ZNF703 mRNA-Targeting Antisense Oligonucleotide Blocks Cell Proliferation and Induces Apoptosis in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
3. Results
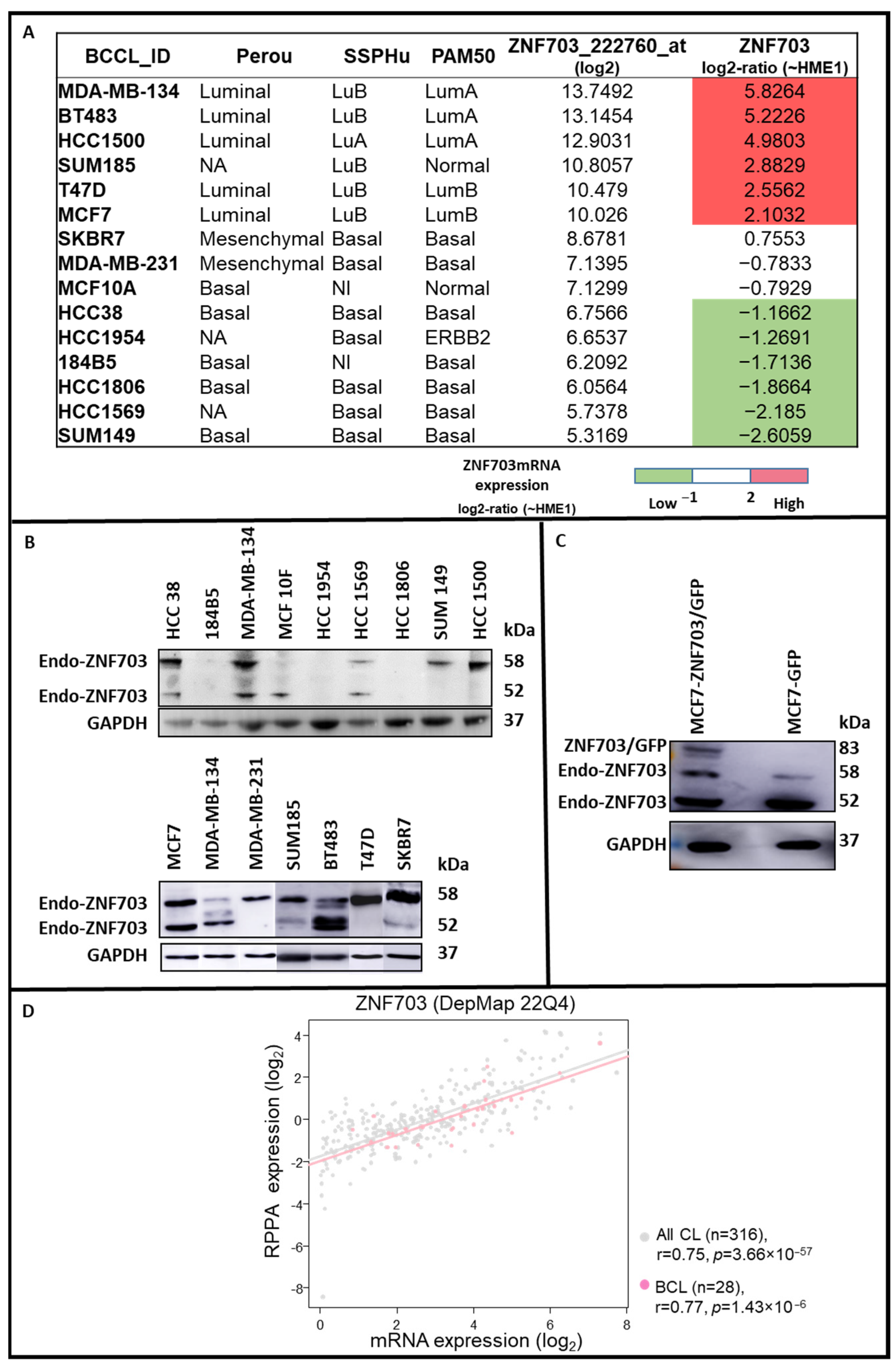
3.1. ZNF703 Expression in Different Breast Cancer Cell Lines
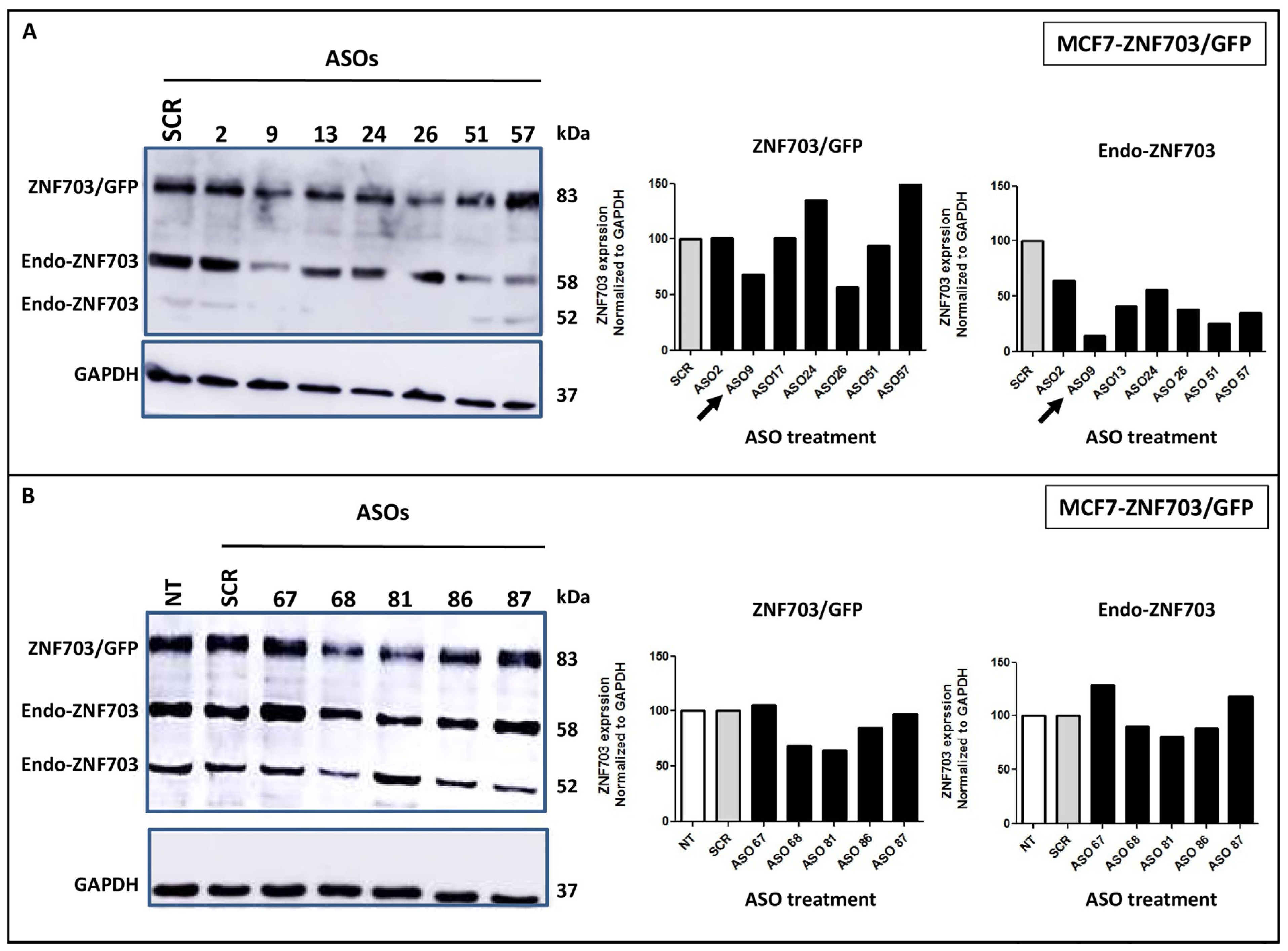
3.2. ASO Design, Synthesis, and Screening for Inhibitory Activity on ZNF703 mRNA
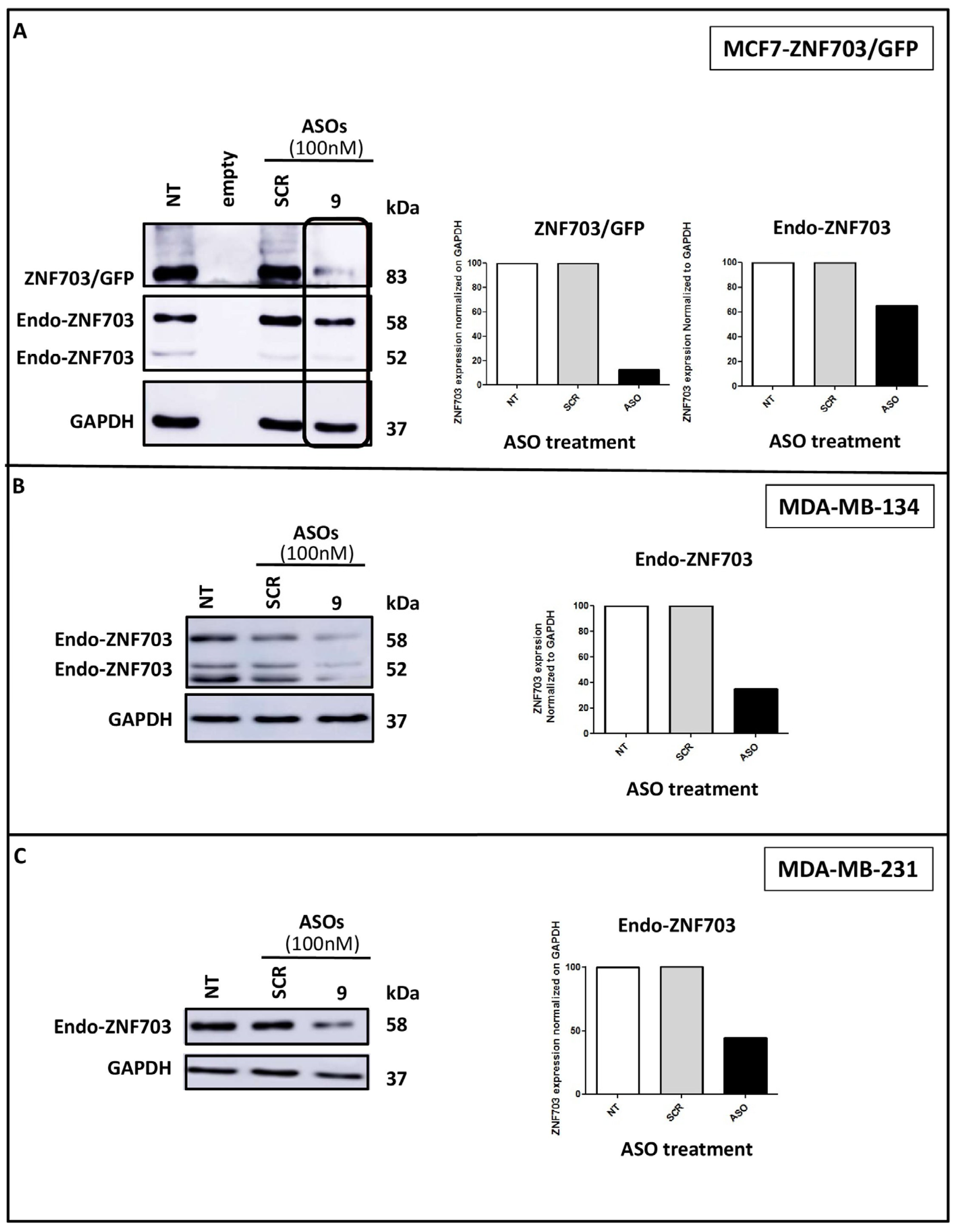
3.3. ZNF703-ASO9 Targeting ZNF703 mRNA Downregulates ZNF703 Protein Expression in BC Cell Lines
3.4. ZNF703-ASO9 Has Antiproliferative Effect in BC Cell Lines
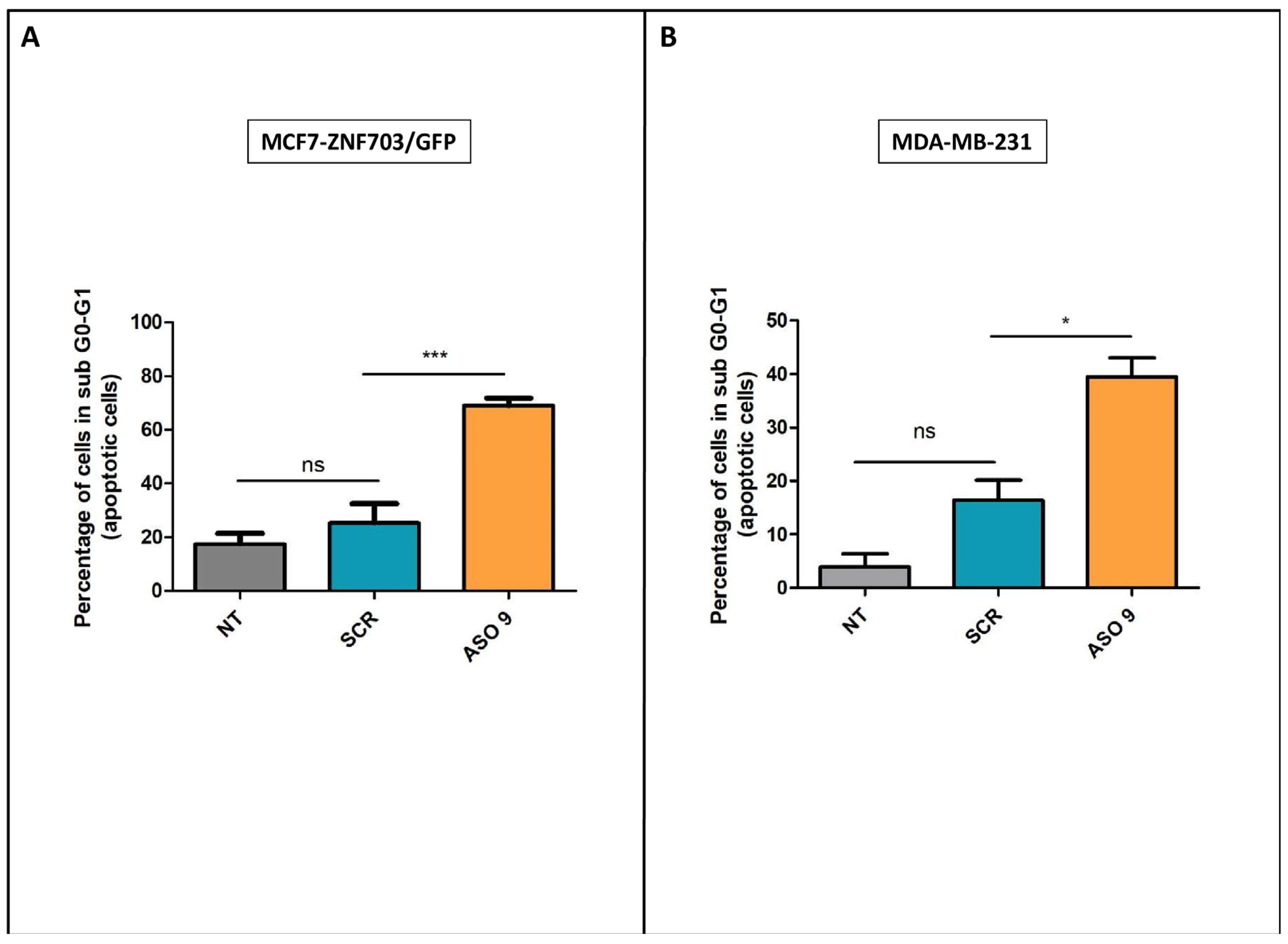
3.5. ASO Inhibition of ZNF703 Induces Cell Death
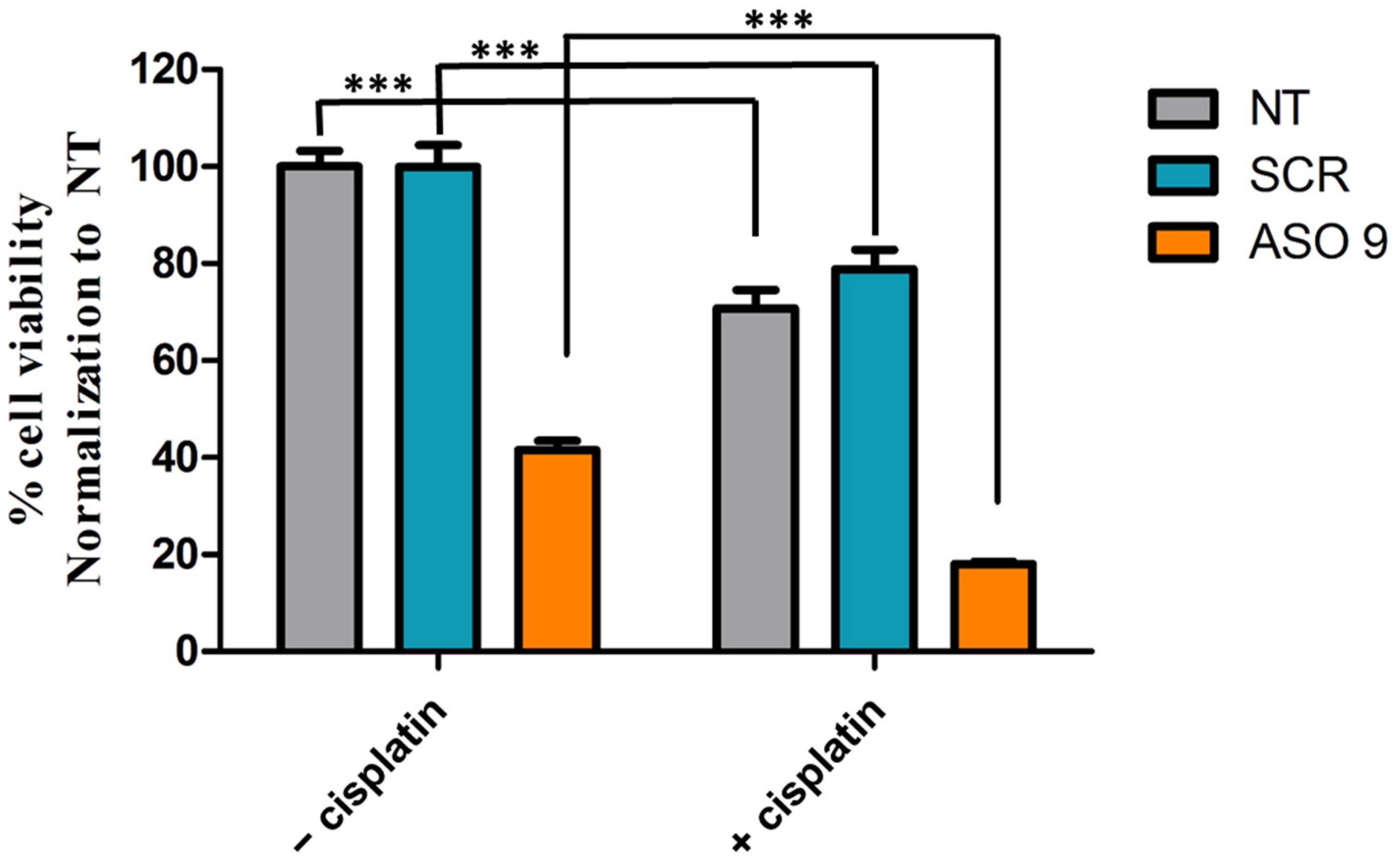
3.6. The Anti-Cancer Effects of ASO9 Are Improved When Combined with Cisplatin in MCF7-ZNF703/GFP BC Cell Line
4. Discussion
4.1. ZNF703 Oncogene Is a Potential Therapeutic Target in Breast and Other Advanced Cancers
4.2. ASO Targeting ZNF703 mRNA May Be an Effective Strategy for Advanced BCs
4.3. ZNF703 mRNA-Targeting May Be Used in Combination with Chemo- and Hormone-Therapy of Advanced Breast Cancers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 2009, 360, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Børresen-Dale, A.-L. Systems biology and genomics of breast cancer. Cold Spring Harb. Perspect. Biol. 2011, 3, a003293. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.-M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.-M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sotiriou, C. Luminal breast cancer: From biology to treatment. Nat. Rev. Clin. Oncol. 2013, 10, 494–506. [Google Scholar] [CrossRef]
- Fleurot, E.; Goudin, C.; Hanoux, V.; Bonnamy, P.-J.; Levallet, J. Estrogen receptor α regulates the expression of syndecan-1 in human breast carcinoma cells. Endocr. Relat. Cancer 2019, 26, 615–628. [Google Scholar] [CrossRef]
- Meijer, D.; Sieuwerts, A.M.; Look, M.P.; van Agthoven, T.; Foekens, J.A.; Dorssers, L.C.J. Fibroblast growth factor receptor 4 predicts failure on tamoxifen therapy in patients with recurrent breast cancer. Endocr. Relat. Cancer 2008, 15, 101–111. [Google Scholar] [CrossRef]
