A New Biomaterial Derived from Aloe vera—Acemannan from Basic Studies to Clinical Application
Abstract
1. Introduction
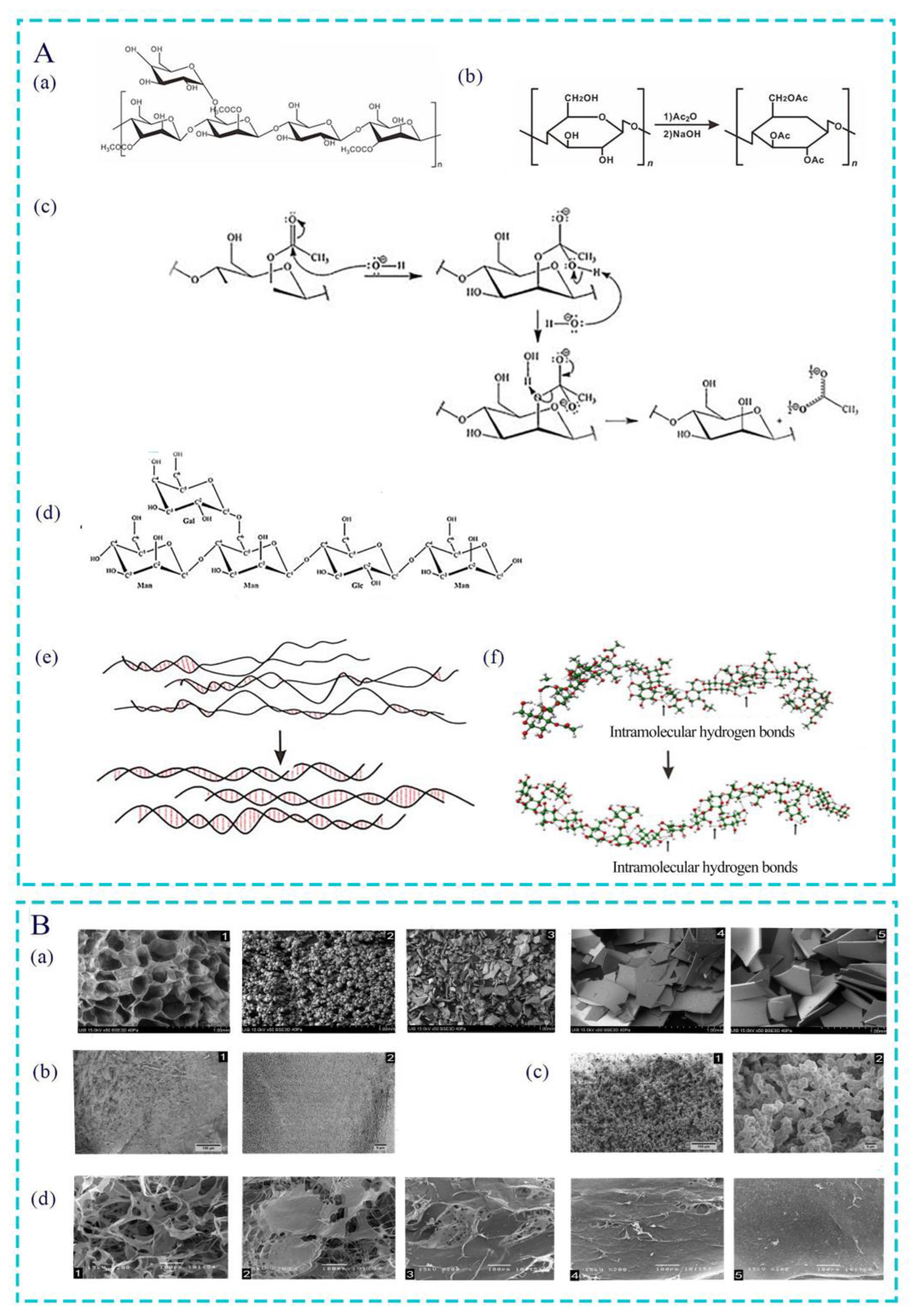
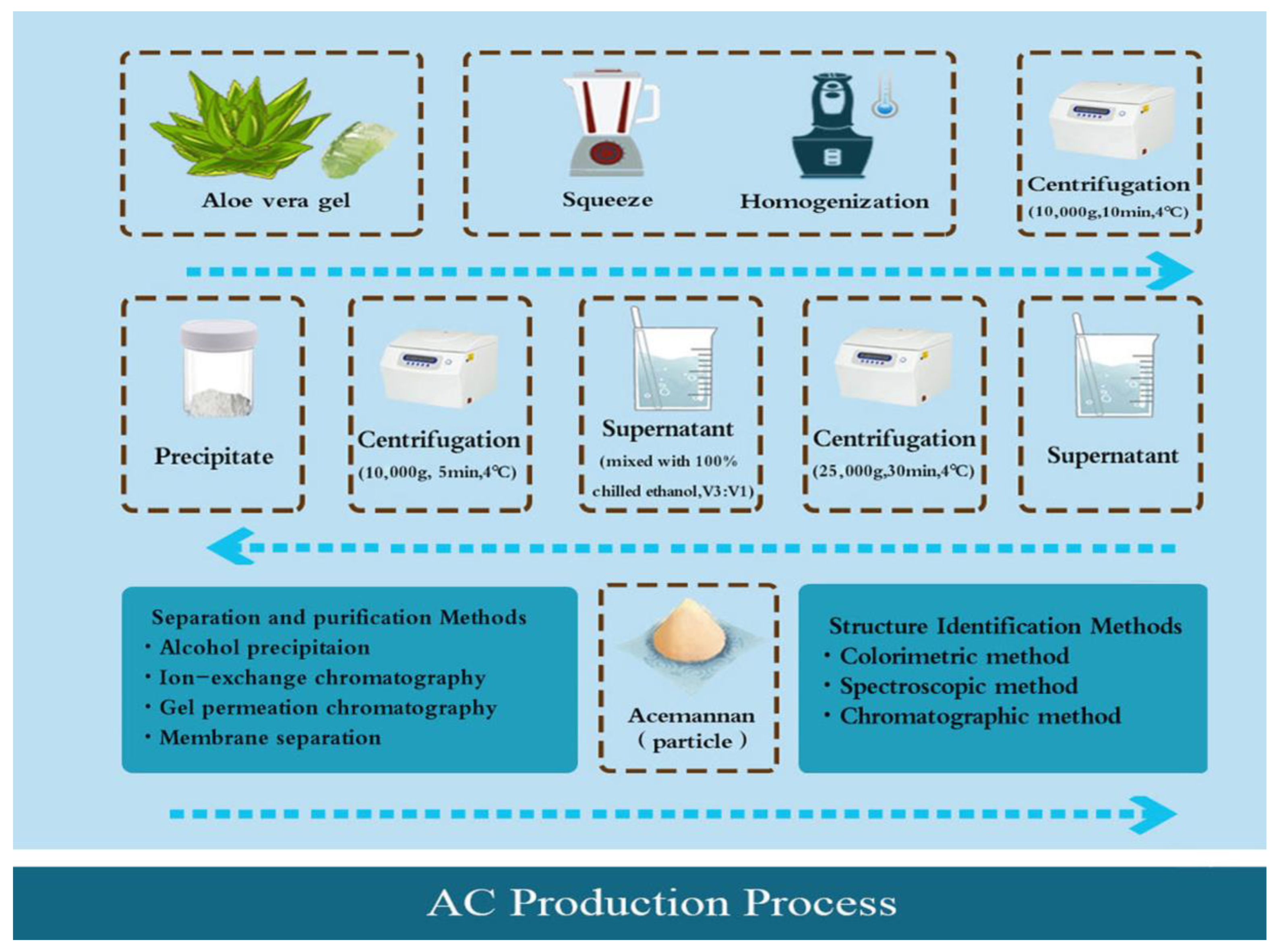
2. Manufacturing Process of AC Products
2.1. Crude Extraction
2.2. Separation and Purification
2.3. Structure Identification
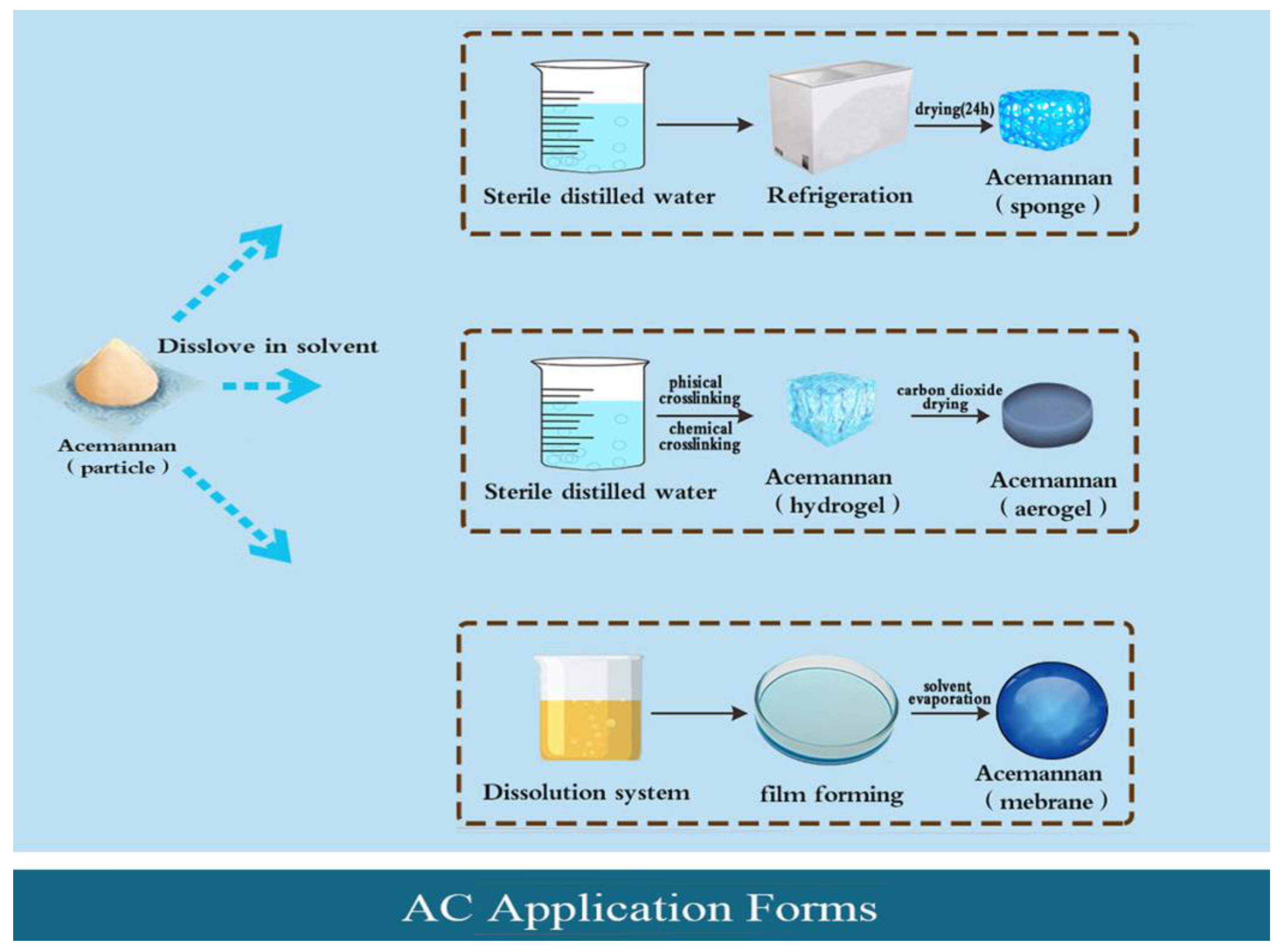
3. Application Forms of AC
3.1. AC Particle
3.2. AC Sponge
3.3. AC Hydrogel and Aerogel
3.4. AC Film
4. Effect of Acetyl Group on Bioactivity of AC
4.1. Acetylation Modification of Polysaccharide
4.2. Effect of Acetyl Groups on Biological Activity of Polysaccharides
4.3. Effect of Different Degrees of Acetylation Modification on AC
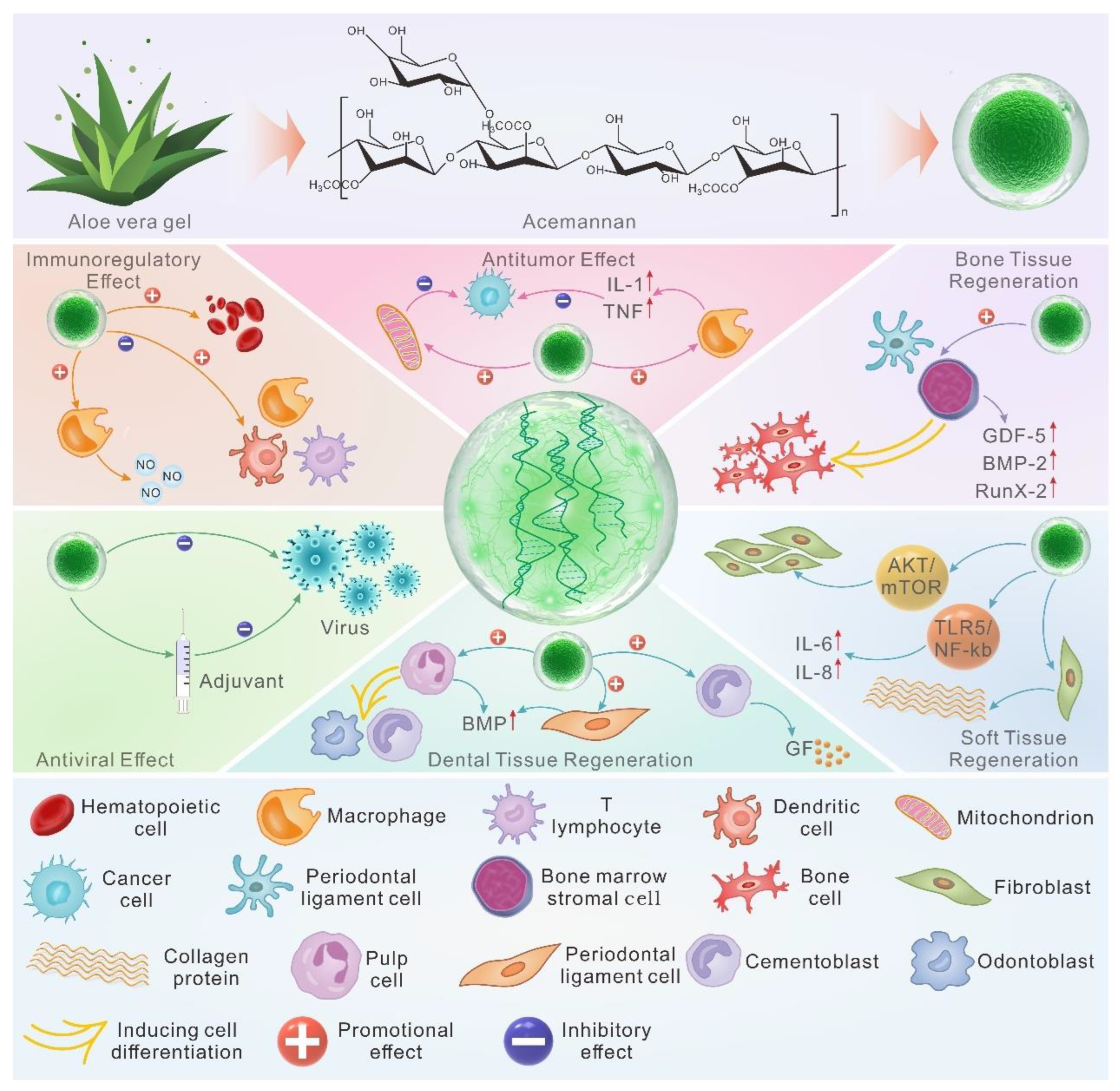
5. Biological Functions of AC
5.1. Immunoregulation
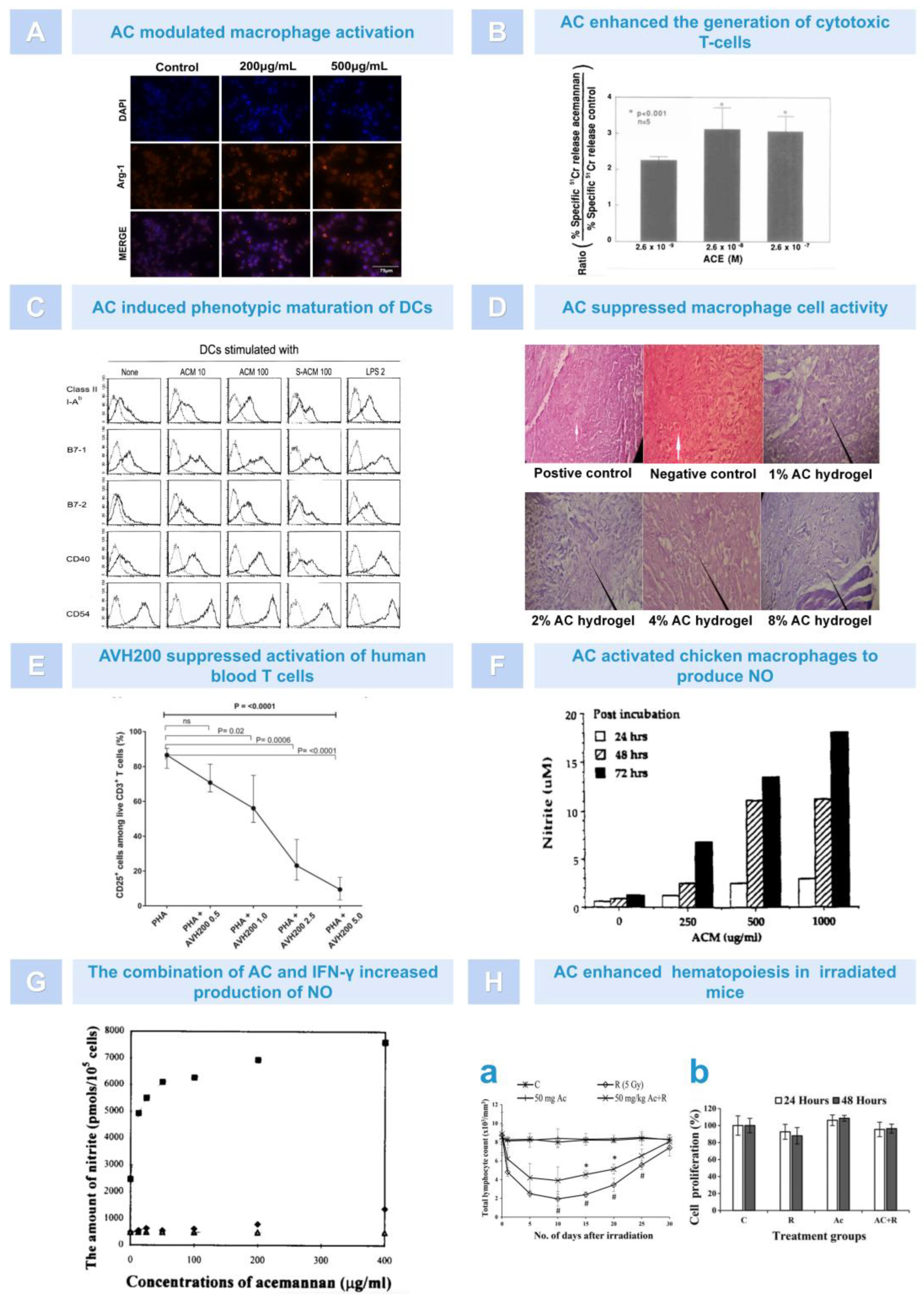
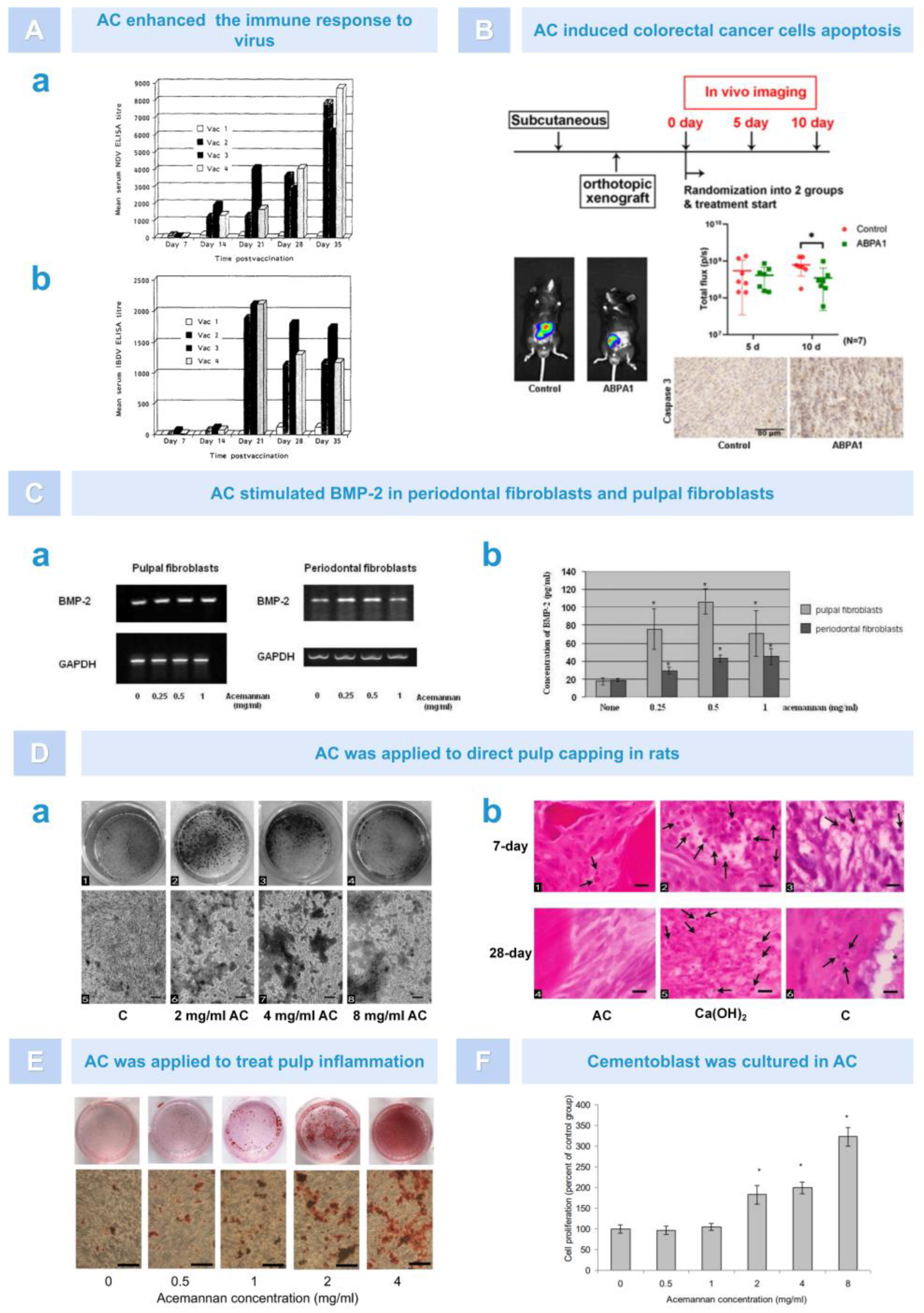
5.2. Antiviral Effect
5.3. Anti-Tumor Effect
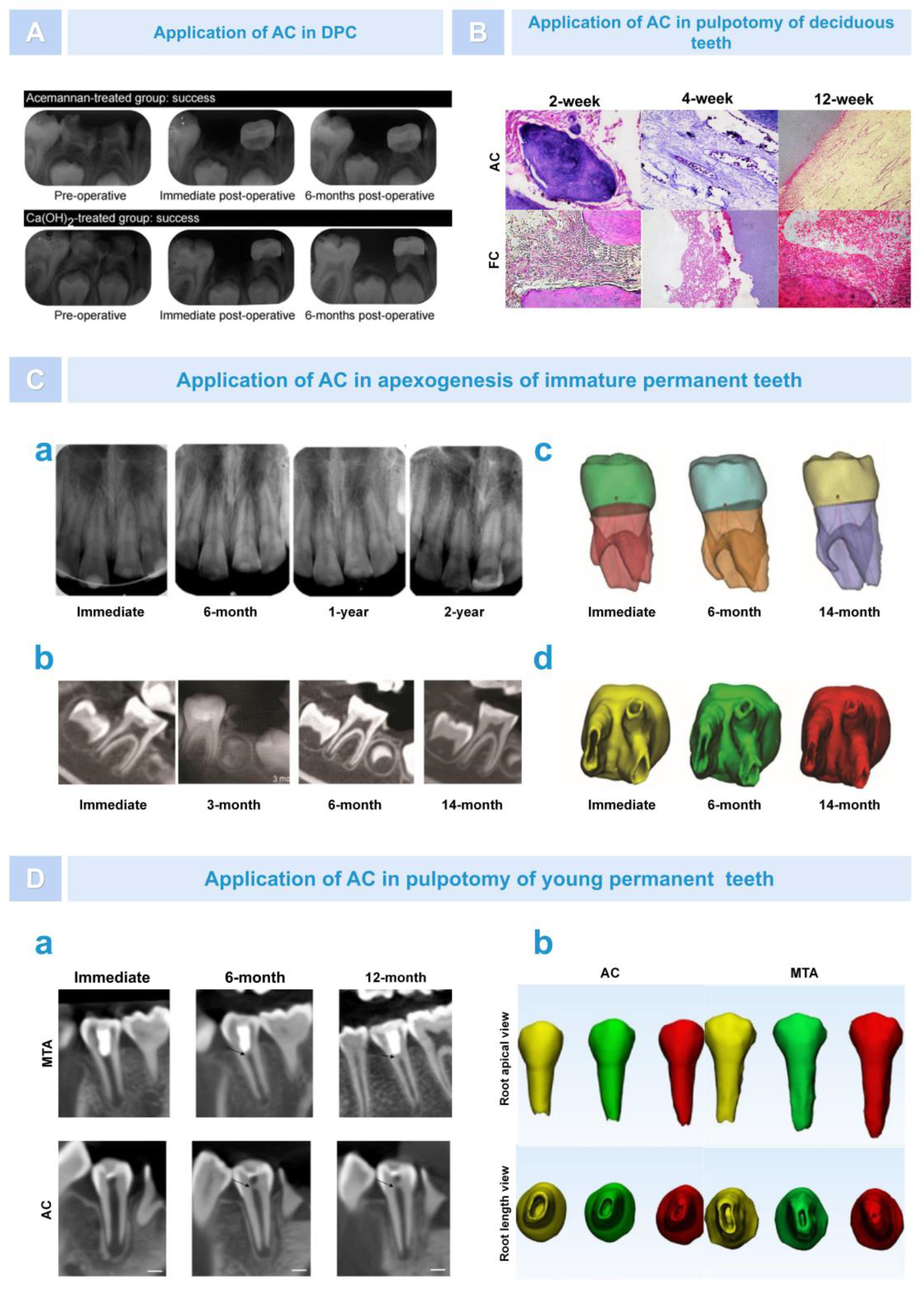
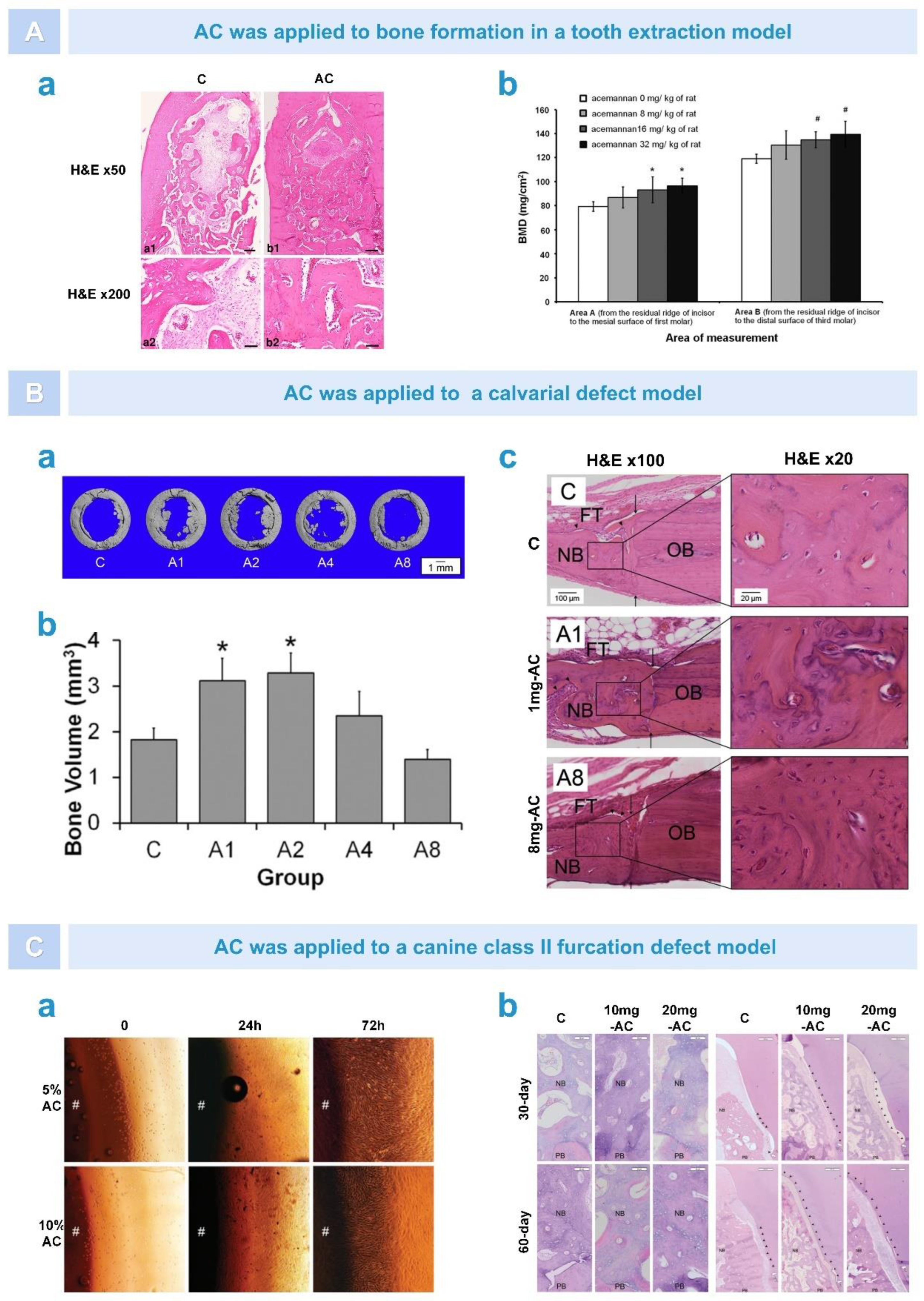
5.4. Dental Tissue Regeneration
5.5. Osteogenesis
5.6. Soft Tissue Healing
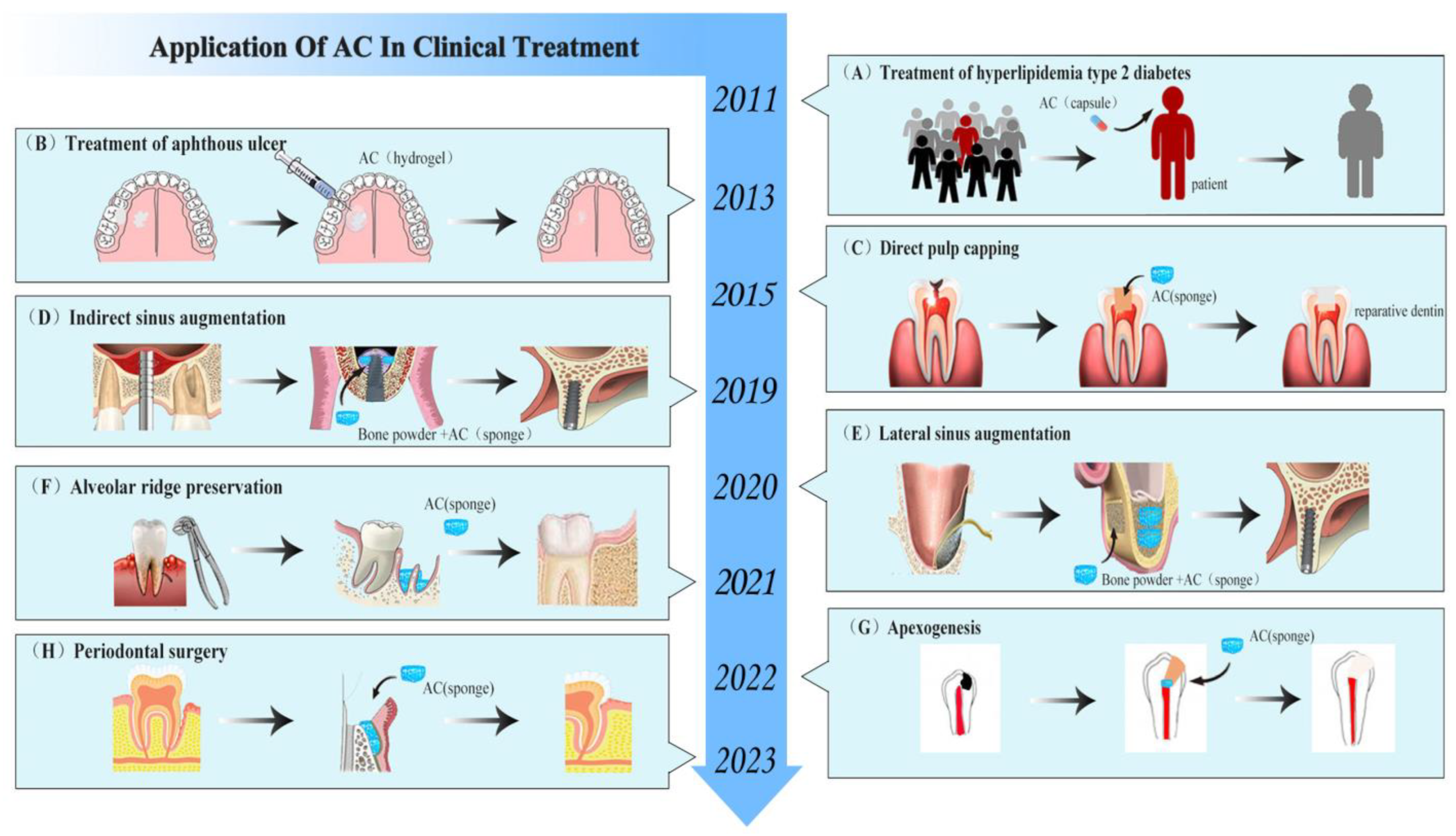
6. Advances in the Clinical Application of AC
6.1. Regeneration of Dental Pulp–Dentin Complex
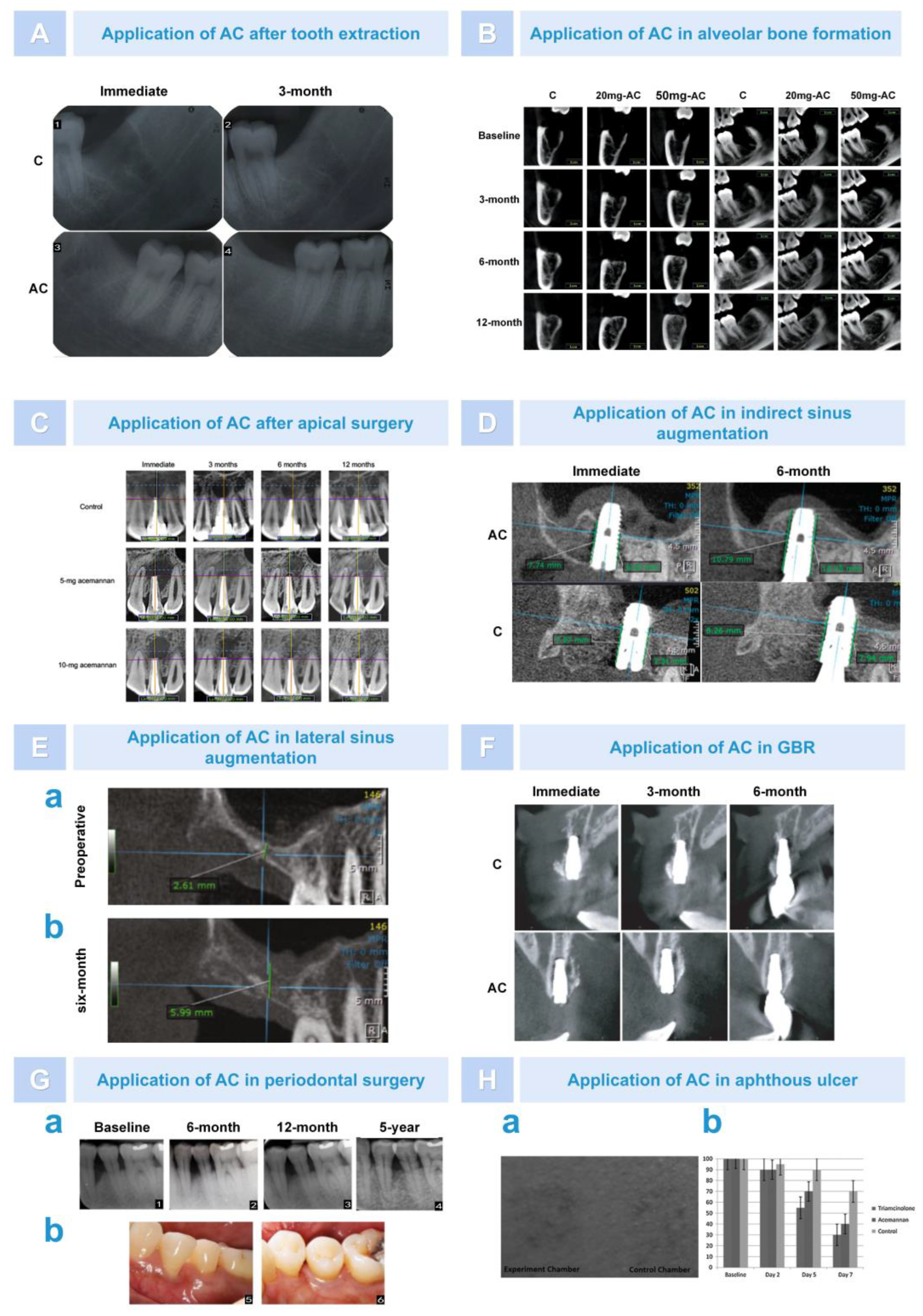
6.2. Bone Regeneration
6.2.1. Osteogenesis after Alveolar Surgery
6.2.2. Bone Augmentation in Oral Implantation Area
6.2.3. Repair of Periodontal Tissue after Treatment of Periodontitis
6.3. Treatment of Skin and Mucosal Disease
6.4. Reduction in Blood Sugar and Blood Lipids
7. Combined Application of AC and Other Compounds
7.1. Combining AC with Polysaccharide
7.1.1. Chitosan (CS)
7.1.2. Alginate (ALG)
7.1.3. Glycosaminoglycan (GAG)
7.2. Combining AC with Collagen (Col)
7.3. Combining AC with Lipids
7.4. Combining AC with Plants
7.5. Combining AC with Other Compounds
8. Conclusions and Prospects
8.1. Using Computer-Aided Drug Design (CADD) Systems for Drug Efficacy Analysis
8.2. Druggability Analysis of AC
8.3. Toxicity Analysis of AC
8.4. Exploring the Relationship between Surface Structure and Molecular Structure and Drug Activity
8.5. Limitations of AC Application in Tissue Engineering
8.6. Developing and Perfecting the Application Form of AC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Acemannan |
| CS | Chitosan |
| DeAC | Deacetylated acemannan |
| FD | Freeze-drying |
| IFD | Industrial freeze-drying |
| SD | Spray-drying |
| RWD | Refractance window drying |
| RZD | Radiant zone drying |
| SEM | Scanning electron microscopy |
| PDLCs | Periodontal ligament cells |
| PVA | Polyving akohol |
| LPS | Lipopolysaccharide |
| TCs | T cells |
| NO | Nitric oxide |
| INF-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| IL-1 | Interleukin-1 |
| HIV | Human immunodeficiency virus |
| FIV | Feline immunodeficiency virus |
| BRM | Biological response modulators |
| CVB3 | Coxsackievirus B3 |
| NDV | Newcastle disease virus |
| IBDV | Infectious bursal disease virus |
| PAG | processed Aloe vera gel |
| BMP-2 | Bone morphogentic protein-2 |
| MTA | Mineral trioxide aggregate |
| PDPCs | Primary human dental pulp cells |
| TLR-2 | Toll-like receptor 2 |
| COL-1 | Type I collagen |
| FC | Formocresol |
| BMSCs | Bone marrow mesenchymal stem cells |
| Runx2 | Runt-related transcription factor 2 |
| GDF-5 | Growth differentiation factor 5 |
| DPC | Direct pulp capping |
| CBCT | Cone-beam computed tomography |
| GBR | Guided bone regeneration |
| DBB | Deproteinized bovine bone |
| ISQ | Implant stability quotient |
| AV | Aloe vera |
| ALN | Alendronate |
| mSBI | Modified gingival sulcus bleeding index |
| GTR | Guided tissue regeneration |
| GAG | Glycosaminoglycan |
| ALG | Alginate |
| Col | Collagen |
| SA | Stearic acid |
| SLN | Solid lipid nanoparticles |
| AZT | Zidovudine |
| ACY | Acyclovir |
| CADD | Computer aided drug design |
| NOEL | No observed effect level |
References
- Zhang, X.; Williams, D. Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cao, D.; Ding, J.J. Recent advances in regenerative biomaterials. Regen. Biomater. 2022, 9, rbac098. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-assisted regenerative medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Sierra-García, G.D.; Castro-Ríos, R.; González-Horta, A.; Lara-Arias, J.; Chávez-Montes, A.J. Acemannan, an extracted polysaccharide from Aloe vera: A literature review. Nat. Prod. Commun. 2014, 9, 1934578X1400900836. [Google Scholar] [CrossRef]
- Esua, M.F.; Rauwald, J.-W. Novel bioactive maloyl glucans from Aloe vera gel: Isolation, structure elucidation and in vitro bioassays. Carbohydr. Res. 2006, 341, 355–364. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from Aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, J.R.; Rodríguez-González, V.M.; González-Laredo, R.F.; Eim, V.; González-Centeno, M.R.; Femenia, A. Effect of different drying procedures on the bioactive polysaccharide acemannan from Aloe vera (Aloe barbadensis Miller). Carbohydr. Polym. 2017, 168, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Miramon-Ortíz, D.A.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Goycoolea, F.M.; Lizardi-Mendoza, J. Acemannan Gels and Aerogels. Polymers 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Jales, S.T.L.; Barbosa, R.D.M.; da Silva, G.R.; Severino, P.; de Lima Moura, T.F.A. Natural polysaccharides from Aloe vera L. gel (Aloe barbadensis Miller): Processing techniques and analytical methods. In Polysaccharides: Properties and Applications; Inamuddin, M.I.A., Boddula, R., Altalhi, T., Eds.; John Wiley & Sons: New York, NY, USA, 2021; pp. 1–22. [Google Scholar]
