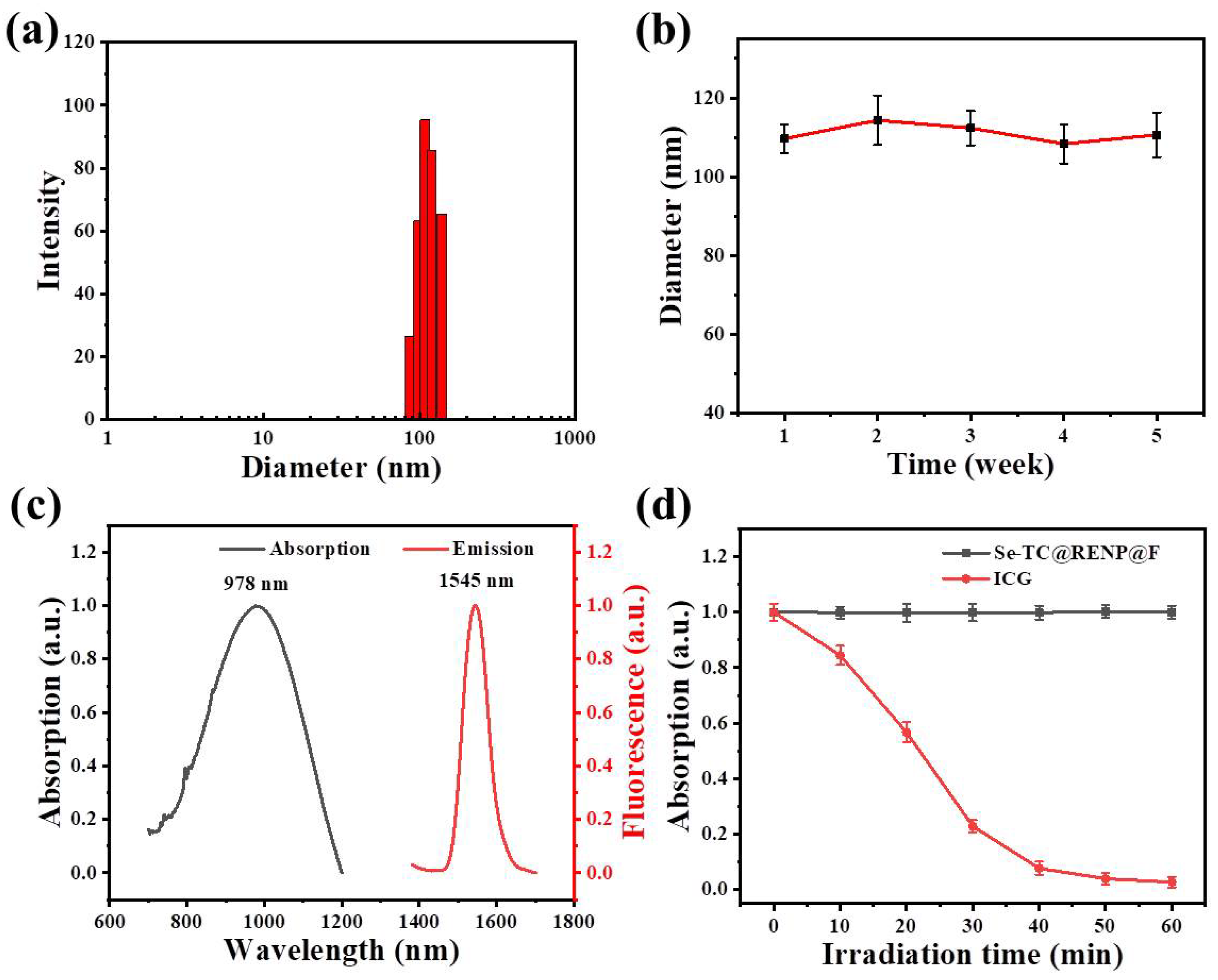
3.1. Preparation and Characterization of Se-TC@RENP@F
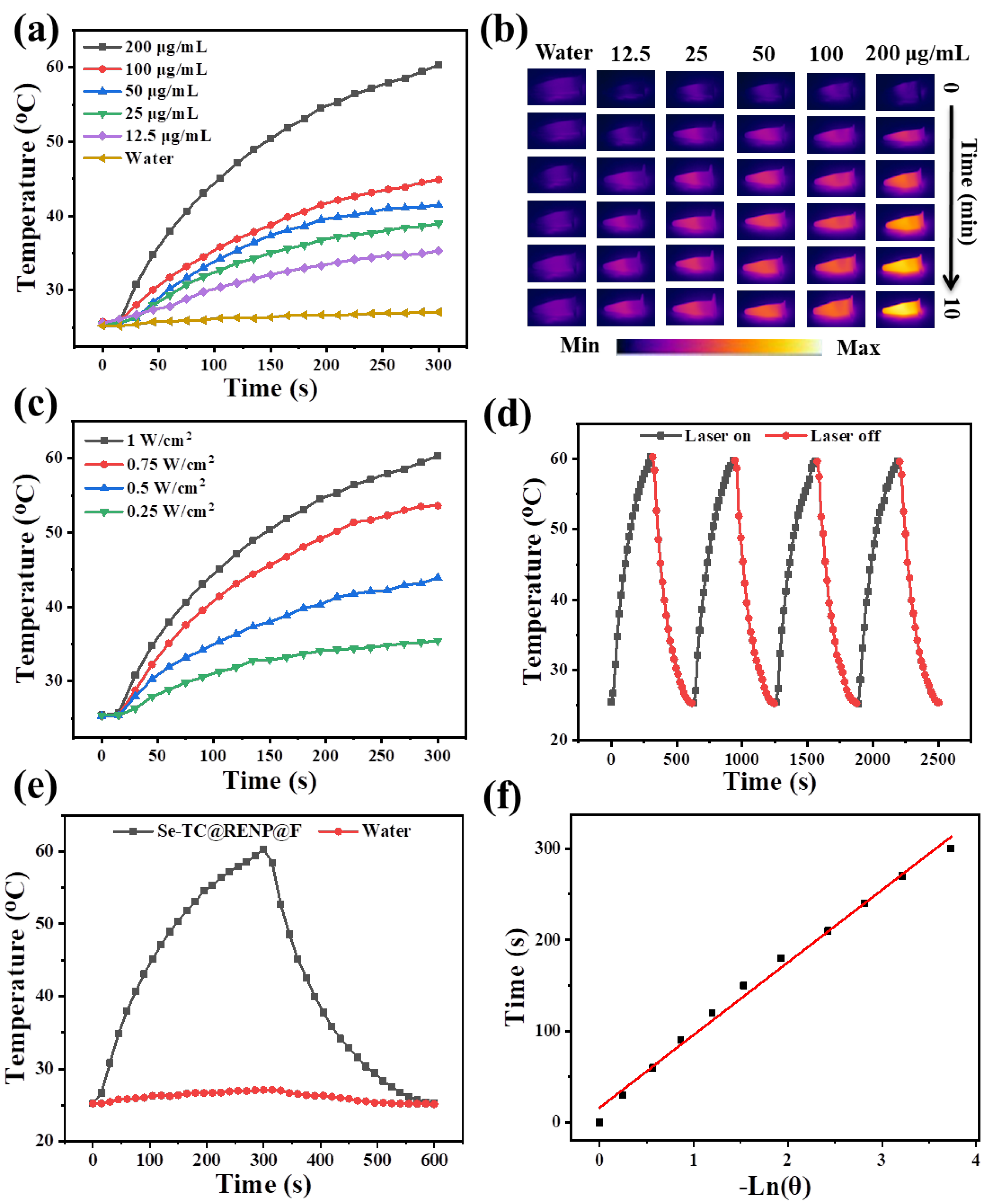
3.2. Photothermal Performance of Se-TC@RENP@F
3.3. Cytotoxicity Assay of Se-TC@RENP@F
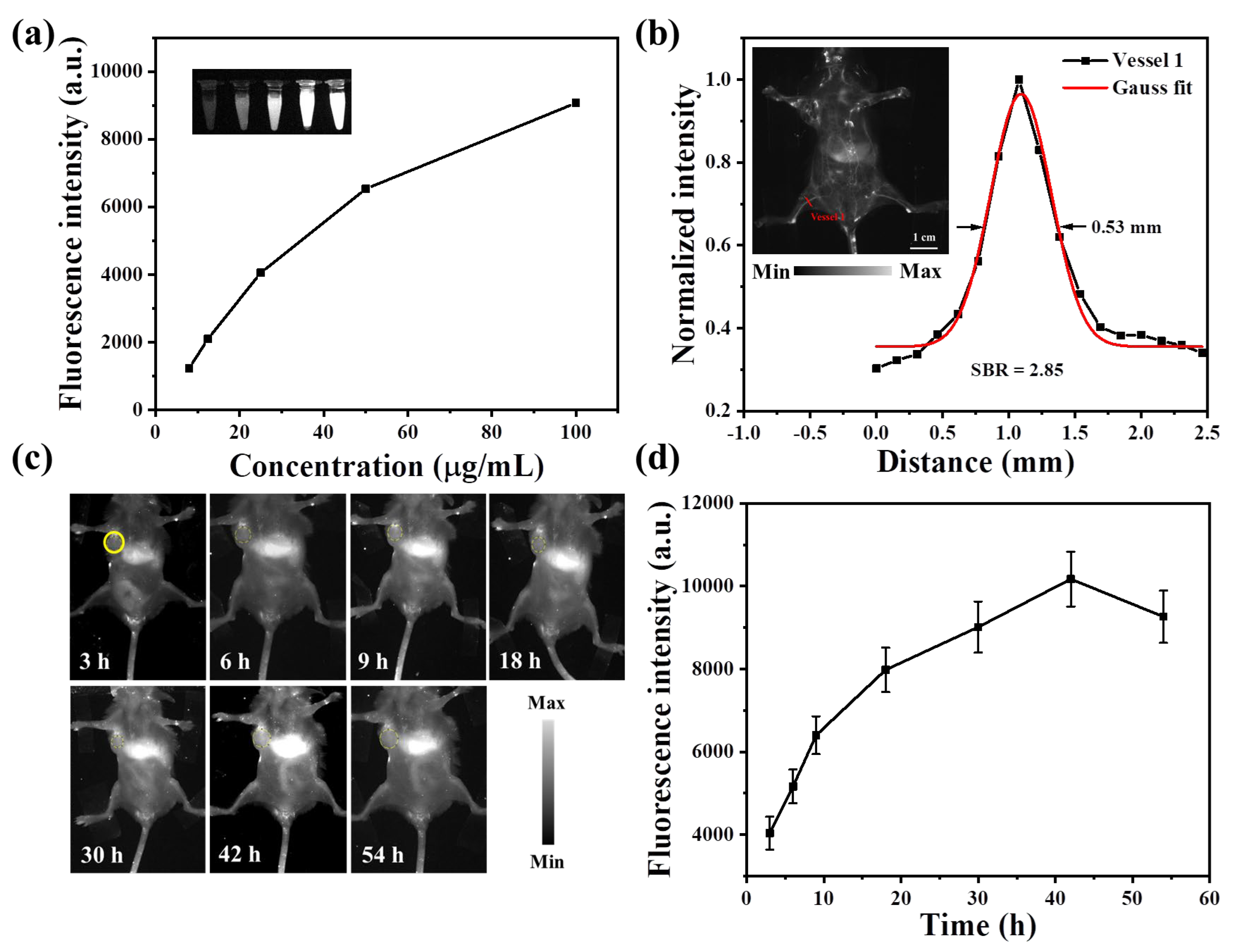
3.4. In Vivo NIR-IIb FLI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliver. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Ma, H.; Altenberg, G.A.; Liang, H. Nanodiscs: A versatile nanocarrier platform for cancer diagnosis and treatment. Chem. Soc. Rev. 2022, 51, 1702–1728. [Google Scholar] [CrossRef]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Bernal, A.; Calcagno, C.; Mulder, W.J.M.; Pérez-Medina, C. Imaging-guided nanomedicine development. Curr. Opin. Chem. Biol. 2021, 63, 78–85. [Google Scholar] [CrossRef]
- Allard, C.; Schue, L.; Fossard, F.; Recher, G.; Nascimento, R.; Flahaut, E.; Loiseau, A.; Desjardins, P.; Martel, R.; Gaufres, E. Confinement of Dyes inside Boron Nitride Nanotubes: Photostable and Shifted Fluorescence down to the Near Infrared. Adv. Mater. 2020, 32, 2001429. [Google Scholar] [CrossRef]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Sekar, R.; Basavegowda, N.; Thathapudi, J.J.; Sekhar, M.R.; Joshi, P.; Somu, P.; Baek, K.-H. Recent Progress of Gold-Based Nanostructures towards Future Emblem of Photo-Triggered Cancer Theranostics: A Special Focus on Combinatorial Phototherapies. Pharmaceutics 2023, 15, 433. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Walker, E.; Springer, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Chemoradiotherapy of Prostate Cancer Using Gold Nanoclusters with Protease Activatable Monomethyl Auristatin E. ACS Appl. Mater. Interfaces 2022, 14, 14916–14927. [Google Scholar] [CrossRef] [PubMed]
- Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Tumac, A.C.; Brohlin, O.R.; Wijesundara, Y.H.; Adlooru, A.V.; Benjamin, C.; Lee, H.; Parsamian, P.; et al. PhotothermalPhage: A Virus-Based Photothermal Therapeutic Agent. J. Am. Chem. Soc. 2021, 143, 16428–16438. [Google Scholar] [CrossRef] [PubMed]
