Exploring the Enhanced Antiproliferative Activity of Turmeric Oil and 6-Mercaptopurine in a Combined Nano-Particulate System Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of Turmeric Oil Based SNEDDS
2.3. Characterization of the Prepared SNEDDS Formulations
2.4. Solubility of 6-MP in the Prepared SNEDDS Formulations
2.5. Evaluation of Cell Viability of the Treated HepG2 and MCF-7
2.6. Assessment of Apoptosis via Annexin V
2.7. Assessment of Cell Cycle
2.8. Statistical Analysis
3. Results and Discussion
3.1. Development and Characterization of Turmeric Oil-Based SNEDDS
3.2. Effect of the Prepared Formulation on the Anti-Tumor Activity and Cell Morphology
3.3. Apoptosis of HepG2 Treated with Pure 6-MP and SNEDDS Formulations
3.4. Cell Cycle Analysis of HepG2 Treated with Pure 6-MP and SNEDDS Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orellana-Paucar, A.M.; Machado-Orellana, M.G. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules 2022, 27, 5055. [Google Scholar] [CrossRef] [PubMed]
- Kuttan, R.; Liju, V.B.; Jeena, K. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Barthwal, M.K.; Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Prakash, P.; Malasoni, R.; Dwivedi, A.K.; Dikshit, M. Curcuma oil ameliorates insulin resistance & associated thrombotic complications in hamster & rat. Indian J. Med. Res. 2015, 141, 823–832. [Google Scholar] [CrossRef]
- Hastak, K.; Lubri, N.; Jakhi, S.; More, C.; John, A.; Ghaisas, S.; Bhide, S. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997, 116, 265–269. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Chemopreventive Activity of Turmeric Essential Oil and Possible Mechanisms of Action. Asian Pac. J. Cancer Prev. 2014, 15, 6575–6580. [Google Scholar] [CrossRef]