- Zhang, Y.; Bao, Z.; Ye, X.; Xie, Z.; He, K.; Mergens, B.; Li, W.; Yatcilla, M.; Zheng, Q. Chemical Investigation of Major Constituents in Aloe vera Leaves and Several Commercial Aloe Juice Powders. J. AOAC Int. 2018, 101, 1741–1751. [Google Scholar] [CrossRef]
- Campestrini, L.; Silveira, J.; Duarte, M.; Koop, H.; Noseda, M. NMR and rheological study of Aloe barbadensis partially acetylated glucomannan. Carbohydr. Polym. 2013, 94, 511–519. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rosselló, C.; Rodríguez-González, V.; González-Laredo, R.; Gallegos-Infante, J.; Medina-Torres, L. Effect of different drying procedures on physicochemical properties and flow behavior of Aloe vera (Aloe barbadensis Miller) gel. LWT 2016, 74, 378–386. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Calderas, F.; Minjares, R.; Femenia, A.; Sánchez-Olivares, G.; Gónzalez-Laredo, F.; Santiago-Adame, R.; Ramirez-Nuñez, D.; Rodríguez-Ramírez, J.; Manero, O. Structure preservation of Aloe vera (barbadensis Miller) mucilage in a spray drying process. LWT 2016, 66, 93–100. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q.J. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part II. Fine structures of O-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, B.; Qi, X.-M.; Peng, F.; Yao, C.-L.; Sun, R.-C. Fractionation of bamboo hemicelluloses by graded saturated ammonium sulphate. Carbohydr. Polym. 2015, 129, 201–207. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Hu, X.; Guo, Q.; Kang, J.; Yada, R. Extraction, fractionation and physicochemical characterization of water-soluble polysaccharides from Artemisia sphaerocephala Krasch seed. Carbohydr. Polym. 2011, 86, 831–836. [Google Scholar] [CrossRef]
- Xing, J.-M.; Li, F.-F. Separation and purification of aloe polysaccharides by a combination of membrane ultrafiltration and aqueous two-phase extraction. Appl. Biochem. Biotechnol. 2009, 158, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.-M.; Li, F.-F. Purification of aloe polysaccharides by using aqueous two-phase extraction with desalination. Nat. Prod. Res. 2009, 23, 1424–1430. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rodríguez-González, V.M. Compositional and structural features of the main bioactive polysaccharides present in the Aloe vera plant. J. AOAC Int. 2018, 101, 1711–1719. [Google Scholar] [CrossRef]
- Ray, A.; Aswatha, S.M. An analysis of the influence of growth periods on physical appearance, and acemannan and elemental distribution of Aloe vera L. gel. Ind. Crop. Prod. 2013, 48, 36–42. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Enferadi, S.T. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. BMC Chem. 2012, 2, 33. [Google Scholar] [CrossRef]
- Kiran, P.; Rao, P.S. Development and characterization of reconstituted hydrogel from Aloe vera (Aloe barbadensis Miller) powder. J. Food Meas. Charact. 2016, 10, 411–424. [Google Scholar] [CrossRef]
- Shi, X.-D.; Nie, S.-P.; Yin, J.-Y.; Que, Z.-Q.; Zhang, L.-J.; Huang, X.-J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocoll. 2017, 73, 176–183. [Google Scholar] [CrossRef]
- Mandal, G.; Das, A.J. Structure of the glucomannan isolated from the leaves of Aloe barbadensis Miller. Carbohydr. Res. 1980, 87, 249–256. [Google Scholar] [CrossRef]
- Ahl, L.I.; Grace, O.M.; Pedersen, H.L.; Willats, W.G.T.; Jørgensen, B.; Rønsted, N. Analyses of Aloe Polysaccharides Using Carbohydrate Microarray Profiling. J. AOAC Int. 2018, 101, 1720–1728. [Google Scholar] [CrossRef]
- Trinh, H.A.; Le, B.; Pittayapat, P.; Thunyakitpisal, P. Indirect sinus augmentation with and without the addition of a biomaterial: A randomized controlled clinical trial. Implant. Dent. 2019, 28, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.J.D.; Chokboribal, J.; Pauwels, R.; Banlunara, W.; Sangvanich, P.; Jaroenporn, S.; Thunyakitpisal, P. Acemannan increased bone surface, bone volume, and bone density in a calvarial defect model in skeletally-mature rats. J. Dent. Sci. 2018, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Songsiripradubboon, S.; Banlunara, W.; Sangvanich, P.; Trairatvorakul, C.; Thunyakitpisal, P. Clinical, radiographic, and histologic analysis of the effects of acemannan used in direct pulp capping of human primary teeth: Short-term outcomes. Odontology 2015, 104, 329–337. [Google Scholar] [CrossRef]
- Jansisyanont, P.; Tiyapongprapan, S.; Chuenchompoonut, V.; Sangvanich, P.; Thunyakitpisal, P. The effect of acemannan sponges in post-extraction socket healing: A randomized trial. J. Oral Maxillofac. Surgery, Med. Pathol. 2016, 28, 105–110. [Google Scholar] [CrossRef]
- Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J. Periodontal Res. 2013, 49, 164–178. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, M.; Wang, T.; Chen, X.; Li, Q.; Zhao, X. Preparation of aloe polysaccharide/honey/PVA composite hydrogel: Antibacterial activity and promoting wound healing. Int. J. Biol. Macromol. 2022, 211, 249–258. [Google Scholar] [CrossRef]