- Roque Iii, J.A.; Cole, H.D.; Barrett, P.C.; Lifshits, L.M.; Hodges, R.O.; Kim, S.; Deep, G.; Francés-Monerris, A.; Alberto, M.E.; Cameron, C.G.; et al. Intraligand Excited States Turn a Ruthenium Oligothiophene Complex into a Light-Triggered Ubertoxin with Anticancer Effects in Extreme Hypoxia. J. Am. Chem. Soc. 2022, 144, 8317–8336. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, Q.; Jiang, Y.; Wu, X.; Yue, X.; Geng, Y.; Shen, J.; Ding, D. Semiconducting Polymer Nanoparticles with Intramolecular Motion-Induced Photothermy for Tumor Phototheranostics and Tooth Root Canal Therapy. Adv. Mater. 2022, 34, 2200179. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, F.; Zhang, L.; Li, M.; Wang, G.; Wang, W.; Xu, Y.; Wei, W. Manipulating Host-Guest Charge Transfer of a Water-Soluble Double-Cavity Cyclophane for NIR-II Photothermal Therapy. Angew. Chem. Int. Ed. 2023, 62, 202301267. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Z.C.; Ji, Y.C.; Zhou, S.D.; Jia, B.Y.; Zhang, Y.H.; Wang, J.; Ding, Y.; Wang, Y.; Yao, Y.; et al. Icing on the cake: Combining a dual PEG-functionalized pillararene and an A-D-A small molecule photosensitizer for multimodal phototherapy. Sci. China Chem. 2022, 65, 1134–1141. [Google Scholar] [CrossRef]
- Jo, G.; Park, Y.; Park, M.H.; Hyun, H. Near-Infrared Fluorescent Hydroxyapatite Nanoparticles for Targeted Photothermal Cancer Therapy. Pharmaceutics 2023, 15, 1374. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Jo, G.; Kim, E.J.; Hyun, H. Enhanced Tumor Accumulation of Low-Molecular-Weight Hyaluronic Acid/Chitosan Nanocomplexes for Photothermal Therapy. Pharmaceutics 2023, 15, 613. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Y.; Yuan, Z. Breast Cancer Cell Membrane Camouflaged Lipid Nanoparticles for Tumor-Targeted NIR-II Phototheranostics. Pharmaceutics 2022, 14, 1367. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, H.; Xiong, Y.; Zhang, X.; Cai, J.; Chen, C.; Gao, Y.; Lu, F.; Fan, Q. Simple Preparation of Near-infrared-II Organic Small Molecule-based Phototheranostics by Manipulation of the Electron-donating Unit. Acta Chim. Sin. 2022, 80, 1485–1493. [Google Scholar] [CrossRef]
