Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases
Abstract
1. Introduction
2. Retinal Diseases and Current Treatment Strategies
3. Nanomedicine Approaches for Retinal Diseases
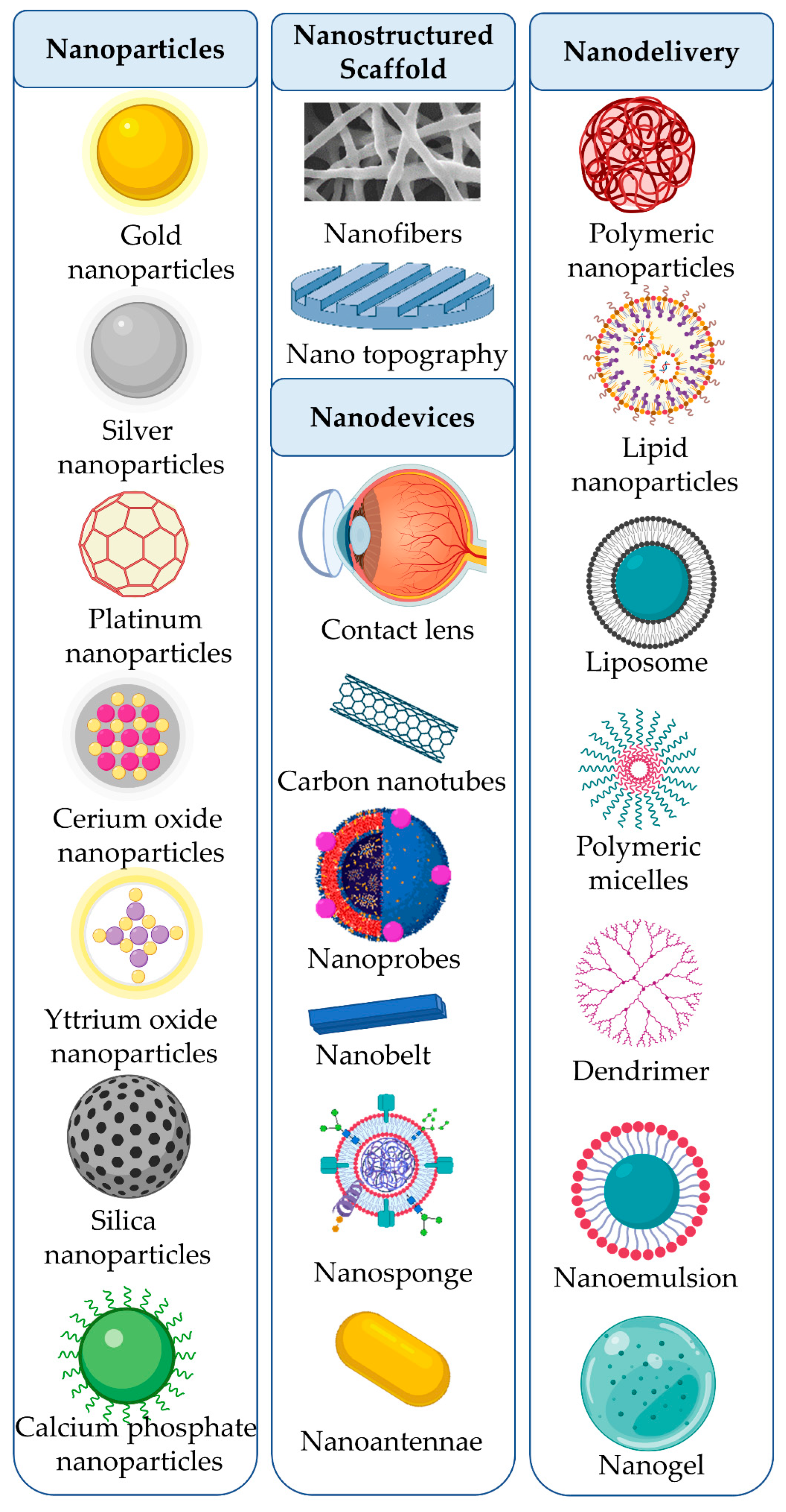
3.1. Nanoparticles
3.1.1. Metallic Nanoparticles
3.1.2. Non-Metallic Nanoparticles
3.2. Nanostructured Scaffolds
3.2.1. Nanofibers
3.2.2. Nanotopography
3.3. Nanodevices
3.4. Nanostructure-Based Drug Delivery Systems
3.4.1. Polymeric Nanoparticles
3.4.2. Lipid Nanoparticles
3.4.3. Liposomes
3.4.4. Polymeric Micelles
3.4.5. Dendrimers
3.4.6. Nanoemulsions
3.4.7. Nanogels
4. The Future of Nanomedicines in the Management of Retinal Diseases
4.1. Overcoming Blood–Ocular Barriers
4.2. Cell-Specific Delivery in the Retina
4.3. Safety and Efficacy
4.4. Drug Delivery Systems
4.5. Gene Therapy
4.6. Precision Nanomedicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Drug delivery to the retina: Challenges and opportunities. Expert Opin. Biol. Ther. 2003, 3, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Khanal, S.; Tan, H.S.; Thapa, S.S.; van Rens, G.H.M.B. Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study. Clin. Ophthalmol. 2020, 14, 2109. [Google Scholar]
- Mahaling, B.; Low, S.W.Y.; Beck, M.; Kumar, D.; Ahmed, S.; Connor, T.B.; Ahmad, B.; Chaurasia, S.S. Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders. Int. J. Mol. Sci. 2022, 23, 2591. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Rosenblatt, T.R.; Vail, D.; Saroj, N.; Boucher, N.; Moshfeghi, D.M.; Moshfeghi, A.A. Increasing Incidence and Prevalence of Common Retinal Diseases in Retina Practices Across the United States. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Mahaling, B.; Srinivasarao, D.A.; Raghu, G.; Kasam, R.K.; Bhanuprakash Reddy, G.; Katti, D.S. A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale 2018, 10, 16485–16498. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Fong, D.S.; Girach, A.; Boney, A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: A literature review. Retina 2007, 27, 816–824. [Google Scholar] [CrossRef]
- Schachat, A.P.; Oyakawa, R.T.; Michels, R.G.; Rice, T.A. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology 1983, 90, 522–530. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Mandal, K. Treatment of retinopathy of prematurity. Early Hum. Dev. 2008, 84, 95–99. [Google Scholar] [CrossRef]
- Honda, S.; Hirabayashi, H.; Tsukahara, Y.; Negi, A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221. [Google Scholar] [CrossRef]
- Wubben, T.J.; Besirli, C.G.; Zacks, D.N. Pharmacotherapies for Retinal Detachment. Ophthalmology 2016, 123, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Fahim, A. Retinitis pigmentosa: Recent advances and future directions in diagnosis and management. Curr. Opin. Pediatr. 2018, 30, 725–733. [Google Scholar] [CrossRef]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Mahaling, B.; Baruah, N.; Ahamad, N.; Maisha, N.; Lavik, E.; Katti, D.S. A non-invasive nanoparticle-based sustained dual-drug delivery system as an eyedrop for endophthalmitis. Int. J. Pharm. 2021, 606, 120900. [Google Scholar] [CrossRef]
- Mester, U.; Volker, B.; Kroll, P.; Berg, P. Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2000 eyes. Ophthalmic Surg. 1988, 19, 482–484. [Google Scholar] [CrossRef]
- Retinitis Pigmentosa|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinitis-pigmentosa (accessed on 14 March 2022).