- Susanto, C.; Wijaya, C.D.; Wijaya, E.P. The Effect of Acemannan Hydrogel on Collagen Expression in Gingival Tissue of Diabetes Mellitus Animal Model. Biosci. Med. J. Biomed. Transl. Res. 2022, 6, 2774–2778. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Silva, S.S.; Reis, R.L. Acemannan-based films: An improved approach envisioning biomedical applications. Mater. Res. Express 2019, 6, 095406. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Fernandes, E.M.; Ribeiro, A.R.; Ribeiro, A.P.; Silva, S.S.; Reis, R.L. Physicochemical features assessment of acemannan-based ternary blended films for biomedical purposes. Carbohydr. Polym. 2021, 257, 117601. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Broaders, K.E.; Cohen, J.A.; Beaudette, T.T.; Bachelder, E.M.; Fréchet, J.M.J. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc. Natl. Acad. Sci. USA 2009, 106, 5497–5502. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Biduski, B.; Prietto, L.; Castilhos, D.D.; Zavareze, E.D.R.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2017, 221, 1614–1620. [Google Scholar] [CrossRef]
- Wu, C.; Su, H.; Tang, S.; Bumgardner, J.D. The stabilization of electrospun chitosan nanofibers by reversible acylation. Cellulose 2014, 21, 2549–2556. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Zhang, Y.; Duan, L.; Zhou, C.; Ni, Y.; Liao, X.; Li, Q.; Hu, X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Zhao, M.; Qi, H. O-acetylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Int. J. Biol. Macromol. 2014, 69, 39–45. [Google Scholar] [CrossRef]
- Niu, Y.; Li, Q.; Xie, R.; Liu, S.; Wang, R.; Xing, P.; Shi, Y.; Wang, Y.; Dong, L.; Wang, C. Modulating the phenotype of host macrophages to enhance osteogenesis in MSC-laden hydrogels: Design of a glucomannan coating material. Biomaterials 2017, 139, 39–55. [Google Scholar] [CrossRef]
- Feng, Y.; Mu, R.; Wang, Z.; Xing, P.; Zhang, J.; Dong, L.; Wang, C. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat. Commun. 2019, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Lv, Z.; Ye, L.; Yang, Y.; Tang, Q. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food Chem. 2014, 143, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, X.; Zhang, J.; Duan, Y.; Wang, X.; Zhang, Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J. Biol. Macromol. 2012, 50, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Nasri, R.; Ben Amor, I.; Li, S.; Gargouri, J.; Nasri, M. Structural features, anti-coagulant and anti-adhesive potentials of blue crab (Portunus segnis) chitosan derivatives: Study of the effects of acetylation degree and molecular weight. Int. J. Biol. Macromol. 2020, 160, 593–601. [Google Scholar] [CrossRef]
- Yang, J.; Cai, J.; Wu, K.; Li, D.; Hu, Y.; Li, G.; Du, Y. Preparation, characterization and anticoagulant activity in vitro of heparin-like 6-carboxylchitin derivative. Int. J. Biol. Macromol. 2012, 50, 1158–1164. [Google Scholar] [CrossRef]
- Salah, F.; El Ghoul, Y.; Mahdhi, A.; Majdoub, H.; Jarroux, N.; Sakli, F. Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Ind. Crop. Prod. 2017, 103, 13–18. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Role of acemannan O-acetyl group in murine radioprotection. Carbohydr. Polym. 2019, 207, 460–470. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Luo, Y.; Lao, C.; Tong, X.; Du, J.; Huang, B.; Li, D.; Chen, J.; Ye, H.; et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr. Polym. 2022, 297, 120032. [Google Scholar] [CrossRef]
- Womble, D.; Helderman, J. The Impact of Acemannan on the Generation and Function of Cytotoxic T-Lymphocytes. Immunopharmacol. Immunotoxicol. 1992, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, M.K.; Yun, Y.-P.; Kim, Y.; Kim, J.S.; Kim, Y.S.; Kim, K.; Han, S.S.; Lee, C.-K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharmacol. 2001, 1, 1275–1284. [Google Scholar] [CrossRef]
- Muharraran, F.; Rusip, G.; Dalimunthe, R.P. Potential of Hydrogel Acemannan Aloe Vera (Aloe vera) on Wound Healing After Tooth Extraction In vivo Via Regulation of Inflammatory Response. Biosci. Med. J. Biomed. Transl. Res. 2022, 6, 1908–1913. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Magnusson, M.K.; Isaksson, S.; Larsson, F.; Öhman, L. Effects of Aloe barbadensis Mill. extract (AVH200®) on human blood T cell activity in vitro. J. Ethnopharmacol. 2016, 179, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.; Sharma, J.; Nordgren, R. Nitric oxide production by chicken macrophages activated by acemannan, a complex carbohydrate extracted from Aloe Vera. Int. J. Immunopharmacol. 1995, 17, 183–188. [Google Scholar] [CrossRef]
- Zhang, L.; Tizard, I.R. Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera gel. Immunopharmacology 1996, 35, 119–128. [Google Scholar] [CrossRef]
- Djeraba, A.; Quere, P. In vivo macrophage activation in chickens with Acemannan, a complex carbohydrate extracted from Aloe vera. Int. J. Immunopharmacol. 2000, 22, 365–372. [Google Scholar] [CrossRef]
- Egger, S.F.; Brown, G.S.; Kelsey, L.S.; Yates, K.M.; Rosenberg, L.J.; Talmadge, J.E. Studies on optimal dose and administration schedule of a hematopoietic stimulatory β-(1, 4)-linked mannan. Int. J. Immunopharmacol. 1996, 18, 113–126. [Google Scholar] [CrossRef]