- Li, H.; Dai, H.; Mei, A.; Ruan, X.; Wang, W.; Yang, D.; Wang, W.; Zhang, Q.; Dong, X.; Shao, J. Triphenylamine flanked boron difluoride formazanate for NIR-II fluorescence imaging-guided photothermal therapy. Dye. Pigment. 2022, 205, 110478. [Google Scholar] [CrossRef]
- Xu, M.K.; Zhang, C.; Zeng, Z.L.; Pu, K.Y. Semiconducting Polymer Nanoparticles as Activatable Nanomedicines for Combinational Phototherapy. ACS Appl. Polym. Mater. 2021, 3, 4375–4389. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Xi, D.; Xiao, M.; Cao, J.; Zhao, L.; Xu, N.; Long, S.; Fan, J.; Shao, K.; Sun, W.; Yan, X.; et al. NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy. Adv. Mater. 2020, 32, 1907855. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhao, F.; Xue, J.; Huang, L. NIR-II absorbing organic nanoagents for photoacoustic imaging and photothermal therapy. BMEMat 2023, 1, 12009. [Google Scholar] [CrossRef]
- Guo, X.; Yang, J.; Li, M.; Zhang, F.; Bu, W.; Li, H.; Wu, Q.; Yin, D.; Jiao, L.; Hao, E. Unique Double Intramolecular and Intermolecular Exciton Coupling in Ethene-Bridged aza-BODIPY Dimers for High-Efficiency Near-Infrared Photothermal Conversion and Therapy. Angew. Chem. Int. Ed. 2022, 61, 202211081. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Na, W.; Ge, W.; Huang, H.; Gao, F.; Zhong, L.; Zhao, Y.; Dong, X. Biodegradable Charge-Transfer Complexes for Glutathione Depletion Induced Ferroptosis and NIR-II Photoacoustic Imaging Guided Cancer Photothermal Therapy. Angew. Chem. Int. Ed. 2021, 60, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Bai, H.; Li, S.; Xiao, Y.; Cui, X.; Li, X.; Tan, J.; Huang, Z.; Shen, D.; Liu, W.; et al. Water-Soluble Organic Nanoparticles with Programable Intermolecular Charge Transfer for NIR-II Photothermal Anti-Bacterial Therapy. Angew. Chem. Int. Ed. 2021, 60, 11758–11762. [Google Scholar] [CrossRef]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window. Nano-Micro Lett. 