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Laradji, A.; Karakocak, B.B.; Kolesnikov, A.V.; Kefalov, V.J.; Ravi, N. Hyaluronic Acid-Based Gold Nanoparticles for the Topical Delivery of Therapeutics to the Retina and the Retinal Pigment Epithelium. Polymers 2021, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Kim, K.W.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar] [CrossRef]
- Chan, C.M.; Hsiao, C.Y.; Li, H.J.; Fang, J.Y.; Chang, D.C.; Hung, C.F. The Inhibitory Effects of Gold Nanoparticles on VEGF-A-Induced Cell Migration in Choroid-Retina Endothelial Cells. Int. J. Mol. Sci. 2019, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.B.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Kalishwaralal, K.; Venkataraman, D.; Eom, S.H.; Park, J.; Gurunathan, S. Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009, 7, 8. [Google Scholar] [CrossRef]
- Clark, A.; Zhu, A.; Sun, K.; Petty, H.R. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J. Nanopart. Res. 2011, 13, 5547. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Mitra, R.N.; Merwin, M.J.; Han, Z.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free Radic. Biol. Med. 2014, 75, 140–148. [Google Scholar] [CrossRef]
- Yanai, A.; Häfeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Giannini, M.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A.; Dente, L.; Raffa, V. Magnetic nanoparticles as intraocular drug delivery system to target retinal pigmented epithelium (RPE). Int. J. Mol. Sci. 2014, 15, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Tabatabaei, M.S.; Girouard, H.; Martel, S. Hyperthermia of magnetic nanoparticles allows passage of sodium fluorescein and Evans blue dye across the blood-retinal barrier. Int. J. Hyperth. 2016, 32, 657–665. [Google Scholar] [CrossRef]
- Li, H.; Ma, M.; Zhang, J.; Hou, W.; Chen, H.; Zeng, D.; Wang, Z. Ultrasound-Enhanced Delivery of Doxorubicin-Loaded Nanodiamonds from Pullulan-all-trans-Retinal Nanoparticles for Effective Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20341–20349. [Google Scholar] [CrossRef]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomed. 2018, 13, 4379–4389. [Google Scholar] [CrossRef]
- Shimanovskaya, E.V.; Nikol’skaya, I.I.; Binevskii, P.V.; Beznos, O.V.; Klyachko, N.L.; Pavlenko, T.A.; Chesnokova, N.B.; Kost, O.A. Lisinopril in the composition of calcium phosphate nanoparticles as a promising antiglaucoma agent. Nanotechnol. Russ. 2014, 9, 219–226. [Google Scholar] [CrossRef]
- Warnke, P.H.; Alamein, M.; Skabo, S.; Stephens, S.; Bourke, R.; Heiner, P.; Liu, Q. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013, 9, 9414–9422. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, B.; Liu, X.; Li, X.; Zeng, C.; Shi, H.; Xu, X.; Lin, T.; Dai, L.; Liu, Y. Aligned Nanofibers from Polypyrrole/Graphene as Electrodes for Regeneration of Optic Nerve via Electrical Stimulation. ACS Appl. Mater. Interfaces 2016, 8, 6834–6840. [Google Scholar] [CrossRef]
- Phelan, M.A.; Kruczek, K.; Wilson, J.H.; Brooks, M.J.; Drinnan, C.T.; Regent, F.; Gerstenhaber, J.A.; Swaroop, A.; Lelkes, P.I.; Li, T. Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Tissue Eng. Part C Methods 2020, 26, 433–446. [Google Scholar] [CrossRef]
- Piret, G.; Perez, M.T.; Prinz, C.N. Neurite outgrowth and synaptophysin expression of postnatal CNS neurons on GaP nanowire arrays in long-term retinal cell culture. Biomaterials 2013, 34, 875–887. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef]
- Johnen, S.; Meißner, F.; Krug, M.; Baltz, T.; Endler, I.; Mokwa, W.; Walter, P. Properties of Retinal Precursor Cells Grown on Vertically Aligned Multiwalled Carbon Nanotubes Generated for the Modification of Retinal Implant-Embedded Microelectrode Arrays. J. Ophthalmol. 2016, 2016, 2371021. [Google Scholar] [CrossRef] [PubMed]
- Watterson, W.J.; Moslehi, S.; Rowland, C.; Zappitelli, K.M.; Smith, J.H.; Miller, D.; Chouinard, J.E.; Golledge, S.L.; Taylor, R.P.; Perez, M.T.; et al. The Roles of an Aluminum Underlayer in the Biocompatibility and Mechanical Integrity of Vertically Aligned Carbon Nanotubes for Interfacing with Retinal Neurons. Micromachines 2020, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Peng, Y.; Ge, C.; Bu, Y.; Liu, H.; Huang, H.; Ou, S.; Xue, Y.; Dai, L. A turn-on fluorescent lysine nanoprobe based on the use of the Alizarin Red aluminum(III) complex conjugated to graphene oxide, and its application to cellular imaging of lysine. Microchim. Acta 2017, 184, 3521–3528. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, A.; Song, W.; Wang, M.; Gu, X.; Zhang, Y.; Yu, M.; Zhao, X.; Dai, M.; Yan, L.; et al. Biocompatible single-crystal selenium nanobelt based nanodevice as a temperature-tunable photosensor. J. Nanomater. 2012, 2012, 384671. [Google Scholar] [CrossRef]
- Coburn, P.S.; Miller, F.C.; LaGrow, A.L.; Land, C.; Mursalin, H.; Livingston, E.; Amayem, O.; Chen, Y.; Gao, W.; Zhang, L.; et al. Disarming Pore-Forming Toxins with Biomimetic Nanosponges in Intraocular Infections. mSphere 2019, 4, e00262-19. [Google Scholar] [CrossRef]