- Egger, S.F.; Brown, G.S.; Kelsey, L.S.; Yates, K.M.; Rosenberg, L.J.; Talmadge, J.E. Hematopoietic augmentation by a β-(1, 4)-linked mannan. Cancer Immunol. Immunother. 1996, 43, 195–205. [Google Scholar] [CrossRef]
- Kumar, S.; Tiku, A.B. Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food Agric. Immunol. 2016, 27, 72–86. [Google Scholar] [CrossRef]
- Españo, E.; Kim, J.; Kim, J.-K. Utilization of Aloe Compounds in Combatting Viral Diseases. Pharmaceuticals 2022, 15, 599. [Google Scholar] [CrossRef]
- Kahlon, J.B.; Kemp, M.C.; Carpenter, R.H.; McAnalley, B.H.; McDaniel, H.R.; Shannon, W.M. Inhibition of AIDS virus replication by acemannan in vitro. Mol. Biother. 1991, 3, 127–135. [Google Scholar] [PubMed]
- Yates, K.; Rosenberg, L.; Harris, C.; Bronstad, D.; King, G.; Biehle, G.; Walker, B.; Ford, C.; Hall, J.; Tizard, I. Pilot study of the effect of acemannan in cats infected with feline immunodeficiency virus. Veter-Immunol. Immunopathol. 1992, 35, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sheets, M.; Unger, B.A.; Giggleman Jr, G.F.; Tizard, I. Studies of the effect of acemannan on retrovirus infections: Clinical stabilization of feline leukemia virus-infected cats. Mol. Biother. 1991, 3, 41–45. [Google Scholar] [PubMed]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Gauntt, C.J.; Wood, H.J.; McDaniel, H.R.; McAnalley, B.H. Aloe polymannose enhances anti-coxsackievirus antibody titres in mice. Phytotherapy Res. 2000, 14, 261–266. [Google Scholar] [CrossRef]
- Chinnah, A.D.; Baig, M.A.; Tizard, I.R.; Kemp, M.C. Antigen dependent adjuvant activity of a polydispersed β-(1, 4)-linked acetylated mannan (acemannan). Vaccine 1992, 10, 551–557. [Google Scholar] [CrossRef]
- Im, S.-A.; Oh, S.-T.; Song, S.; Kim, M.-R.; Kim, D.-S.; Woo, S.-S.; Jo, T.H.; Park, Y.I.; Lee, C.-K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Norman, J.; Curtin, G.; Corrier, D.; McDaniel, H.R.; Busbee, D. Decreased mortality of Norman murine sarcoma in mice treated with the immunomodulator, Acemannan. Mol. Biother. 1991, 3, 79–87. [Google Scholar] [PubMed]
- Im, S.-A.; Kim, J.-W.; Kim, H.-S.; Park, C.-S.; Shin, E.; Do, S.-G.; Park, Y.I.; Lee, C.-K. Prevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by processed Aloe vera gel. Int. Immunopharmacol. 2016, 40, 428–435. [Google Scholar] [CrossRef]
- Tong, X.; Lao, C.; Li, D.; Du, J.; Chen, J.; Xu, W.; Li, L.; Ye, H.; Guo, X.; Li, J. An acetylated mannan isolated from Aloe vera induce colorectal cancer cells apoptosis via mitochondrial pathway. Carbohydr. Polym. 2022, 291, 119464. [Google Scholar] [CrossRef]
- Thesleff, I.; Vaahtokari, A.; Vainio, S.; Jowett, A. Molecular mechanisms of cell and tissue interactions during early tooth development. Anat. Rec. Off. Publ. Am. Assoc. Anat. 1996, 245, 151–161. [Google Scholar] [CrossRef]
- Ruehle, M.A.; Krishnan, L.; Vantucci, C.E.; Wang, Y.; Stevens, H.Y.; Roy, K.; Guldberg, R.E.; Willett, N. Effects of BMP-2 dose and delivery of microvascular fragments on healing of bone defects with concomitant volumetric muscle loss. J. Orthop. Res. 2019, 37, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Jittapiromsak, N.; Jettanacheawchankit, S.; Lardungdee, P.; Sangvanich, P.; Thunyakitpisal, P.D. Effect of acemannan on BMP-2 expression in primary pulpal fibroblasts and periodontal fibroblasts, in vitro study. J. Oral Tissue Eng. 2007, 4, 149–154. [Google Scholar]
- Jittapiromsak, N.; Sahawat, D.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Acemannan, an Extracted Product from Aloe Vera, Stimulates Dental Pulp Cell Proliferation, Differentiation, Mineralization, and Dentin Formation. Tissue Eng. Part A 2010, 16, 1997–2006. [Google Scholar] [CrossRef]
- Songsiripradubboon, S.; Kladkaew, S.; Trairatvorakul, C.; Sangvanich, P.; Soontornvipart, K.; Banlunara, W.; Thunyakitpisal, P. Stimulation of Dentin Regeneration by Using Acemannan in Teeth with Lipopolysaccharide-induced Pulp Inflammation. J. Endod. 2017, 43, 1097–1103. [Google Scholar] [CrossRef]
- Sahawat, D.; Kanthasuwan, S.; Sangvanich, P.; Takata, T.; Kitagawa, M.; Thunyakitpisal, P. Acemannan induces cementoblast proliferation, differentiation, extracellular matrix secretion, and mineral deposition. J. Med. Plants Res. 2012, 6, 4069–4076. [Google Scholar]
- Boonyagul, S.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Effect of acemannan, an extracted polysaccharide from Aloe vera, on BMSCs proliferation, differentiation, extracellular matrix synthesis, mineralization, and bone formation in a tooth extraction model. Odontology 2014, 102, 310–317. [Google Scholar] [CrossRef]
- Xing, W.; Guo, W.; Zou, C.-H.; Fu, T.-T.; Li, X.-Y.; Zhu, M.; Qi, J.-H.; Song, J.; Dong, C.-H.; Li, Z.; et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J. Dermatol. Sci. 2015, 79, 101–109. [Google Scholar] [CrossRef]
- Thunyakitpisal, P.; Ruangpornvisuti, V.; Kengkwasing, P.; Chokboribal, J.; Sangvanich, P. Acemannan increases NF-κB/DNA binding and IL-6/-8 expression by selectively binding Toll-like receptor-5 in human gingival fibroblasts. Carbohydr. Polym. 2017, 161, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan Stimulates Gingival Fibroblast Proliferation; Expressions of Keratinocyte Growth Factor-1, Vascular Endothelial Growth Factor, and Type I Collagen; and Wound Healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef]