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Yu, D.; Wang, M.; Wang, D.; Wang, B. Quinoid Conjugated Polymer Nanoparticles with NIR-II Absorption Peak Toward Efficient Photothermal Therapy. Chem. Eur. J. 2023, 29, 202202930. [Google Scholar] [CrossRef]
- Oliinyk, O.S.; Ma, C.; Pletnev, S.; Baloban, M.; Taboada, C.; Sheng, H.; Yao, J.; Verkhusha, V.V. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat. Methods 2023, 20, 70–74. [Google Scholar] [CrossRef]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, B.; Li, J.; Liu, Y.; Dong, J.; Peng, X.; Zhang, R. Multifunctional MnO2/Ag3SbS3 Nanotheranostic Agent for Single-Laser-Triggered Tumor Synergistic Therapy in the NIR-II Biowindow. ACS Appl. Mater. Interfaces 2022, 14, 4980–4994. [Google Scholar] [CrossRef]
- Shanmugam, M.; Kuthala, N.; Vankayala, R.; Chiang, C.-S.; Kong, X.; Hwang, K.C. Multifunctional CuO/Cu2O Truncated Nanocubes as Trimodal Image-Guided Near-Infrared-III Photothermal Agents to Combat Multi-Drug-Resistant Lung Carcinoma. ACS Nano 2021, 15, 14404–14418. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, M.; Lan, S.; Li, J.; Zhang, X.; Zhang, D.; Liu, X.; Liu, J. Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. 2018, 54, 13599–13602. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Chen, J.; Liu, S.; Deng, S.; Liu, C.; McCulloch, I.; Yue, W.; Cheng, D. Co-delivery of NIR-II semiconducting polymer and pH-sensitive doxorubicin-conjugated prodrug for photothermal/chemotherapy. Acta Biomater. 2022, 137, 238–251. [Google Scholar] [CrossRef]
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z.; et al. Rational Design of Conjugated Small Molecules for Superior Photothermal Theranostics in the NIR-II Biowindow. Adv. Mater. 2020, 32, 2001146. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Ma, D.; Zhang, X.; Xin, K.; Yang, Y.; Peng, X.; Xiao, Y. Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 nm Laser toward Deep-Seated Tumors. Small 2021, 17, 2100398. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, C.; Wang, X.; Yan, M.; Ling, Z.; Chen, Y.; Liu, Z. A Borondifluoride-Complex-Based Photothermal Agent with an 80 % Photothermal Conversion Efficiency for Photothermal Therapy in the NIR-II Window. Angew. Chem. Int. Ed. 2021, 60, 22376–22384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Z.; Liang, Z.; Wang, T.; Chen, Y.; Liu, Z. Discovery of BODIPY J-aggregates with absorption maxima beyond 1200 nm for biophotonics. Sci. Adv. 2022, 8, eadd5660. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Lu, S.; Wang, Z.; Liu, S.; Yu, X.; Li, X.; Sun, Y. Construction of emissive ruthenium(II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nat. Commun. 2022, 13, 2009. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.K.; Liu, W.; Brennan, S.P.; de Lourdes Betancourt-Mendiola, M.; Smith, B.D. Non-Covalent Assembly Method that Simultaneously Endows a Liposome Surface with Targeting Ligands, Protective PEG Chains, and Deep-Red Fluorescence Reporter Groups. Chem. Eur. J. 2017, 23, 12646–12654. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, H.; Liang, Y.; Dai, H. Rational Design of High Brightness NIR-II Organic Dyes with S-D-A-D-S Structure. Acc. Mater. Res. 2021, 2, 170–183. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, G.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, A.; Dai, R.; Li, Y.; Chen, X.; Qin, Y.; Ren, S.; Li, R.; Cheng, Z.; Zhang, R. A “Dual-Source, Dual-Activation” Strategy for an NIR-II Window Theranostic Nanosystem Enabling Optimal Photothermal-Ion Combination Therapy. Small 2022, 18, 2201179. [Google Scholar] [CrossRef]
- Li, C.; Jiang, G.; Yu, J.; Ji, W.; Liu, L.; Zhang, P.; Du, J.; Zhan, C.; Wang, J.; Tang, B.Z. Fluorination Enhances NIR-II Emission and Photothermal Conversion Efficiency of Phototheranostic Agents for Imaging-Guided Cancer Therapy. Adv. Mater. 2023, 35, 2208229. [Google Scholar] [CrossRef]