- van Agthoven, T.; Sieuwerts, A.M.; Veldscholte, J.; Meijer-van Gelder, M.E.; Smid, M.; Brinkman, A.; den Dekker, A.T.; Leroy, I.M.; van Ijcken, W.F.J.; Sleijfer, S.; et al. CITED2 and NCOR2 in anti-oestrogen resistance and progression of breast cancer. Br. J. Cancer 2009, 101, 1824–1832. [Google Scholar] [CrossRef]
- Tran, B.; Bedard, P.L. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- van Agthoven, T.; Godinho, M.F.E.; Wulfkuhle, J.D.; Petricoin, E.F.; Dorssers, L.C.J. Protein pathway activation mapping reveals molecular networks associated with antiestrogen resistance in breast cancer cell lines. Int. J. Cancer 2012, 131, 1998–2007. [Google Scholar] [CrossRef]
- Salmans, M.L.; Zhao, F.; Andersen, B. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: A potential drug target and biomarker. Breast Cancer Res. 2013, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Levine, K.M.; Priedigkeit, N.; Basudan, A.; Tasdemir, N.; Sikora, M.J.; Sokol, E.S.; Hartmaier, R.J.; Ding, K.; Ahmad, N.Z.; Watters, R.J.; et al. FGFR4 overexpression and hotspot mutations in metastatic ER+ breast cancer is enriched in the lobular subtype. NPJ Breast Cancer 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Arruabarrena-Aristorena, A.; Maag, J.L.V.; Kittane, S.; Cai, Y.; Karthaus, W.R.; Ladewig, E.; Park, J.; Kannan, S.; Ferrando, L.; Cocco, E.; et al. FOXA1 Mutations Reveal Distinct Chromatin Profiles and Influence Therapeutic Response in Breast Cancer. Cancer Cell 2020, 38, 534–550.e9. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Thennavan, A.; East, M.P.; Parker, J.S.; Cejalvo, J.M.; Garay, J.P.; Hollern, D.P.; He, X.; Mott, K.R.; Galván, P.; et al. FGFR4 regulates tumor subtype differentiation in luminal breast cancer and metastatic disease. J. Clin. Investig. 2020, 130, 4871–4887. [Google Scholar] [CrossRef]
- Jin, X.; Ge, L.-P.; Li, D.-Q.; Shao, Z.-M.; Di, G.-H.; Xu, X.-E.; Jiang, Y.-Z. LncRNA TROJAN promotes proliferation and resistance to CDK4/6 inhibitor via CDK2 transcriptional activation in ER+ breast cancer. Mol. Cancer 2020, 19, 87. [Google Scholar] [CrossRef]