- Iacopetti, I.; Perazzi, A.; Martinello, T.; Gemignani, F.; Patruno, M. Hyaluronic acid, Manuka honey and Acemannan gel: Wound-specific applications for skin lesions. Res. Veter- Sci. 2020, 129, 82–89. [Google Scholar] [CrossRef]
- Islam, R.; Islam, M.R.R.; Tanaka, T.; Alam, M.K.; Ahmed, H.M.A.; Sano, H. Direct pulp capping procedures–Evidence and practice. Jpn. Dent. Sci. Rev. 2023, 59, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.F.; Sübay, R.K.; Ostro, E.; Suzuki, S.; Suzuki, S.H. Tunnel defects in dentin bridges: Their formation following direct pulp capping. Oper. Dent. 1996, 21, 4–11. [Google Scholar]
- Kogan, P.; He, J.; Glickman, G.N.; Watanabe, I. The Effects of Various Additives on Setting Properties of MTA. J. Endod. 2006, 32, 569–572. [Google Scholar] [CrossRef]
- Donnelly, A.; Foschi, F.; McCabe, P.; Duncan, H.F. Pulpotomy for treatment of complicated crown fractures in permanent teeth: A systematic review. Int. Endod. J. 2022, 55, 290–311. [Google Scholar] [CrossRef]
- Gonna, S.; Deraz, E.; Ghoname, N.; Kabbash, A.; Yagi, A. Acemannan and Formocresol Pulpotomies in Primary Teeth: A Comparative Histopathological Study. J. Gastroenterol. Hepatol. Res. 2017, 6, 2386–2391. [Google Scholar] [CrossRef]
- Vu, T.T.; Nguyen, M.T.; Sangvanich, P.; Nguyen, Q.N.; Thunyakitpisal, P. Acemannan Used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced Continued Root Formation. Pharmaceutics 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Nguyen, M.T.; Sangvanich, P.; Thunyakitpisal, P. Pulse Oximetry and Three-Dimensional Analysis in Evaluating Immature Permanent Teeth Apexogenesis: Two Case Reports. Open Dent. J. 2022, 16, e187421062112271. [Google Scholar] [CrossRef]
- Vu, N.B.; Chuenchompoonut, V.; Jansisyanont, P.; Sangvanich, P.; Pham, T.H.; Thunyakitpisal, P. Acemannan-induced tooth socket healing: A 12-month randomized controlled trial. J. Dent. Sci. 2021, 16, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Le Van, C.; Thu, H.P.T.; Sangvanich, P.; Chuenchompoonut, V.; Thunyakitpisal, P. Acemannan induces rapid early osseous defect healing after apical surgery: A 12-month follow-up of a randomized controlled trial. J. Dent. Sci. 2020, 15, 302–309. [Google Scholar] [CrossRef]
- Poor, M.R.; Hall, J.E.; Poor, A.S. Reduction in the incidence of alveolar osteitis in patients treated with the SaliCept patch, containing Acemannan hydrogel. J. Oral Maxillofac. Surg. 2002, 60, 374–379. [Google Scholar] [CrossRef]
- Otero, A.I.P.; Fernandes, J.C.H.; Borges, T.; Nassani, L.; Castilho, R.D.M.; Fernandes, G.V.D.O. Sinus Lift Associated with Leucocyte-Platelet-Rich Fibrin (Second Generation) for Bone Gain: A Systematic Review. J. Clin. Med. 2022, 11, 1888. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.A.; Dam, V.V.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Acemannan Induced Bone Regeneration in Lateral Sinus Augmentation Based on Cone Beam Computed Tomographic and Histopathological Evaluation. Case Rep. Dent. 2020, 2020, 1675653. [Google Scholar] [CrossRef] [PubMed]
- Amaral Valladão, C.A.; Freitas Monteiro, M.; Joly, J.C. Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts: A retrospective clinical study. Int. J. Implant. Dent. 2020, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Deesricharoenkiat, N.; Jansisyanont, P.; Chuenchompoonut, V.; Mattheos, N.; Thunyakitpisal, P. The effect of acemannan in implant placement with simultaneous guided bone regeneration in the aesthetic zone: A randomized controlled trial. Int. J. Oral Maxillofac. Surg. 2022, 51, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ipshita, S.; Kurian, I.G.; Dileep, P.; Kumar, S.; Singh, P.; Pradeep, A.R. One percent alendronate and aloe vera gel local host modulating agents in chronic periodontitis patients with class II furcation defects: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2018, 9, e12334. [Google Scholar] [CrossRef]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Chansamart, R.; Sangvanich, P.; Thunyakitpisal, P. Clinical and Radiographic Evaluation of Combined Acemannan and Periodontal Surgery Induced-Periodontal Regeneration: 5-Year Follow-up Case Report. Open Dent. J. 2023, 17, e187421062301231. [Google Scholar] [CrossRef]
- Bhalang, K.; Thunyakitpisal, P.; Rungsirisatean, N. Acemannan, a polysaccharide extracted from Aloe vera, is effective in the treatment of oral aphthous ulceration. J. Altern. Complement. Med. 2013, 19, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, A.; Subhani Sajjad, S. Aloe vera: An ancient herb for modern dentistry—A literature review. J. Dent. Surg. 2014, 2014, 210463. [Google Scholar] [CrossRef]
- Chithra, P.; Sajithlal, G.; Chandrakasan, G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J. Ethnopharmacol. 1998, 59, 179–186. [Google Scholar] [CrossRef]
- Thomas, D.R.; Goode, P.S.; LaMaster, K.; Tennyson, T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Adv. Wound Care J. Prev. Health 1998, 11, 273–276. [Google Scholar]
- Deora, N.; Venkatraman, K. Aloe vera in diabetic dyslipidemia: Improving blood glucose and lipoprotein levels in pre-clinical and clinical studies. J. Ayurveda Integr. Med. 2022, 13, 100675. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.H.; Kianbakht, S.; Hajiaghaee, R.; Afkhami, A.M.; Bonakdaran, A.; Hashem, D.F. Aloe vera leaf gel in treatment of advanced type 2 diabetes mellitus needing insulin therapy: A randomized double-blind placebo-controlled clinical trial. J. Med. Plants 2012, 11, 19–27. [Google Scholar]