- Zan, X.J.; Rong, D.Y.; Tu, Y.H.; Xue, Y.C.; Ye, Z.Y.; Kang, Y.Q.; Zhou, Y.; Cao, Y. Effect of turmeric volatile oil on proliferation and apoptosis of human skin SCC A431 cells. China J. Chin. Mater. Medica 2016, 41, 2883–2887. [Google Scholar]
- Wang, S.; Li, Y.; Li, W.; Zhang, K.; Yuan, Z.; Cai, Y.; Xu, K.; Zhou, J.; Du, Z. Curcuma oil ameliorates benign prostatic hyperplasia through suppression of the nuclear factor-kappa B signaling pathway in rats. J. Ethnopharmacol. 2021, 279, 113703. [Google Scholar] [CrossRef]
- Villegas, I.; Sánchez-Fidalgo, S.; de la Lastra, C.A. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1040–1061. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Elgemeie, G. Thioguanine, Mercaptopurine: Their Analogs and Nucleosides as Antimetabolites. Curr. Pharm. Des. 2005, 9, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, A.A.; Marchetti-Laurent, C.; Poindessous, V.; Antonio, S.; Laurent-Puig, P.; Bortoli, S.; Loriot, M.-A.; Pallet, N. 6-mercaptopurine promotes energetic failure in proliferating T cells. Oncotarget 2017, 8, 43048–43060. [Google Scholar] [CrossRef] [PubMed]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.S.; Lee, J.; Kotov, N.A. Gold Nanoparticles Enhance the Anti-Leukemia Action of a 6-Mercaptopurine Chemotherapeutic Agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Yen, C.-C.; Hsu, M.-C.; Wu, Y.-T. Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin. Molecules 2020, 25, 3119. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Felimban, R.I.; Tayeb, H.H.; Rizg, W.Y.; Alnadwi, F.H.; Alotaibi, H.A.; Alhakamy, N.A.; Abd-Allah, F.I.; Mohamed, G.A.; Zidan, A.S.; et al. Development of Multi-Compartment 3D-Printed Tablets Loaded with Self-Nanoemulsified Formulations of Various Drugs: A New Strategy for Personalized Medicine. Pharmaceutics 2021, 13, 1733. [Google Scholar] [CrossRef]