- Augereau, P.; Patsouris, A.; Bourbouloux, E.; Gourmelon, C.; Abadie Lacourtoisie, S.; Berton Rigaud, D.; Soulié, P.; Frenel, J.S.; Campone, M. Hormonoresistance in advanced breast cancer: A new revolution in endocrine therapy. Ther. Adv. Med. Oncol. 2017, 9, 335–346. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Lin, L.; Snider, J.; Prat, A.; Parker, J.S.; Luo, J.; DeSchryver, K.; Allred, D.C.; et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J. Clin. Oncol. 2011, 29, 2342–2349. [Google Scholar] [CrossRef]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Goncalves, R.; Sanati, S.; Creighton, C.J.; DeSchryver, K.; Crouch, E.; Brink, A.; Watson, M.; et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J. Clin. Oncol. 2017, 35, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gluz, O.; Christgen, M.; Kates, R.E.; Braun, M.; Küemmel, S.; Schumacher, C.; Potenberg, J.; Kraemer, S.; Kleine-Tebbe, A.; et al. De-Escalation Strategies in Human Epidermal Growth Factor Receptor 2 (HER2)–Positive Early Breast Cancer (BC): Final Analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early BC HER2- and Hormone Receptor–Positive Phase II Randomized Trial—Efficacy, Safety, and Predictive Markers for 12 Weeks of Neoadjuvant Trastuzumab Emtansine With or Without Endocrine Therapy (ET) Versus Trastuzumab Plus ET. J. Clin. Oncol. 2017, 35, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Formisano, L.; Lu, Y.; Servetto, A.; Hanker, A.B.; Jansen, V.M.; Bauer, J.A.; Sudhan, D.R.; Guerrero-Zotano, A.L.; Croessmann, S.; Guo, Y.; et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat. Commun. 2019, 10, 1373. [Google Scholar] [CrossRef]
- Ono, M.; Oba, T.; Shibata, T.; Ito, K.-I. The mechanisms involved in the resistance of estrogen receptor-positive breast cancer cells to palbociclib are multiple and change over time. J. Cancer Res. Clin. Oncol. 2021, 147, 3211–3224. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Procter, M.; de Azambuja, E.; Leyland-Jones, B.; Gelber, R.D.; Dowsett, M.; Loi, S.; Saini, K.S.; Cameron, D.; Untch, M.; et al. Magnitude of trastuzumab benefit in patients with HER2-positive, invasive lobular breast carcinoma: Results from the HERA trial. J. Clin. Oncol. 2013, 31, 1954–1960. [Google Scholar] [CrossRef]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; de Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef]