- Roy, S.; Bag, N.; Bardhan, S.; Hasan, I.; Guo, B. Recent progress in NIR-II fluorescence imaging-guided drug delivery for cancer theranostics. Adv. Drug Deliver. Rev. 2023, 197, 114821. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Shao, Y.; Zhao, Y.; Liu, Z. Complement-Opsonized NIR-IIb Emissive Immunotracers for Dynamically Monitoring Neutrophils in Inflammation-Related Diseases. Adv. Mater. 2022, 34, 2203477. [Google Scholar] [CrossRef]
- Yuan, Y.; Feng, Z.; Li, S.; Huang, Z.; Wan, Y.; Cao, C.; Lin, S.; Wu, L.; Zhou, J.; Liao, L.S.; et al. Molecular Programming of NIR-IIb-Emissive Semiconducting Small Molecules for In Vivo High-Contrast Bioimaging Beyond 1500 nm. Adv. Mater. 2022, 34, 2201263. [Google Scholar] [CrossRef]
- Wanderi, K.; Cui, Z. Organic fluorescent nanoprobes with NIR-IIb characteristics for deep learning. Exploration 2022, 2, 20210097. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, Y.; Zhao, Y.; Huang, W.; Zhang, F.; Zhu, S.; Wu, Q.; Fu, S.; Tang, B.Z.; Wang, D. Molecular engineering of AIE luminogens for NIR-II/IIb bioimaging and surgical navigation of lymph nodes. Matter 2022, 5, 2847–2863. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, X.; Zeng, S.; Chen, G. Clearable Shortwave-Infrared-Emitting NaErF4 Nanoparticles for Noninvasive Dynamic Vascular Imaging. Chem. Mater. 2020, 32, 3365–3375. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, D.; Gao, D.; Yi, Z.; Zheng, H.; Sheng, Z.; Liu, X. High-Specificity In Vivo Tumor Imaging Using Bioorthogonal NIR-IIb Nanoparticles. Adv. Mater. 2021, 33, 2102950. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Fan, Y.; El-Toni, A.M.; Alhoshan, M.S.; Li, D.; Zhang, F. In Vivo High-resolution Ratiometric Fluorescence Imaging of Inflammation Using NIR-II Nanoprobes with 1550 nm Emission. Nano Lett. 2019, 19, 2418–2427. [Google Scholar] [CrossRef]