- LaGrow, A.L.; Coburn, P.S.; Miller, F.C.; Land, C.; Parkunan, S.M.; Luk, B.T.; Gao, W.; Zhang, L.; Callegan, M.C. A Novel Biomimetic Nanosponge Protects the Retina from the Enterococcus faecalis Cytolysin. mSphere 2017, 2, e00335-17. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bao, J.; Zhang, Y.; Li, Z.; Zhou, X.; Wan, C.; Huang, L.; Zhao, Y.; Han, G.; Xue, T. Mammalian Near-Infrared Image Vision through Injectable and Self-Powered Retinal Nanoantennae. Cell 2019, 177, 243–255.e15. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, X.; Hirano, Y.; Tyagi, P.; Barabás, P.; Uehara, H.; Miya, T.R.; Singh, N.; Archer, B.; Qazi, Y.; et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano 2013, 7, 3264–3275. [Google Scholar] [CrossRef]
- Marano, R.J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005, 12, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.G.; Augustine, R.; Zhang, Y.; Hong, S.C.; Kim, I. Synthesis of Stimuli-Responsive Heterofunctional Dendrimer by Passerini Multicomponent Reaction. ACS Omega 2019, 4, 6660–6668. [Google Scholar] [CrossRef]
- Iwase, T.; Fu, J.; Yoshida, T.; Muramatsu, D.; Miki, A.; Hashida, N.; Lu, L.; Oveson, B.; Lima E Silva, R.; Seidel, C.; et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J. Control. Release 2013, 172, 625–633. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005, 511, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhukova, V.; Semyonkin, A.; Osipova, N.; Malinovskaya, Y.; Maksimenko, O.; Chernikov, V.; Sokolov, M.; Grigartzik, L.; Sabel, B.A.; et al. Release kinetics of fluorescent dyes from PLGA nanoparticles in retinal blood vessels: In vivo monitoring and ex vivo localization. Eur. J. Pharm. Biopharm. 2020, 150, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.K.; Durairaj, C.; Kompella, U.B.; Agwu, O.; Oliver, S.C.N.; Quiroz-Mercado, H.; Mandava, N.; Olson, J.L. Comparison of Long-Acting Bevacizumab Formulations in the Treatment of Choroidal Neovascularization in a Rat Model. J. Ocul. Pharmacol. Ther. 2011, 27, 219. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, Y.; Li, F.H.; Du, L.F.; Duan, Y.R. Enhanced delivery of biodegradable mPEG-PLGA-PLL nanoparticles loading Cy3-labelled PDGF-BB siRNA by UTMD to rat retina. J. Biosci. 2017, 42, 299–309. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wang, A.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Clunies-Ross, A.J.M.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Rodríguez, A.; Delgado, D.; Gascón, A.R.; Solinís, M.Á. Lipid nanoparticles as drug/gene delivery systems to the retina. J. Ocul. Pharmacol. Ther. 2013, 29, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Apaolaza, P.S.; del Pozo-Rodríguez, A.; Solinís, M.A.; Rodríguez, J.M.; Friedrich, U.; Torrecilla, J.; Weber, B.H.F.; Rodríguez-Gascón, A. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef]
- Amadio, M.; Pascale, A.; Cupri, S.; Pignatello, R.; Osera, C.; D’Agata, V.; D’Amico, A.G.; Leggio, G.M.; Ruozi, B.; Govoni, S.; et al. Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat. Pharmacol. Res. 2016, 111, 713–720. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Control. Release 2019, 298, 177–185. [Google Scholar] [CrossRef]
- Nahire, R.; Haldar, M.K.; Paul, S.; Mergoum, A.; Ambre, A.H.; Katti, K.S.; Gange, K.N.; Srivastava, D.K.; Sarkar, K.; Mallik, S. Polymer-coated echogenic lipid nanoparticles with dual release triggers. Biomacromolecules 2013, 14, 841–853. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Tozuka, Y.; Shimazawa, M.; Hara, H.; Takeuchi, H. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release 2009, 136, 247–253. [Google Scholar] [CrossRef]
- Shimazaki, H.; Hironaka, K.; Fujisawa, T.; Tsuruma, K.; Tozuka, Y.; Shimazawa, M.; Takeuchi, H.; Hara, H. Edaravone-Loaded Liposome Eyedrops Protect against Light-Induced Retinal Damage in Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7289–7297. [Google Scholar] [CrossRef]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Matsuo, T.; Masuda, I.; Yasuda, T.; Matsuo, N. Gene transfer to the retina of rat by liposome eye drops. Biochem. Biophys. Res. Commun. 1996, 219, 947–950. [Google Scholar] [CrossRef]
- Cholkar, K.; Gunda, S.; Earla, R.; Pal, D.; Mitra, A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech 2015, 16, 610. [Google Scholar] [CrossRef]
- Iriyama, A.; Oba, M.; Ishii, T.; Nishiyama, N.; Kataoka, K.; Tamaki, Y.; Yanagi, Y. Gene Transfer Using Micellar Nanovectors Inhibits Choroidal Neovascularization In Vivo. PLoS ONE 2011, 6, 28560. [Google Scholar] [CrossRef]
- Ma, F.; Nan, K.; Lee, S.; Beadle, J.R.; Hou, H.; Freeman, W.R.; Hostetler, K.Y.; Cheng, L. Micelle formulation of hexadecyloxypropyl-cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015, 89, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Pan, H.; Liu, L.; Ji, M.; Sheng, N.; Wang, C.; Cai, L.; Ma, Y. Retinal-conjugated pH-sensitive micelles induce tumor senescence for boosting breast cancer chemotherapy. Biomaterials 2016, 83, 219–232. [Google Scholar] [CrossRef]