- Cornen, S.; Guille, A.; Adélaïde, J.; Addou-Klouche, L.; Finetti, P.; Saade, M.-R.; Manai, M.; Carbuccia, N.; Bekhouche, I.; Letessier, A.; et al. Candidate luminal B breast cancer genes identified by genome, gene expression and DNA methylation profiling. PLoS ONE 2014, 9, e81843. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.M.; Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Choi, W.J. Association of BRCA Mutation Types, Imaging Features, and Pathologic Findings in Patients with Breast Cancer with BRCA1 and BRCA2 Mutations. Am. J. Roentgenol. 2017, 209, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Addou-Klouche, L.; Adélaïde, J.; Finetti, P.; Cervera, N.; Ferrari, A.; Bekhouche, I.; Sircoulomb, F.; Sotiriou, C.; Viens, P.; Moulessehoul, S.; et al. Loss, mutation and deregulation of L3MBTL4 in breast cancers. Mol. Cancer 2010, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Sircoulomb, F.; Nicolas, N.; Ferrari, A.; Finetti, P.; Bekhouche, I.; Rousselet, E.; Lonigro, A.; Adélaïde, J.; Baudelet, E.; Esteyriès, S.; et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol. Med. 2011, 3, 153–166. [Google Scholar] [CrossRef]
- Finetti, P.; Guille, A.; Adelaide, J.; Birnbaum, D.; Chaffanet, M.; Bertucci, F. ESPL1 is a candidate oncogene of luminal B breast cancers. Breast Cancer Res. Treat. 2014, 147, 51–59. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Orsetti, B.; Cervera, N.; Finetti, P.; Sircoulomb, F.; Rougé, C.; Lasorsa, L.; Letessier, A.; Ginestier, C.; Monville, F.; et al. Comprehensive profiling of 8p11-12 amplification in breast cancer. Mol. Cancer Res. 2005, 3, 655–667. [Google Scholar] [CrossRef]
- Adélaïde, J.; Finetti, P.; Bekhouche, I.; Repellini, L.; Geneix, J.; Sircoulomb, F.; Charafe-Jauffret, E.; Cervera, N.; Desplans, J.; Parzy, D.; et al. Integrated profiling of basal and luminal breast cancers. Cancer Res. 2007, 67, 11565–11575. [Google Scholar] [CrossRef]
- Holland, D.G.; Burleigh, A.; Git, A.; Goldgraben, M.A.; Perez-Mancera, P.A.; Chin, S.-F.; Hurtado, A.; Bruna, A.; Ali, H.R.; Greenwood, W.; et al. ZNF703 is a common Luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol. Med. 2011, 3, 167–180. [Google Scholar] [CrossRef]
- Song, X.; Ji, J.; Rothstein, J.H.; Alexeeff, S.E.; Sakoda, L.C.; Sistig, A.; Achacoso, N.; Jorgenson, E.; Whittemore, A.S.; Klein, R.J.; et al. MiXcan: A framework for cell-type-aware transcriptome-wide association studies with an application to breast cancer. Nat. Commun. 2023, 14, 377. [Google Scholar] [CrossRef]