- Huseini, H.F.; Kianbakht, S.; Hajiaghaee, R.; Dabaghian, F.H. Anti-hyperglycemic and Anti-hypercholesterolemic Effects of Aloe vera Leaf Gel in Hyperlipidemic Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Planta Medica 2011, 78, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Lozano, A.Y.; Domard, A.; Velázquez, C.A.; Goycoolea, F.M.; Argüelles-Monal, W.M. Physical properties and antibacterial activity of chitosan/acemannan mixed systems. Carbohydr. Polym. 2015, 115, 707–714. [Google Scholar] [CrossRef]
- Banerjee, D.; Bose, S. Effects of Aloe Vera Gel Extract in Doped Hydroxyapatite-Coated Titanium Implants on in Vivo and in Vitro Biological Properties. ACS Appl. Bio Mater. 2019, 2, 3194–3202. [Google Scholar] [CrossRef]
- Suciati, T.; Rachmawati, P.; Soraya, E.; Mahardhika, A.B.; Hartarti, R.; Anggadiredja, K. A novel acemannan-chitosan modified lipid nanoparticles as intracellular delivery vehicles of antibiotic. J. Appl. Pharm. Sci. 2018, 8, 001–011. [Google Scholar]
- Gofur, A.R.P.; Fajriyani, A.N.L.; Anggraeni, R.P.; Winias, S.J.B.; Archives, C. The potential combination of PLDSCs with acemannan in chitosan-composites scaffold for regeneration in defect Ameloblastoma. Biochem. Cell Arch. 2020, 20, 2979–2982. [Google Scholar]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Luna-Zapién, E.A.; Minjares-Fuentes, R.; Sierra-Campos, E.; Marszalek, J.E.; Barraza-Guerrero, S.I.; Meza-Velázquez, J.A. Conservation of commercial quality and bioactive compounds of guava pieces by application of an alginate-acemannan coating. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13080. [Google Scholar] [CrossRef]
- Huang, M.; Lou, C.; Wu, Z.; Li, W.; Lee, P.W.; Tang, Y.; Liu, G. In silico prediction of UGT-mediated metabolism in drug-like molecules via graph neural network. J. Cheminf. 2022, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, V.; Jacobson, K.A. In Silico Drug Design for Purinergic GPCRs: Overview on Molecular Dynamics Applied to Adenosine and P2Y Receptors. Biomolecules 2020, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Sularsih, S.; Mulawarmanti, D.; Rahmitasari, F.; Siswodihardjo, S. In Silico Analysis of Glycosaminoglycan-Acemannan as a Scaffold Material on Alveolar Bone Healing. Eur. J. Dent. 2022, 16, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kresnoadi, U.; Rahayu, R.P.; Rubianto, M.; Sudarmo, S.M.; Budi, H.S. TLR2 Signaling Pathway in Alveolar Bone Osteogenesis Induced by Aloe vera and Xenograft (XCB). Braz. Dent. J. 2017, 28, 281–286. [Google Scholar] [CrossRef]
- Anders, H.-J.; Schaefer, L. Beyond tissue injury—Damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1387–1400. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Andonegi, M.; Irastorza, A.; Izeta, A.; De La Caba, K.; Guerrero, P. Physicochemical and Biological Performance of Aloe Vera-Incorporated Native Collagen Films. Pharmaceutics 2020, 12, 1173. [Google Scholar] [CrossRef]
- Thant, A.A.; Ruangpornvisuti, V.; Sangvanich, P.; Banlunara, W.; Limcharoen, B.; Thunyakitpisal, P. Characterization of a bioscaffold containing polysaccharide acemannan and native collagen for pulp tissue regeneration. Int. J. Biol. Macromol. 2023, 225, 286–297. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Bharadwaj, R.; Ranganath, P.; Silverman, N.; Agrawal, G. Biodegradable disulfide crosslinked chitosan/stearic acid nanoparticles for dual drug delivery for colorectal cancer. Carbohydr. Polym. 2022, 294, 119833. [Google Scholar] [CrossRef]
- Pereira, P.; Barreira, M.; Cruz, C.; Tomás, J.; Luís, Â.; Pedro, A.Q.; Queiroz, J.A.; Sousa, F. Brain-targeted delivery of pre-miR-29b using lactoferrin-stearic acid-modified-chitosan/polyethyleneimine polyplexes. Pharmaceuticals 2020, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Shy, J.K.; Sharma, C.P.; Kalarikkal, N.; Sandeep, K.; Thomas, S.; Pothen, L.A. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with Aloe Vera in glioma cells. Mater. Sci. Eng. C 2016, 66, 40–50. [Google Scholar]
- Gomes, J.M.M. Biocompatible Ionic Liquids as Processing Tools for Biomaterials to Be Applied as Novel Therapeutic Platforms. Available online: https://repositorium.sdum.uminho.pt/handle/1822/83141 (accessed on 18 June 2023).
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mittal, P.; Yadav, A.; Mishra, A.K.; Hazari, P.P.; Sharma, R.K. Sustained activity of stimuli-responsive curcumin and acemannan based hydrogel patches in wound healing. ACS Appl. Bio Mater. 2022, 5, 598–609. [Google Scholar] [CrossRef]
- Pachimalla, P.R. An in vitro Study to Evaluate the Bioactivity of Osteoblast Cells on the Titanium Disk Coated with the Hydro Gel formulated from Acemannan and Curcuminoids. Int. J. Prosthodont. Restor. Dent. 2018, 8, 22–27. [Google Scholar]
- Chan, W.-H.; Wu, H.-Y.; Chang, W.H. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem. Toxicol. 2006, 44, 1362–1371. [Google Scholar] [CrossRef]