- Osipova, O.; Sharoyko, V.; Zashikhina, N.; Zakharova, N.; Tennikova, T.; Urtti, A.; Korzhikova-Vlakh, E. Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. Triamcinolone Acetonide-Loaded PEGylated Microemulsion for the Posterior Segment of Eye. ACS Omega 2020, 5, 7928–7939. [Google Scholar] [CrossRef]
- Huang, L.; Liang, W.; Zhou, K.; Wassel, R.A.; Ridge, Z.D.; Ma, J.X.; Wang, B. Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization. Biology 2021, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Hagigit, T.; Abdulrazik, M.; Valamanesh, F.; Behar-Cohen, F.; Benita, S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: An in-vivo study in rats and mice. J. Control. Release 2012, 160, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lin, S.; Zhang, Z.; Hu, J.; Liu, G.; Tu, Y.; Yang, Y.; Zou, H.; Mo, Y.; Miao, L. pH-responsive nanoemulsions for controlled drug release. Biomacromolecules 2014, 15, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Laradji, A.M.; Kolesnikov, A.V.; Karakoçak, B.B.; Kefalov, V.J.; Ravi, N. Redox-Responsive Hyaluronic Acid-Based Nanogels for the Topical Delivery of the Visual Chromophore to Retinal Photoreceptors. ACS Omega 2021, 6, 6172–6184. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Omar, M.M.; El Sisi, A.M. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int. J. Nanomed. 2019, 14, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, S.N.; Chaudhary, V.; Garg, S.J. Complications of intravitreal injections: 2022. Curr. Opin. Ophthalmol. 2022, 33, 137–146. [Google Scholar] [CrossRef]
- Sharma, A.; Tandon, A.; Tovey, J.C.K.; Gupta, R.; Robertson, J.D.; Fortune, J.A.; Klibanov, A.M.; Cowden, J.W.; Rieger, F.G.; Mohan, R.R. Polyethylenimine-Conjugated Gold Nanoparticles: Gene Transfer Potential and Low Toxicity in the Cornea. Pharm. Fac. Artic. Res. 2011, 7, 505–513. [Google Scholar] [CrossRef]
- Sun, J.G.; Jiang, Q.; Zhang, X.P.; Shan, K.; Liu, B.H.; Zhao, C.; Yan, B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef]
- Mahaling, B.; Verma, M.; Mishra, G.; Chaudhuri, S.; Dutta, D.; Sivakumar, S. Fate of GdF3 nanoparticles-loaded PEGylated carbon capsules inside mice model: A step toward clinical application. Nanotoxicology 2020, 14, 577–594. [Google Scholar] [CrossRef]
- Chu, T.C.; He, Q.; Potter, D.E. Biodegradable calcium phosphate nanoparticles as a new vehicle for delivery of a potential ocular hypotensive agent. J. Ocul. Pharmacol. Ther. 2002, 18, 507–514. [Google Scholar] [CrossRef]
- Rajendran Nair, D.S.; Seiler, M.J.; Patel, K.H.; Thomas, V.; Camarillo, J.C.M.; Humayun, M.S.; Thomas, B.B. Tissue Engineering Strategies for Retina Regeneration. Appl. Sci. 2021, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Chan, B.Q.Y.; Liu, Z.; Parikh, B.H.; Zhang, K.; Lin, Q.; Su, X.; Kai, D.; Choo, W.S.; Young, D.J.; et al. Electrospun pectin-polyhydroxybutyrate nanofibers for retinal tissue engineering. ACS Omega 2017, 2, 8959–8968. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Zhu, W.; Zhong, Z.; Moran, A.; Wang, W.; Zhang, K.; Chen, S. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting 2018, 11, e00029. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chuang, J.H.; Buddhakosai, W.; Wu, W.J.; Lee, C.J.; Chen, W.S.; Yang, Y.P.; Li, M.C.; Peng, C.H.; Chen, S.J. Elongation of Axon Extension for Human iPSC-Derived Retinal Ganglion Cells by a Nano-Imprinted Scaffold. Int. J. Mol. Sci. 2017, 18, 2013. [Google Scholar] [CrossRef]
- Wang, D.; Luo, M.; Huang, B.; Gao, W.; Jiang, Y.; Li, Q.; Nan, K.; Lin, S. Localized co-delivery of CNTF and FK506 using a thermosensitive hydrogel for retina ganglion cells protection after traumatic optic nerve injury. Drug Deliv. 2020, 27, 556. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Fabrication of micro-structures of poly [(R)-3-hydroxybutyric acid] by electro-spraying/-spinning: Understanding the influence of polymer concentration and solvent type. J. Mater. Sci. 2014, 49, 4246–4260. [Google Scholar] [CrossRef]
- Cellot, G.; La Monica, S.; Scaini, D.; Rauti, R.; Bosi, S.; Prato, M.; Gandolfi, S.; Ballerini, L. Successful regrowth of retinal neurons when cultured interfaced to carbon nanotube platforms. J. Biomed. Nanotechnol. 2017, 13, 559–565. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in ocular drug delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.; Verestiuc, L.; Nevell, T.G.; Tsibouklis, J. Polymeric materials for ophthalmic drug delivery: Trends and perspectives. J. Mater. Chem. 2006, 16, 3439–3443. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Colucci, P.; Giannaccini, M.; Baggiani, M.; Kennedy, B.N.; Dente, L.; Raffa, V.; Gabellini, C. Neuroprotective Nanoparticles Targeting the Retina: A Polymeric Platform for Ocular Drug Delivery Applications. Pharmaceutics 2023, 15, 1096. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Suri, R.; Nag, T.C.; Mehra, N.; Neupane, Y.R.; Shafi, S.; Sharma, D.; Sharma, K.; Sultana, Y.; Kohli, K. Sirolimus Loaded Chitosan Functionalized PLGA Nanoparticles Protect Against Sodium Iodate-Induced Retinal Degeneration. J. Drug Deliv. Sci. Technol. 2023, 82, 104369. [Google Scholar] [CrossRef]