- Slorach, E.M.; Chou, J.; Werb, Z. Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Genes Dev. 2011, 25, 471–484. [Google Scholar] [CrossRef]
- Reynisdottir, I.; Arason, A.; Einarsdottir, B.O.; Gunnarsson, H.; Staaf, J.; Vallon-Christersson, J.; Jonsson, G.; Ringnér, M.; Agnarsson, B.A.; Olafsdottir, K.; et al. High expression of ZNF703 independent of amplification indicates worse prognosis in patients with luminal B breast cancer. Cancer Med. 2013, 2, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mu, X.; Huang, O.; Wang, Z.; Chen, J.; Chen, D.; Wang, G. ZNF703 promotes triple-negative breast cancer cells through cell-cycle signaling and associated with poor prognosis. BMC Cancer 2022, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Bi, L.; Yang, G.; Zhang, M.; Liu, C.; Zhao, Y.; Wang, Y.; Wang, J.; Bai, Y.; Zhang, Y. ZNF703 promotes tumor cell proliferation and invasion and predicts poor prognosis in patients with colorectal cancer. Oncol. Rep. 2014, 32, 1071–1077. [Google Scholar] [CrossRef]
- Yang, G.; Ma, F.; Zhong, M.; Fang, L.; Peng, Y.; Xin, X.; Zhong, J.; Yuan, F.; Gu, H.; Zhu, W.; et al. ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol. Rep. 2014, 31, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, W.Q.; Cao, Y.; Sun, Y.A.; Wei, J.; An, X.; Zhang, Y.C.; Song, M.; Wang, S.S.; Yuan, Z.Y.; et al. Elevated ZNF703 Protein Expression Is an Independent Unfavorable Prognostic Factor for Survival of the Patients with Head and Neck Squamous Cell Carcinoma. Dis. Markers 2015, 2015, 640263. [Google Scholar] [CrossRef]
- Baykara, O.; Dalay, N.; Kaynak, K.; Buyru, N. ZNF703 Overexpression may act as an oncogene in non-small cell lung cancer. Cancer Med. 2016, 5, 2873–2878. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Han, J.; Lan, Y.; Xie, C.; Pan, S.; Liu, L. Overexpression of ZNF703 facilitates tumorigenesis and predicts unfavorable prognosis in patients with cholangiocarcinoma. Oncotarget 2016, 7, 76108–76117. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Zang, L.; Li, D.; Yu, F.; Xiang, X.; Li, W. ZNF703 is Overexpressed in Papillary Thyroid Carcinoma Tissues and Mediates K1 Cell Proliferation. Pathol. Oncol. Res. 2020, 26, 355–364. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Li, W.; Zang, L.; Li, D.; Wang, Q.; Yu, F.; Xiang, X. Silencing of zinc finger protein 703 inhibits medullary thyroid carcinoma cell proliferation in. vitro and in vivo. Oncol. Lett. 2020, 19, 943–951. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Hu, Y.; Li, X.; Jin, S.; Liu, O.; Gou, R.; Zhuang, Y.; Guo, Q.; Nie, X.; et al. ZNF703 promotes tumor progression in ovarian cancer by interacting with HE4 and epigenetically regulating PEA15. J. Exp. Clin. Cancer Res. 2020, 39, 264. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Ma, F.; Zhan, M.; Yang, X.; Hua, S.; Li, W.; Li, Y.; Lu, L. Zinc finger protein 703 induces EMT and sorafenib resistance in hepatocellular carcinoma by transactivating CLDN4 expression. Cell Death Dis. 2020, 11, 225. [Google Scholar] [CrossRef]
- Crooke, S.T. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017, 27, 70–77. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, P.; So, A.; Kojima, S.; Signaevsky, M.; Beraldi, E.; Fazli, L.; Hurtado-Coll, A.; Yamanaka, K.; Gleave, M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004, 64, 6595–6602. [Google Scholar] [CrossRef]
- Rocchi, P.; Beraldi, E.; Ettinger, S.; Fazli, L.; Vessella, R.L.; Nelson, C.; Gleave, M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005, 65, 11083–11093. [Google Scholar] [CrossRef] [PubMed]
- Baylot, V.; Katsogiannou, M.; Andrieu, C.; Taieb, D.; Acunzo, J.; Giusiano, S.; Fazli, L.; Gleave, M.; Garrido, C.; Rocchi, P. Targeting TCTP as a new therapeutic strategy in castration-resistant prostate cancer. Mol. Ther. 2012, 20, 2244–2256. [Google Scholar] [CrossRef]
- Karaki, S.; Benizri, S.; Mejías, R.; Baylot, V.; Branger, N.; Nguyen, T.; Vialet, B.; Oumzil, K.; Barthélémy, P.; Rocchi, P. Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J. Control Release 2017, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Le, T.K.; Paris, C.; Cherif, C.; Audebert, S.; Udu-Ituma, S.O.; Benizri, S.; Barthélémy, P.; Bertucci, F.; Taïeb, D.; et al. Antisense Oligonucleotide-Based Therapeutic against Menin for Triple-Negative Breast Cancer Treatment. Biomedicines 2021, 9, 795. [Google Scholar] [CrossRef]
- Cherif, C.; Nguyen, D.T.; Paris, C.; Le, T.K.; Sefiane, T.; Carbuccia, N.; Finetti, P.; Chaffanet, M.; Kaoutari, A.E.; Vernerey, J.; et al. Menin inhibition suppresses castration-resistant prostate cancer and enhances chemosensitivity. Oncogene 2022, 41, 125–137. [Google Scholar] [CrossRef]