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Ali, E.M.; Elashkar, A.A.; El-Kassas, H.Y.; Salim, E.I. Methotrexate loaded on magnetite iron nanoparticles coated with chitosan: Biosynthesis, characterization, and impact on human breast cancer MCF-7 cell line. Int. J. Biol. Macromol. 2018, 120, 1170–1180. [Google Scholar] [CrossRef]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC—Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [CrossRef]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef]
- Gaylord Chemicals Company LLC. Dimethyl Sulfoxide (DMSO) Health and Safety; Gaylord Chemicals Company LLC: Slidell, LA, USA, 2010. [Google Scholar]
- de Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, In Vitro and In Vivo Assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Chavda, H.; Patel, J.; Chavada, G.; Dave, S.; Patel, A.; Patel, C. Self-Nanoemulsifying Powder of Isotretinoin: Preparation and Characterization. J. Powder Technol. 2013, 2013, 108569. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Alay, A.M.S.; Okbazghi, S.Z.; Alhakamy, N.A. Two-Step Optimization to Develop a Transdermal Film Loaded with Dapoxetine Nanoparticles: A Promising Technique to Improve Drug Skin Permeation. Dose-Response 2020, 18, 1559325820923859. [Google Scholar] [CrossRef]
- Yu, C.; Li, C.; Pan, H.; Li, T.; He, S. Preparation of 2-Methoxyestradiol Self-emulsified Drug Delivery System and the Effect on Combination Therapy with Doxorubicin Against MCF-7/ADM Cells. AAPS PharmSciTech 2022, 23, 147. Available online: https://pubmed.ncbi.nlm.nih.gov/35585431/ (accessed on 24 June 2023). [CrossRef]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef]