- Radwan, S.E.S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef]
- Chittasupho, C.; Kengtrong, K.; Chalermnithiwong, S.; Sarisuta, N. Anti-angiogenesis by dual action of R5K peptide conjugated itraconazole nanoparticles. AAPS PharmSciTech 2020, 21, 74. [Google Scholar] [CrossRef]
- Lou, X.; Hu, Y.; Zhang, H.; Liu, J.; Zhao, Y. Polydopamine nanoparticles attenuate retina ganglion cell degeneration and restore visual function after optic nerve injury. J. Nanobiotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Delrish, E.; Ghassemi, F.; Jabbarvand, M.; Lashay, A.; Atyabi, F.; Soleimani, M.; Dinarvand, R. Biodistribution of Cy5-labeled Thiolated and Methylated Chitosan-Carboxymethyl Dextran Nanoparticles in an Animal Model of Retinoblastoma. J. Ophthalmic Vis. Res. 2022, 17, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2149–2160. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.H.; Ji, T.; Mehta, M.; Wang, W.; Marino, E.; Chen, J.; Kohane, D.S. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed. Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez de Lara, M.J.; Singh, M.; Kaur, I.P. Correction to: Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 1531. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.Á.; Pedraz, J.L.; Echevarría, E.; Rodríguez, J.M.; Gascón, A.R. Solid lipid nanoparticles as potential tools for gene therapy: In vivo protein expression after intravenous administration. Int. J. Pharm. 2010, 385, 157–162. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Gu, Z.; Chen, Z.; Li, H.; Cheng, Z.; Li, H.; Zou, L. Co-delivery of microRNA-150 and quercetin by lipid nanoparticles (LNPs) for the targeted treatment of age-related macular degeneration (AMD). J. Control. Release 2023, 355, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Upadhya, A.; Tripathi, S.; Dighe, V.; Singh, K. Liposome-polyethylenimine complexes for the effective delivery of HuR siRNA in the treatment of diabetic retinopathy. Drug Deliv. Transl. Res. 2023, 13, 1675–1698. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Kumar, B.; Chitranshi, N.; Gupta, V.K.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; Vishwas, S.; et al. Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: A new dimension to an anticancer drug. Int. J. Biol. Macromol. 2023, 224, 810–830. [Google Scholar] [CrossRef]
- Christensen, G.; Urimi, D.; Lorenzo-Soler, L.; Schipper, N.; Paquet-Durand, F. Ocular permeability, intraocular biodistribution of lipid nanocapsule formulation intended for retinal drug delivery. Eur. J. Pharm. Biopharm. 2023, 187, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Himawan, E.; Belhadj, S.; Pérez García, R.O.; Paquet Durand, F.; Schipper, N.; Buzgo, M.; Simaite, A.; Marigo, V. Efficient Delivery of Hydrophilic Small Molecules to Retinal Cell Lines Using Gel Core-Containing Solid Lipid Nanoparticles. Pharmaceutics 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Wang, K.; Zhu, X.; Tharkar, P.; Shu, W.; Zhang, T.; Zeng, S.; Zhu, L.; Murray, M.; et al. Compritol solid lipid nanoparticle formulations enhance the protective effect of betulinic acid derivatives in human Müller cells against oxidative injury. Exp. Eye Res. 2022, 215, 108906. [Google Scholar] [CrossRef]
- Gautam, M.; Ryals, R.C.; Sahay, G. Novel lipid nanoparticle variants for in-vivo mRNA delivery to photoreceptors. Investig. Ophthalmol. Vis. Sci. 2022, 63, 69-A0042. [Google Scholar]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Cai, D.; Guo, Q.; Wang, J.; Lei, L.; Li, X.; Shi, S. Penetrating-peptide-mediated non-invasive Axitinib delivery for anti-neovascularisation. J. Control. Release 2022, 347, 449–459. [Google Scholar] [CrossRef]
- Urquhart, A.J.; Eriksen, A.Z. Recent developments in liposomal drug delivery systems for the treatment of retinal diseases. Drug Discov. Today 2019, 24, 1660–1668. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef]
- Ribeiro, M.C.S.; de Miranda, M.C.; Cunha, P.d.S.; Andrade, G.F.; Fulgêncio, G.d.O.; Gomes, D.A.; Fialho, S.L.; Pittella, F.; Charrueau, C.; Escriou, V.; et al. Neuroprotective effect of siRNA entrapped in hyaluronic acid-coated lipoplexes by intravitreal administration. Pharmaceutics 2021, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Normando, E.M.; Davis, B.; Brenton, J.; Guo, L.; Cordeiro, M.F. Retinal Neuroprotective Effect of a Liposomal Formulation of Rosiglitazone in a Rat Model of Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4406. [Google Scholar]