- Le, T.K.; Cherif, C.; Omabe, K.; Paris, C.; Lannes, F.; Audebert, S.; Baudelet, E.; Hamimed, M.; Barbolosi, D.; Finetti, P.; et al. DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer. Mol. Ther. 2023, 31, 471–486. [Google Scholar] [CrossRef]
- Forozan, F.; Veldman, R.; Ammerman, C.A.; Parsa, N.Z.; Kallioniemi, A.; Kallioniemi, O.-P.; Ethier, S.P. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br. J. Cancer 1999, 81, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Monville, F.; Finetti, P.; Adélaïde, J.; Cervera, N.; Fekairi, S.; Xerri, L.; Jacquemier, J.; Birnbaum, D.; et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene 2006, 25, 2273–2284. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Wu, Z.; Jaffee, H.A. Comparison of Affymetrix GeneChip expression measures. Bioinformatics 2006, 22, 789–794. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, C.; Oh, D.S.; Marron, J.S.; He, X.; Qaqish, B.F.; Livasy, C.; Carey, L.A.; Reynolds, E.; Dressler, L.; et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genom. 2006, 7, 96. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., 3rd; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Crooke, S.T.; Vickers, T.A.; Liang, X.H. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, V. Divide and conquer: The genetic basis of molecular subclassification of breast cancer. EMBO Mol. Med. 2011, 3, 183–185. [Google Scholar] [CrossRef]
- Sharma, V.K.; Watts, J.K. Oligonucleotide therapeutics: Chemistry, delivery and clinical progress. Future Med. Chem. 2015, 7, 2221–2242. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef] [PubMed]
- Chery, J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc. J. 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Tazawa, S.; Yawei, Z.; Katayama, H.; Kato, Y.; Nishiwaki, K.; Yokohama, Y.; Ishikawa, M. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int. J. Oncol. 2006, 28, 931–938. [Google Scholar] [CrossRef]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Hassiotou, F.; Blancafort, P.; Filgueira, L. Cisplatin Induces Differentiation of Breast Cancer Cells. Front. Oncol. 2013, 3, 134. [Google Scholar] [CrossRef]