- Bai, F.; Du, W.; Liu, X.; Su, L.; Li, Z.; Chen, T.; Ge, X.; Li, Q.; Yang, H.; Song, J. A NO-Responsive Ratiometric Fluorescent Nanoprobe for Monitoring Drug-Induced Liver Injury in the Second Near-Infrared Window. Anal. Chem. 2021, 93, 15279–15287. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, H.; Xie, X.; Wan, Y.; Li, Q.; Wu, F.; Yang, R.; Wang, W.; Kong, X. Bright Tm3+-based downshifting luminescence nanoprobe operating around 1800 nm for NIR-IIb and c bioimaging. Nat. Commun. 2023, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, B.; Wu, Y.; He, H.; Zhu, X.; Zhang, H.; Dou, C.; Feng, L.; Fan, Y.; Zhang, F. A Tumor-Microenvironment-Responsive Lanthanide-Cyanine FRET Sensor for NIR-II Luminescence-Lifetime In Situ Imaging of Hepatocellular Carcinoma. Adv. Mater. 2020, 32, 2001172. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, R.; Zhang, Y.; Liu, B.; Ramakrishna, S. Anionic benzothiadiazole containing polyfluorene and oligofluorene as organic sensitizers for dye-sensitized solar cells. Chem. Commun. 2008, 3789–3791. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, R.; Yang, W. Polymer Containing Heterocyclic Se Compound and Its Application in Preparing Luminous material. Chinese Patent CN1389488 A, 8 January 2003. [Google Scholar]
- Liang, P.; Tang, Q.; Cai, Y.; Liu, G.; Si, W.; Shao, J.; Huang, W.; Zhang, Q.; Dong, X. Self-quenched ferrocenyl diketopyrrolopyrrole organic nanoparticles with amplifying photothermal effect for cancer therapy. Chem. Sci. 2017, 8, 7457–7463. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Yang, L.; Ding, Y.; Zhu, J.-J. Highly Emissive Nd3+-Sensitized Multilayered Upconversion Nanoparticles for Efficient 795 nm Operated Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 4778–4785. [Google Scholar] [CrossRef]
- He, J.; Ai, L.; Liu, X.; Huang, H.; Li, Y.; Zhang, M.; Zhao, Q.; Wang, X.; Chen, W.; Gu, H. Plasmonic CuS nanodisk assembly based composite nanocapsules for NIR-laser-driven synergistic chemo-photothermal cancer therapy. J. Mater. Chem. B 2018, 6, 1035–1043. [Google Scholar] [CrossRef]