- Fujisawa, T.; Miyai, H.; Hironaka, K.; Tsukamoto, T.; Tahara, K.; Tozuka, Y.; Ito, M.; Takeuchi, H. Liposomal diclofenac eye drop formulations targeting the retina: Formulation stability improvement using surface modification of liposomes. Int. J. Pharm. 2012, 436, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.; Chen, Y.; Urimi, D.; Zizmare, L.; Trautwein, C.; Schipper, N.; Paquet-Durand, F. Pyruvate-conjugation of PEGylated liposomes for targeted drug delivery to retinal photoreceptors. Biomed. Pharmacother. 2023, 163, 114717. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, H.; Liu, C.; Wang, C.; Zeng, X.; Yan, J.; Sun, Y. Efficiency co-delivery of ellagic acid and oxygen by a non-invasive liposome for ameliorating diabetic retinopathy. Int. J. Pharm. 2023, 641, 122987. [Google Scholar] [CrossRef]
- Nishida, S.; Takashima, Y.; Udagawa, R.; Ibaraki, H.; Seta, Y.; Ishihara, H. A Multifunctional Hybrid Nanocarrier for Non-Invasive siRNA Delivery to the Retina. Pharmaceutics 2023, 15, 611. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Puranen, J.; Bahrpeyma, S.; Lautala, V.E.; Karumo, S.; Lajunen, T.; del Amo, E.M.; Ruponen, M.; Urtti, A. Liposomal sunitinib for ocular drug delivery: A potential treatment for choroidal neovascularization. Int. J. Pharm. 2022, 620, 121725. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Li, J.; Cheng, Y.; Zhang, X.; Qu, Y. Triamcinolone acetonide-chitosan coated liposomes efficiently treated retinal edema as eye drops. Exp. Eye Res. 2019, 188, 107805. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Managit, C.; Phanaksri, T.; Treesuppharat, W.; Fuongfuchat, A. Formulation development and in vitro evaluation of transferrin-conjugated liposomes as a carrier of ganciclovir targeting the retina. Int. J. Pharm. 2020, 577, 119084. [Google Scholar] [CrossRef]
- Lee, J.; Goh, U.; Lee, H.J.; Kim, J.; Jeong, M.; Park, J.H. Effective retinal penetration of lipophilic and lipid-conjugated hydrophilic agents delivered by engineered liposomes. Mol. Pharm. 2017, 14, 423–430. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Arnarson, T.; Elworthy, P.H. Effects of structural variations of non-ionic surfactants on micellar properties and solubilization: Surfactants based on erucyl and behenyl (C22) alcohols. J. Pharm. Pharmacol. 2011, 32, 381–385. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.F.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-Crosslinked Polymeric Micelles: Principles, Preparation, Biomedical Applications and Clinical Translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Kabanov, V.A. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjug. Chem. 1995, 6, 7–20. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Ke, L.; Wang, L.J.; Wu, C.; Li, C.; Li, Z.; Wu, Y.L. Flexible polymeric nanosized micelles for ophthalmic drug delivery: Research progress in the last three years. Nanoscale Adv. 2021, 3, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, W.; Wang, B.; Wang, X.; Liu, G.; Tao, Y.; Qi, R. Efficient siRNA Delivery Using PEG-conjugated PAMAM Dendrimers Targeting Vascular Endothelial Growth Factor in a CoCl2-induced Neovascularization Model in Retinal Endothelial Cells. Curr. Drug Deliv. 2016, 13, 590–599. [Google Scholar] [CrossRef]
- Aurelia Chis, A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Yavuz, B.; Bozdaǧ Pehlivan, S.; Ünlü, N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013, 2013, 13. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. PEGylated microemulsion for dexamethasone delivery to posterior segment of eye. J. Biomater. Sci. Polym. Ed. 2020, 31, 1071–1090. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turkish J. Pharm. Sci. 2021, 18, 652. [Google Scholar] [CrossRef]
- Wu, Y.; Tao, Q.; Xie, J.; Lu, L.; Xie, X.; Zhang, Y.; Jin, Y. Advances in Nanogels for Topical Drug Delivery in Ocular Diseases. Gels 2023, 9, 292. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, Y.; Feng, W.; Yang, M.; Shao, B.; Li, J.; Ye, F. NIR-triggered upconversion nanoparticles@thermo-sensitive liposome hybrid theranostic nanoplatform for controlled drug delivery. RSC Adv. 2021, 11, 29065–29072. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Sun, J.; Zi, P.; Jin, X.; Zhang, C. Thermosensitive micelles-hydrogel hybrid system based on poloxamer 407 for localized delivery of paclitaxel. J. Pharm. Sci. 2013, 102, 2707–2717. [Google Scholar] [CrossRef]
- Ito, T.; Yoshida, C.; Murakami, Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng. C 2013, 33, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.G.; Liu, D.Q.; Yu, X.L.; Zhang, L.X.; Zhu, J.; Lu, S.; Liu, R.T. A Conditionally Releasable “Do not Eat Me” CD47 Signal Facilitates Microglia-Targeted Drug Delivery for the Treatment of Alzheimer’s Disease. Adv. Funct. Mater. 2020, 30, 1910691. [Google Scholar] [CrossRef]
- Montes-Cobos, E.; Ring, S.; Fischer, H.J.; Heck, J.; Strauß, J.; Schwaninger, M.; Reichardt, S.D.; Feldmann, C.; Lühder, F.; Reichardt, H.M. Targeted delivery of glucocorticoids to macrophages in a mouse model of multiple sclerosis using inorganic-organic hybrid nanoparticles. J. Control. Release 2017, 245, 157–169. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller cell alterations during retinal degeneration in a transgenic rat model of retinitis pigmentosa. Front. Cell. Neurosci. 2015, 9, 484. [Google Scholar] [CrossRef]