- Chen, S.H.; Chang, J.Y. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int. J. Mol. Sci. 2019, 20, 4136. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.W.; Kelly, C.M. Optimal Choice of Neoadjuvant Chemotherapy for HER2-Negative Breast Cancer: Clinical Insights. Cancer Manag. Res. 2022, 14, 2493–2506. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, X.; Huang, O.; Xie, Z.; Jiang, M.; Geng, M.; Shen, K. Luminal breast cancer cell lines overexpressing ZNF703 are resistant to tamoxifen through activation of Akt/mTOR signaling. PLoS ONE 2013, 8, e72053. [Google Scholar] [CrossRef]
- Yi, J.; Wang, L.; Hu, G.S.; Zhang, Y.Y.; Du, J.; Ding, J.C.; Ji, X.; Shen, H.F.; Huang, H.H.; Ye, F.; et al. CircPVT1 promotes ER-positive breast tumorigenesis and drug resistance by targeting ESR1 and MAVS. EMBO J. 2023, 42, e112408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udu-Ituma, S.; Adélaïde, J.; Le, T.K.; Omabe, K.; Finetti, P.; Paris, C.; Guille, A.; Bertucci, F.; Birnbaum, D.; Rocchi, P.; et al. ZNF703 mRNA-Targeting Antisense Oligonucleotide Blocks Cell Proliferation and Induces Apoptosis in Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1930. https://doi.org/10.3390/pharmaceutics15071930
Udu-Ituma S, Adélaïde J, Le TK, Omabe K, Finetti P, Paris C, Guille A, Bertucci F, Birnbaum D, Rocchi P, et al. ZNF703 mRNA-Targeting Antisense Oligonucleotide Blocks Cell Proliferation and Induces Apoptosis in Breast Cancer Cell Lines. Pharmaceutics. 2023; 15(7):1930. https://doi.org/10.3390/pharmaceutics15071930
Chicago/Turabian StyleUdu-Ituma, Sandra, José Adélaïde, Thi Khanh Le, Kenneth Omabe, Pascal Finetti, Clément Paris, Arnaud Guille, François Bertucci, Daniel Birnbaum, Palma Rocchi, and et al. 2023. "ZNF703 mRNA-Targeting Antisense Oligonucleotide Blocks Cell Proliferation and Induces Apoptosis in Breast Cancer Cell Lines" Pharmaceutics 15, no. 7: 1930. https://doi.org/10.3390/pharmaceutics15071930
APA StyleUdu-Ituma, S., Adélaïde, J., Le, T. K., Omabe, K., Finetti, P., Paris, C., Guille, A., Bertucci, F., Birnbaum, D., Rocchi, P., & Chaffanet, M. (2023). ZNF703 mRNA-Targeting Antisense Oligonucleotide Blocks Cell Proliferation and Induces Apoptosis in Breast Cancer Cell Lines. Pharmaceutics, 15(7), 1930. https://doi.org/10.3390/pharmaceutics15071930