- Notoya, M.; Tsukamoto, Y.; Nishimura, H.; Woo, J.-T.; Nagai, K.; Lee, I.-S.; Hagiwara, H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur. J. Pharmacol. 2004, 485, 89–96. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, H.C. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998, 124, 1029–1040. [Google Scholar] [CrossRef]
- Ekambaram, R.; Saravanan, S.; Selvam, N.; Dharmalingam, S. Statistical optimization of novel acemannan polysaccharides assisted TiO2 nanorods based nanofibers for skin cancer application. Carbohydr. Polym. Technol. Appl. 2021, 2, 100048. [Google Scholar] [CrossRef]
- Pachimalla, P.R.; Mishra, S.K.; Chowdhary, R. Evaluation of hydrophilic gel made from Acemannan and Moringa oleifera in enhancing osseointegration of dental implants. A preliminary study in rabbits. J. Oral Biol. Craniofacial Res. 2020, 10, 13–19. [Google Scholar] [CrossRef]
- Yahya, F.A.; Perdana, N.R.G. Delight diabeto: Puding mangostana nata de aloe vera sebagai neutraceutical food untuk membantu mengontrol kadar gula darah pada penderita diabetes melitus. Majalahkesehatan 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Yates, D. Time-kill kinetics of a novel antimicrobial silver wound gel against select wound pathogens. Wounds 2015, 27, 336–346. [Google Scholar]
- Kahlon, J.B.; Kemp, M.C.; Yawei, N.; Carpenter, R.H.; Shannon, W.M.; McAnalley, B.H. In vitro evaluation of the synergistic antiviral effects of acemannan in combination with azidothymidine and acyclovir. Mol. Biother. 1991, 3, 214–223. [Google Scholar]
- Fogleman, R.W.; Chapdelaine, J.M.; Carpenter, R.H.; McAnalley, B.H. Toxicologic evaluation of injectable acemannan in the mouse, rat and dog. Veter- Hum. Toxicol. 1992, 34, 201–205. [Google Scholar]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of aloeandongensis extract, aloe andongensis leaf juice, aloe arborescens leaf extract, aloe arborescens leaf juice, aloe arborescens leaf protoplasts, aloe barbadensis flower extract, aloe barbadensis leaf, aloe barbadensis leaf extract, aloe barbadensis leaf juice, aloe barbadensis leaf polysaccharides, aloe barbadensis leaf water, aloe ferox leaf extract, aloe ferox leaf juice, and aloe ferox leaf juice extract. Int. J. Toxicol. 2007, 26, 1–50. [Google Scholar]
- López, Z.; Zúñiga, M.N.S.; Femenia, A.; Acevedo-Hernández, G.J.; Flores, J.A.G.; Cano, M.E.; Knauth, P. Dry but Not Humid Thermal Processing of Aloe vera Gel Promotes Cytotoxicity on Human Intestinal Cells HT-29. Foods 2022, 11, 745. [Google Scholar] [CrossRef]
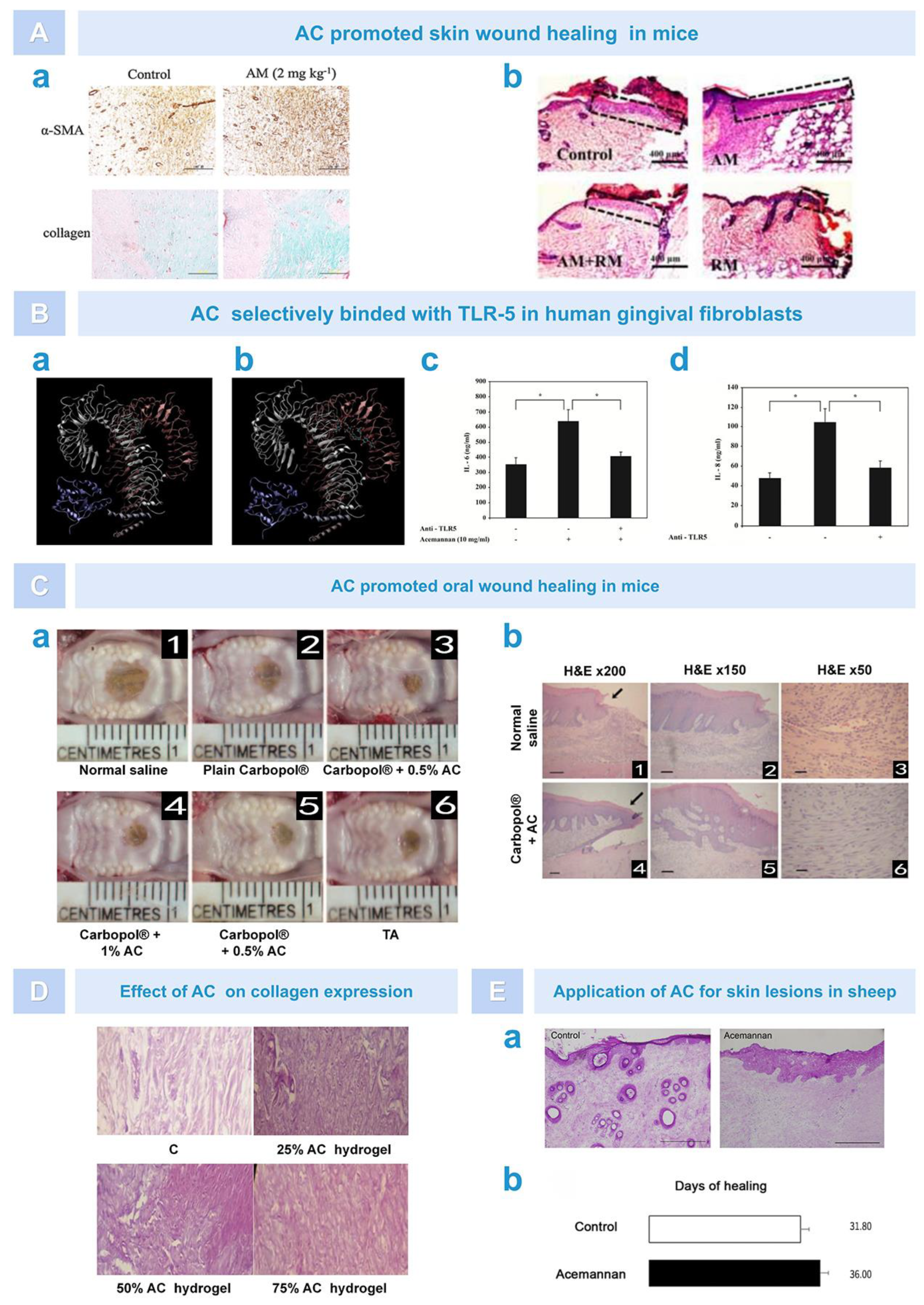
| Function | Source | Dose/Form | Cell/Animal | Results | Ref |
|---|---|---|---|---|---|
| Immunomodulation | Freeze-dried gel | 0.5% (solution) | T cells from human PBMC |
| [60] |
| Freeze-dried gel | 100 μg/mL (solution) | Immature dendritic cells (mouse) |
| [61] | |
| Fresh gel | 1–8 mg/mL (hydrogel) | Macrophage cells (rats) |
| [62] | |
| Freeze-dried gel | 0.5–5.0 mg/mL (gel) | T cells from human PBMC |
| [63] | |
| Fresh gel | 2 mg/mL (solution) | Splenocytes and macrophages (chicken) |
| [65] | |
| Fresh gel | 100 μg/mL (solution) | RAW 264.7 cells (mouse) |
| [66] | |
| Fresh gel | 500 μg/mL (solution) | Macrophage cells (chicken) |
| [67] | |
| Fresh gel | 1–2 mg/mL (solution) | Hematopoietic progenitors (mouse) |
| [68,69] | |
| Fresh gel | 50 mg/kg (pellet) | Splenocytes (mouse) |
| [70] | |
| Antiviral effect | Fresh gel | 31.25–62.5 mg/mL (solution) | CEM-SS1 and MT-2(2) cells |
| [72] |
| Fresh gel | 2–100 mg/kg (solution) | Lymphocytes (cats) |
| [73] | |
| Lyophilized powder | 0.5 mg/kg (solution) | Mouse |
| [76] | |
| Lyophilized powder | 0.1–0.5 mg/mL (solution) | Chicken |
| [77] | |
| Anti-tumor effect | Fresh gel | Solution | Macrophages (mouse) |
| [79] |
| Fresh gel | 200–400 mg/kg (gel) | Mouse |
| [80] | |
| Fresh gel | - | Mouse |
| [81] | |
| Regeneration of dental tissue | Fresh gel | 0.25–1 mg/mL (solution) | Periodontal fibroblasts and pulpal fibroblasts |
| [84] |
| Fresh gel | 1–8 mg/mL (sponge) | Dental pulp cells (rats) |
| [85] | |
| Fresh gel | 0.5–4 mg/mL (Sponge) | Dental pulpal cells (rats) |
| [86] | |
| Fresh gel | 0.5–8 mg/mL (solution) | Cementoblasts |
| [87] | |
| Bone formation | Fresh gel | 2–8 mg/mL (sponge) | BMSCs (rats) |
| [88] |
| Fresh gel | Sponge | Rats |
| [35] | |
| Fresh gel | 0.25–4 mg/mL (sponge) | PDLCs (dogs) |
| [38] | |
| Soft tissue healing | Fresh gel | 150 μg/mL (solution) | Skin fibroblasts (mouse) |
| [89] |
| Fresh gel | 0.01–10 mg/mL (solution) | Human gingival fibroblasts |
| [90] | |
| Fresh gel | 2–16 mg/mL (solution) | GFs (rats) |
| [91] | |
| Fresh gel | 25–75% (hydrogel) | Rats |
| [40] | |
| Fresh gel | Gel | Sheep |
| [92] |
| Clinical Application | Year | Application Field | Sample Size (Unit) | Follow-Up Time | Form/Dose | Control Group | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| Regeneration of pulp–dentin complex | 2022 | Apexogenesis | 2 (people) | 12 months | Sponge | - | Preserved pulp vitality and form apical stop. | [100] |
| 2020 | Pulpotomy (young permanent teeth) | 50 (tooth) | 12 months | Sponge | MTA | Induced continued root formation. | [99] | |
| 2017 | Pulpotomy (deciduous teeth) | 46 (tooth) | 12 weeks | Sponge | FC | Promoted dentin bridge formation. | [98] | |
| 2015 | DPC (deciduous teeth) | 42 (tooth) | 6 months | Sponge (0.4 mg) | calcium hydroxide | Promoted dentin bridge and soft tissue formation. | [36] | |