- Mohammed, F.; Rashid-Doubell, F.; Taha, S.; Cassidy, S.; Fredericks, S. Effects of curcumin complexes on MDA-MB-231 breast cancer cell proliferation. Int. J. Oncol. 2020, 57, 445–455. [Google Scholar] [CrossRef]
- Jacob, J.N.; Toloue, M. Biological studies of turmeric oil, part 1: Selective in vitro anticancer activity of turmeric oil (TO) and TO-paclitaxel combination. Nat. Prod. Commun. 2013, 8, 807–810. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.; Wu, W.; Long, Z.; Xiao, H.; Luo, F.; Shen, Y.; Lin, Q. Elaboration of curcumin-loaded rice bran albumin nanoparticles formulation with increased in vitro bioactivity and in vivo bioavailability. Food Hydrocoll. 2018, 77, 834–842. [Google Scholar] [CrossRef]
- Shao, Z.-M.; Shen, Z.-Z.; Liu, C.-H.; Sartippour, M.R.; Go, V.L.; Heber, D.; Nguyen, M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer 2002, 98, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-I.; Ke, Y.-F.; Ko, Y.-C.; Lin, J.-K. Curcumin Inhibits SK-Hep-1 Hepatocellular Carcinoma Cell Invasion in vitro and Suppresses Matrix Metalloproteinase-9 Secretion. Oncology 1998, 55, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef]
- Jamal, A.; Asseri, A.H.; Ali, E.M.M.; El-Gowily, A.H.; Khan, M.I.; Hosawi, S.; Alsolami, R.; Ahmed, T.A. Preparation of 6-Mercaptopurine Loaded Liposomal Formulation for Enhanced Cytotoxic Response in Cancer Cells. Nanomaterials 2022, 12, 4029. [Google Scholar] [CrossRef]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kay, N.E.; Secreto, C.R.; Shanafelt, T.D. Curcumin Inhibits Prosurvival Pathways in Chronic Lymphocytic Leukemia B Cells and May Overcome Their Stromal Protection in Combination with EGCG. Clin. Cancer Res. 2009, 15, 1250–1258. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Lai, C.-W.; Shen, C.-J.; Lai, Y.-W.; Chen, C.-M. Curcumin reduces pulmonary tumorigenesis in vascular endothelial growth factor (VEGF)-overexpressing transgenic mice. Mol. Nutr. Food Res. 2011, 55, 1036–1043. [Google Scholar] [CrossRef]
- Cao, J.; Jia, L.; Zhou, H.-M.; Liu, Y.; Zhong, L.-F. Mitochondrial and Nuclear DNA Damage Induced by Curcumin in Human Hepatoma G2 Cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Chen, J.; Shi, Y.; Jia, J.; Zhang, Y. Curcumin-induced histone hypoacetylation: The role of reactive oxygen species. Biochem. Pharmacol. 2005, 69, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Aggarwal, B.B.; Bishayee, A. Curcumin and Liver Cancer: A Review. Curr. Pharm. Biotechnol. 2011, 13, 218–228. [Google Scholar] [CrossRef] [PubMed]



| Run | Composition | Characterization | |||||
|---|---|---|---|---|---|---|---|
| TO | Tween 80 | DMSO | Maximum Solubility | Particle Size | PDI | Zeta Potential | |
| (w/v) | (w/v) | (w/v) | (mg/mL) | (nm) | (mV) | ||
| F1 | 15 | 10 | 75 | 20 | 303.6 ± 19.3 | 0.431 | −12.35 ± 0.12 |
| F2 | 25 | 10 | 65 | 11.11 | 333.5 ± 9.7 * | 0.429 | −27.70 ± 0.46 * |
| F3 | 35 | 10 | 55 | 9.09 | 360.3 ± 6.2 * | 0.692 | −30.05 ± 0.07 * |
| F4 | 15 | 20 | 65 | 19.4 | 319.2 ± 39.3 | 0.662 | −12.35 ± 0.15 |
| F5 | 25 | 20 | 55 | 9.02 | 399.3 ± 4.0 * | 0.461 | −25.19 ± 0.16 * |
| F6 | 35 | 20 | 45 | 7.69 | 425.7 ± 7.4 * | 0.401 | −34.10 ± 0.06 * |
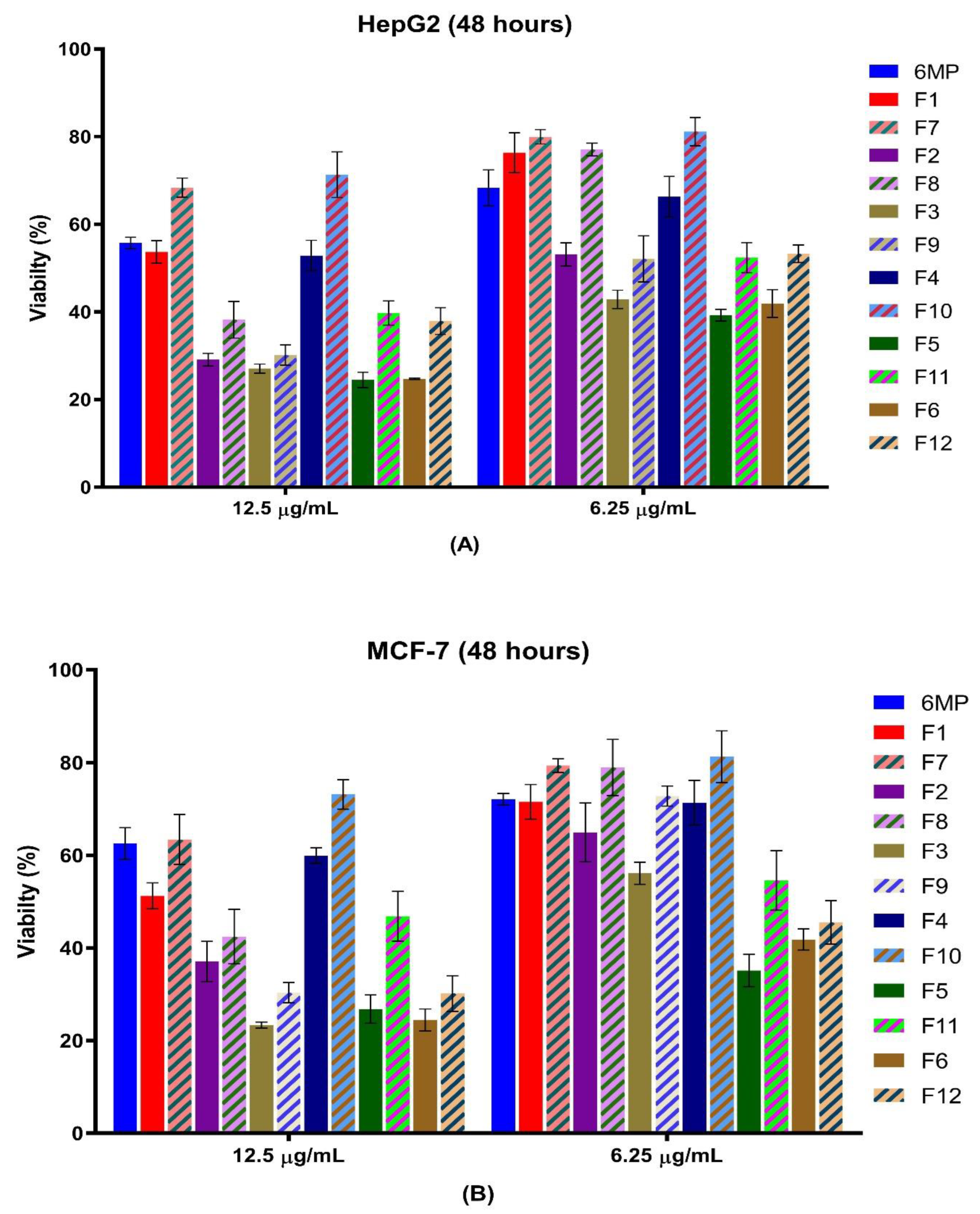
| Code | HepG2 Cell Line | MCF-7 Cell Line | ||||||