- Jimnez, J.L.; Clemente, M.I.; Weber, N.D.; Sanchez, J.; Ortega, P.; De La Mata, F.J.; Gmez, R.; Garca, D.; Lpez-Fernndez, L.A.; Muoz-Fernndez, M.N. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs 2010, 24, 331–343. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef]
- Glassman, P.M.; Myerson, J.W.; Ferguson, L.T.; Kiseleva, R.Y.; Shuvaev, V.V.; Brenner, J.S.; Muzykantov, V.R. Targeting drug delivery in the vascular system: Focus on endothelium. Adv. Drug Deliv. Rev. 2020, 157, 96–117. [Google Scholar] [CrossRef]
- Bourges, J.L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.C.; Gurny, R.; BenEzra, D.; Behar-Cohen, F.F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, L.; Ruhanen, E.; Kinnarinen, T.; Rönkkö, S.; Pelkonen, J.; Urtti, A.; Peltonen, S.; Järvinen, K. Starch acetate microparticles for drug delivery into retinal pigment epithelium—In vitro study. J. Control. Release 2004, 98, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, G.; Li, J.; Fu, Y.; Mavlyutov, T.A.; Yao, A.; Nickells, R.W.; Gong, S.; Guo, L.W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release 2017, 247, 153–166. [Google Scholar] [CrossRef]
- Farjo, R.; Skaggs, J.; Quiambao, A.B.; Cooper, M.J.; Naash, M.I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 2006, 1, e38. [Google Scholar] [CrossRef]
- Patel, D.M.; Patel, N.N.; Patel, J.K. Nanomedicine scale-up technologies: Feasibilities and challenges. In Emerging Technologies for Nanoparticle Manufacturing; Springer International Publishing: Cham, Switzerland, 2021; pp. 511–539. [Google Scholar] [CrossRef]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.Q.; Sun, W.; Naderi, A.; Kern, T.; Palczewski, K.; Lu, Z.R. Stable Retinoid Analogue Targeted Dual pH-Sensitive Smart Lipid ECO/ pDNA Nanoparticles for Specific Gene Delivery in the Retinal Pigment Epithelium. ACS Appl. Bio Mater. 2020, 3, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Tajima, S.; Takeoka, S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J. Control. Release 2010, 142, 267–276. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.K.; Sharma, A.; Tandon, A. Gene therapy in the Cornea: 2005–present. Prog. Retin. Eye Res. 2012, 31, 43–64. [Google Scholar] [CrossRef]
- Donnelly, K.S.; Giuliano, E.A.; Sharma, A.; Tandon, A.; Rodier, J.T.; Mohan, R.R. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet. Ophthalmol. 2014, 17, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.W.; Yau, K.W. In vivo gene delivery in the retina using polyethylenimine. Biotechniques 2007, 42, 285–288. [Google Scholar] [CrossRef]
- Tandon, A.; Sharma, A.; Rodier, J.T.; Klibanov, A.M.; Rieger, F.G.; Mohan, R.R. BMP7 Gene Transfer via Gold Nanoparticles into Stroma Inhibits Corneal Fibrosis In Vivo. PLoS ONE 2013, 8, e66434. [Google Scholar] [CrossRef]
- Erickson, K.K.; Sundstrom, J.M.; Antonetti, D.A. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 2007, 10, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Sen, H.A.; Berkowitz, B.A.; Wilson, C.A.; Juan, E. Localization and quantitation of blood-retinal barrier breakdown in experimental proliferative vitreoretinopathy. Arch. Ophthalmol. 1994, 112, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xu, C.; Tian, H.; Chen, X. A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials 2019, 197, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef]
| Retinal Diseases | Current Treatment Strategies | Limitations |
|---|---|---|
| Diabetic retinopathy (DR) and ischemic retinopathy (IR) | Intravitreal injection of anti-VEGF * | Complexities and treatment burden (monthly visits) associated with multiple intravitreal injections and treatment non-responders [7,8,9] |
| Laser photocoagulation | Causes permanent damage to the retinal cells, can leave scotomas/blind spots [7,10] | |
| Vitrectomy | Surgical and anesthesia risks, post-operative infection/inflammation or retinal detachment [7,11] | |
| Age-related macular degeneration (AMD) | Lutein + zeaxanthin | Daily administration may lead to adherence problems, cost can be a concern [12] |
| Intravitreal injection of anti-VEGF | Risk and treatment burden associated with multiple intravitreal injections [9] | |
| Photodynamic therapy | Limited efficacy [13] | |
| Retinopathy of prematurity (ROP) and proliferative vitreoretinopathy (PVR) | Cryotherapy and laser photocoagulation | Cornea, iris, and lens burns, hyphema [14] |
| Intravitreal injection of anti-VEGF | Complexities associated with multiple intravitreal injections and retinal detachment [9,14,15] | |
| Vitrectomy | Vitreous or subretinal hemorrhage [16] | |
| Retinopathy of prematurity (ROP) and inherited retinal diseases (IRDs) | Pre-retinal membrane removal or retinal reattachment surgery | Reoccurrence of retinal fibrosis [17] |
| No treatment available, gene therapy and stem cell therapy are under investigation | Issues related to viral gene therapy. Direct administration of stem cells may have difficulty with integration [18] |