- Tong, S.; Hou, S.; Ren, B.; Zheng, Z.; Bao, G. Self-Assembly of Phospholipid–PEG Coating on Nanoparticles through Dual Solvent Exchange. Nano Lett. 2011, 11, 3720–3726. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, M.; He, Z.; Zhao, Y.; Wang, J.; Lu, Y. Photoactive Oligomer with an Acceptor–Donor–Acceptor-Conjugated Structure for Single Near-Infrared Light-Triggered Photothermal/Photodynamic Synergistic Therapy of Tumors. ACS Appl. Polym. Mater. 2023, 5, 1530–1538. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, Q.; Song, J.; Li, S.; Li, X.-L.; Kang, B.K.; Chen, H.-Y.; Xu, J.-J. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. Int. Ed. 2023, 62, 202213922. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, W.; Sun, W.; Du, J.; Fan, J.; Peng, X. Highly Inoxidizable Heptamethine Cyanine–Glucose Oxidase Conjugate Nanoagent for Combination of Enhanced Photothermal Therapy and Tumor Starvation. Adv. Funct. Mater. 2022, 32, 2111853. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, X.; Tang, Y.; Xiong, Y.; Wang, X.; Li, C.; Xiao, T.; Lu, F.; Xu, M. High-Performance Hybrid Phototheranostics for NIR-IIb Fluorescence Imaging and NIR-II-Excitable Photothermal Therapy. Pharmaceutics 2023, 15, 2027. https://doi.org/10.3390/pharmaceutics15082027
Wang Q, Zhang X, Tang Y, Xiong Y, Wang X, Li C, Xiao T, Lu F, Xu M. High-Performance Hybrid Phototheranostics for NIR-IIb Fluorescence Imaging and NIR-II-Excitable Photothermal Therapy. Pharmaceutics. 2023; 15(8):2027. https://doi.org/10.3390/pharmaceutics15082027
Chicago/Turabian StyleWang, Qi, Xinmin Zhang, Youguang Tang, Yanwei Xiong, Xu Wang, Chunlai Li, Tangxin Xiao, Feng Lu, and Mengze Xu. 2023. "High-Performance Hybrid Phototheranostics for NIR-IIb Fluorescence Imaging and NIR-II-Excitable Photothermal Therapy" Pharmaceutics 15, no. 8: 2027. https://doi.org/10.3390/pharmaceutics15082027
APA StyleWang, Q., Zhang, X., Tang, Y., Xiong, Y., Wang, X., Li, C., Xiao, T., Lu, F., & Xu, M. (2023). High-Performance Hybrid Phototheranostics for NIR-IIb Fluorescence Imaging and NIR-II-Excitable Photothermal Therapy. Pharmaceutics, 15(8), 2027. https://doi.org/10.3390/pharmaceutics15082027