|---|---|---|---|---|---|---|---|---|
| 12.5 µg/mL | p-Value # | 6.25 µg/mL | p-Value # | 12.5 µg/mL | p-Value # | 6.25 µg/mL | p-Value # | |
| 6-MP | 55.70 ± 1.33 | 68.3 ± 4.09 | 62.53 ± 3.4 | 72.13 ± 1.2 | ||||
| F1 | 53.68 ± 2.58 | 0.001715 * | 76.32 ± 4.54 | 0.262352 | 51.24 ± 2.78 | 0.025611 * | 71.53 ± 3.75 | 0.028023 * |
| F2 | 29.08 ± 1.44 | 0.023803 * | 53.11 ± 2.64 | 0.000162 * | 37.10 ± 4.33 | 0.274345 | 64.93 ± 6.34 | 0.050307 |
| F3 | 27.03 ± 1.05 | 0.100611 | 42.8 ± 2.07 | 0.047353 * | 23.36 ± 0.66 | 0.006331 * | 56.11 ± 2.37 | 0.000836 * |
| F4 | 52.84 ± 3.46 | 0.006941 * | 66.28 ± 4.64 | 0.010462 * | 59.94 ± 1.67 | 0.003056 * | 71.36 ± 4.8 | 0.080496 |
| F5 | 24.50 ± 1.75 | 0.001281 * | 39.2 ± 1.34 | 0.003569 * | 26.78 ± 3.07 | 0.00497 * | 35.13 ± 3.47 | 0.010065 * |
| F6 | 24.71 ± 0.17 | 0.001767 * | 41.91 ± 3.17 | 0.006454 * | 24.46 ± 2.36 | 0.095437 * | 41.79 ± 2.29 | 0.286597 |
| F7 | 68.32 ± 2.20 | 79.32 ± 1.63 | 63.42 ± 5.4 | 79.36 ± 1.45 | ||||
| F8 | 38.20 ± 4.21 | 77.1 ± 1.45 | 42.43 ± 5.87 | 78.95 ± 6.06 | ||||
| F9 | 30.17 ± 2.32 | 52.07 ± 5.26 | 30.31 ± 2.20 | 72.8 ± 2.16 | ||||
| F10 | 71.30 ± 5.22 | 81.11 ± 3.23 | 73.45 ± 3.16 | 81.28 ± 5.59 | ||||
| F11 | 39.75 ± 2.75 | 52.37 ± 3.45 | 46.84 ± 5.37 | 54.55 ± 6.45 | ||||
| F12 | 37.89 ± 3.07 | 53.23 ± 2.00 | 30.144 ± 3.85 | 45.5 ± 4.67 | ||||
| Treatment | % Viable Cells | % Necrosis | % Early Apoptosis | % of Late Apoptosis | Fold of Changes in Late Apoptosis vs. Control | % Total Apoptosis |
|---|---|---|---|---|---|---|
| Control | 82.3 | 1.6 | 0.3 | 15.8 | 16.1 | |
| 6-MP | 36.5 | 0.7 | 0.3 | 62.4 | 3.95 | 62.7 |
| F1 | 26.2 | 1.9 | 1.9 | 70.1 | 4.43 | 72.0 |
| F4 | 48.1 | 1.2 | 1.7 | 49.0 | 3.1 | 50.7 |