| Nanomedicines | Applications | Refs. |
|---|---|---|
| Gold nanoparticles |
| [25,26,27,28] |
| Silver nanoparticles |
| [29,30] |
| Platinum, cerium oxide and yttrium oxide nanoparticles |
| [31,32,33] |
| Iron oxide nanoparticles |
| [34,35,36] |
| Silica nanoparticles, carbon nanospheres, calcium phosphate nanoparticles |
| [37,38,39,40] |
| Nanofibers |
| [4,41,42,43] |
| Nano topography |
| [42,44] |
| Contact lens |
| [45] |
| Carbon nanotubes |
| [46,47] |
| Nanoprobes |
| [48] |
| Selenium nanobelts |
| [49] |
| Nanosponge |
| [50,51] |
| Nanoantennae |
| [52] |
| Polymeric nanoparticles Dendrimers |
| [8,53,54,55,56,57,58,59,60,61,62,63] |
| Lipid nanoparticles Liposomes |
| [64,65,66,67,68,69,70,71,72,73] |
| Polymeric micelles |
| [74,75,76,77,78,79] |
| Nanoemulsion Nanogels |
| [80,81,82,83,84,85,86] |
| Polymers | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| N-isopropylacrylamide and acrylamide | Radical polymerization |
|
| [108] |
| PLGA-PEG | Double emulsion solvent evaporation |
|
| [109] |
| Chitosan-coated PLGA | Oil-in-water emulsion |
|
| [110] |
| Chitosan functionalized PLGA | Nanoprecipitation |
|
| [111] |
| Bovine serum albumin, hyaluronic acid. | Desolvation |
|
| [112] |
| PLGA-hyaluronic acid | Solvent displacement |
|
| [113] |
| Polydopamine | Classical Stober method |
|
| [114] |
| Thiolated and methylated chitosan carboxymethyl dextran | Coacervation |
|
| [115] |
| Lipid Nanoparticle | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| Dioctadecyl dimethyl ammonium bromide, glyceryl monostearate, soy phosphatidylcholine, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)- PEG2000-maleimide | Film-ultrasonic |
|
| [124] |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), hydrogenated soy phosphocholine, methoxy PEG2000-DSPE | Thin-film hydration |
|
| [125] |
| Chitosan, glyceryl monostearate, linoleoyl polyoxyl-6-glycerides (Labrafil M 2125 CS) | Melt emulsification–ultrasonication |
|
| [126] |
| Medium-chain triglycerides, dioctadecyl-3,3,3,3 tetramethylindodicarbocyanine (DiO), polyethylene glycol (15)-hydroxy stearate, hydrogenated phosphatidylcholine | Phase inversion |
|
| [127] |
| Poloxamers 407/188, glycerol tripalmitate (GTP), soybean lecithin, stearic acid, PLGA | Emulsification |
|
| [128] |
| Glyceryl di behenate (Compritol 888), behenoyl polyoxyl-8 glycerides (Compritol HD5 ATO) | Microemulsion |
|
| [129] |
| Di Stearoyl phosphatidylcholine (DSPC), sterols, 1,2-dimyristoyl-rac-glycero-3-methoxy polyethylene glycol (DMG-PEG) | Rapid microfluidic mixing |
|
| [130] |
| Lauroyl PEG -32 glycerides, propylene glycol monocaprylate, hydrogenated coco glycerides | Hot-melt emulsification and ultrasonication |
|
| [131] |
| DSPE-PEG, PLGA, soybean lecithin | Membrane hydration and high-power ultrasonic method |
|
| [132] |
| Liposomes | Method of the Preparation | Characterization | Applications | Refs. |
|---|---|---|---|---|
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocoline (POPC), DSPE-mPEG2000, DSPE-N-[maleimide (PEG)2000], cholesterol, monocarboxylate | Thin-film hydration |
|
| [140] |
| DSPE-PEG2000-NHS, DSPE-PEG2000-TAT, egg phosphatidylcholine, cholesterol | Thin-film evaporation |
|
| [141] |
| 1,2-oleoyl-3-trimethylammonium-propane (DOTAP), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, LipoTrust Ex Oligo (short oligonucleotides) | Thin-film evaporation |
|
| [142] |
| DSPC, DOPC, DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) | Thin-film hydration |
|
| [143] |
| Soybean phosphatidylcholine, diacetyl phosphate, cholesterol, chitosan | Hydration |
|
| [144] |
| DSPC,DSPE-PEG2000, DSPE-PEG-Mal, cholesterol | Reverse-phase evaporation technique |
|
| [145] |
| DSPE-PEG2000, HSPC (hydrogenated soy phosphatidylcholine), DMPC, DOTAP | Thin-film hydration |
|
| [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahaling, B.; Low, S.W.Y.; Ch, S.; Addi, U.R.; Ahmad, B.; Connor, T.B.; Mohan, R.R.; Biswas, S.; Chaurasia, S.S. Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases. Pharmaceutics 2023, 15, 2005. https://doi.org/10.3390/pharmaceutics15072005
Mahaling B, Low SWY, Ch S, Addi UR, Ahmad B, Connor TB, Mohan RR, Biswas S, Chaurasia SS. Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases. Pharmaceutics. 2023; 15(7):2005. https://doi.org/10.3390/pharmaceutics15072005
Chicago/Turabian StyleMahaling, Binapani, Shermaine W. Y. Low, Sanjay Ch, Utkarsh R. Addi, Baseer Ahmad, Thomas B. Connor, Rajiv R. Mohan, Swati Biswas, and Shyam S. Chaurasia. 2023. "Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases" Pharmaceutics 15, no. 7: 2005. https://doi.org/10.3390/pharmaceutics15072005
APA StyleMahaling, B., Low, S. W. Y., Ch, S., Addi, U. R., Ahmad, B., Connor, T. B., Mohan, R. R., Biswas, S., & Chaurasia, S. S. (2023). Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases. Pharmaceutics, 15(7), 2005. https://doi.org/10.3390/pharmaceutics15072005