| Bone formation | 2023 | Periodontal surgery | 3 (tooth) | 5 years | Sponge | - | Reduced probing pocket depth, increased clinical attachment level, and bone density. | [110] |
| 2022 | GBR | 20 (people) | 6 months | Particle | DBB | Enhanced dimensional stability of the regenerated tissue. | [107] | |
| 2021 | Alveolar ridge preservation | 35 (people) | 12 months | Sponge (20, 50 mg) | Spontaneous blood-clotting | Reduced tooth socket volume. | [101] | |
| 2020 | Lateral sinus augmentation | 1 (people) | 6 months | Sponge (150 mg) | - | Increased bone height significantly. | [105] | |
| 2019 | Indirect sinus augmentation | 30 (people) | 6 months | Sponge (50 mg) | No-treatment control | Enhanced endo-sinus bone formation greatly. | [34] | |
| 2019 | Apical surgery | 22 (tooth) | 12 months | Sponge (5, 10 mg) | Spontaneous blood-clotting | Enhanced early bone healing. | [102] | |
| 2018 | Periodontitis with furcation defects | 90 (people) | 12 months | Gel | ALN | Improved periodontal pocket depth and attachment loss. | [108] | |
| 2016 | Alveolar ridge preservation | 99 (people) | 3 months | Sponge (50 mg) | Spontaneous blood-clotting | Increased the bone density and tooth socket healing. | [37] | |
| 2002 | Alveolar osteitis | 1194 (people) | 7 days | Hydrogel | Gelfoam | Reduced the incidence of alveolar osteitis. | [103] | |
| Treatment of skin and mucosal diseases | 2013 | Aphthous ulcer | 100 (people) | 7 days | Gel (0.5%) | Triamcinolone | Reduced ulcer size and pain. | [111] |
| 1998 | Pressure ulcers | 30 (people) | 10 weeks | Hydrogel | Moist saline gauze | Promoted ulcer healing. | [114] | |
| Improvement of blood sugar and lipids | 2011 | Advanced Type 2 Diabetes | 35 (people) | 2 months | Capsule (300 mg) | Placebo capsules | Lowered the blood levels of fasting glucose and glycosylated hemoglobin significantly. | [116] |
| 2011 | Hyperlipidemic Type 2 Diabetes | 67 (people) | 2 months | Capsule (300 mg) | Placebo capsules | Lowered the fasting blood glucose, HbA1c, total cholesterol, and LDL levels significantly. | [117] |
| Drug | Application Field | Application Form | Result/Function | Ref |
|---|---|---|---|---|
| AC + CS | Wound healing | Hydrogel | Weakened the mechanical strength and biological activity of the CS gel with increasing AC. | [118] |
| AC + CS | Wound healing | Film | Resulted in strong synergistic effects and leaded to mixed junction zones formation. | [41] |
| AC + CS | Osseointegration | Solution | Improved osseointegration with a seamless implant interface. | [119] |
| AC + CS | Carrier modification | Solution | Enhanced drug loading capacity. | [120] |
| AC + CS | Tissue regeneration | Scaffold | Repaired tissue defects caused by ameloblastoma. | [121] |
| AC + ALG | Wound healing | Film | Leaded to more resistant and stable structures. | [41] |
| AC + ALG | Antioxidation | Film | Enhanced the antioxidant capacity. | [123] |
| AC + ALG + CS | Wound healing | Film | Retained and created a moist environment around the wound to promote its healing. | [42] |
| AC + GAG | Bone healing | Scaffold | Binded with a TLR-2 target receptor. | [126] |
| Ac + COL | Pulp regeneration | Scaffold | Increased expression of dentin extracellular matrix proteins. | [132] |
| AC + SA | Carrier modification | Nanoparticle | Improved hydrophilicity to enhance drug absorption. | [135] |
| AC + Caffeate + ALG | Treatment of osteoarthritis | Bead | Promoted ATDC5 chondrocyte-like cell growth and cartilage-like extracellular matrix formation. | [136] |
| AC + Curcumin | Wound healing | Hydrogel | Reduced wound healing days greatly. | [138] |
| AC + Curcumin | Osseointegration | Hydrogel | Inhibited osteoblast differentiation. | [139] |
| AC + Resveratrol | Treatment of skin cancer | Scaffold | Inhibited the growth of A431 skin cancer cells and skin pathogens (e.g., Staphylococcus aureus and Pseudomonas aeruginosa). | [143] |
| AC + Moringa oleifera | Osseointegration | Hydrophilic gel | Increased bone contact with implant. | [144] |
| AC + Silver salt | Wound healing | Gel | Showed broad-spectrum antimicrobial activity. | [146] |
| AC + Honey | Wound healing | Hydrogel | Inhibited the growth of Staphylococcus aureus, Escherichia coli, and candida albicans. | [39] |
| AC + AZT or ACY | Antiviral | - | Inhibited the replication of HIV-1 and HSV-1. | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Niu, Y.; Qin, S.; Ma, G. A New Biomaterial Derived from Aloe vera—Acemannan from Basic Studies to Clinical Application. Pharmaceutics 2023, 15, 1913. https://doi.org/10.3390/pharmaceutics15071913
Bai Y, Niu Y, Qin S, Ma G. A New Biomaterial Derived from Aloe vera—Acemannan from Basic Studies to Clinical Application. Pharmaceutics. 2023; 15(7):1913. https://doi.org/10.3390/pharmaceutics15071913
Chicago/Turabian StyleBai, Yingjie, Yimeng Niu, Shengao Qin, and Guowu Ma. 2023. "A New Biomaterial Derived from Aloe vera—Acemannan from Basic Studies to Clinical Application" Pharmaceutics 15, no. 7: 1913. https://doi.org/10.3390/pharmaceutics15071913
APA StyleBai, Y., Niu, Y., Qin, S., & Ma, G. (2023). A New Biomaterial Derived from Aloe vera—Acemannan from Basic Studies to Clinical Application. Pharmaceutics, 15(7), 1913. https://doi.org/10.3390/pharmaceutics15071913