| F7 | 56.1 | 0.9 | 1.7 | 41.4 | 2.6 | 43.1 |
| F10 | 67.6 | 1.1 | 1.8 | 29.5 | 1.87 | 31.3 |
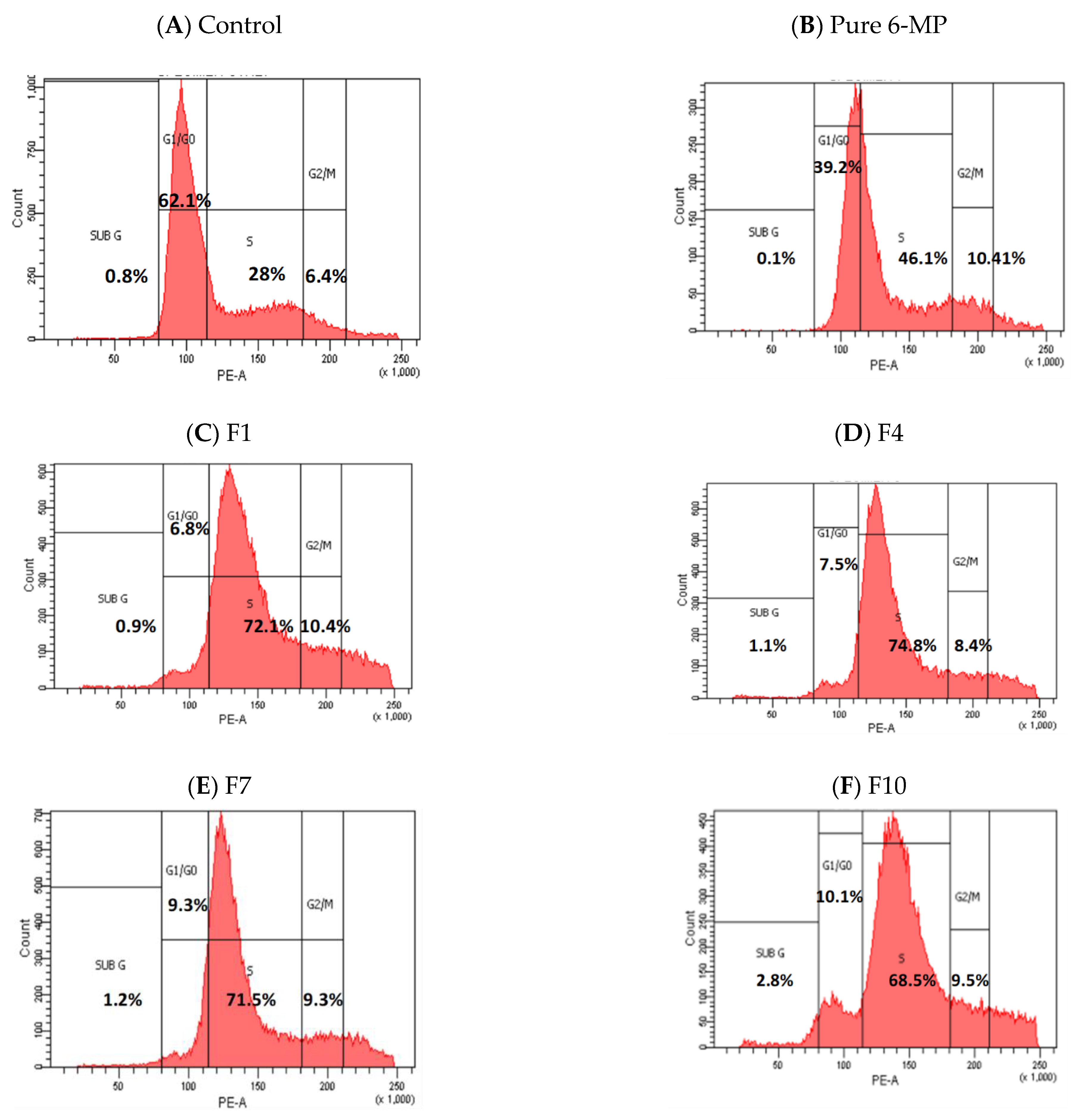
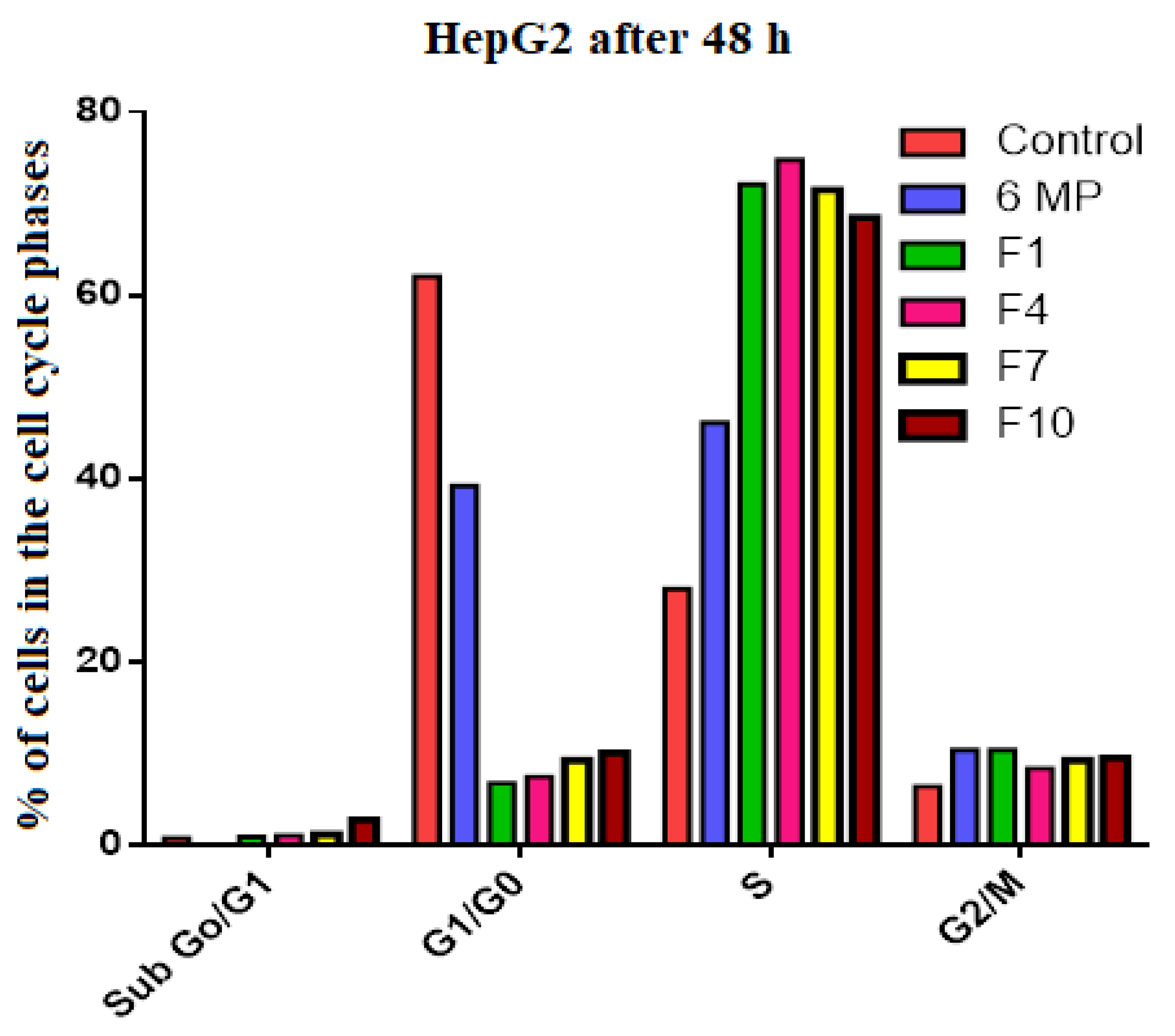
| Control | 6-MP | F1 | F4 | F7 | F10 | |
|---|---|---|---|---|---|---|
| Sub G1 | 0.8 | 0.1 | 0.9 | 1.1 | 1.2 | 2.8 |
| G1/G0 | 62.1 | 39.2 | 6.8 | 7.5 | 9.3 | 10.1 |
| S | 28.0 | 46.1 | 72.1 | 74.8 | 71.5 | 68.5 |
| G2/M | 6.4 | 10.4 | 10.4 | 8.4 | 9.3 | 9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, T.A.; Ali, E.M.M.; Kalantan, A.A.; Almehmady, A.M.; El-Say, K.M. Exploring the Enhanced Antiproliferative Activity of Turmeric Oil and 6-Mercaptopurine in a Combined Nano-Particulate System Formulation. Pharmaceutics 2023, 15, 1901. https://doi.org/10.3390/pharmaceutics15071901
Ahmed TA, Ali EMM, Kalantan AA, Almehmady AM, El-Say KM. Exploring the Enhanced Antiproliferative Activity of Turmeric Oil and 6-Mercaptopurine in a Combined Nano-Particulate System Formulation. Pharmaceutics. 2023; 15(7):1901. https://doi.org/10.3390/pharmaceutics15071901
Chicago/Turabian StyleAhmed, Tarek A., Ehab M. M. Ali, Abdulaziz A. Kalantan, Alshaimaa M. Almehmady, and Khalid M. El-Say. 2023. "Exploring the Enhanced Antiproliferative Activity of Turmeric Oil and 6-Mercaptopurine in a Combined Nano-Particulate System Formulation" Pharmaceutics 15, no. 7: 1901. https://doi.org/10.3390/pharmaceutics15071901
APA StyleAhmed, T. A., Ali, E. M. M., Kalantan, A. A., Almehmady, A. M., & El-Say, K. M. (2023). Exploring the Enhanced Antiproliferative Activity of Turmeric Oil and 6-Mercaptopurine in a Combined Nano-Particulate System Formulation. Pharmaceutics, 15(7), 1901. https://doi.org/10.3390/pharmaceutics15071901








